?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Protein was extracted from the seeds of Kleinhovia hospita Linn., which is being a nonconventional source. Extraction of K. hospita seed protein at various pH values in aqueous solution and at pH 7, different salt concentrations were done. Fractionation of protein from seeds was performed to separate albumin, globulin, prolamin, and glutelin. The amino acid compositions of total protein isolate (TPI) and the fractions were determined. A total of 15 amino acids were identified including 9 essential amino acids. Gel filtration by Sephadex G-100 revealed the presence of three components in the TPI. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis of TPI and fractions showed different polypeptide bands having molecular weights ranging from 12 to 42 kDa approximately. Scanning electron microscopic study of TPI and fractions revealed the surface topology of the protein.
Introduction
Food is the basic need of human beings containing carbohydrates, fats, proteins, minerals, water, and roughage as major components. Among these, proteins are most important compounds because its deficiency causes malnutrition.[Citation1] Proteins are nitrogen containing compounds composed of mainly 20 different amino acids linked together by peptide bond. The increasing demand for protein is the major acute problem in developing countries where the average protein intake is less than that required. Due to insufficient supply of proteins from the sources like animal origin, government organization and researchers are searching for new sources of protein as a food supplement which must be cheap, reliable, and good quality.[Citation2] These factors inspired the authors to search protein sources from nonconventional plants origin in our country. The abundant unutilized plants can be used to meet the increasing demands for edible proteins. Plant proteins also play significant roles in human nutrition.
With this objective of finding nonconventional protein sources, work on the seeds of Kleinhovia hospita L. was undertaken. K. hospita is an important evergreen, tropical tree growing up to 20 m high, native to the tropical parts of Asia, Africa, and Australia. It is monotypic and the only species representing of its genus (family: Sterculiaceae earlier, it is now included in the family Malvaceae).[Citation3,Citation4] Plant parts of K. hospita have been used as folk medicine for the treatment of pruritus, tetter, epatitis, and scabies.[Citation5] Different compounds such as alkaloid, terpenoid, coumarin, and steroid are isolated and characterized from the different parts of this plant.[Citation6–Citation10] The leaf of K. hospita shows a good composition of fatty acids.[Citation11] Antioxidant compounds with their antioxidant activity are also investigated from the leaves of this plant.[Citation12,Citation13] Other biological parameters like hepatoprotective, antibacterial, cytotoxic, and tyrosinase inhibitory activities are also determined from various parts of the plant.[Citation6,Citation8,Citation11,Citation13–Citation15]
Materials and methods
Plant materials and chemicals
Fresh and matured fruits of K. hospita were collected from a particular geographical area during the month of December–January in Research Farm (23°53′N latitude and 83°25′E longitude), Burdwan University campus, Burdwan, W. Bengal, India. The specimen was authenticated by Prof. Ambarish Mukherjee, Department of Botany, The University of Burdwan, and voucher specimens MCD3 for fruits of this plant have been deposited at the herbarium (BURD) of the Department of Botany. All the chemicals and solvents used in this experiment were of analytical grade. Sephadex G-100, BSA, pepsin, lysozyme, and ovalbumin were purchased from Sigma Aldrich (USA) and protein standard from Fischer Thermoscientific (USA).
