ABSTRACT
The volatile compounds of 10 Piper species cultivated in Hainan Island, China, have been investigated. Eighty compounds were profiled after headspace–solid phase microextraction (HS–SPME) with gas chromatography–mass spectrometry (GC–MS). Mean Bray–Curtis similarity value was only 22.78 ± 1.98% among the different Piper species. The volatile compounds were largely grouped as hydrocarbons, aldehydes, alcohols, acids, ketones, esters, and phenols. The main compounds comprised benzaldehyde, cinnamaldehyde, β-caryophyllene, ocimene, lavandulol, myrcene, cubebene, terpinene, linalool, α-caryophyllene, β-elemene, and germacrene. Principal component analysis revealed that Piper laetispicum, Piper longum, Piper hainanense, Piper betle, and Piper flaviflorum were characterized by high contents of β-caryophyllene, α-caryophyllene, germacrene, and β-pinene. Piper puberulum and Piper cathayanum were associated with high contents of linalool, myrcene, and germacrene D. On the other hand, Piper pseudofuligineum and Piper retrofractum were related with high contents of ocimene. Finally, Piper auritum was associated with high content of cinnamaldehyde. Volatile profiling of Piper species by HS–SPME–GC–MS and the interrelationship investigated among the volatiles can be used as a roadmap for future resource utilization or biotechnological applications.
Introduction
Piper is the largest genus in the family Piperaceae, which is distributed throughout the tropical regions, with major concentrations occurring in Central and South Americas, Caribbean, Africa, Asia, and some Pacific Islands.[Citation1,Citation2] The aroma emitted by its leaf/fruit/root has caused Piper species to be widely utilized as flavoring ingredient in cooking.[Citation3–Citation5] Among Piper species, Piper nigrum is characterized as the “king of spice” and is incredibly popular since ancient times; its yields can reach 461,452 t, and the corresponding profits may exceed 100 billion dollars.[Citation6] Piper laetispicum, P. nigrum, Piper tuberculatum, Piper retrofractum, Piper longum, Piper hainanense, and Piper puberulum have been applied in folk medicine for invigorating the circulation, reducing stasis, detumescence, and as analgesics.[Citation7] In accordance with the “Flora of China[Citation8],” many Piper species, such as Piper betle, P. hainanense, Piper hancei, and Piper Yunnanense, present high economical and ethnobotanical benefits given their use as spices, vegetation, fragrance, and traditional medicines.
Aroma is one of the essential components of spice and medicine quality. Aroma is also one of the essential factors that attract consumers. Compared with other spices, such as saffron[Citation9], chili peppers[Citation10], and thyme[Citation11], several studies have focused on volatile aroma compounds in Piper species although some investigations have shown that Piper species are remarkably rich in volatile compounds, of which more than 270 compounds have been identified.[Citation12,Citation13] However, most of such studies only focused on the analysis of volatile compounds released from Piper nigrum.[Citation14,Citation15] Limonene, α-pinene, d-3-carene, β-pinene, 4-carene, terpinolene, α-copaene, β-caryophyllene, α-caryophyllene, and d-elemene have been identified as important compounds to the overall flavor of Piper nigrum and its essential oil.[Citation14–Citation18] Recently, several studies reported that aroma is specific to species and exhibits unique potential flavor characteristics.[Citation19,Citation20] Topul et al.[Citation21] concluded that Piper aduncum contains significantly higher amounts of dillapiole than Piper gibbilimbum species, whereas P. gibbilimbum contains more gibbilimbols than P. aduncum.
In this study, headspace–solid phase microextraction (HS–SPME) combined with gas chromatography–mass spectrometry (GC–MS) was applied to study the characteristic volatiles of 10 Piper species for food and flavor. This study essentially focused on comparing aroma composition and content, particularly among Piper species that have not been extensively studied. These plants include Chinese local species and a number of species from other countries in order to acquire information for further breeding programs, cultivation, and finding new food sources.
Materials and methods
Plant materials
All fresh Piper leaves were obtained from the Germplasm Repository of Black Pepper of the Spice and Beverage Research Institute, CATAS, Hainan Province, China. Approximately 20 g of raw leaves was collected at 8:00–10:00 a.m., onAugust 8–10, 2016, depending on 10 different Piper species, P. betle, P. auritum, P. retrofractum, P. hainanense, Piper pseudofuligineum, P. laetispicum, Piper flaviflorum, Piper cathayanum, P. puberulum, and P. longum. Following collections, the leaves were moisturized and transported to the laboratory immediately. In all experiments, leaves were sliced by using a knife into similarly thin slices to enable their placement in the headspace bottle (volume: ~5 mL).
