?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The interaction of trypsin with acteoside was studied using ultraviolet visible absorption, fluorescence, synchronous fluorescence, circular dichroism techniques, along with molecular docking method. The fluorescence experiments indicated that acteoside quenched the intrinsic fluorescence of trypsin via a combined quenching process (static and dynamic quenching). The binding constant of acteoside to trypsin obtained was 2.50 × 105 L mol−1 at 298 K and the number of binding site was about one under the same experimental condition. The thermodynamic functions ΔH° and ΔS° of the binding process were 8.79 kJ mol−1 and 132.58 J mol−1 K−1, respectively, which indicated that the hydrophobic force was the main acting force between them. Ultraviolet–visible, synchronous fluorescence together with circular dichroism spectra studies demonstrated that the interaction of acteoside with trypsin lead to a loosening and unfolding of the protein backbone with partial β-sheet structures being transformed into α-helix structures. All these experimental results were validated and explained reasonably by docking studies. And the molecular docking results further illustrated that besides hydrophobic forces, hydrogen bonds also played an important role in the stabilization of the acteoside–trypsin complex. Results from this study should be helpful to make full use of acteoside in the food industry and be useful to the design of the drugs for the diseases related to trypsin.
Introduction
Herbs have attracted a great deal of interests as a source for the development of drugs and one of the key ingredients in functional foods. Herbal medicines derived from plant extracts are increasingly being utilized to treat a wide variety of clinical diseases.[Citation1] Acteoside (see ) contains the hydroxyphenylethyl and caffeoyl moieties which are well-known antioxidants and was also reported to possess various biological activities, such as anti-stress and immunosuppressive properties, anti-nephritis, cardioactive, and antitumor activities.[Citation2]
Proteases are a kind of important proteins which can cleave the covalent peptide bond between amino acids.[Citation3] The water-soluble globular protein, trypsin (EC 3.4.21.4), belongs to serine protease family and exists in form of trypsinogen in the pancreas of all vertebrates. Trypsin contains two domains of nearly equal size and consists of 223 amino acid residues with molecular weight of 23,300 Da.[Citation4] The major constituent of each domain is a set of six antiparallel strands of polypeptide chain laced together into a β-sheet unit by a network of H-bonds.[Citation5] Trypsin plays important roles in some biological processes in vertebrates, such as digestion and thrombin in blood coagulation cascade,[Citation6,Citation7] and that is why it is intimately related to human health and illness. Due to the importance of trypsin in the digestive system of human, it is often used as a model of the digestive proteases to investigate the interactions of small molecules with proteins in food and medicinal herbs.[Citation8] On the other hand, studies also showed that the excessive activity of trypsin could induce acute pancreatitis, inflammation, and tumor.[Citation9] Therefore, compounds that interact with serine proteinases might be used in the treatment of some diseases related to trypsin.
Besides traditional spectroscopic methods including ultraviolet visible,[Citation10,Citation11] fluorescence, infrared, and circular dichroism (CD),[Citation12,Citation13] some other techniques such as isothermal titration calorimetry[Citation14] and molecular simulation[Citation15,Citation16] have also been proposed to assess the drug-binding ability of biological macromolecules.
In this study, the interaction between trypsin and acteoside was studied using multiple spectroscopic methods and molecular docking technique. Many attempts were made to explore the binding mechanism, the special binding site, and the effect of acteoside on the conformational change of trypsin. The study could provide the basic data for the pharmacokinetics and pharmacodynamics of acteoside in the human body in vitro and should be useful to make full use of acteoside in the food industry. This study might also be helpful to the design of drugs for the diseases related to trypsin.
Materials and methods
Materials
Trypsin (from bovine pancreas, potency ≥3000 units mg−1) was purchased from Aladdin Reagent Co. Ltd. (Shanghai, China) and was used for the present study without any further purification. Its molecular weight was assumed to be 23,300. A phosphate buffer was used to keep the pH value at 8.0 to provide physiological conditions for the chemical reactions in all the solutions. Trypsin was dissolved in this buffer to form the trypsin solution with a concentration of 2.50 × 10−7 mol L−1. Acteoside (purity >98 wt.%) was obtained from College of Medical Science of Shenzhen University. Methods for extraction and purification of acteoside were described in Ref..[Citation17] The stock solution of acteoside (1.00 × 10−3 mol L−1) was also prepared in the buffer mentioned above. All solutions were stored in refrigerator at 4°C in dark. All other chemicals were of analytical grade, and deionized water was used throughout all experiments.