Extraction and nitrogen solubility of protein
Seeds obtained after decoating of fruits were dried in air and then crushed to finely powdered. The finely powered seeds (200 g) were deoiled by extracting with petroleum ether (2 L, 40–60°C) in a Soxhlet apparatus for 72 h. The seed flour was then washed twice with chloroform:methanol mixture (3:1), air dried, and finally stored in a refrigerator at 4°C for further use. The protein solubility of de-oiled seed flour was determined at different pHs (2–12) using distilled water (1:20, w/v) with an electrical stirrer at room temperature (20°C) for 30 min and in presence of different molar concentrations (0.1–1.0 M) of salts like sodium chloride (NaCl), potassium chloride (KCl), magnesium chloride hexahydrate (MgCl2, 6H2O), and magnesium sulfate heptahydrate (MgSO4, 7H2O) at pH 7.[Citation16–Citation18] The nitrogen content of each extract was determined by micro-Kjeldhal method.[Citation19]
Preparation of TPI
Total protein isolate (TPI) from de-oiled seed flour was extracted by distilled water (1: 20, w/v) at pH 7 for 30 min (20°C). The suspension was first centrifuged at 5000 rpm for 15 min and then, supernatant was recovered. The residue was re-extracted twice more with same solvent using same procedure. The pH of the total supernatant was lowered to 4 (nearly isoelectric point of protein; minimum solubility of protein) by 1 N trichloroacetic acid (TCA). The precipitate thus obtained was recovered by centrifugation at 5000 rpm for 15 min. The recovered precipitate was dissolved in distilled water at pH 7 and finally dialyzed against distilled water for 48 h at 4°C. The extracted total protein was then freeze dried and stored in a refrigerator for further analysis.
Proximate chemical analysis
Both nitrogen and protein contents of seed and de-oiled seed were determined by micro-Kjeldhal method. Moisture and ash contents of the above seeds were subsequently determined using standard methods of analysis.[Citation19]
Fractionation of seed protein
The protein fractionations of de-oiled seed flour were isolated using modified Osborne method.[Citation20,Citation21] Defatted and dried seed sample (25 g) was stirred in 250 ml of distilled water (w/v = 1:10) with a magnetic stirrer at room temperature (20°C) for 30 min. The suspension was then centrifuged at 5000 rpm for 15 min to give the albumin extract. The residue was extracted with 250 ml of 0.5 M NaCl for 2 h and centrifuged at 5000 rpm for 15 min to give the globulin extract. Then, the residue was extracted with 250 ml of 70% ethanol for 2 h followed by centrifugation to give the prolamin extract. The residue left was extracted again with 250 ml of 0.1 M NaOH for 2 h to give the glutelin extract. Similarly, each extraction step was performed twice to recover the most of the protein. The albumin, globulin, prolamin, and glutelin fractions were obtained by precipitating the respective supernatant with 1 N TCA at pH 4 followed by keeping the supernatant overnight in a refrigerator. Each precipitate thus isolated was successively washed (three times) and dialyzed (48 h at 4°C) with distilled water (pH 7). The seed protein fractions were then freeze dried and used for determination of amino acid composition, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) electrophoresis, and scanning electron microscopic (SEM) analysis.
Amino acid analysis
Five milligram of each protein samples (TPI and four fractions) were dissolved in 2 ml of 6N HCl and the mixtures were hydrolyzed in a boiling water bath for 24 h. Then, solutions were centrifuged at 3500 rpm for 15 min. The supernatants were filtered and neutralized with 1N NaOH. Then, the filtered solutions were diluted to 1:100 (v/v) with Milli-Q water and were allowed for separation of amino acids by HPLC method (Agilent 1100 HP-HPLC).[Citation22] The peak areas were recorded and calibrated with respect to the standard chromatogram.