Extraction of volatile compounds
HS–SPME was used to extract the volatile compounds and evaluate their concentrations. For each extraction sample, 1.5 g of leaf slices was weighted and placed in a 5-mL capped SPME vial and allowed to stand for 30 min at ambient room temperature ([22 ± 3]°C). Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS) fibers were applied in this study for assaying, and film thickness was 50/30 μm (Supelco, Bellefonte, PA, USA). Through manual penetration of the silicone septum, SPME products can be inserted into the vial. Then, after 40 min, the fibers will be exposed to the headspace of sliced leaves. After extraction, the operator of SPME device will set the needle to 0.5 cm for GC injector and then desorbed the fiber for 10 min. During the experiment, each step of sampling or analysis was finished in triplicate. This study also used empty bottles as control for analysis.
GC–MS conditions
Applications of 7890A/5975C GC–MS system (Agilent Technologies, USA), coupled with a DB-5MS column, were carried out under the following conditions: temperature of line heater of MS transfer of 280°C, injector temperature of 250°C, and those of corresponding operations should be carried out in split-less mode. The initial temperature of the oven should be set at 50°C and kept for 5 min. Afterward, the temperature was increased as follows: 50–80°C at the rate of 10°C/min, 80–100°C at the rate of 2°C/min, 100–140°C at the rate of 8°C/min, 140–200°C at the rate of 6°C/min, and 200–280°C at the rate of 15°C/min. Helium was applied as carrier gas at a flow rate of 1.0 mL/min. The mass spectrometer Agilent 5975C was operated at 70 eV in electron ionization mode. Source temperature was set to 230°C, the quadrupole temperature was set to 150°C, and the mass range m/z 30–500 amu was conducted in a full-scan mode.
Volatile compounds were identified according to their linear retention indices (LRIs) and by comparing their mass spectra with the MS database via the NIST 2014 library, Volatile Compounds in Food 15.1, and Pherobase (database of pheromones and semi-chemicals). We also compared the fragmentation patterns in the mass spectra with those reported in the literature. LRIs were determined via alkane series (C7–C30). Compounds were quantified as area percentages of total volatiles.
Statistical analysis
Bray–Curtis similarity (BCS), an indicator of the composition similarity between different species, was applied to identify whether these species were the same. This index not only incorporates the existence of components but also incorporates their relative contents. Analysis of volatile compounds verified in the 10 species of Piper was carried out by principal component analysis (PCA) via the R package (2.12.2) under Windows environment.
Results and discussion
Volatile compounds of different piper species
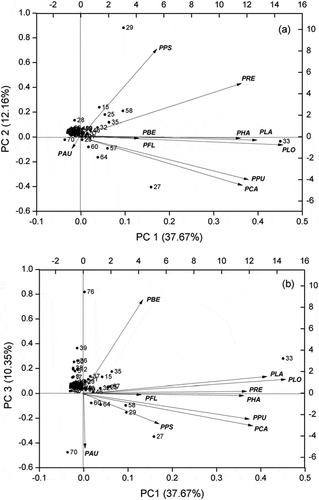
lists the volatile compounds identified in our HSSPME–GC–MS platform and the relative levels for the analyzed 10 Piper species: P. betle, P. auritum, P. retrofractum, P. hainanense, P. pseudofuligineum, P. laetispicum, P. flaviflorum, P. cathayanum, P. puberulum, and P. longum. A total of 80 volatile compounds have been identified: 44 hydrocarbons, 14 alcohols, 9 aldehydes, 7 esters, 3 acids, 2 ketones, and 1 phenol (). The main compounds comprised benzaldehyde, cinnamaldehyde, β-caryophyllene, ocimene, lavandulol, myrcene, cubebene, terpinene, linalool, α-caryophyllene, β-elemene, and germacrene. Analyzing the volatile component profile of P. auritum, 19 components were detected with a strong majority comprising aldehydes (cinnamaldehyde: 77.98%). Other compounds, such as hydrocarbons (terpinene: 5.88%, terpinolene: 5.11%, α-terpinene: 1.89%, and β-caryophyllene: 1.88%) and acids (ethanedioic acid 0.49%), were also detected ().
