ABSTRACT
The objective of this work was to contribute to the knowledge of the relationship between phenolic composition and the temperature of maceration/fermentation on Petit Verdot red wines, which were made with three treatments (17°C, 21°C, and 25°C). The phenolic compounds of Vitis vinifera cv. Petit Verdot wines elaborated at different maceration/fermentation temperatures were determined and compared by high-performance liquid chromatography coupled with diode array detector and electrospray ionization mass spectrometry. A total of 45 phenolic compounds were detected in all wines. The phenolic composition was affected by the temperature of maceration. The increment of maceration/fermentation temperature had a positive effect on the total concentration of the phenolic compounds and chromatic characteristics of these wines. The colour of Petit Verdot young red wines showed more colour intensity and their luminosity descended due to the increase in temperature. The highest total content of anthocyanins was determined in wines macerated at 21°C. However, other groups of phenolic compounds (flavonols, proanthocyanidins, and stilbenes) increased their total content by raising the maceration/fermentation temperature, reaching maximum values at 25°C.
Introduccion
The phenolic content of red wine is responsible for the colour, mouthfeel, and ageing potential of the wine.[Citation1,Citation2] The phenolic content of the grape is distributed throughout the various tissues of the berry, with the skin, seed, and pulp each possessing a distinct phenolic content. In the winemaking process, phenolic compounds are extracted from grapes into the wine during the maceration step.[Citation3] The diffusion of the different polyphenols from grape to must-wine and, consequently, their extractability and final concentration at the end of the maceration process largely depends on their location in the berry and the characteristics of the different grape varieties, especially those related to the phenolic composition content and the extractability of these polyphenols.[Citation4]
Most phenolic compounds found in red wines may be grouped into two classes based on fundamental chemical structures: the flavonoids and the nonflavonoids.[Citation5] Flavonoids, located in grape skins, seeds, and stems, include anthocyanins, flavan-3-ol monomers, oligomeric and polymeric proanthocyanidins, flavonols, flavanonols, and flavones. Nonflavonoids, which derive primarily from the pulp and skins of grape berries, include hydroxycinnamic and hydroxybenzoic acids, resveratrol, and its derivatives. All are important constituents of both grapes and wine due to their direct and indirect contribution to wine sensorial properties such as colour, flavour, astringency, bitterness, and structure of the wines.[Citation6]
The phenolic compound concentration and the distribution of the individual phenolic species in each class vary across different wines and are affected by the grape variety, growing conditions, and the winemaking practices employed.[Citation7] Although many fermentation parameters and winemaking techniques affect phenolic extraction, it is generally agreed that one of the prime factor is fermentation temperature.[Citation2] Fermentation temperature[Citation8,Citation9] and maceration duration[Citation7] also impact significantly in red wine style and quality.
Elevating fermentation temperature has frequently been used to get a good extraction of phenolic compounds from the skins and to obtain wines with colour, body, and structure,[Citation9] increasing the total polyphenol content in wines.[Citation8] When the temperature at the end of the fermentation is increased, a promotion of colour extraction and an increase in the concentration of phenolic compounds have been observed in Pinot noir wines, but, at the same time, the wine is exposed to the risk of increasing the concentration of volatile phenols.[Citation10] Nevertheless, studies demonstrated that Cabernet Sauvignon wines elaborated at low fermentation temperature (15°C) showed higher total phenolic content and better chromatic parameters due to the maceration time increase.[Citation11] The objective of this work was to contribute to the knowledge of the relationship between phenolic composition and the temperature of maceration/fermentation. The effects of three maceration/fermentation temperatures (17°C, 21°C, and 25°C) in red wine phenolic composition have been studied.
Materials and methods
Fermentation assays
The experimental work was carried out with Petit Verdot grapes from Mancha Region (Spain), harvested during crop 2014 on the optimal timing of technological maturity: 25.80 Brix, 6.02 g/L total acidity, 3.21 pH, and 1.29 g/L L-malic acid. Just after the grapes were crushed, 50 mg/L of SO2 were added to musts.
Three maceration/fermentation temperatures were studied: 17°C, 21°C, and 25°C and all the experiments were carried out in triplicate using 100 L stainless steels tanks equipped with a wrap stainless steel cooling jacket and thermometer connected to a control temperature system. Alcoholic fermentation was induced by inoculating S. cerevisiae VN® (Uvaferm VN® Lallemand Inc., Zug, Switzerland), according to the manufacturer’s instructions, and daily tanks were manually punched down. The evolution of the fermentation was followed by daily measurement of density and wine was pressed when a density of 995 g/L was reached. Alcoholic fermentation was then finished at room temperature, as determined by glucose and fructose levels.
At the end of alcoholic fermentation, wines were decanted, free SO2 was adjusted to 30 mg/L to prevent the development of the malolactic fermentation, and all wines were stored at 5°C for 30 days. Subsequently, wines were decanted again and cold stabilized at −5°C for 15 days, free SO2 level was again adjusted to 30 mg/L, and the wines were filtered with 0.2 micras, bottled, and stored at 18 ± 2°C for 3 months.