Gel filtration
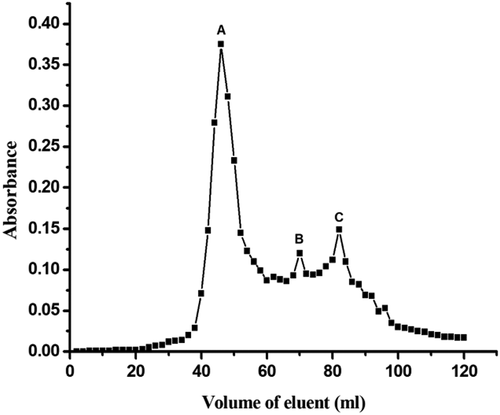
Gel filtration chromatography for molecular weight determination was performed by slight modification of Whitaker method.[Citation23,Citation24] A sephadex G-100 column (2.5 cm i.d. × 30 cm) was used for this method at 20°C. 0.01 M phosphate buffer (pH 7) containing 0.2 M NaCl was used as an eluting buffer. Void volume of the column was determined using blue dextran. A volume of 2 ml of each protein sample was injected in the column from the stock of 4 mg/ml. The standard samples and the protein sample (TPI) were applied in this column separately. Fractions of 2 ml were collected at the flow rate of 0.4 ml/min and monitored with a Shimadzu UV–visible spectrophotometer (Model No 1601, Kyoto, Japan). The molecular weights were also calculated for each component thus obtained (A–C) by the following equation:
where V and V0 are the elution and the void volumes, respectively.
PAGE
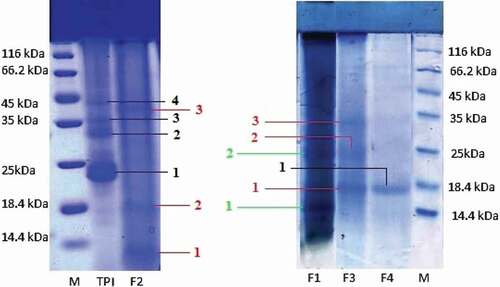
Gel electrophoresis studies of TPI and protein fractions were done according to Laemmli method.[Citation25] Ten percentage of sodium dodecyl sulfate–polyacrylamide gel (Biorad) was used for molecular mass and purity analysis of purified proteins. An amount of 20 µg of each protein sample was loaded to the lane of the gel in a similar way and the electrophoresis experiment was performed at a constant voltage (150 V) in 10% SDS running buffer. After electrophoresis, the gel was stained with 0.006% coomassie blue in 10% (v/v) acetic acid and 90% (v/v) double-distilled water solution for 30 min placed on a rocker. Then, it was destained by 45% (v/v) methanol and 10% (v/v) acetic acid aqueous solution on a rocker at room temperature. After that, gels were transferred into water for storage. The polypeptide bands thus obtained were compared with protein molecular weight marker (mixture of seven native proteins, 14.4–116 kDa).
SEM studies
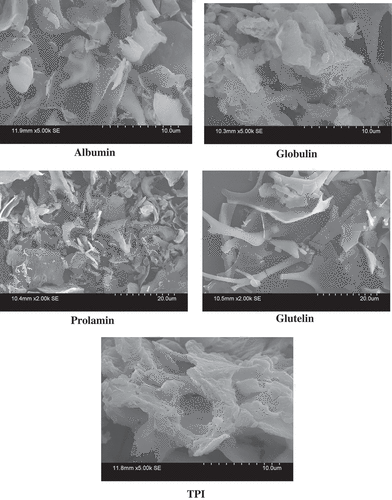
SEM images of the lyophilized TPI and the protein fractions were performed. Each of the samples was mounted on circular aluminum stubs with double sticky tape and coated with 20 nm of gold using an IB2 ion coater. The samples were examined and photographed using a scanning electron microscope (model S-530; Hitachi Ltd., Tokyo, Japan) at an accelerating potential of 15 kV.
Results and discussion
Nitrogen solubility of seed protein
The solubility of protein in water at different pHs was placed in . The maximum nitrogen solubility of protein was found to be 92.96% at pH 12, whereas the minimum solubility (48.67%) at pH 4. The major forces involved in this process are electrostatic, hydrophobic, and hydrogen bonding. In an isoelectric state of protein molecules, the solubility goes to minimum (zero electrostatic repulsion). TPI also showed minimum solubility at pH 4 although it was a mixture of proteins. On the other hand, solubility patterns of seed protein in presence of different salt concentrations (0.1–1.0 M) at pH 7 were investigated (). Some common salts like uni-univalent, bi-univalent, and bi-bivalent were used for the study of solubility profile. Nitrogen solubilities for KCl, MgCl2, and MgSO4 showed almost similar patterns whereas, for NaCl, the pattern was different. Maximum nitrogen solubilities at 0.6 M were 54.93%, 71.12%, and 79.89% for KCl, MgCl2, and MgSO4, respectively, whereas for NaCl, it was 51.46% at 1.0 M. The solubility pattern of protein was higher in bivalent salts than the monovalent salts due to high-charge potential of bivalent cations.[Citation16–Citation18]
Table 1. Nitrogen solubility of K. hospita seed protein in aqueous solution at different pHs.