Table 1. Volatile compounds identified in 10 different species of Piper using HS–SPME–GC–MS.
P. betle exhibited a major complex volatile profile. This plant consisted of 31 compounds, among which 20 were hydrocarbons (cubebene: 9.36%, β-caryophyllene: 8.54%, myrcene D: 6.27%), 6 were alcohols (leaf alcohol: 3.74%), 2 were acids (acetic acid: 4.16%, and benzoic acid: 2.14%), 2 were esters (leaf acetate: 4.11% and (Z)-2-pentenyl ester: 0.50%), and 1 was aldehyde (benzaldehyde: 23.56%).
In P. hainanense, 21 components were detected (). The main compounds can be grouped as follows: 10 hydrocarbons (β-elemene 10.49%, β-pinene 8.37%, (E)-β-farnesene 7.23%, and α-pinene 7.14%), 5 aldehydes (leaf aldehyde 0.69%, benzaldehyde 0.26%), 1 acid (acetic acid 0.14%), and 1 ester (methyl benzoate 0.13%).
In P. retrofractum, 21 compounds were detected (): 14 hydrocarbons (ocimene: 15.6%, linalool: 12.9%, α-caryophyllene: 9.61%, and germacrene D: 7.15%), 4 alcohols (linalool: 12.9% and trans-hex-3-en-1-ol: 1.78%), 1 ester (octyl salicylate: 0.34%), 1 phenol (catechol: 2.48%), and 1 ketone (2-octanone: 0.29%).
As for P. laetispicum, 20 components were detected with a strong majority of hydrocarbons (76.9%). Other compounds, such as alcohols (9.04%), ketones (1.22%), and aldehydes (0.83%), were also detected (). The most abundant compound was β-caryophyllene, accounting for 26.15% of the total GC peak area, followed by germacrene (15.28%), α-caryophyllene (9.98%), nerolidol (8.84%), and α-pinene (6.09%).
The volatile compound profile of P. pseudofuligineum comprised 16 components (): 11 hydrocarbons (β-pinene: 11.95%, α-pinene: 6.73%, cinene: 4.64%, and myrcene: 2.76%), 3 esters (diethyl phthalate: 0.42% and isopentyl salicylate: 0.25%), and 2 alcohols (linalool: 1.62% and 4-methylbenzyl alcohol: 0.33%).
P. flaviflorum contained major alcohol compounds. The plant was composed of 24 components (), among which 7 were alcohols (lavandulol: 28.69%, leaf alcohol: 9.66%, nerolidol: 8.07%). Other compounds, such as hydrocarbons (30.95%), acids (3.49%), ketones (4%), and aldehydes (0.21%), were also detected.
P. cathayanum possessed major hydrocarbons compounds (). The plant consisted of 19 components, among which 15 were hydrocarbons (70.57%). The most abundant compound was myrcene, accounting for 16.06% of the total GC peak area, followed by β-caryophyllene (13.23%), sabinene (10.46%), germacrene (6.8%), and β-pinene (6.1%). Other compounds, such as aldehydes (2.03%), ester (1.1%), and alcohols (0.88%), were also detected.
Similar to P. cathayanum species, P. puberulum also featured major hydrocarbons compounds (). The plant was composed of 14 components, among which 12 were hydrocarbons (86.03%). The most abundant compound was myrcene, accounting for 34.92% of the total GC peak area, followed by β-caryophyllene (22.51%), cyclohexene (14.26%), β-elemene (4.82%), and trans-β-ocimene (4.08%). Other compounds, such as alcohols (1.46%) and aldehydes (0.47%), were also detected.
In P. longum, 21 components were identified (): 16 hydrocarbons (52.78%), 3 alcohols (4.94%), and 1 ketone (3.2%). The most abundant compound was β-caryophyllene, accounting for 27.7% of the total GC peak area, followed by β-pinene (4.39%), cadinene (4.08%), α-pinene (3.06%), and 2-nonanone (3.2%).
