ABSTRACT
Breast cancer is one of the most important causes of cancer related morbidity and mortality in the world. Along with genetic, environmental factors also play a multifaceted role in the development of disease. Breast contains several bacterial species performing specialized functions. Probiotics, as functional food, play pivotal role against breast cancer development in vivo and in vitro. Current review summarized all the available data related to diet, probiotics, and their association with breast cancer risk along with underlying mechanisms. Presently, it was believed that many of the commercially available probiotic products were safe to use and had some beneficial health effects for the host. Probiotics had a potential to act against breast cancer progression evidenced by many animal model and cell-based experiments. Some probiotics strains may be useful as an adjuvant therapy for breast cancer prevention or treatment, by modulating immune response or breast microbial community. However, large-scale clinical trials and intense research are mandatory to explore probiotics-related metabolic and molecular mechanisms in breast cancer.
Introduction
Breast cancer is the most common malignancy among women and accounts for 29% of cancer cases diagnosed in women. According to an estimate, approximately 2.5 million breast cancer cases were registered in 2016.[Citation1] Diverse variations in breast cancer incidence was thought to be outcome of differences in hormonal factors, reproductive patterns, and detection strategies at different stages.[Citation2,Citation3] Advancement in the screening and treatment of breast cancer have steadily decreased its mortality particularly for HER-2 and luminal cancers but for triple negative cancers, it remains at higher rates.[Citation4] However, regardless of this extensive progress, all the ethnic/racial groups have not been equally benefitted because of number of reasons including stage distribution among patients, ethnic rate of survival, and mortality.[Citation1,Citation5] Environmental and genetic factors together involve a complex interplay in breast cancer etiology. Older age at menarche, greater parity, younger age at the first birth, and long breast-feeding duration were associated with decreased risk of breast cancer.[Citation4,Citation6,Citation7] Whereas alcohol consumption, high glycemic diet, higher body mass index, familial history of breast cancer, menopause age, and menopausal hormonal therapy were established risk factors for breast cancer.[Citation8–Citation11]
Diverse microbiota is associated with several human body parts such as gastrointestinal tract, mouth, and skin. The composition of this microbiota chiefly is bacteria, though, fungi, viruses, and protozoans also exist.[Citation12] Human microbiota consists of enormous species of bacteria and majority of them are beneficial to the host. Some of them prevent pathogens to clink with gut wall and preserve immunity of gut mucosa.[Citation13] In large intestine of humans, gut microbiota is in abundance approximately one hundred billion organisms per intestinal content gram.[Citation14] Number of gut bacteria exceed the total number of host’s eukaryotic cells and impart various health benefits.[Citation15] This gut flora mainly consists of probiotic bacteria and migrates to other parts of body especially in breast of lactating females but the transport mechanism from gut to breast is still unknown.[Citation16]
Probiotics means “for life” or an antonym for antibiotics [Citation17], when taken in sufficient amount, viable and non-disease causing microorganisms (bacteria or yeasts) impart health benefits via preclusion (prevention) and cure of particular pathological disorders or can lessen the possibility of disease to host are referred as probiotics.[Citation18,Citation19]
Various microorganisms are used as probiotics especially lactic acid bacteria (LAB). LAB includes most of Lactobacillus species, some species of Bifidobacteria, Enterococcus, and Streptococcus.[Citation20] A non-LAB probiotic, i.e., Escherichia coli Nissle 1917, was established to be operational in the treatment of infectious intestinal disorders.[Citation21] Most of these bacterial species reside in human intestine.[Citation15] The only probiotic yeast employed is the nonpathogenic Saccharomyces boulardii.[Citation22,Citation23] LAB and certain non-LAB probiotics are generally recognized as safe organisms which can be securely used for medical or veterinary purpose.[Citation24]
Numerous aspects of biological function are controlled by usual gut microflora including cancer prognosis.[Citation25] Normally, probiotic bacteria are like natural inhabitants (bacteria) of the human gut specifically those of breastfed infants that are showing natural fortification against a number of diseases and infections.[Citation12] There are vast, evidences that support effectiveness of probiotics for different illness including antibiotic associated diarrhea, traveler’s diarrhea, ulcerative colitis, Cohn’s disease, etc. Abdominal cramps, flatulence, bloating, and watery diarrhea could be the result of incomplete lactose absorption by gut wall. This occurs due to lactase reduction (β-galactosidase) in intestinal mucosa and might be cured by probiotics (LAB) resulting in lactase production.[Citation12]
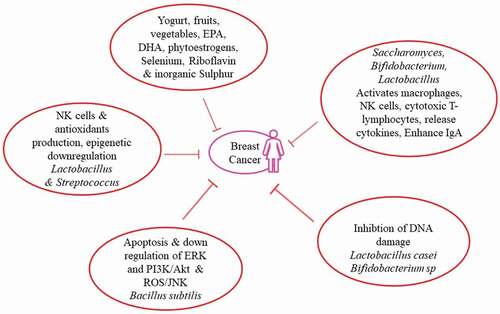
Health benefits derived from probiotic food intake (LAB fermented food, probiotic drinks, etc.) containing Lactobacillus spp., Enterococcus spp., Bifidobacterium spp., and Lactococcus spp., etc. are documented.[Citation26–Citation42] Numerous health benefits related to probiotics consumption are explored but its involvement in the breast cancer etiology is still not fully understood. Therefore, current study is designed to explore the association of probiotics with risk of breast cancer, as illustrated in .