Ultraviolet visible spectra
The UV absorption spectra of trypsin solution in the wavelength ranging from 200 to 400 nm were recorded on a UV-2501PC spectrophotometer (Shimadzu, Kyoto, Japan). The phosphate buffer was used for blank correction. The concentration of trypsin was fixed at 2.50 × 10−7 mol L−1, while the concentration of acteoside was varied from 0 to 6.67 × 10−6 mol L−1 by the successive addition of the stock solution of acteoside (1.00 × 10−3 mol L−1).
Fluorescence spectra
The fluorescence measurements were performed on a F-4500 spectrofluorimeter (Hitachi, Tokyo, Japan) in the wavelength range of 250–500 nm at different temperatures (288, 298, and 310 K) with an excitation wavelength at 280 nm. AMP-10C water bath (Yiheng, China) was used to maintain the temperature of the experimental procedure. A 3.0-mL solution containing 2.50 × 10−7 mol L−1 trypsin was titrated by successive additions of 1.00 × 10−3 mol L−1 acteoside solution and the concentration of acteoside varied from 0 to 6.67 × 10−6 mol L−1. To eliminate the inner filter effect caused by the compounds which were present in the solution, Eq. (1) was used to correct the fluorescence intensity of the protein.[Citation18]
where F and Fobs are the corrected and observed fluorescence intensities, respectively. A1 and A2 are the absorbances at the excitation and emission wavelengths, respectively. All the fluorescence intensities measured in this study were corrected using Eq. (1).
The fluorescence quenching that may occur via static or dynamic mechanism can be described by the Stern–Volmer equation (Eq. 2).[Citation19]
where F and F0 are the fluorescence intensities of trypsin in the presence and absence of acteoside, respectively, Kq is the bimolecular quenching constant, τ0 is the average lifetime of the fluorophore of trypsin in absence of acteoside, [Q] is the concentration of acteoside, and Ksv is the Stern–Volmer quenching constant. The τ0 of trypsin is 2.80 ns according to the reference.[Citation20]
The binding constant Ka and the number of binding sites n for trypsin–acteoside complex were evaluated using the following equation (Eq. 3).[Citation21]
where [Qt] and [Pt] are the total concentrations for the quencher and the protein, respectively.
To analyze the thermodynamics of the interaction of acteoside with trypsin, the Van’t Hoff equation (Eq. (4)) and the Gibbs–Helmholtz equation (Eq. (5)) were used to calculate the thermodynamic parameters for the binding of acteoside to trypsin: enthalpy change (ΔH°), entropy change (ΔS°), and free energy change (ΔG°).
where T is the experimental temperature, and R is the gas constant.
Synchronous fluorescence spectra
The preparation of solutions and the spectrophotometer used were the same as those for above fluorescence spectral measurements. The difference between excitation wavelength and emission wavelength (Δλ) was fixed at 15 and 60 nm, respectively. The synchronous fluorescence measurements thus can provide the characteristic information for Tyr residues and Trp residues within trypsin.
Circular dichroism spectra
CD spectra were measured on a J-810 spectropolarimeter (Jasco, Tokyo, Japan) in a 1-mm path quartz cell at room temperature. The CD spectra of trypsin solution in absence and presence of acteoside were recorded in the wavelength ranging from 200 to 250 nm at a scanning speed of 100 nm min−1. The concentration of trypsin was 2.50 × 10−7 mol L−1. The concentration ratios of acteoside to trypsin were prepared to be 0:1, 3:1, and 7:1, respectively. The secondary structure contents were calculated by use of CDNN Program (Applied Photophysics Ltd., Surrey, UK).[Citation22]
Molecular docking
Docking studies were carried out using Sybyl-X 2.0 software (Tripos, St. Louis, MO, USA). The structure of trypsin was downloaded from the RCSB Protein Data Bank (PDB ID 2PTN). The three-dimensional structure of acteoside was generated from PM3 semiempirical calculations using Chem3D Ultra 6.0 (PerkinElmer Informatics Co., Waltham, MA, USA). The Surflex-Dock program was used to build the possible interaction modes between acteoside and trypsin. The best docked pose was visualized by Chimera molecular graphic software (http://www.cgl.ucsf.edu/chimera/) and Ligplot (http://www.ebi.ac.uk/thornton-srv/software/LigPlus/), respectively.