Diverse volatile compounds were observed in the different Piper species. In accordance with Varughese et al.[Citation22] and Srivastave et al.,[Citation23] the total profile of volatile compounds in the 10 Piper species revealed the predominance of esters, hydrocarbons, aldehydes, and alcohols. Volatile assemblages differed considerably among the different Piper species (BCS = 22.78% ± 1.98%, range: 3.15–58.83%, n = 45 comparisons, ). Volatile compositions of P. laetispicum were more similar to those of P. longum (BCS = 58.83%) than those of P. retrofractum (35.67%), P. hainanense (34.53%), P. cathayanum (34.47%), P. puberulum (31.90%), P. flaviflorum (30.39%), P. pseudofuligineum (20.07%), and P. betle (17.94%). P. auritum showed significant dissimilarity to the other nine Piper species (BCS = 4.38% ± 0.12%, range: 3.15%–6.09%, n = 9 comparisons), especially to that of P. hainanense (3.15%). This pattern indicated high diversity in volatile compounds of Piper at the species level.
Table 2. The Bray–Curtis similarity values (%) among different species of Piper.
Relative abundance of different classes of volatile compounds in different piper species
Compositions and relative contents of volatiles in the different Piper species varied significantly (). Forty-four hydrocarbons were identified, and these hydrocarbons accounted for 20.10–86.03% of the total volatiles. The sum of hydrocarbons in P. retrofractum, P. pseudofuligineum, P. laetispicum, P. cathayanum, and P. puberulum was significantly high at over 60% (). However, P. auritum presented low hydrocarbon content (20.1%). Some hydrocarbons, such as β-caryophyllene, myrcene, ocimene and β-pinene, are linked to pleasant woody, fruit, fragrance of flowers, and pine odor, respectively.[Citation24–Citation29] In this study, β-caryophyllene, myrcene, ocimene, and β-pinene were the major hydrocarbon compounds, accounting for more than 50% of the total hydrocarbons. P. puberulum yielded the highest β-caryophyllene, myrcene, and ocimene contents at 22.51 ± 0.36%, 34.92 ± 0.36%, and 0.94 ± 0.03%, respectively, followed by P. pseudofuligineum at 2.32 ± 0.10%, 2.76 ± 0.12%, and 31.48 ± 0.55% (). However, in P. auritum, contents of these compounds were very low, with mean content of 0.45 ± 0.029% in myrcene and 1.88 ± 0.031% in β-caryophyllene.
Table 3. Relative abundance of difference classes of volatile compounds in 10 Piper species.
Fourteen alcohols were detected in the 10 Piper species. The highest alcohol content was observed in P. flaviflorum (50.18%), showing significant differences from the remaining Piper species, whereas no alcohol was detected in P. auritum. Alcohols are described to possess “fruity” notes.[Citation30] For alcohols, lavandulol, linalool, and 2-pentanol are described to feature “fruity,” “floral” notes.[Citation31] These compounds also contribute to the fruity and floral odors in fine Piper. In the current study, contents of 2-pentanol were significantly higher in P. betle and P. hainanense species than in the remaining studied plants. P. retrofractum presented high levels of linalool, accounting for 12.9% of the total compounds. However, linalool contents of P. auritum and P. cathayanum were very low.
Aldehyde compounds of the 10 Piper species contributed 0.00–78.06% of the total volatile content. As major components, aldehydes can result from lipid oxidation or self-oxidation and degradation of fatty acids.[Citation24,Citation32] The sum of aldehydes was significantly higher in P. auritum than the other Piper plants. Cinnamaldehyde and benzaldehyde were detected as the major aldehyde compounds, accounting for more than 90% of the total aldehydes. The relative content of cinnamaldehyde in P. auritum was very high, reaching 77.98% (). Cinnamaldehyde, which is the main component of volatile oil of the traditional Chinese medicine cinnamon, features a special cinnamon flavor.[Citation30,Citation31] Cinnamaldehyde exerts pharmacological effects, including antipyretic, analgesic, antibacterial, and antiviral properties.[Citation32]
The current study detected 7 esters, 3 acids, 2 ketones, and 1 phenol compound in 10 Piper species. Volatiles such as 2-nonanone (ketone) and leaf acetate (ester) are described to manifest a fresh and sweet odor and fruity taste.[Citation10] P. flaviflorum contained the highest 2-nonanone content (4.00%), followed by P. longum (3.20%) and P. laetispicum (1.22%). Leaf acetate was present only in P. betle and P. cathayanum species (). No significant differences in the total contents of acids, esters, and phenols were detected among P. laetispicum, P. puberulum, and P. longum.