Diet and breast cancer
Various epidemiological studies in animals and human have reported a well-established role of diet in breast cancer prevention or development.[Citation43,Citation44] Diet is one of the most modifiable breast cancer risk factor. Changes in the dietary habits not only related to reduced cancer risk but also patients already diagnosed and treated with breast cancer can improve their overall health and increase survival rate by opting healthier diet and better life style.[Citation45] Many studies have provided evident that obesity and breast cancer are linked with each other.[Citation46] Being overweight means a person is at a risk of having breast cancer. Diet rich in glycemic load was significantly associated with increased risk of breast cancer.[Citation11] Certain approaches can help to prevent weight gain that might not only decrease breast cancer risk but also improve treatment outcomes in breast cancer patients.[Citation47]
Several studies suggested that intake of omega-6 and omega-3 particularly marine fatty acids, i.e., eicosapentaenoic and docosahexaenoic was linked with better breast cancer prognosis.[Citation43,Citation48,Citation49] In-vitro inhibition of cancer cells and reduced tumor growth in breast cancer rat models by canola oil suggested its inverse relationship with breast cancer progression.[Citation50,Citation51] Intake of vegetables and fruit rich diet was positively associated with reduced breast cancer risk.[Citation52] It was well documented that yoghurt consumption was negatively associated with breast cancer risk.[Citation12,Citation53]
Dietary fiber intake was also associated with decreased risk of breast cancer. It was reported that dietary phytoestrogens can increase ERα positive breast cancer risk, and it was linked with their estrogenic effects illustrated in vivo and in vitro. The proliferative consequence of soy isoflavones was primarily experienced in tumor bearing animal models. But inconsistently, phytoestrogens ingestion has also been associated with decreased breast cancer risk.[Citation54] Controversy related to soy isoflavones effect on risk of breast cancer was investigated and it was confirmed that soy isoflavone phase II metabolism is different between rodents and humans. Therefore, it should be considered important whether to use results obtained from rodents for humans or not. Soy consumption is associated with reduced breast cancer risk among Asians.[Citation55] Researchers are interested to explore the exact hormonal and non-hormonal isoflavones mechanism by which they can exert some beneficial effects. Soy isoflavones shared similar chemical structure with estrogens and considered as probable selective estrogen receptor modulators.[Citation56] So, they can mimic estrogen and bind to estrogen receptors and stimulate or inhibit its functions in tissues. Montales et al., using estrogen receptor-negative and receptor-positive human breast cancer cells, reported that sera of mice ingesting a diet containing blueberry polyphenol and isoflavone genistein changed mammosphere formation.[Citation57] Breast cancer prevention by genistein was linked with regulation of breast adiposity.[Citation58]
Role of folate or folic acid is still controversial and is investigated both in animal models and breast cancer patients. Some of the epidemiological studies showed inverse relationship between folic acid consumption and breast cancer risk.[Citation43,Citation59] It was observed that useful effects of folate are associated with a genetic polymorphism of MTHFR (folate-metabolizing enzyme, methylenetetrahydrofolate reductase) in some populations. Different studies have reported that MTHFR C677T polymorphism might be able to modify the association between risk of breast cancer and dietary intake of folic acid.[Citation60–Citation62] A systematic review illustrated that European and American women are at greater risk of breast cancer because of increased consumption of folate or folic acid in the form of fortified foods.[Citation63]
Selenium, essential micronutrient, was investigated to be inversely associated with breast cancer risk in animal models.[Citation64] Riboflavin was also reported to be associated with reduced risk of breast cancer.[Citation65] Inorganic sulfur showed markedly decreased proliferation of human breast cancer cells due to reduced ErbB2 and ErbB3 mRNA expression and protein, stressing ErbB-Akt pathway.[Citation66] There is still a need to do more work in order to investigate the association of diet with breast cancer risk and fulfil the gaps.
Association of bacteria with breast cancer
It was reported that health encouraging bacteria, Lactobacillus and Streptococcus, are more predominant in healthy breast than the cancerous one. Both these bacterial groups possess anticarcinogenic properties, i.e., they involved in the production of natural killer (NK) cells to regulate tumor progression. Immune cells reduction is directly linked with increased breast cancer risk. Antioxidants produced by Streptococcus thermophilus are linked with decreased DNA damage, and thus, cancer by neutralizing reactive oxygen species (ROS).[Citation18]
In healthy breast, Lactococcus and Streptococcus bacteria are more prevalent because bacteria were thought to have anticancer property. Bacteria from cancerous females were grown on human breast cancer cells to check their ability of triggering DNA damage. Proliferation of these cancerous cells showed alarming situation of double-stranded DNA breaks caused by three different bacterial strains, i.e., Enterobacteriaceae, E. coli, and Staphylococcus. Probiotic bacteria, Lactobacillus, can break down carcinogens. Antioxidants that decrease DNA damage are produced by probiotic strain S. thermophilus.[Citation67]
Kinjo et al.[Citation68] working with association of microbial dysbiosis with breast cancer reported that normal breast tissue was enriched with Sphingomonas yanoikuyae as compared to the diseased one, suggesting its potential role as a probiotic. Remarkably, S. yanoikuyae exhibit glycosphingolipid ligands, dynamic activators of iNKT (invariant NKT) cells. iNKTs are central mediators in cancer immunosurveillance and have fundamental role in controlling breast cancer metastasis.[Citation69,Citation70] Future studies are aimed to explore S. yanoikuyae’s prospective role in the development and progression of breast cancer.
Lactobacillus crispatus and Lactobacillus acidophilus were reported to have antiproliferative activity against breast cancer cells. Additionally, lactobacilli can decrease transcriptional activity of four different cancer-testis antigens. It was reported that expression of cancer-testis antigens involves epigenetic regulations. Therefore, lactobacilli may cause epigenetic downregulation of this expression. Expression of cancer-testis antigens was linked with poor prognosis and high-grade tumors, so expressional downregulation by lactobacilli may open up new era of clinical research applications.[Citation71]
Enzyme activity
Bacillus subtilis CSY191, a potential probiotic and biosurfactant (surfactin) producer, was isolated from doenjang, (traditional fermented soybean paste of Korean origin). Surfactin hinders MCF-7 growth in dose dependent manner. Apoptotic induction and cell cycle arrest through the downregulation of ERK and PI3K/Akt (cell survival regulating signals) are responsible for this growth hindrance via surfactin. Surfactin induces apoptosis and stop proliferation of MCF-7 human breast cancer cells through mitochondrial/caspase ROS/JNK-mediated pathway. Production of ROS caused by surfactin induce regulation of stress-induced apoptosis through activation of ERK1/2 and JNK. Apoptotic cell death induction is a promising approach for cancer treatment.[Citation72]
Cytotoxicity
It was reported that tumor development was delayed and sometimes blocked as well when a mouse with breast cancer was given fermented milk containing probiotic bacterium Lactobacillus casei (CRL 431). It was associated with tumor triggered modulation of immune response. Immune response can be improved by daily intake of probiotic strain L. casei, thus decreasing tumor growth and increasing endurance.[Citation12,Citation73]
Reduced NK cells and their cytotoxicity were correlated with cancer development. Different probiotic bacteria have ability to enhance NK cell activities. Three weeks consumption of fermented milk containing L. casei was reported to elevate the activity of NK cells in aged people and disappeared after 6 weeks.[Citation74] Number of NK cells were restored to original in smokers simply by 3 weeks daily intake of L. casei.[Citation75] Therefore, probiotics positively effects NK cells functioning. But with the passage of time, probiotics efficiency seem to decrease, gathering scientist’s attention.[Citation76]
Oral administration of fermented milk with Lactobacillus helveticus R389 demonstrated immunoregulatory response in breast cancer bearing mice and suggested its use as immune adjuvant therapy to protect against malignancies.[Citation77] Yazdi et al. not only reported an increase in inferon (IFN)-γ and IL-2 levels but also shows enhanced NK cell activity in selenium nanoparticles enriched Lactobacillus plantarum-treated mice. These results illustrated that this administration modulated immune responses. IFN-γ/IL-4 rise upon administration of selenium nanoparticles enriched L. plantarum may highlight a Th1 bias of immune response. Reduced tumor growth along with increased survival rate in 4T1 breast cancer mice model showed that selenium nanoparticles enriched L. plantarum may persuade a more effective antitumor response among them.[Citation78] Furthermore, oral administration of selenium nanoparticles enriched Lactobacillus brevis is suggested to be related with better disease prognosis among highly metastatic breast cancer bearing mice.[Citation79]
Other studies also reported similar kind of results in breast cancer bearing mice that administration of L. acidophilus altered cytokine production in a Th1 protective manner, promising antitumor immunity. Therefore, there is a possibility to use lactobacilli or other probiotic microorganisms as adjuvant treatment in anticancer chemotherapy.[Citation80,Citation81] To date, data representing correlation between probiotics and NK cells are quite insufficient, therefore, advanced studies are still required to explore the underlying mechanisms.