Table 2. Nitrogen solubility of K. hospita seed protein in different salt concentration at pH 7.0.
Physicochemical properties of seed protein
Nitrogen, total protein, ash, and moisture contents of both seed and de-oiled seed were given in . The protein content of de-oiled seed was 14.75%, comparable to the protein (17.10%) reported for hyacinth bean.[Citation26] After fractionation, four fractions such as albumin, globulin, prolamin, and glutelin were obtained from de-oiled seed. The abundances of albumin (26%) and glutelin (57%) were higher compare to that of globulin (14%) and prolamin (3%). This result showed that water and NaOH-soluble protein fractions were the predominant in K. hospita seed.
Table 3. Proximate chemical analysis of the K. hospita seed and de-oiled seed.
Amino acid composition
The amino acid compositions of TPI and the four fractions were shown in including the data of soybean protein as a reference. Each of the TPI and the protein fractions contained 15 amino acids in which 9 were essential. The E/T (%) of TPI, albumin, globulin, prolamin, and glutelin was 40.50%, 39.71%, 41.73%, 43.61%, and 38.37%, respectively (Fig. S1), which were comparable to soybean protein. Thus, among the four protein factions, prolamin could be most nutritious whereas glutelin is least nutritious with respect to soybean protein.[Citation27]
Table 4. Amino acid composition of K. hospita seed protein.
Molecular weight determination by gel filtration
Two different methods were used for determination of molecular weight of K. hospita seed protein. Three components () were obtained by gel filtration chromatography (). According to the Whitaker’s equation, the molecular weights for three components (A–C) were calculated as 126,599, 33,009, and 18,435 Da, respectively. From the standard curve, the molecular weights of the above components were also determined as 125,893, 31,989, and 18,197 Da, respectively, using standard proteins (BSA, ovalbumin, pepsin and lysozyme) (Fig. S2). Thus, we may conclude that there were three components of polypeptide present in the TPI of the seed.
Table 5. Molecular weights of K. hospita seed proteins, as determined by a gel filtration procedure.
Electrophoresis results
SDS–PAGE analysis of TPI and protein fractions of K. hospita seed were shown in . TPI showed four bands approximately at 23, 32, 35, and 42 kDa, whereas albumin contained two bands at 15 and 25 kDa. The globulin fraction showed three bands approximately at 12, 18.4, and 25 kDa where as prolamin fraction displayed three bands at 18, 25, and 32 kDa. The last fraction (glutelin) contained one band at 18 kDa. Most of the polypeptides were noticed to bind SDS with a constant ratio such that they had basically the same charge densities and migrated in the gel according to their molecular weights. The results showed that the seed protein of K. hospita was simple in structure and characterized by the presence of all the types of protein fractions.
SEM for micromorphological studies
Surface morphological pattern of TPI and protein fractions showed distinct surface structure (). The TPI showed flaky plate structure whereas albumin fraction displayed as crystal plate structure. Globulin fraction appeared as a spiral like structure with dissimilar way. Crystal plates with various shapes were observed in the prolamin fraction. The glutelin fraction showed plate-like structure with small flake. Thus, SEM studies explain the different characteristics of protein which may contribute to the physicochemical and functional properties of seed protein.