PCA of volatile compounds
PCA was used to analyze the data for the 80 volatile compounds obtained from 10 Piper species. The first three components of PCA explained 37.67%, 12.16%, and 10.35% of variation, explaining ~60.19% of combined variance (). As shown in , the 10 Piper species can be divided into 4 groups based on their position in the score scatter plot of PCA. The first group was laid on the positive side of PC 1 and included P. laetispicum, P. longum, P. hainanense, P. betle, and P. flaviflorum species, which were characterized as possessing high concentrations of β-caryophyllene, α-caryophyllene, germacrene, and β-pinene. P. auritum species sighted on the negative side of PC 1 formed the second group. P. auritum was characterized by extremely high cinnamaldehyde content. The third group contained P. puberulum and P. cathayanum, which were on the negative side of PC 2 and were characterized as species with high concentrations of myrcene, linalool, and germacrene D. The fourth group contained P. pseudofuligineum and P. retrofractum, which were on the positive side of PC 2 and were characterized as species with high concentration of ocimene. The remaining 71 volatiles, which include general components, (E)-β-farnesene, α-farnesene, benzyl alcohol, leaf aldehyde, copaene, and trans-β-ocimene, and relatively rare volatiles, such as 4-methylbenzyl alcohol, cineole, and 2-nonanone, showed no relationship with the first 3 components.
Figure 1. The composition of volatile compounds in different species of Piper by HS–SPME–GC–MS. PBE: P. betle; PAU: P. auritum; PRE: P. retrofractum; PHA: P. hainanense; PPS: P. pseudofuligineum; PLA: P. laetispicum; PFL: P. flaviflorum; PCA: P. cathayanum; PPU: P. puberulum; PLO: P. longum.
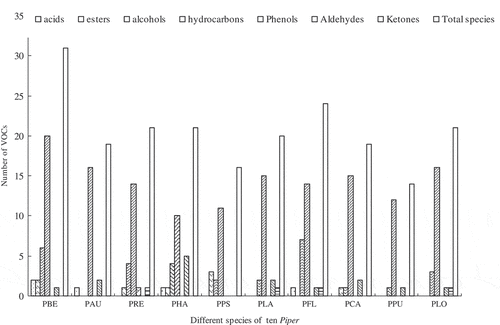
Figure 2. Principal components analysis biplot showing relationship between the differences species of Piper and the volatile compounds: PC1 versus PC2 plots (a) and PC1 versus PC3 plots (b). PBE: P. betle; PAU: P. auritum; PRE: P. retrofractum; PHA: P. hainanense; PPS: P. pseudofuligineum; PLA: P. laetispicum; PFL: P. flaviflorum; PCA: P. cathayanum; PPU: P .puberulum; PLO: P. longum. Black dots represent distribution of 80 volatile compounds in 10 Piper species (numbers correspond to those in ).
Conclusion
Volatiles composition and content depended largely upon Piper species background. A total of 80 volatile compounds were identified, and the predominant volatile compounds comprised hydrocarbons and aldehydes, followed by alcohols, acids, ketones, esters, and phenols. P. laetispicum, P. longum, P. hainanense, P. betle, and P. flaviflorum, were characterized by abundant β-caryophyllene, α-caryophyllene, germacrene, and β-pinene; P. puberulum and P. cathayanum were characterized by high concentrations of linalool, myrcene, and germacrene D; P. pseudofuligineum and P. retrofractum were associated with extremely high contents of ocimene. Specifically, P. auritum featured high content of cinnamaldehyde. The volatile components of Piper not only had been used as flavor ingredients but also had contained terpenoids with biological activities. This research showed significance for the comprehensive utilization of Piper germplasm and provides an important reference for further research.
Additional information
Funding
References
- Mabberley, D. J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, their Classification and Uses, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 20–22.
- Quijano-Abril, M. A.; Callejas-Posada, R.; Miranda-Esquivel, D. R. Areas of Endemism and Distribution Patterns for Neotropical Piper Species (Piperaceae). Journal of Biogeography 2006, 33, 1266–1278. DOI: 10.1111/jbi.2006.33.issue-7.
- Shi, Y. N.; Xin, Y.; Ling, Y.; Li, X. C.; Hao, C. Y.; Zhu, H. T.; Wang, D.; Yang, C. R.; Xu, M.; Zhang, Y. J. Chemical Constituents from Piper Hainanense and Their Cytotoxicities. Journal of Asian Natural Products Research 2016, 18, 730–736. DOI: 10.1080/10286020.2016.1158709.