Reduction of DNA damage by probiotics
Cell susceptibility to DNA damage and capability for restoring this damage is essential for cancer induction, elevation, and evolution. Increased DNA damage was observed in breast cancer patients as compared to normal healthy controls.[Citation82,Citation83] BRCA1, PTEN, and TP53 genes are involved in DNA repair mechanism but any alteration would lead to increased breast cancer risk.[Citation84–Citation86] Loss of heterozygosity, followed by loss of expression of genes for DNA damage response or alteration of cell cycle control, results in inherited breast cancer in pre-menopausal years.[Citation83,Citation85,Citation86]
In-vivo and in-vitro studies showed that certain probiotics procured with antigenotoxic activity. L. casei Shirota inhibits DNA damage in the rat model on exposure to mutagen (N-methyl-N-nitro, N-nitrosoguanidine).[Citation87] Different lactobacilli species showed antigenotoxicity in rats against 1,2-dimethyl hydrazine (carcinogen) in specie specific manner. L. acidophilus when treated with heat lost antigenotoxic effect thus showing viable organism as a liability. In-vitro studies showed antimutagenic activity of consortium of probiotic strains including E. coli, L. casei, and Bifidobacterium longum.[Citation88] DNA damage was expressively reduced by Bifidobacterium sp. 420, Bifidobacterium Bb12, Enterococcusfaecium, and Lactobacillus bulgaricus. Bifidobacterium Bb12 was highly defensive against DNA damage. Incubating the different concentrations of Bifidobacterium Bb12 and L. plantarumin faecal water showed reduced genotoxicity. Heat-treated (nonviable) probiotics had no effect on faecal water genotoxicity.[Citation89]
It was thought that breast cancer risk can be reduced in perimenopausal women with probiotic supplementation. But it was reported that short-term probiotic and soy supplementation did not have distinct effect on the hormonal profile of these women.[Citation90] Combined long-term exposure of L. casei and soy milk proved to be beneficial in breast cancer prevention among chemically treated rats.[Citation91] In Japanese women, regular consumption of L. casei and soy isoflavone since adolescence was significantly associated with decreased breast cancer risk.[Citation92] So, long-term exposure was required to accomplish chemopreventive effect on cancer development. Therefore, it might open a new era in cancer treatment particularly using natural sources of probiotics along with some bacterial strains.
Immunomodulatory functions of probiotics
Probiotics played an influential role in modulating gastrointestinal health. Most of the reported mechanisms for this modulation were suppression of pathogenic bacteria by producing competitor environment.[Citation93,Citation94] Potential applications of probiotics are being expanded beyond alleviating gastrointestinal disorders to include benefits involving antihypertension, immunomodulation, improving serum lipid profiles, and alleviation of postmenopausal disorders. All the aforementioned benefits are explored in cell-based experiments and scientists are looking for in-vivo justifications.
Probiotic organisms are not only meant for immune systems encouragement but have many other useful properties. Saccharomyces cerevisiae Boulardii, (Biocodex) Bifidobacterium animalis Bb-12 (Chr. Hansen), L. casei Shirota (Yakult), Bifidobacterium lactis DR10, Lactobacillus johnsonii La1, and Lactobacillus rhamnosus GG (Valio, Culturelle) are known for their immunomodulatory characteristics.[Citation34,Citation95,Citation96] Probiotics probably elevates immunologic barrier via inflammatory responses and enhancing intestinal immunoglobulin A (IgA).[Citation94,Citation97,Citation98] Probiotics can enhance cellular, nonspecific immune response by activation of macrophages, NK cells, antigen-specific cytotoxic T-lymphocytes, release of various cytokines in dosage dependent manner strain specific manner. Supplementation of probiotic organism at earlier ages could help to avoid immune-mediated illnesses. Probiotics also played pivotal role in pregnancy. They may have positive effects on fetal immunity particularly on transforming growth factor-β1 levels, cord blood IFN-γ levels and breast milk IgA. Yogurt or fermented milk may also deliver probiotics to improve mucosal immune system of the gut via cytokine-producing cells and enhancing number of IgA+ cells in effector wall of intestine.[Citation38,Citation96]
Inhibitory effects of probiotics
Probiotics can be used as natural cancer therapeutic agents due to little cytotoxic activity. Probiotics also produce specific growth inhibitors targeting no other than cancer cells.[Citation99,Citation100] Probiotic strains including B. animalis, L. acidophilus, Bifidobacterium infantis, Lactobacillus paracasei, Bifidobacterium bifidum were observed to reduce cancer cell growth in MCF7 cells.[Citation101,Citation102] L. helveticus R389 was involved in increased immunity booster; cytokines (including IL-10 and IL-4) and decreased growth of tumor cells when receive 4T1 mouse mammary adenocarcinoma cells injection subcutaneous to murine model (BALB/c).[Citation77,Citation102]
Enterococcus lactis IW5 produce metabolites having cytotoxic potential on various cancer cells. These metabolites after 24 h of incubation reduce the growth of breast cancer cells. These cytotoxic metabolites had antiproliferative effect positively different from E. lactis IW5 treated group as compared to the untreated control group. Antiproliferative properties of proteinaceous postbiotic metabolites (PPM) produced by L. plantarum I-UL4 on MFC7 cancerous cells were evaluated. Probiotic bacteria were grown on modified MRS (De Man, Rogosa and Sharpe agar) media supplemented with Tween 80 and the metabolites (PPM) produced were cytotoxic toward MFC7 cells in dosage and time-dependent manner. The greatest intoxicating antiproliferative effect showed by the lowest viable cell count was witnessed at 24 h of incubation. Moreover 80.87% of apoptotic MCF7 cells were observed at 48 h incubation. Results showed that the probiotic strain L. plantarum I-UL4 producing PPM significantly reduce the number of cancer cells, in the media supplemented with Tween 80.[Citation103] Probiotic strains also showed antineoplastic activities inhibiting mammary tumor in animal models, emphasizing hypothetically universal effects of probiotics as anticarcinogenic agents.[Citation104–Citation107]
Prebiotics, synbiotics, and breast cancer