Chemical analysis
The wines were analytically characterized. The following parameters were determined: alcohol content, total acidity (expressed as tartaric acid), pH, volatile acidity (expressed as acetic acid), L-malic acid, colour intensity, and tonality following the official analytical methods established by the International Organisation of Vine and Wine.[Citation12]
Colour parameters were obtained following the OIV method for the determination of chromatic characteristics according to CIELab (Resolution Oeno 1/2006) Method OIV-MA AS2-11 using an Agilent 8453 diode array spectrophotometer (Agilent Technologies, Santa Clara CA, USA), with a homemade program for spectra treatment. The measuring conditions were transmittance between 770 and 380 nm at 5-nm intervals, 1-mm cuvettes, D65 illuminant, and a 10 reference pattern observer. Results expressed were referred to 1-cm optical length.[Citation12]
Sample preparation for flavonols analysis
PCX SPE cartridges (500 mg, 6 mL; Bond Elut Plexa, Agilent, Palo Alto, CA, USA) allowed the isolation of non-anthocyanin phenolic compounds from wines, and these anthocyanin-free fractions were used to analyse flavonols. To carry out this separation, 3 mL of wine were diluted with 3 mL of HCl 0.1 N, and the prepared samples were then passed through the solid phase extraction (SPE) cartridges that had been previously conditioned with 5 mL of methanol and 5 mL of water. After the cartridges were washed with 5 mL of HCl 0.1 N and 5 mL of water, the anthocyanin-free fraction was eluted with 3 × 5 mL of methanol. This eluate was dried in a rotary evaporator (35°C) and redissolved in 1.5 mL of 20% methanol in water and directly injected into the HPLC equipment.
Analysis of monomeric phenolic compounds in wines by HPLC
Individual phenolic compounds were determined by high-performance liquid chromatography coupled with diode array detector and electrospray ionization mass spectrometry following the conditions of previously described methods[Citation13] to the use of narrow-bore, smaller particle size, chromatography columns. Analysis was performed on an Agilent 1100 series system equipped with a photodiode array detector and a LC/MSD Trap VL electrospray ionization mass spectrometry (ESI-MS/MS), both coupled to an Agilent ChemStation for data processing.
For anthocyanin analysis, wines were filtered (0.2 µm CHROMAFIL PET, Macherey-Nagel, Düren, Germany), and injection volume was 10 µL. Separation was achieved on a narrow-bore column Zorbax Eclipse XDB-C18 (2.1 × 150 mm; 3.5-µm particle; Agilent), with pre-column Zorbax Eclipse XDB-C8 (2.1 × 12.5 mm; 5-µm particle; Agilent), both thermostated at 40°C. Eluents used were (a) acetonitrile/water/formic acid (3:88.5:8.5 v/v/v) and (b) acetonitrile/water/formic acid (50:41.5:8.5 v/v/v). The linear solvents’ gradient for anthocyanin analysis was as follows: 0 min, 6% B; 10 min, 30% B; 30 min, 50% B; 34 min, 100% B; 36 min, 100% B; 42 min, 4% B; and 50 min, 4% B.
For non-anthocyanin analysis, free-anthocyanin fractions (see sample preparation for flavonols analysis) were filtered (0.2 µm CHROMAFIL PET, Macherey-Nagel, Düren, Germany), and injection volume was 20 µL. The same column was used while eluents were (a) acetonitrile/water/formic acid (3:88.5:8.5 v/v/v), (b) acetonitrile/water/formic acid (50:41.5:8.5 v/v/v), and (c) methanol/water/formic acid (90:1.5:8.5 v/v/v). The linear solvents’ gradient for non-anthocyanin analysis was as follows: 0 min, 2% B and 0% C; 8 min, 4% B and 0% C; 37 min, 17% B and 13% C; 51 min, 30% B and 20% C; 51.5 min, 40% B and 30% C; 56 min, 50% B and 50% C; 57 min, 50% B and 50% C; and 64 min, 4% B and 0% C. The use of a narrow-bore column allowed establishing a slow low rate (0.19 mL/min).
For identification, Ion Trap ESI-MS/MS detector was used in both positive (anthocyanins) and negative (flavonols) ion modes, setting the following parameters: dry gas N2, 8 L/min; drying temperature, 325°C; nebulizer, N2, 50 psi; ionization and fragmentation parameters were optimized by direct infusion of appropriate standard solutions (malvidin-3-O-glucoside in positive ionization mode; quercetin-3-O-glucoside in negative ionization mode); scan range, 50–1.200 m/z. Identification was based on spectroscopic data (UV-Vis and MS/MS) obtained from authentic standards, when available, or previously reported data.[Citation13,Citation14] Quantification was made using the diode-array dectector (DAD) chromatograms recorded at 520 nm (anthocyanins), 360 nm (flavonols), and the calibration graphs of the respective standards (R2 > 0.999). Quantification of noncommercial compounds was made according to the calibration graphs of the most similar compounds. Hence, anthocyanins were expressed as mg/L of malvidin-3-O-glucoside and flavonols were expressed as mg/L of quercetin-3-O-glucoside.