Results and discussions
Ultraviolet visible spectral studies
Ultraviolet visible absorption spectroscopy can be used to explore the structural change of a protein and to investigate the formation of a protein–drug complex. The ultraviolet visible absorption spectra of trypsin in the absence and presence of acteoside are shown in . The strong absorption peak at 213 nm reflects the framework conformation of the protein (trypsin), and the weak absorption peak at 278 nm appears because of the aromatic amino acids (Trp, Tyr, and Phe).[Citation23] It can be seen from that with gradual addition of acteoside to trypsin solution, the intensity of the peak at 213 nm decreases with obvious red shifts (from 213 to 217 nm), suggesting that the interaction between acteoside and trypsin leads to a loosening and unfolding of the trypsin skeleton.[Citation24] On the contrary, the intensity of the peak at 278 nm increased with slight blue shifts (from 278 to 277 nm) as the increase of the concentration of acteoside, indicating that acteoside can bind to trypsin and a acteoside–trypsin complex is formed, which results in the increase of the probability of π–π* transition of aromatic amino acid residues in trypsin, and the intensity of absorbance peak around 278 nm.[Citation25]
Fluorescence spectral studies
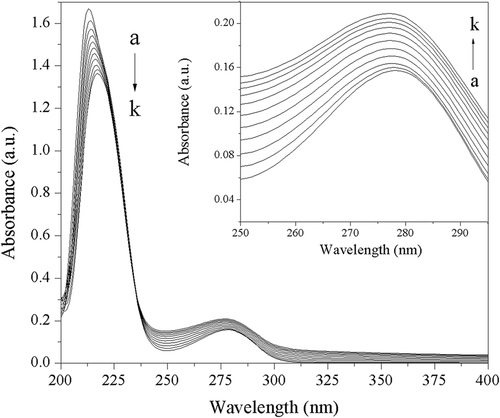
The intrinsic fluorophores of trypsin are four Trp residues, ten Tyr residues, and three Phe residues. Among these aromatic amino acid residues, Trp and Tyr residues are the major contributors of intrinsic fluorescence.[Citation26] The locations of Trp and Tyr residues are shown in ) and ), respectively. The effect of acteoside on fluorescence intensity of trypsin is shown in . It can be seen from that the fluorescence intensity of trypsin decreases regularly with the increase of acteoside concentration.
Figure 3. Positions of tryptophan (a) and tyrosine (b) residues in three-dimensional structure of trypsin.
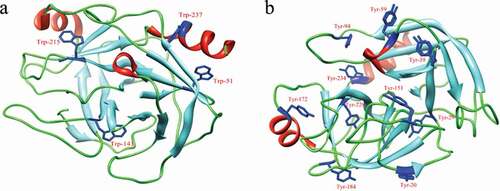
Figure 4. Fluorescence spectra of 2.50 × 10–7 mol L−1 trypsin in absence and presence of acteoside in phosphate buffer (pH 8.0) at 310 K, c(acteoside) (a–k): 0, 0.67, 1.33, 2.00, 2.67, 3.33, 4.00, 4.67, 5.33, 6.00, 6.67 (×10–6 mol L−1), respectively.
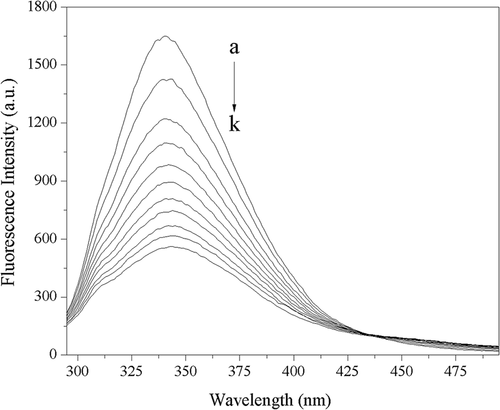
The fluorescence quenching of protein can be divided into three quenching mechanisms, namely, the static quenching due to the formation of the ground-state complex of protein with quenchers, the dynamic quenching caused by the collision between protein and quenchers, and the combined quenching of static quenching and dynamic quenching because of both collision and complex formation with the same quencher.[Citation27] The Stern–Volmer plot of the fluorescence quenching of trypsin by acteoside (figure is not shown) showed an upward curvature, indicating the possibility of a combined quenching (static and dynamic) process.[Citation28] The values of Ksv (L mol−1) at different temperatures were calculated according to Eq. (2) and are listed in . From , it can be found that the Ksv increases with increasing temperature, which suggests that the quenching process is initiated by dynamic quenching.[Citation27] What’s more, the values for Kq at the three different temperatures were all greater than the maximum diffusion collision quenching rate constant (2.0 × 1010 L mol−1 s−1), indicating that the static quenching process is also involved in the system of acteoside and trypsin,[Citation29] which is consistent with the result obtained from the above UV spectral studies. That is to say, the quenching mechanism of trypsin by acteoside is a combined process of both dynamic and static quenchings.
Table 1. Quenching constants for acteoside-trypsin-system at different temperatures.