Prebiotics are low molecular weight carbohydrates (2–10 carbons) which are not easily digested, including certain resistant starches and fibers.[Citation89] Widely describe prebiotics are nondigestible oligosaccharides, e.g., fructo-oligosaccharides. In the human intestine, they are poorly digested reaching unaltered to the colon acting as a substrate for the gut microflora. Number of Lactobacilli and Bifidobacteria were specifically stimulated by these prebiotics at the expense of another pathogenic microflora. Burns and Rowland extensively reviewed the anticarcinogenicity of probiotics and prebiotics.[Citation108,Citation109] Supplements containing probiotics and prebiotics had shielding effects against a wide range of procedures including induction of DNA damage in the colonic mucosa of rats.[Citation89,Citation110]
With probable anticancer properties, identifying probiotics, prebiotics, and synbiotics is expensive and time consuming, due to variety and number of these agents when working on animal models. In-vitro approaches are more practical alternative. The vital importance of DNA damage in the initiation and progression of cancer and reduction in genotoxicity by synbiotics is considered a highly applicable endpoint.[Citation111] Another study suggested preventive role of prebiotics/dietary fibers in chemically induced mammary cancer using mouse model.[Citation109]
Kassayova et al. worked on murine model providing them with a probiotic strain L. plantarum along with its associated prebiotic inulin while inducing mammary carcinogenesis to the animal by 7,12dimethylbena anthracene. Tumor frequency was significantly reduced using a combination of pro and prebiotics. Furthermore, it was observed that L. plantarum is very effective (via immunomodulatory mechanism) against breast cancer even in the absence of prebiotics.[Citation77,Citation109]
Conclusion
Probiotics have a vast range of beneficial properties and gained an increasing medical importance over the last decade. Probiotics enhance immune system and anti-inflammatory activities. Long-term consumption of probiotics was significantly associated with suppression of breast cancer formation and proliferation. Laboratory-based investigations including animal model and human breast cancer cells had shown antitumor effects of probiotics, but further studies involving humans in clinical trials are required to understand insight mechanisms and to use probiotics as cancer therapeutics. Probiotics are more cost effective and have less harmful effects as compared to various pharmacological applications like monoclonal antibodies. Therapeutic application of probiotics gained more interest for the role of gut microbiota in disease and health. Future success of probiotics depends upon continued explication of complex interactions between microbiota and host cells. In general, food containing probiotic producing microorganisms should be the part of routine diet to practice healthy life style.
References
- Siegel, R. L.; Miller, K. D.; Jemal, A. Cancer Statistics. CA: A Cancer Journal for Clinicians 2016, 66(1), 7–30.
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer Statistics. CA: A Cancer Journal for Clinicians 2010, 60(5), 277–300.
- Anderson, K. N.; Schwab, R. B.; Martinez, M. E. Reproductive Risk Factors and Breast Cancer Subtypes: A Review of the Literature. Breast Cancer Research Treatment 2014, 144(1), 1–10.
- Barnard, M. E.; Boeke, C. E.; Tamimi, R. M. Established Breast Cancer Risk Factors and Risk of Intrinsic Tumor Subtypes. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 2015, 1856(1), 73–85.
- Malik, S. S.; Masood, N.; Yasmin, A. Prostate Cancer and Glutathione S-Transferase Deletions. EXCLI J 2015, 14, 1049.
- Xing, P.; Li, J.; Jin, F. A Case–Control Study of Reproductive Factors Associated with Subtypes of Breast Cancer in Northeast China. Medical Oncology 2010, 27(3), 926–931.
- Tamimi, R. M.; Colditz, G. A.; Hazra, A.; Baer, H. J.; Hankinson, S. E.; Rosner, B.; Marotti, J.; Connolly, J. L.; Schnitt, S. J.; Collins, L. C. Traditional Breast Cancer Risk Factors in Relation to Molecular Subtypes of Breast Cancer. Breast Cancer Research Treatment 2012, 131(1), 159–167.
- Saxena, T.; Lee, E.; Henderson, K. D.; Clarke, C. A.; West, D.; Marshall, S. F.; Deapen, D.; Bernstein, L.; Ursin, G. Menopausal Hormone Therapy and Subsequent Risk of Specific Invasive Breast Cancer Subtypes in the California Teachers Study. Cancer Epidemiology Biomarkers and Prevention 2010, 19(9), 1055–9965.
- Gaudet, M. M.; Press, M. F.; Haile, R. W.; Lynch, C. F.; Glaser, S. L.; Schildkraut, J.; Gammon, M. D.; Thompson, W. D.; Bernstein, J. L. Risk Factors by Molecular Subtypes of Breast Cancer across a Population-Based Study of Women 56 Year or Younger. Breast Cancer Research and Treatment 2011, 130(2), 587–597.
- Razzaghi, H.; Troester, M. A.; Gierach, G. L.; Olshan, A. F.; Yankaskas, B. C.; Millikan, R. C. Association between Mammographic Density and Basal-Like and Luminal A Breast Cancer Subtypes. Breast Cancer Research 2013, 15(5), 76.
- Sieri, S.; Pala, V.; Brighenti, F.; Agnoli, C.; Grioni, S.; Berrino, F.; Scazzina, F.; Palli, D.; Masala, G.; Vineis, P.; et al. High Glycemic Diet and Breast Cancer Occurrence in the Italian EPIC Cohort. Nutrition, Metabolism and Cardiovascular Diseases 2013, 23(7), 628–634.
- Khan, A. A.; Khurshid, M.; Khan, S.; Alshamsan, A. Gut Microbiota and Probiotics: Current Status and Their Role in Cancer Therapeutics. Drug Development Research 2013, 74, 365–375.
- Maldonado Galdeano, C.; de Moreno de LeBlanc, A.; Vinderola, G.; Bibas Bonet, M. E.; Perdigo, G. Proposed Model: Mechanisms of Immunomodulation Induced by Probiotic Bacteria. Clinical and Vaccine Immunology 2007, 14(5), 485–492. DOI:10.1128/CVI.00406-06.
- Ranadheera, R. D. C. S.; Baines, S. K.; Adams, M. C. Importance of Food in Probiotic Efficacy. Food Res., Intl 2010, 43, 1–7.
- Fariq, A.; Production, S. A. Biomedical Applications of Probiotic Biosurfactants. Current Microbiology 2016, 72, 489–495.
- Jost, T.; Lacroix, C.; Braegger, C. P.; Rochat, F.; Chassard, C. Vertical Mother–Neonate Transfer of Maternal Gut Bacteria via Breastfeeding. Environmental Microbiology 2014, 16(9). 2891–2904. DOI:10.1111/1462-2920.12238.
- Gibson, G. R.; Fuller, R. Aspects of In-Vitro and In-Vivo Research Approaches Directed Towards Identifying Probiotics and Probiotics for Human Use. Journal of Nutrition 2000, 130, 391–395.
- Kumar, M.; Kumar, A.; Nagpal, R.; Mohania, D.; Behare, P.; Verma, V.; Kumar, P.; Poddari, D.; Aggarwal, P. K.; Henry, C. J. K.; et al. Attributes of Probiotics: An Update. IInternational Journal of Food Sciences and Nutrition 2010, 61(5), 473–496.