- Andrade, E. H. A.; Zoghbi, M. D. G. Volatile Constituents of the Leaves and Stems of Piper Glandulosissimum Yunck. Journal of Essential Oil Research 2007, 19, 401–402. DOI: 10.1080/10412905.2007.9699935.
- Oliveira, J. C. S. D.; Dias, I. J. M.; Camara, C. A. G. D.; Schwartz, M. O. E. Volatile Constituents of the Leaf Oils of Piper aduncum L. From Different Regions of Pernambuco (Northeast of Brazil). Journal of Essential Oil Research 2006, 18, 557–559. DOI: 10.1080/10412905.2006.9699166.
- Hao, C. Y.; Xia, Z. Q.; Fan, R.; Tan, L. H.; Hu, L. S.; Wu, B. D.; Wu, H. S. De Novo Transcriptome Sequencing of Black Pepper (Piper nigrum L.) And an Analysis of Genes Involved in Phenylpropanoid Metabolism in Response to Phytophthora capsici. BMC Genomics. 2016, 17, 822–835. DOI: 10.1186/s12864-016-3155-7.
- Gajurel, P. R.; Rethy, P.; Kumar, Y. A New Species of Piper (Piperaceae) from Arunachal Pradesh, North-Eastern India. Botanical Journal of the Linnean Society 2001, 137, 417–419. DOI: 10.1111/boj.2001.137.issue-4.
- Flora of China Editorial Committee. Flora of China; Science Press & Missouri Botanical Garden: Beijing, 1995; Vol. 4. http://foc.eflora.cn/
- Condurso, C.; Cincotta, F.; Tripodi, G.; Verzera, A. Bioactive Volatiles in Sicilian (South Italy) Saffron: Safranal and Its Related Compounds. Journal of Essential Oil Research 2017, 29, 221–227. DOI: 10.1080/10412905.2016.1244115.
- Junior, S. B.; Tavares, A. M.; Filho, J. T.; Zini, C. A.; Godoy, H. T. Analysis of the Volatile Compounds of Brazilian Chilli Peppers (Capsicum spp.) At Two Stages of Maturity by Solid Phase Micro-Extraction and Gas Chromatography-Mass Spectrometry. Food Research International 2012, 48, 98–107. DOI: 10.1016/j.foodres.2012.02.005.
- Condurso, C.; Verzera, A.; Ragusa, S.; Tripodi, G.; Dima, G. Volatile Composition of Italian Thymus capitatus (L.) Hoffmanns. Et Link Leaves. Journal of Essential Oil Research 2013, 25, 239–243. DOI: 10.1080/10412905.2013.775680.
- Steinhaus, M.; Schieberle, P. Characterization of Odorants Causing an Atypical Aroma in White Pepper Powder (Piper nigrum L.) Based on Quantitative Measurements and Orthonasal Breakthrough Thresholds. Journal of Agricultural and Food Chemistry 2005, 53, 6049–6055. DOI: 10.1021/jf0506030.
- Dominique, F.; Amaral, A. C. F.; Gérzia, M. C.; Leon, A. L. L.; Silva, J. R. D. A. Chemical and Biological Analyses of the Essential Oils and Main Constituents of Piper Species. Molecules 2012, 17, 1819–1829. DOI: 10.3390/molecules17021819.
- Jagella, T.; Grosch, W. Flavour and Off-Flavour Compounds of Black and White Pepper (Piper nigrum L.) I. Evaluation of Potent Odorants of Black Pepper by Dilution and Concentration Techniques. European Food Research and Technology 1999, 209, 16–21. DOI: 10.1007/s002170050449.
- Jagella, T.; Grosch, W. Flavour and Off-Flavour Compounds of Black and White Pepper (Piper nigrum L.) II. Odour Activity Values of Desirable and Undesirable Odorants of Black Pepper. European Food Research and Technology 1999, 209, 22–26. DOI: 10.1007/s002170050450.
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E.; Tognolini, M.; Maldonado, M. E.; Bruni, R. Bioactivities of Piper aduncum L. And Piper Obliquum Ruiz & Pavon (Piperaceae) Essential Oils from Eastern Ecuador. Environmental Toxicology and Pharmacology 2009, 27, 39–48. DOI: 10.1016/j.etap.2008.08.002.