The binding constants (Ka) and the number of binding sites (n) of acteoside to trypsin were calculated from the intercepts and the slopes of the double-logarithm curves of log[(F0−F)/F] versus log(1/([Qt]−(F0 − F)[Pt]/F0)) at three different temperatures and are listed in . From , it can be seen that the binding constant (Ka) increases with increase in temperature, revealing that the stability of trypsin–acteoside complex increases with the increase of temperature and that the formation of the complex is an endothermic reaction. Furthermore, the number of binding sites calculated for the trypsin–acteoside system was approximately equal to one. This means that there is only one binding site on the trypsin for acteoside.[Citation30]
Table 2. Binding parameters of acteoside to trypsin at different temperatures.
Thermodynamic studies
The acting forces between drugs and biomolecules are composed of weak interactions including hydrogen bond, van der Waals force, electrostatic, and hydrophobic interaction.[Citation31] The thermodynamic parameters, enthalpy change (ΔH°) and entropy change (ΔS°) of binding reactions, are the main evidences to speculate their binding pattern. The signs and magnitudes of thermodynamic parameters account for the main forces contributing to the stability of the protein–drug complex. According to the data obtained for changes in enthalpy (ΔH°) and entropy (ΔS°), the main acting forces stabilizing the drug–protein complex can be evaluated as follows: (1) ΔH°> 0 and ΔS°> 0, hydrophobic forces; (2) ΔH°< 0 and ΔS°< 0, van der Waals interactions and hydrogen bonds; (3) ΔH°< 0 and ΔS°> 0, electrostatic interactions.[Citation32]
The temperature dependence of binding constant was studied using the experimental data at three different temperatures (288, 298, and 310 K). Because the temperature range was not too wide, trypsin would not undergo any structural degradation, and the reaction enthalpy change can be regarded as a constant within the experimental temperature range. The values of ΔH° and ΔS° can thus be determined from the slope and intercept of the graph of ln Ka versus 1/T ()). Accordingly, the free energy change ΔG° can then be obtained from the Gibbs–Helmholtz equation (Eq. (5)). The calculated thermodynamic parameters are displayed in ).
Figure 5. (a) Van’t Hoff plots of lnKa versus 1/T; (b) bar diagram showing thermodynamic parameters (T = 298 K) for binding of acteoside to trypsin.
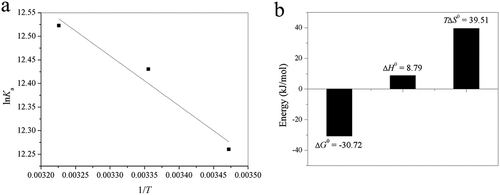
From ), the negative sign for free energy (ΔG°) indicates that the binding process is spontaneous. The ΔH° and ΔS° for the binding interaction between acteoside and trypsin were calculated to be 8.79 kJ mol−1 and 132.58 J mol−1 K−1, respectively. According to the above thermodynamic standpoint, hydrophobic interactions play the main role in binding of acteoside to trypsin.
Synchronous fluorescence spectral studies
The synchronous fluorescence spectra can provide information on the molecular microenvironment in the vicinity of the fluorophores. To understand the conformational change of trypsin induced by acteoside, synchronous fluorescence spectra of trypsin at different values of Δλ with fixed concentration of trypsin and various concentrations of acteosdie were recorded and are shown in .
Figure 6. Synchronous fluorescence spectra of trypsin with different concentrations of acteoside, (a) Δλ = 15 nm, (b) Δλ = 60 nm, pH = 8.0, c(trypsin) = 2.50 × 10–7 mol L−1, c(acteoside) (a–k): 0, 0.67, 1.33, 2.00, 2.67, 3.33, 4.00, 4.67, 5.33, 6.00, 6.67 (×10–6 mol L−1); (c) Quenching of trypsin synchronous fluorescence by acteoside, pH = 8.0, c(trypsin) = 2.50 × 10–7 mol L−1, x-axis represents the concentration of acteoside.