Conclusion
The chemical investigation of K. hospita seed protein was performed for the first time. The solubility of seed protein in water is very high. The seed protein contains high amount of albumin and glutelin fractions. TPI and the protein fractions show that seed of K. hospita contains most of essential amino acids in a considerable amount. Both the gel filtration study and SDS–PAGE analysis reflect the molecular weights of TPI and the protein fractions. Considering all these facts and high availability of the seed in India, it may be concluded that K. hospita seed protein may be used as a food supplement after proper toxicological screening.
Supplemental_file.docx
Download ()Acknowledgments
One of the authors (M. C. Dey) is grateful to The University of Burdwan, Burdwan-713104, W. Bengal, India, for providing fellowship. The authors have no potential conflict of interest.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
References
- Mauron, J.; Some Current Problems in Protein Nutrition. In Rod, J. W. G., Rolls, B. A., Eds Protein in Human Nutrition. Academic Press: New York, 1973; pp. 1–9.
- Adebowale, K. O.; Lawal, O. S. Comparative Study of the Functional Properties of Bambarra Groundnut (Voandzeia Subterranean), Jack Bean (Canavalia Ensiformis) and Mucuna Bean (Mucuna Pruriens) Flours. Food Research International 2004, 376, 355–365. DOI: 10.1016/j.foodres.2004.01.009.
- C.S.I.R. New Delhi. The Wealth of India: Raw Materials. A dictionary of Indian raw materials and industrial products. 1959, 5, p. 321.
- Chopra, R. N.; Nayar, S. L.; Chopra, I. C. Glossary of Indian Medicinal Plants; PID: New Delhi, 1956. pp. 147.
- (a) Latiff, A. In Plant Resources of South-East Asia, Faridah, H. I., Van Der Maesen, L. J. G. Eds.; In Prosea Foundation: Bogor, 1997; Vol. 11, pp. 166–167. (b) Editorial Committee of the Administration Bureau of Traditional Chinese Medicine. Chinese Materia Medica (Zhonghua Bencao); Shanghai Science & Technology Press: Shanghai. 1999, 5, pp. 387–388.
- Gan, L. S.; Ren, G.; Mo, J. X.; Zhang, X. Y.; Yao, W.; Zhou, C. X. Cycloartanetriterpenoids from Kleinhovia Hospita. Journal of Natural Products 2009, 72, 1102–1105. DOI: 10.1021/np900029z.
- Zhou, C. X.; Zou, L.; Gan, L. S.; Cao, Y. L. Kleinhospitines A–D, New Cycloartanetriterpenoid Alkaloids from Kleinhovia Hospita. Organic Letters 2013, 15, 2734–2737. DOI: 10.1021/ol401066j.
- Mo, J. X.; Bai, Y.; Liu, B.; Zhou, C. X.; Zou, L.; Gan, L. S. Two New Cycloartane Triterpenoids from Kleinhovia Hospita. Helvetica Chimica Acta 2014, 97, 887–894. DOI: 10.1002/hlca.201300331.
- Rativanich, T.; Dietrichs, H. Alkaloids from Thai Tree Used in Folk Medicine. Natural History Bulletin - Siam Society 1971, 24, 145–151.
- Soekamto, N. H.; Noor, A.; Dini, I.; Rudiyansyah; Garson, M. Coumarin and Steroid Compound from Stem Bark of Kleinhovia Hospita Linn. Proceeding International Seminar on Chemistry 2008, 231–234.
- Dey, M. C.; Roy, R. N.; Sinhababu, A. Fatty Acid Composition and Antibacterial Activity of the Leaf Oil of Kleinhovia Hospita Linn. International Journal of Chemical Technology and Research 2017, 10, 378–384.
- Arung, E. T.; Kusuma, I. W.; Kim, Y. U.; Shimizu, K.; Kondo, R. Antioxidative Compounds from Leaves of Tahongai (Klienhovia Hospita). Journal of Wood Science 2012, 58, 77–80. DOI: 10.1007/s10086-011-1217-7.