- Santos, P. R. D.; Moreira, D. L.; Guimarães, E. F.; Kaplan, M. A. C. Essential Oil Analysis of 10 Piperaceae Species from the Brazilian Atlantic Forest. Phytochemistry. 2001, 58, 547–551. DOI: 10.1016/S0031-9422(01)00290-4.
- Maia, J. G. S.; Andrade, E. H. A. Database of the Amazon Aromatic Plants and Their Essential Oils. Quimica Nova 2009, 32, 595–622. DOI: 10.1590/S0100-40422009000300006.
- Dominique, F.; Amaral, A. C. F.; Machado, G. M. C.; Leon, L. L.; Silva, J. R. D. A. Chemical and Biological Analyses of the Essential Oils and Main Constituents of Piper Species. Molecules 2012, 17, 1819–1829. DOI: 10.3390/molecules17021819.
- Mazida, M. M.; Salleh, M. M.; Osman, H. Analysis of Volatile Aroma Compounds of Fresh Chilli (Capsicum annuum) during Stages of Maturity Using Solid Phase Micro Extraction (SPME). Journal of Food Composition and Analysis 2005, 18, 427–437. DOI: 10.1016/j.jfca.2004.02.001.
- Topul, R.; Wossa, S. W.; Leach, D. N.; Waterman, P. G. Volatile Chemical Constituents of Piper aduncum L and Piper gibbilimbum C.D.C (Piperaceae) from Papua New Guinea. Molecules 2007, 12, 389–394. DOI: 10.3390/12030389.
- Varughese, T.; Unnikrishnan, P. K.; Deepak, M.; Balachandran, I.; Shree, A. B. S. Chemical Composition of the Essential Oils from Stem, Root, Fruit and Leaf of Piper longum Linn. Journal of Essential Oil Bearing Plants 2016, 19, 52–58. DOI: 10.1080/0972060X.2015.1119065.
- Srivastave, S.; Gupta, M. M.; Kumar, S. Volatile Constituents of the Essential Oil of Piper mullesua from Manipur (India). Journal of essential Oil Research 1999, 11, 563–564. DOI: 10.1080/10412905.1999.9701214.
- Qin, X. W.; Lai, J. X.; Tan, L. H.; Hao, C. Y.; Li, F. P.; He, S. Z.; Song, Y. H. Characterization of Volatile Compounds in Criollo, Forastero, and Trinitario Cocoa Seeds (Theobroma cacao L.) In China. International Journal of Food Properties 2017, 20, 2261–2275. DOI: 10.1080/10942912.2016.1236270.
- Arctander, S.;. Perfume and Flavour Chemicals; Arctander: Elizabeth, NJ, 1969.
- Ziegleder, G.;. Linalool Contents as Characteristic of Some Flavour Grade Cocoas. Z. Lebensm. Food Research 1990, 191, 306–309. DOI: 10.1007/BF01202432.
- Werkhoff, P.; Gûntert, M.; Kramme, G.; Sommer, H.; Kaulen, J. Vacuum Headspace Method in Aroma Research: Flavor Chemistry of Yellow Passion Fruits. Journal of Agricultural and Food Chemistry 1998, 46, 1076–1093. DOI: 10.1021/jf970655s.
- Hadi, M. A. M. E.; Zhang, F. J.; Wu, F. F.; Zhou, C. H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. DOI: 10.3390/molecules18078200.
- Blank, I.; Grosch, W. Evaluation of Potent Odorants in Dill Seed and Dill Herb (Anethum graveolens L.) By Aroma Extract Dilution Analysis. Journal of Food Science 1991, 56, 63–67. DOI: 10.1111/jfds.1991.56.issue-1.
- Saltini, R.; Akkerman, R.; Frosch, S. Optimizing Chocolate Production through Traceability: A Review of the Influence of Farming Practices on Cocoa Bean Quality. Food Control 2013, 29, 167–187. DOI: 10.1016/j.foodcont.2012.05.054.
- Cerny, C.; Fay, L. B. Mechanism of Formation of Alkylpyrazine in the Maillard Reaction. Journal of Agricultural and Food Chemistry 1995, 43, 2818–2822. DOI: 10.1021/jf00059a009.
- Krysiak, W.; Majda, T.; Nebesny, E. An Effect of Relative Air Humidity on the Content of Volatile Compounds in Roasting Cocoa Beans. In Focus on Food Engineering Research and Development; Vivian, N.P., Eds.; Nova Science, Inc.: Vivian, N.P., 2007; pp. 467–482.