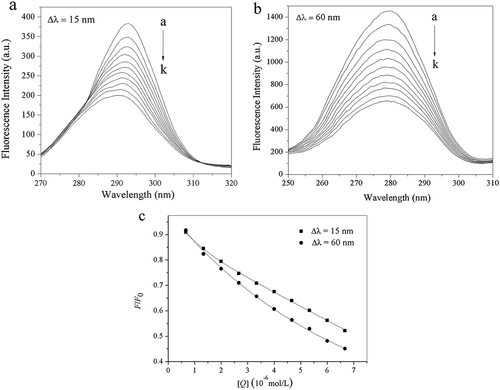
It is indicated from that the fluorescence intensity of the tryptophan residues is much stronger than that of tyrosine residues, suggesting that the main contribution to the fluorescence of trypsin comes from tryptophan residues.[Citation33] Generally, a red-shift of the emission peak is indicative of an increase in the hydrophilicity around the fluorophore, while a blue-shift should be due to an increase in the hydrophobicity around the fluorophore moiety.[Citation34] From , it can be seen that the emission peak of tyrosine residues shows an obvious blue shift (from 293 to 290 nm) after addition of acteoside, indicating that the conformational change of trypsin induced by acteoside is accompanied with a decrease in polarity and an increase in hydrophobicity of microenvironment around tyrosine residues.[Citation35] For tryptophan residues, their emission peak did not show any obvious shift after interacting with acteoside, suggesting that acteoside exerts little effect on the microenvironment of tryptophan residues.[Citation36] In addition, fluorescence intensities of tryptophan (Δλ = 60 nm) and tyrosine (Δλ = 15 nm) residues of trypsin were decreased by 54.92% and 47.85%, respectively, when the concentration of acteoside was increased to 6.67 × 10−6 mol L−1, which implies that Trp residues contribute more to the intrinsic fluorescence quenching of trypsin than Tyr residues and that the binding site of acteoside may be closer to Trp residues compared to Tyr residues.[Citation37]
Circular dichroism spectral studies
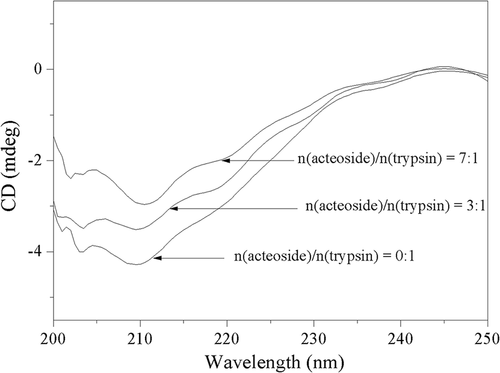
To investigate the secondary structural change of trypsin induced by acteoside, the CD spectra of trypsin in the presence of acteoside at different concentrations were measured and are shown in . It can be seen from that there are two negative bands at around 208 and 220 nm, respectively, which is compatible with a normally folded active enzyme.[Citation38] The calculated contents of different secondary structures are listed in . According to , with the addition of acteoside, the content of α-helix increases while the β-sheet content decreases. These results elucidate that the interaction between acteoside and trypsin results in a conformational change of trypsin with partial β-sheet structures being transformed into α-helix structures, which is concordant with the experimental results obtained from previous UV spectral studies.
Table 3. Effect of acteoside on secondary structure content of trypsin.
Molecular docking studies
Molecular docking was performed by molecular modeling software Sybyl-X 2.0 to obtain an idea of the binding location of acteoside on trypsin. The stereo view of the best energy ranked result of the binding mode between acteoside and trypsin is shown in ).
Figure 8. (a) Best docked conformation of trypsin–acteoside complex, acteoside is shown as stick model; (b) two-dimensional illustration (generated by LIGPLOT) of interaction of amino acid residues with acteoside.
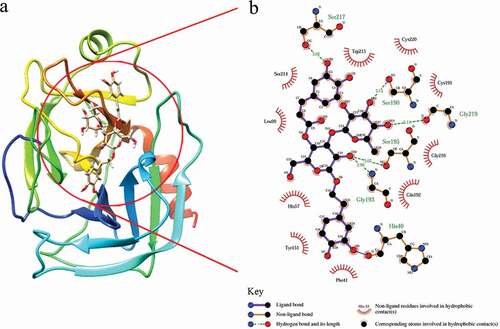
The docking result suggests that acteoside exhibits a strong complexing activity with trypsin and that the hydrophobic interactions exist widely within the acteoside–trypsin complex, which matches the result from thermodynamic studies very well. In addition, acteoside contains hydroxyl groups, which can form hydrogen bond with the polar groups (NH2, OH, and SH group) of amino acids of trypsin.[Citation39] Accordingly, hydrogen-binding interactions of acteoside with Ser-217, Ser-190, Gly-219, Ser-195, and Gly-193 of trypsin were observed and are shown as olive green dashed lines in ). Herein, docking result further shows that besides hydrophobic interactions, hydrogen bonds also help stabilize the acteoside–trypsin complex. Furthermore, it is noticed that the calculated binding distance between Trp-215 and acteoside (2.293 Å) was shorter than that between Tyr-151 and acteoside (2.600 Å), that is, acteoside is closer to Trp residue comparing to Tyr residue, which is accordant with the result from previous synchronous fluorescence studies.