- Arung, E. T.; Kusuma, I. W.; Purwatiningsih, S.; Roh, S. S.; Yang, C. H.; Jeon, S.; Kim, Y. U.; Sukaton, E.; Susilo, J.; Astuti, Y.; et al. Antioxidant Activity and Cytotoxicity of the Traditional Indonesian Medicine Tahongai (Kleinhovia Hospita L Extract.). Journal of Acupuncture and Meridian Studies 2009, 2, 306–308. DOI: 10.1016/S2005-2901(09)60073-X.
- Yuliana; Herawati, S. Phytochemical Content and Protective Effect of Kleinhovia Hospita Leaves Extract on Pancreatic Cytotoxicity in Hyperglycemic Rats. Jurnal Veteriner 2016, 17, 411–417. DOI: 10.19087/jveteriner.2016.17.3.411.
- Arung, E. T.; Kusuma, I. W.; Iskandar, Y. M.; Yasutake, S.; Shimizu, K.; Kondo, R. Screening of Indonesian Plants for Tyrosinase Inhibitory Activity. Journal of Wood Science 2005, 51, 520–525. DOI: 10.1007/s10086-004-0690-7.
- Laskar, S.; Sinhababu, A.; Thakur, S.; Basak, B. Extraction and Chemical Investigation of Lagerstroemia Speciosa Seed Proteins. Journal of American Lab 1998, 30, 22–24.
- Basak, B.; Bhattacharya, U. K.; Sinhababu, A.; Laskar, S. Extraction and Chemical Investigation of Kulthi (Macrotylona Uniflorus Lam.) Seed Protein. Applied Biochemistry and Biotechnology 1994, 49, 281–290. DOI: 10.1007/BF02783062.
- Kundu, P.; Laskar, S. Fractionation and Some Chemical Studies on Ailanthus Excelsa Roxb. Seed Protein. Journal of the American Oil Chemists’ Society 2008, 85, 835–843. DOI: 10.1007/s11746-008-1273-3.
- AOAC. Official Methods of Analysis, 15th; Association of Official Analytical Chemists: Washington, DC, 1990.
- Osborne, T. B.; The Vegetable Proteins; Longmans, Green and Co.: London, 1924. pp. 51–56.
- Chavan, U. D.; Mckenzie, D. B.; Shahidi, F. Protein Classification of Beach Pea (Lathyrus Maritimus L.). Food Chemistry 2001, 75, 145–153. DOI: 10.1016/S0308-8146(01)00122-4.
- Bruckner, H.; Wittner, R.; Godel, H. Fully Automated High-Performance Liquid Chromatographic Separation of DL-amino Acids Derivatized with O-Phthaldialdehyde Together with N-Isobutyryl-Cysteine. application to food samples. Chromatographia 1991, 32, 383–388.
- Whitaker, J. R.; Determination of Molecular Weights of Proteins by Gel Filtration on Sephadex. Analytical Chemistry 1963, 35, 1950–1953. DOI: 10.1021/ac60205a048.
- Whitaker, J. R.; Correction. Determination of Molecular Weights of Proteins by Gel Filtration on Sephadex. Analytical Chemistry 1964, 36, 89. DOI: 10.1021/ac60207a072.
- Laemmli, U. K.; Cleavage of Saturated Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. DOI: 10.1038/227680a0.
- Subagio, A.; Characterization of Hyacinth Bean (Lablab Purpureus L. Sweet) Seeds from Indonesia and Their Protein Isolate. Food Chemistry 2006, 95, 65–70. DOI: 10.1016/j.foodchem.2004.12.042.
- Brul’e, D.; Savoie, L. In Vitro Digestibility of Protein and Amino Acids in Protein Mixture. Journal of the Science of Food and Agriculture 1998, 43, 361–372. DOI: 10.1002/jsfa.2740430409.