- Chong, E. S. L.; Potential, A. Role of Probiotics in Colorectal Cancer Prevention: Review of Possible Mechanisms of Action. World Journal of Microbiology and Biotechnology. 2013. DOI:10.1007/s11274-013-1499-6.
- Argyri, A. A.; Zoumpopoulou, G.; Karatzas, K. G.; Tsakalidou, E.; Nychas, G. E.; Panagou, E. Z.; Tassou, C. C. Selection of Potential Probiotic Lactic Acid Bacteria from Fermented Olives by In-Vitro Tests. Food Microbiology 2013, 33, 282–291.
- Verna, E.; Lucak, S. Use of Probiotics in Gastrointestinal Disorders: What to Recommend?. Therapeutic Advances in Gastroenterology 2010, 3, 307–319.
- Efficacy, K. T. Safety of the Probiotic Saccharomyces Boulardii for the Prevention and Therapy of Gastrointestinal Disorders. Therapeutic Advances in Gastroenterology 2012, 5(2), 111–125.
- Morrow, L. E.; Gogineni, V.; Malesker, M. A. Probiotics in the Intensive Care Unit. Nutrition in Clinical Practice 2012, 27(2), 235–241.
- Hoque, M. Z.; Akter, F.; Hossain, K. M.; Rahman, M. S. M.; Billah, M. M.; Islam, K. M. D.; Isolation, I. Analysis of Probiotic Properties of Lactobacillus Spp. From Selective Regional Yoghurts. World Journal of Dairy & Food Sciences 2010, 5(1), 39–46.
- Khan, A. A.; Shrivastava, A.; Khurshid, M. Normal to Cancer Microbiome Transformation and Its Implication in Cancer Diagnosis. Biochimica et Biophysica Acta (BBA) 2012, 1826, 331–337.
- Lankaputhra, W. E. V.; Shah, N. P. Antimutagenic Properties of Probiotic Bacteria and of Organic Acids. Mutation Research 1998, 397, 169–182.
- Wollowski, I.; Rechkemmer, G.; Pool-Zobel, B. L. Protective Role of Probiotics and Prebiotics in Colon Cancer. The American Journal of Clinical Nutrition 2001, 73, 451–455.
- LeBlanc, J. G.; Matar, C.; Valdez, J. C.; LeBlanc, J.; Perdigon, G. Immunomodulating Effects of Peptidic Fractions Issued from Milk Fermented with Lactobacillus Helveticus. Journal of Dairy Science 2002, 85, 2733–2742.
- Le Leu, R. K.; Brown, I. L.; Hu, Y.; Bird, A. R.; Jackson, M.; Esterman, A.; Young, G. P.; Synbiotic, A. Combination of Resistant Starch and Bifidobacterium Lactis Facilitates Apoptotic Deletion of Carcinogen-Damaged Cells in Rat Colon. Journal of Nutrition 2005, 135, 996–1001.
- Hlivak, P.; Odraska, J.; Ferencik, M.; Ebringer, L.; Jahnova, E.; One-Year, M. Z. Application of Probiotic Strain Enterococcus Faecium M-74 Decreases Serum Cholesterol Levels. Bratislavsk´e l´ek´a¡rske listy 2005, 106, 67–72.
- Hsieh, M. L.; Chou, C. C. Mutagenicity and Antimutagenic Effect of Soymilk Fermented with Lactic Acid Bacteria and Bifidobacteria. International Journal of Food Microbiology 2006, 111, 43–47.
- Maldonado Galdeano, C.; Perdigion, G. The Probiotic Bacterium Lactobacillus Casei Induces Activation of the Gut Mucosal Immune System through Innate Immunity. Clinical and Vaccine Immunology 2006, 13, 219–226.
- Shah, N. P.; Functional Cultures and Health Benefits. In: International- Dairy-Federation Symposium on Rheology and Structure of Fermented Milk, Elsevier Science Ltd, Oxford, England/Sirmione, Italy, 2006; 35–36.
- Shah, N. P. Functional Cultures and Health Benefits. International Dairy Journal 2007, 17, 1262–1277.
- Vasiljevic, T.; Shah, N. P. Fermented Milk-Health Benefits beyond Probiotic Effect. In Handbook of Food Products Manufacturing; Hui, Y. H., Ed..; John Wiley and Sons, Inc: Hoboken, NJ, 2007.
- Amit-Romach, E.; Uni, Z.; Reifen, R. Therapeutic Potential of Two Probiotics in Inflammatory Bowel Disease as Observed in the Trinitrobenzene Sulfonic Acid Model of Colitis. Diseases of the Colon & Rectum 2008, 51, 1828–1836.
- Paineau, D.; Carcano, D.; Leyer, G.; Darquy, S.; Alyanakian, M. A.; Simoneau, G.; Bergmann, J. F.; Brassart, D.; Bornet, F.; Ouwehand, A. C. Effects of Seven Potential Probiotic Strains on Specific Immune Responses in Healthy Adults: A Double-Blind, Randomized, Controlled Trial. FEMS Immunology and Medical Microbiology 2008, 53, 107–113.
- Prescott, S. L.; Wickens, K.; Westcott, L.; Jung, W.; Currie, H.; Black, P. N.; Stanley, T. V.; Mitchell, E. A.; Fitzharris, P.; Siebers, R.; et al. Supplementation with Lactobacillus Rhamnosus or Bifidobacterium Lactis Probiotics in Pregnancy Increases Cord Blood Interferon-γ and Breast Milk Transforming Growth Factor-β and Immunoglobin A Detection. Clinical & Experimental Allergy 2008, 38, 1606–1614.
- Pronio, A.; Montesani, C.; Butteroni, C.; Vecchione, S.; Mumolo, G.; Vestri, A.; Vitolo, D.; Boirivant, M. Probiotic Administration in Patients with Ileal Pouch-Anal Anastomosis for Ulcerative Colitis Is Associated with Expansion of Mucosal Regulatory Cells. Inflammatory Bowel Diseases 2008, 14, 662–668.
- Probiotics, R. G. Prebiotics—Progress and Challenges. International Dairy Journal 2008, 18, 969–975.
- Ryan, K. A.; O’Hara, A. M.; Van Pijkeren, J. P.; Douillard, F. P.; O’Toole, P. W. Lactobacillus Salivarius Modulates Cytokine Induction and Virulence Factor Gene Expression in Helicobacter Pylori. Journal of Medical Microbiology 2009, 58, 996–1005.
- Van Hai, N.; Buller, N.; Fotedar, R. The Use of Customised Probiotics in the Cultivation of Western King Prawns (Penaeus Latisulcatus Kishinouye, 1896). Fish & Shellfish Immunology 2009, 27, 100–104.
- Aragon, F.; Perdigon, G.; De LeBlanc, A. D. Modification in the Diet Can Induce Beneficial Effects against Breast Cancer. World Journal of Clinical Oncology 2014, 5(3), 455.