Conclusion
In this study, the binding interaction of acteoside with trypsin was studied using UV spectroscopy, fluorescence spectroscopy, synchronous fluorescence spectroscopy, CD spectroscopy, and molecular docking. The experimental results reveal that a trypsin–acteoside complex is formed and trypsin fluorescence can be quenched by acteoside through a combined quenching process (static and dynamic quenchings). The experimental results also suggest that hydrophobic forces together with hydrogen bonds play important roles in stabilizing the trypsin–acteoside complex and that the interaction of acteoside with trypsin leads to a loosening and unfolding of the protein backbone with partial β-sheet structures being transformed into α-helix structures. This study can provide the basic data for the pharmacokinetics and pharmacodynamics of acteoside in the human body in vitro and should be useful to minimize its antinutritional effect and make its full use in the food industry. Furthermore, acteoside may be another active compound from Chinese tea that has the potential to be developed into a therapy or health product for the treatment of diseases and conditions related to excessive activity of trypsin.
Additional information
Funding
References
- Lee, K. J.; Woo, E. R.; Choi, C. Y.; Shin, D. W.; Lee, D. G.; You, H. J.; Jeong, H. G. Protective Effect of Acteoside on Carbon Tetrachloride-Induced Hepatotoxicity. Life Sciences 2004, 74, 1051–1064. DOI: 10.1016/j.lfs.2003.07.020.
- Xiong, Q.; Hase, K.; Tezuka, Y.; Namba, T.; Kadota, S. Acteoside Inhibits Apoptosis in D-Galactosamine and Lipopolysaccharide-Induced Liver Injury. Life Sciences 1999, 65, 421–430. DOI: 10.1016/S0024-3205(99)00263-5.
- Arvizu-Flores, A. A.; Quintero-Reyes, I. E.; Felix-Lopez, M.; Islas-Osuna, M. A.; Yepiz-Plascencia, G.; Pacheco-Aguilar, R.; Navare, A.; Fernández, F. M.; Velazquez-Contreras, E. F.; Sotelo-Mundo, R. R.; et al. Thermodynamic Activation and Structural Analysis of Trypsin I from Monterey Sardine (Sardinops Sagax Caerulea). Food Chemistry 2012, 133, 898–904. DOI: 10.1016/j.foodchem.2012.01.111.
- Ghosh, S.;. Interaction of Trypsin with Sodium Dodecyl Sulfate in Aqueous Medium: A Conformational View. Colloids and Surfaces B: Biointerfaces 2008, 66, 178–186. DOI: 10.1016/j.colsurfb.2008.06.011.
- Stroud, R. M.; Kay, L. M.; Dickerson, R. E. The Structure of Bovine Trypsin: Electron Density Maps of the Inhibited Enzyme at 5 Å and at 2·7 Å Resolution. Journal of Molecular Biology 1974, 83, 185–208. DOI: 10.1016/0022-2836(74)90387-8.
- Hörn, H.; Heidland, A. Proteases: Potential Role in Health and Diseases; Plenum Press: New York, 1992.
- Reich, E.; Rifkin, D. B.; Shaw, E. Proteases and Biological Control; Cold Spring Harbor Laboratory: New York, 1975.
- Liu, Y.; Zhang, G.; Zeng, N.; Hu, S. Interaction between 8-Methoxypsoralen and Trypsin: Monitoring by Spectroscopic, Chemometrics and Molecular Docking Approaches. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2017, 173, 188–195. DOI: 10.1016/j.saa.2016.09.015.
- Li, Q.; Wei, Q.; Yuan, E.; Yang, J.; Ning, Z. Interaction between Four Flavonoids and Trypsin: Effect on the Characteristics of Trypsin and Antioxidant Activity of Flavonoids. International Journal of Food Science and Technology 2014, 49, 1063–1069. DOI: 10.1111/ijfs.12401.
- Shen, L.; Xu, H.; Huang, F.; Li, Y.; Xiao, H.; Yang, Z.; Hu, Z.; He, Z.; Zeng, Z.; Li, Y. Investigation on Interaction between Ligupurpuroside A and Pepsin by Spectroscopic and Docking Methods. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015, 135, 256–263. DOI: 10.1016/j.saa.2014.06.087.
- Wang, J.; Chan, C.; Huang, F. W.; Xie, J. F.; Xu, H.; Ho, K. W.; Zheng, S. G.; Hu, Z. L.; Lu, J.; He, Z. D. Interaction Mechanism of Pepsin with a Natural Inhibitor Gastrodin Studied by Spectroscopic Methods and Molecular Docking. Medicinal Chemistry Research 2017, 26, 405–413. DOI: 10.1007/s00044-016-1760-2.
- Ying, M.; Huang, F.; Ye, H.; Xu, H.; Shen, L.; Huan, T.; Huang, S.; Xie, J.; Tian, S.; Hu, Z.; et al. Study on Interaction between Curcumin and Pepsin by Spectroscopic and Docking Methods. International Journal of Biological Macromolecules 2015, 79, 201–208. DOI: 10.1016/j.ijbiomac.2015.04.057.
- Fang, Y.; Xu, H.; Shen, L.; Huang, F.; Yibulayin, S.; Huang, S.; Tian, S.; Hu, Z.; He, Z.; Li, F.; et al. Study on the Mechanism of the Interaction between Acteoside and Pepsin Using Spectroscopic Techniques. Luminescence. 2015, 30, 859–866. DOI: 10.1002/bio.v30.6.
- Xu, H.; Zhu, Q. Q.; Lu, J.; Chen, X. J.; Xiao, J.; Liu, Z. G.; Chen, S. P.; Tong, M. L.; Ji, L. N.; Liang, Y. Studies on Thermodynamic Nature of Steroselectivity for Ruthenium (II) Polypyridyl Complex Binding to DNA. Inorganic Chemistry Communications 2010, 13, 711–714. DOI: 10.1016/j.inoche.2010.03.025.
- Wu, Z.; Shen, L.; Han, Q.; Lu, J.; Tang, H.; Xu, X.; Xu, H.; Huang, F.; Xie, J.; He, Z.; et al. Mechanism and Nature of Inhibition of Trypsin by Ligupurpuroside A, a Ku-Ding Tea Extract, Studied by Spectroscopic and Docking Methods. Food Biophysics 2017, 12, 78–87. DOI: 10.1007/s11483-016-9465-0.
- Hong, W. X.; Huang, F.; Huan, T.; Xu, X.; Han, Q.; Wang, G.; Xu, H.; Duan, S.; Duan, Y.; Long, X.; et al. Comparative Studies on DNA-binding and in Vitro Antitumor Activity of Enantiomeric Ruthenium (II) Complexes. Journal of Inorganic Biochemistry 2018, 180, 54–60. DOI: 10.1016/j.jinorgbio.2017.11.024.
- Wong, I. Y. F.; He, Z. D.; Huang, Y.; Chen, Z. Y. Antioxidative Activities of Phenylethanoid Glycosides from Ligustrum Purpurascens. Journal of Agricultural and Food Chemistry 2001, 49, 3113–3119. DOI: 10.1021/jf0100604.
- Song, W.; Yu, Z.; Hu, X.; Liu, R. Dissection of the Binding of Hydrogen Peroxide to Trypsin Using Spectroscopic Methods and Molecular Modeling. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015, 137, 286–293. DOI: 10.1016/j.saa.2014.08.037.
- Gholami, S.; Bordbar, A. K. Exploring Binding Properties of Naringenin with Bovine β-lactoglobulin: A Fluorescence, Molecular Docking and Molecular Dynamics Simulation Study. Biophysical Chemistry 2014, 187-188, 33–42. DOI: 10.1016/j.bpc.2014.01.003.
- GonçAlves, R.; Mateus, N.; De Freitas, V. Biological Relevance of the Interaction between Procyanidins and Trypsin: A Multitechnique Approach. Journal of Agricultural and Food Chemistry 2010, 58, 11924–11931. DOI: 10.1021/jf1023356.
- Mandal, P.; Ganguly, T. Fluorescence Spectroscopic Characterization of the Interaction of Human Adult Hemoglobin and Two Isatins, 1-Methylisatin and 1-Phenylisatin: A Comparative Study. The Journal of Physical Chemistry B 2009, 113, 14904–14913. DOI: 10.1021/jp9062115.
- Bhomia, R.; Trivedi, V.; Coleman, N. J.; Mitchell, J. C. The Thermal and Storage Stability of Bovine Haemoglobin by Ultraviolet-Visible and Circular Dichroism Spectroscopies. Journal of Pharmaceutical Analysis 2016, 6, 242–248. DOI: 10.1016/j.jpha.2016.02.004.
- Chi, Z.; Liu, R.; Zhang, H. Noncovalent Interaction of Oxytetracycline with the Enzyme Trypsin. Biomacromolecules. 2010, 11, 2454–2459. DOI: 10.1021/bm100633h.
- Hu, X.; Yu, Z.; Liu, R. Spectroscopic Investigations on the Interactions between Isopropanol and Trypsin at Molecular Level. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2013, 108, 50–54. DOI: 10.1016/j.saa.2013.01.072.
- Yu, X.; Yang, Y.; Zou, X.; Tao, H.; Ling, Y.; Yao, Q.; Zhou, H.; Yi, P. Study on the Interaction between Novel Spiro Pyrrolidine and Bovine Serum Albumin by Spectroscopic Techniques. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2012, 94, 23–29. DOI: 10.1016/j.saa.2012.03.050.