- Chajes, V.; Romieu, I. Nutrition and Breast Cancer. Maturitas 2014, 77, 7–11.
- Thomson, C. A. Diet and Breast Cancer: Understanding Risks and Benefits. Nutrition in Clinical Practice 2012, 27(5), 636–650.
- Rodriguez, S. F.; Aguilar, M. A.; Manuel-y-Keenoy, B. Influence of Body Weight on the Prognosis of Breast Cancer Survivors; Nutritional Approach after Diagnosis. Nutricion Hospitalaria 2013, 28(6), 1829–1841.
- Chaudhry, Z. W.; Brown, R. V.; Fawole, O. A.; Wilson, R.; Gudzune, K. A.; Maruthur, N. M.; Segal, J.; Hutfless, S. M. Comparative Effectiveness of Strategies to Prevent Weight Gain among Women with and at Risk for Breast Cancer: A Systematic Review. SpringerPlus 2013, 2(1), 277.
- Patterson, R. E.; Flatt, S. W.; Newman, V. A.; Natarajan, L.; Rock, C. L.; Thomson, C. A.; Caan, B. J.; Parker, B. A.; Pierce, J. P. Marine Fatty Acid Intake Is Associated with Breast Cancer Prognosis. Journal of Nutrition 2011, 141(2), 201–206.
- De Lorgeril, M.; Salen, P. New Insights into the Health Effects of Dietary Saturated and Omega-6 and Omega-3 Polyunsaturated Fatty Acids. BMC Medicine 2012, 10(1), 50.
- Cho, K.; Mabasa, L.; Fowler, A. W.; Walsh, D. M.; Park, C. S. Canola Oil Inhibits Breast Cancer Cell Growth in Cultures and In-Vivo and Acts Synergistically with Chemotherapeutic Drugs. Lipids 2010, 45(9), 777–784.
- Mabasa, L.; Cho, K.; Walters, M. W.; Bae, S.; Park, C. S. Maternal Dietary Canola Oil Suppresses Growth of Mammary Carcinogenesis in Female Rat Offspring. Nutrition and Cancer 2013, 65(5), 695–701.
- Aune, D.; Chan, D. S.; Vieira, A. R.; Rosenblatt, D. N.; Vieira, R.; Greenwood, D. C.; Fruits, N. T. Vegetables and Breast Cancer Risk: A Systematic Review and Meta-Analysis of Prospective Studies. Breast Cancer Research and Treatment 2012, 134(2), 479–493.
- Collaborative Group on Hormonal Factors in Breast Cancer. Alcohol, tobacco and breast cancer–collaborative reanalysis of individual data from. 53 Epidemiological Studies, Including 58 515 Women with Breast Cancer and 95 067 Women without the Disease. British Journal of Cancer 2002, 87(11), 1234.
- Chi, F.; Wu, R.; Zeng, Y. C.; Xing, R.; Liu, Y.; Xu, Z. G. Post-Diagnosis Soy Food Intake and Breast Cancer Survival: A Meta-Analysis of Cohort Studies. Asian Pacific Journal of Cancer Prevention 2013, 14(4), 2407–2412.
- Nagata, C.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Inoue, M.; Tsugane, S.; Sasazuki, S. Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Soy Intake and Breast Cancer Risk: An Evaluation Based on a Systematic Review of Epidemiologic Evidence among the Japanese Population. Japanese Journal of Clinical Oncology 2014, 44(3), 282–295.
- Setchell, K. D. Soy Isoflavones—Benefits and Risks from Nature’s Selective Estrogen Receptor Modulators (Serms). Journal of the American College of Nutrition 2001, 20(5), 354–362.
- Montales, M. T.; Rahal, O. M.; Kang, J.; Rogers, T. J.; Prior, R. L.; Wu, X.; Simmen, R. C. Repression of Mammosphere Formation of Human Breast Cancer Cells by Soy Isoflavone Genistein and Blueberry Polyphenolic Acids Suggests Diet-Mediated Targeting of Cancer Stem-Like/Progenitor Cells. Carcinogenesis 2012, 33(3), 652–660.
- Montales, M. T.; Rahal, O. M.; Nakatani, H.; Matsuda, T.; Simmen, R. C. Repression of Mammary Adipogenesis by Genistein Limits Mammosphere Formation of Human MCF-7 Cells. Journal of Endocrinology 2013, 218(1), 135–149.
- Lajous, M.; Lazcano-Ponce, E.; Hernandez-Avila, M.; Willett, W.; Folate, R. I. Vitamin B6, and Vitamin B12 Intake and the Risk of Breast Cancer among Mexican Women. Cancer Epidemiology, Biomarkers & Prevention 2006, 15(3), 443–448.
- Shrubsole, M. J.; Gao, Y. T.; Cai, Q.; Shu, X. O.; Dai, Q.; Hébert, J. R.; Jin, F.; Zheng, W. MTHFR Polymorphisms, Dietary Folate Intake, and Breast Cancer Risk. Cancer Epidemiology, Biomarkers & Prevention 2004, 13(2), 190–196.
- Gao, C. M.; Tang, J. H.; Cao, H. X.; Ding, J. H.; Wu, J. Z.; Wang, J.; Liu, Y. T.; Li, S. P.; Su, P.; Matsuo, K.; et al. Dietary Folate Intake and Breast Cancer Risk in Chinese Women. Journal of Human Genetics 2009, 54(7), 1–8.
- Alshatwi, A. A. Breast Cancer Risk, Dietary Intake and Methylenetetrahydrofolate Reductase (MTHFR) Single Nucleotide Polymorphisms. Food and Chemical Toxicology 2010, 48(7), 1881–1885.
- Castillo, L. C.; Tur, J. A.; Folate, U. R. Breast Cancer Risk: A Systematic Review. Revista medica de Chile 2012, 140(2), 251–260.
- Yan, L.; DeMars, L. C. Dietary Supplementation with Methylseleninic Acid, but Not Selenomethionine, Reduces Spontaneous Metastasis of Lewis Lung Carcinoma in Mice. International Journal of Cancer 2012, 131(6), 1260–1266.
- Bassett, J. K.; Baglietto, L.; Hodge, A. M.; Severi, G.; Hopper, J. L.; English, D. R.; Giles, G. G. Dietary Intake of B Vitamins and Methionine and Breast Cancer Risk. Cancer Causes Control 2013, 24(8), 1555–1563.
- Ha, A. W.; Hong, K. H.; Kim, H. S.; Kim, W. K. Inorganic Sulfur Reduces Cell Proliferation by Inhibiting of ErbB2 and ErbB3 Protein and mRNA Expression in MDA-MB-231 Human Breast Cancer Cells. Nutrition Research and Practice 2013, 7(2), 89–95.