- Wang, Y. Q.; Tan, C. Y.; Zhuang, S. L.; Zhai, P. Z.; Cui, Y.; Zhou, Q. H.; Zhang, H. M.; Fei, Z. In Vitro and in Silico Investigations of the Binding Interactions between Chlorophenols and Trypsin. Journal of Hazardous Materials 2014, 278, 55–65. DOI: 10.1016/j.jhazmat.2014.05.092.
- Wang, Q.; Huang, C. R.; Jiang, M.; Zhu, Y. Y.; Wang, J.; Chen, J.; Shi, J. H. Binding Interaction of Atorvastatin with Bovine Serum Albumin: Spectroscopic Methods and Molecular Docking. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2016, 156, 155–163. DOI: 10.1016/j.saa.2015.12.003.
- Sahoo, B. K.; Ghosh, K. S.; Dasgupta, S. Molecular Interactions of Isoxazolcurcumin with Human Serum Albumin: Spectroscopic and Molecular Modeling Studies. Biopolymers. 2009, 91, 108–119. DOI: 10.1002/bip.v91:2.
- Abou-Zied, O. K.; Al-Shihi, O. I. K. Characterization of Subdomain IIA Binding Site of Human Serum Albumin in Its Native, Unfolded, and Refolded States Using Small Molecular Probes. Journal of the American Chemical Society 2008, 130, 10793–10801. DOI: 10.1021/ja8031289.
- Bozoğlan, B. K.; Tunç, S.; Duman, O. Investigation of Neohesperidin Dihydrochalcone Binding to Human Serum Albumin by Spectroscopic Methods. Journal of Luminescence 2014, 155, 198–204. DOI: 10.1016/j.jlumin.2014.06.032.
- Cui, F.; Yan, Y.; Zhang, Q.; Yao, X.; Qu, G.; Lu, Y. Characterization of the Interaction between 8-Bromoadenosine with Human Serum Albumin and Its Analytical Application. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2009, 74, 964–971. DOI: 10.1016/j.saa.2009.09.001.
- Shu, Y.; Xue, W.; Xu, X.; Jia, Z.; Yao, X.; Liu, S.; Liu, L. Interaction of Erucic Acid with Bovine Serum Albumin Using a Multi-Spectroscopic Method and Molecular Docking Technique. Food chemistry 2015, 173, 31–37. DOI: 10.1016/j.foodchem.2014.09.164.
- Sarkar, M.; Paul, S. S.; Mukherjea, K. K. Interaction of Bovine Serum Albumin with a Psychotropic Drug Alprazolam: Physicochemical, Photophysical and Molecular Docking Studies. Journal of Luminescence 2013, 142, 220–230. DOI: 10.1016/j.jlumin.2013.03.026.
- Sharma, A. S.; Anandakumar, S.; Ilanchelian, M. A Combined Spectroscopic and Molecular Docking Study on Site Selective Binding Interaction of Toluidine Blue O with Human and Bovine Serum Albumins. Journal of Luminescence 2014, 151, 206–218. DOI: 10.1016/j.jlumin.2014.02.009.
- Jayabharathi, J.; Jayamoorthy, K.; Thanikachalam, V. Docking Investigation and Binding Interaction of Benzimidazole Derivative with Bovine Serum Albumin. Journal of Photochemistry and Photobiology B: Biology 2012, 117, 27–32. DOI: 10.1016/j.jphotobiol.2012.08.018.
- Wang, J.; Liu, R.; Qin, P. Toxic Interaction between Acid Yellow 23 and Trypsin: Spectroscopic Methods Coupled with Molecular Docking. Journal of Biochemical and Molecular Toxicology 2012, 26, 360–367. DOI: 10.1002/jbt.v26.9.
- Wang, Y.; Zhang, G.; Wang, L. Potential Toxicity of Phthalic Acid Esters Plasticizer: Interaction of Dimethyl Phthalate with Trypsin in Vitro. Journal of Agricultural and Food Chemistry 2014, 63, 75–84. DOI: 10.1021/jf5046359.
- Simon, L. M.; Kotorman, M.; Garab, G.; Laczko, I. Structure and Activity of α-Chymotrypsin and Trypsin in Aqueous Organic Media. Biochemical and Biophysical Research Communications 2001, 280, 1367–1371. DOI: 10.1006/bbrc.2001.4282.
- Wu, X.; He, W.; Wang, W.; Luo, X.; Cao, H.; Lin, L.; Feng, K.; Liu, Z. Investigation of the Interaction between (−)-Epigallocatechin-3-Gallate with Trypsin and α-chymotrypsin. International Journal of Food Science and Technology 2013, 48, 2340–2347.