- Reid, G.; Sanders, M. E.; Gaskins, H. R.; Gibson, G. R.; Mercenier, A.; Rastall, R. M.; Roberfroid, M.; Rowland, I.; Cherbut, C.; Klaenhammer, T. R. New Scientific Paradigms for Probiotics and Prebiotics. Journal of Clinical Gastroenterology 2003, 37, 105–118.
- Kinjo, Y.; Wu, D.; Kim, G.; Guo-Wen, X. Recognition of Bacterial Glycosphingolipids by Natural Killer T Cells. Nature 2005, 434(7032), 520.
- Terabe, M.; Berzofsky, J. A. N. K. T. Cells in Immunoregulation of Tumor Immunity: A New Immunoregulatory Axis. Trends in Immunology 2007, 28(11), 491–496.
- Hix, L. M.; Shi, Y. H.; Brutkiewicz, R. R.; Stein, P. L.; Wang, C. R.; Zhang, M. CD1d-Expressing Breast Cancer Cells Modulate NKT Cell-Mediated Antitumor Immunity in a Murine Model of Breast Cancer Metastasis. PLOS One 2011, 6(6), 202–207.
- Modarressi, R. E.; Daneshvar, M.; Beigom, M.; Mobasheri, E. M. Lactobacillus Acidophilus and Lactobacillus Crispatus Culture Supernatants Downregulate Expression of Cancer-Testis Genes in the MDA-MB-231 Cell Line. Asian Pacific Journal of Cancer Prevention 2014, 15(10), 4255–4259.
- Lee, J. H.; Nam, S. H.; Seo, W. T.; Yun, H. D.; Hong, S. Y.; Kim, M. K.; Cho, K. M. The Production of Surfactin during the Fermentation of Cheonggukjang by Potential Probiotic Bacillus Subtilis CSY191 and the Resultant Growth Suppression of MCF-7 Human Breast Cancer Cells. Food Chemistry 2012, 131, 1347–1354.
- Soltan Dallal, M. M.; Yazdi, M. H.; Holakuyee, M.; Hassan, Z. M.; Abolhassani, M.; Mahdavi, M. Lactobacillus Casei Ssp.Casei Induced Th1 Cytokine Profile and Natural Killer Cells Activity in Invasive Ductal Carcinoma Bearing Mice. Iranian Journal of Allergy, Asthma and Immunology 2012, 11, 183–189.
- Takeda, K.; Okumura, K. Effects of a Fermented Milk Drink Containing Lactobacillus Casei Strain Shirota on the Human NK Cell Activity. Journal of Nutrition 2007, 137(3), 791–793.
- Morimoto, K.; Takeshita, T.; Nanno, M.; Tokudome, S.; Nakayama, K. Modulation of Natural Killer Cell Activity by Supplementation of Fermented Milk Containing Lactobacillus Casei in Habitual Smokers. Preventive Medicine 2005, 40(5), 589–594.
- Ho, Y. H.; Lu, Y. C.; Chang, H. C.; Lee, S. Y.; Tsai, M. F.; Huang, Y. T.; Hsu, T. Y. Daily Intake of Probiotics with High IFN-γ/IL-10; ratio increases the cytotoxicity of human natural killer cells: A personalized probiotic approach. Journal of Immunology Research, 2014, 1–7. https://www.hindawi.com/journals/jir/2014/721505/
- de Moreno de LeBlanc, A.; Matar, C.; LeBlanc, N.; Perdigon, G. Effects of Milk Fermented by Lactobacillus Helveticus R389 on a Murine Breast Cancer Model. Breast Cancer Research 2005, 7, 477–486.
- Yazdi, M. H.; Mahdavi, M.; Kheradmand, E.; Shahverdi, A. R. The Preventive Oral Supplementation of a Selenium Nanoparticle Enriched Probiotic Increases the Immune Response and Lifespan of 4T1 Breast Cancer Bearing Mice. Arzneimittel-Forschung 2012, 62, 525–531.
- Yazdi, M. H.; Mahdavi, M.; Setayesh, N.; Esfandyar, M.; Shahverdi, A. R.; Nanoparticle-Enriched, S. Lactobacillus Brevis Causes More Efficient Immune Responses In-Vivo and Reduces the Liver Metastasis in Metastatic Form of Mouse Breast Cancer. DARU Journal of Pharmaceutical Sciences 2013, 21(1), 33.
- Maroof, H.; Hassan, Z. M.; Mobarez, A. M.; Mohamadabadi, M. A. Lactobacillus Acidophilus Could Modulate the Immune Response against Breast Cancer in Murine Model. Journal of Clinical Immunology 2012, 32(6), 1353–1359.
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A. Y.; Potential, P. Biotherapeutic Effects of Newly Isolated Vaginal Lactobacillus Acidophilus 36YL Strain on Cancer Cells. Anaerobe 2014, 28, 29–36.
- Synowiec, E.; Stefanska, J.; Morawiec, Z.; Blasiak, J.; Wozniak, K. Association between DNA Damage, DNA Repair Genes Variability and Clinical Characteristics in Breast Cancer Patients. Mutation Research 2008, 648, 65–72.
- Davis, P. J.; Davis, F. B.; Mousa, S. A.; Luidens, M. K.; Lin, H. Y. Membrane Receptor for Thyroid Hormone: Physiologic and Pharmacologic Implications. Annual Review of Pharmacology and Toxicology 2011, 51, 99–115.
- Walsh, M.; Gardiner, G.; Hart, O.; Lawlor, P.; Daly, M.; Lynch, B. Predominance of a Bacteriocin-Producing Lactobacillus Salivarius Component of a Five-Strain Probiotic in the Porcine Ileum and Effects on Host Immune Phenotype. FEMS Microbiology and Ecology 2008, 64(2), 317–327. DOI:10.1111/j.1574-6941.2008.00454.x.
- Jara, L.; Dubois, K.; Gaete, D.; De Mayo, T.; Ratkevicius, N.; Bravo, T.; Margarit, S.; Blanco, R.; Gómez, F.; Waugh, E.; et al. Variants in DNA Double-Strand Break Repair Genes and Risk of Familial Breast Cancer in a South American Population. Breast Cancer Research and Treatment 2010, 122, 813–822.
- Gonzalez-Angulo, A. M.; Timms, K. M.; Liu, S.; Chen, H.; Litton, J. K.; Potter, J.; Lanchbury, J. S.; Stemke-Hale, K.; Hennessy, B. T.; Arun, B. K.; et al. Outcome of BRCA Mutations in Unselected Patients with Triple Receptor-Negative Breast Cancer. Clinical Cancer Research 2011, 17, 1082–1089.
- Dolara, P.; Luceri, C.; De Filippo, C.; Femia, A. P.; Giovannelli, L.; Caderni, G.; Cresci, A. Red Wine Polyphenols Influence Carcinogenesis, Intestinal Microflora, Oxidative Damage and Gene Expression Profiles of Colonic Mucosa in F344 Rats. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2005, 591(1), 237–246.
- Commane, D.; Hughes, R.; Shortt, C.; Rowland, I. The Potential Mechanisms Involved in the Anti-Carcinogenic Action of Probiotics. Mutation Research 2005, 591, 276–289.
- Burns, A. J.; Rowland, I. R. Antigenotoxicity of Probiotics and Prebiotics on Faecal Water-Induced DNA Damage in Human Colon Adenocarcinoma Cells. Mutation Research 2004, 551, 233–243.
- McMullen, M. H.; Hamilton-Reeves, J. M.; Bonorden, M. J.; Wangen, K. E.; Phipps, W. R.; Feirtag, J. M.; Kurzer, M. S. Consumption of Lactobacillus Acidophilus and Bifidobacterium Longum Does Not Alter Phytoestrogen Metabolism and Plasma Hormones in Men: A Pilot Study. Journal of Alternative and Complementary Medicine 2006, 12(9), 887–894.
- Kaga, C.; Takagi, A.; Kano, M.; Kado, S.; Kato, I.; Sakai, M.; Miyazaki, K.; Nanno, M.; Ishikawa, F.; Ohashi, Y.; et al. Lactobacillus Casei Shirota Enhances the Preventive Efficacy of Soymilk in Chemically Induced Breast Cancer. Cancer Science 2013, 104(11), 1508–1514.
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Hozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, S.; et al. Probiotic Beverage with Soy Isoflavone Consumption for Breast Cancer Prevention: A Case-Control Study. Current Nutrition and Food Science 2013, 9(3), 194–200.
- Liong, M. T. Probiotics: A Critical Review of Their Potential Role as Antihypertensives, Immune Modulators, Hypocholesterolemics, and Perimenopausal Treatments. Nutrition Reviews 2007, 65(7), 316–328.
- Donkor, O. N.; Shah, N. P.; Apostolopoulos, V.; Vasiljevic, T. Development of Allergic Responses Related to Microorganisms Exposure in Early Life. International Dairy Journal 2010, 20, 373–385.
- Kopp, M.; Hennemuth, I.; Heinzmann, A.; Randomized, U. R. Double-Blind, Placebo-Controlled Trial of Probiotics for Primary Prevention: No Clinical Effects of Lactobacillus GG Supplementation. Pediatrics 2008, 121(4), 850–856.
- Ashraf, R.; Shah, N. P. Immune System Stimulation by Probiotic Microorganisms. Critical Reviews in Food Science and Nutrition 2014, 54(7), 938–956.
- Galdeano, C.; de Moreno de LeBlanc, A.; Vinderola, G.; Bibas-Bonet, M. E.; Perdigón, G.; Proposal Model:, A. Mechanisms of Immunomodulation Induced by Probiotic Bacteria. Review. Clinical and Vaccine Immunology 2007, 14, 485–492.
- Dogi, C. A.; Weill, F.; Perdigon, G. Immune Response of Nonpathogenic Gram (+) and Gram (−) Bacteria in Inductive Sites of the Intestinal Mucosa Study of the Pathway of Signaling Involved. Immunobiology 2010, 215, 60–69.
- Choi, S. S.; Kim, Y.; Han, K. S.; You, S.; Oh, S.; Kim, S. H. Effects of Lactobacillus Strains on Cancer Cell Proliferation and Oxidative Stress In-Vitro. Letters in Applied Microbiology 2006, 42, 452–458.
- Hassan, Z.; Mustafa, S.; Rahim, R. A.; Isa, N. M. Anti-Breast Cancer Effects of Live, Heat-Killed and Cytoplasmic Fractions of Enterococcus Faecalis and Staphylococcus Hominis Isolated from Human Breast Milk. In Vitro Cellular and Developmental Biology – Animal 2016, 52, 337–348.
- Hirayama, K.; Rafter, J. The Role of Probiotic Bacteria in Cancer Prevention. Microbes and Infection 2000, 2(6), 681–686.
- Kim, P. I.; Jung, M. Y.; Chang, Y. H.; Kim, S.; Kim, S. J.; Park, Y. H. Probiotic Properties of Lactobacillus and Bifidobacterium Strains Isolated from Porcine Gastrointestinal Tract. Applied Microbiology and Biotechnology 2007, 74(5), 1103–1111.
- Tan, H. K.; Foo, H. L.; Loh, T. C.; Alitheen, N. B. M.; Rahim, R. A. Cytotoxic Effect of Proteinaceous Postbiotic Metabolites Produced by Lactobacillus Plantarum I-UL4 Cultivated in Different Media Composition on MCF-7 Breast Cancer Cell. Malaysian Journal of Microbiology 2015, 11(2), 207–214.
- Saarela, M.; Mogensen, G.; Fonden, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic Bacteria: Safety, Functional and Technological Properties. Journal of Biotechnology 2000, 84(3), 197–215.
- Lim, B. K.; Mahendran, R.; Lee, Y. K.; Bay, B. H. Chemopreventive Effect of Lactobacillus Rhamnosus on Growth of a Subcutaneously Implanted Bladder Cancer Cell Line in the Mouse. Japanese Journal of Cancer Research 2002, 93, 36–41.
- de Moreno de LeBlanc, A.; Matar, C.; Farnworth, E.; Perdigon, G. Study of Immune Cells Involved in the Antitumor Effect of Kefir in a Murine Breast Cancer Model. Journal of Dairy Science 2007, 90, 1920–1928.
- Iyer, C.; Kosters, A.; Sethi, G.; Kunnumakkara, A. B.; Aggarwal, B. B.; Probiotic, V. J. Lactobacillus Reuteri Promotes TNF-induced Apoptosis in Human Myeloid Leukemia-Derived Cells by Modulation of NF-kB and MAPK Signaling. Cellular Microbiology 2008, 10(7), 1442–1452.
- Burns, A. J. R.; Rowland, I. R. Anti-Carcinogenicity of Probiotics and Prebiotics. Intestinal Microbiology 2000, 1(1), 13–24.
- Kassayova, M.; Bobrov, N.; Strojny, L.; Kiskova, T.; Mikes, J.; Demeckova, V. Preventive Effects of Probiotic Bacteria Lactobacillus Plantarum and Dietary Fiber in Chemically-Induced Mammary Carcinogenesis. Anticancer Research 2014, 34(9), 4969–4975.
- De Preter, V.; Raemen, H.; Cloetens, L.; Houben, E.; Rutgeerts, P.; Verbeke, K. Effect of Dietary Intervention with Different Pre-and Probiotics on Intestinal Bacterial Enzyme Activities. European Journal of Clinical Nutrition 2008, 62(2), 225.
- Oberreuther-Moschner, D. L.; Jahreis, G.; Rechkemmer, G.; Pool-Zobel, B. L. Dietary Intervention with the Probiotics Lactobacillus Acidophilus 145 and Bifidobacterium Longum 913 Modulates the Potential of Human Faecal Water to Induce Damage in HT29clone19A Cells. British Journal of Nutrition 2004, 91(6), 925–932.