Sample preparation for flavan-3-ols analysis
Flavan-3-ols (monomers, B-type dimers, and polymeric proanthocyanidins) were isolated from wines using SPE on C18 cartridges (Sep-pak Plus C18, Waters Corp., Milford, MA; cartridges filled with 820 mg of adsorbent). A mixture of 2 mL of each wine with 6 mL of water was then passed through the C18 cartridge, which had been previously conditioned with methanol (5 mL) and water (5 mL); after the cartridge was dried under reduced pressure, methanol (15 mL) and ethyl acetate (5 mL) were added in order to recover adsorbed phenolics; after the solvent was evaporated in a rotary evaporator (35°C), the residue was dissolved in methanol (2 mL) and stored at −18°C until analysis.
Identification and quantification flavan-3-ols and stilbenes using multiple reaction monitoring HPLC-ESI-MS/MS
The analysis was carried out using a HPLC Agilent 1200 series system equipped with DAD (Agilent, Germany) and coupled to an AB Sciex 3200 TRAP (Applied Biosystems) with triple quadrupole, turbo spray ionization (electrospray assisted by a thermonebulization) mass spectroscopy system (ESI-MS/MS). The chromatographic system was managed and the mass spectra data was processed using the Analyst MSD software (Applied Biosystems, version 1.5).
Structural information concerning the proanthocyanidins was obtained using the pyrogallol-induced acid-catalyzed depolymerization method.[Citation15] The reaction consisted of adding 0.50 mL of the pyrogallol solution (100 g/L pyrogallol plus 20 g/L of ascorbic acid in 0.3 N HCl) to 0.25 mL of the sample in methanol and incubating 40 min at 30°C. The hydrolysis reaction was stopped by adding 2.25 mL of sodium acetate (67 mM). An aliquot of 2 mL of the reacted sample was placed in a vial and injected directly into the equipment for analysis.
The samples, before and after the acid-catalyzed depolymerization reaction, were injected (20 μL) into an Ascentis C18 reversed-phase column (150 mm × 4.6 mm with 2.7 μm of particle size) (Supelco, Bellefonte, USA), with the temperature controlled at 16°C. The solvents and gradients used for this analysis and the multiple reaction monitoring settings as well as all the mass transitions (m/z) for identification and quantitation were according to the methodology reported by Lago-vanzela.[Citation14]
Statistical analysis
Data were subjected to the Student–Newman–Keuls test to identify any statistically significant differences between different maceration temperatures. The SPSS 12.0 software statistical package for Windows (Chicago, USA) was used for this analysis. Multivariate data analysis (principal component analysis (PCA)) was used to obtain an overview of the chemical and phenolic compounds analysed and to investigate possible correlations between the samples. SPSS 12.0 software (IBM, USA) was used for both analyses.
Results and discussion
Oenological parameters and colour
The oenological parameters are shown in . All wines presented glucose + fructose concentrations bellow 0.5 g/L (data not shown). Wines produced at 25°C showed significantly lower ethanol contents than the corresponding at 17°C and 21°C. Higher ethanol concentration at lower maceration/fermentation temperature has been previously documented.[Citation16,Citation17] In contrast, an increase in the content of glycerine is observed with increase in maceration/fermentation temperature. Several parameters have been shown to influence the final glycerol levels in wine, among them is the maceration/fermentation temperature.[Citation18]
Table 1. Oenological and colour parameters at the end of alcoholic fermentation of Petit Verdot wines elaborated at different temperatures of maceration and fermentation.
Oenological parameters of these wines were similar to those obtained in Petit Verdot wines elaborated in other regions.[Citation19] Total and volatile acidity were lower in the wine obtained by the maceration at lower temperature (17°C) compared to wines made at higher temperature (21 and 25°C). Several studies have reported increased total and volatile acidity with increasing fermentation temperature.[Citation9,Citation17] Winemaking variables and techniques are known to affect the colour of red wines. Less colour intensity was obtained in the wines made at 17°C. In this way, higher maceration/fermentation temperatures have been reported to increase phenolic extraction.[Citation7] In the Cielab parameters, although having not found statistically significant differences, it is observed that when the maceration/fermentation temperature decreased, the wines showed lower colour intensity and tonality described the change of red colour toward orange tones (higher values of a* and b* coordinates).
Phenolic composition of wines
Anthocyanins
shows the concentrations (mg/L as malvidin-3-O-glucoside) of the anthocyanin monomers identified in the wines at different maceration/fermentation temperature. The completed series of nonacylated, acetylated, and p-coumaroylated anthocyanins of the five anthocyanidins usually found in V. vinifera grape varieties (delphinidin, cyanidin, petunidin, peonidin, and malvidin) were detected in these wines, as well as the 3-O-caffeoyl glucosides of malvidin and peonidin.[Citation20,Citation21] Trisubstituted anthocyanins (delphinidin, petunidin, and malvidin) predominated over disubstituted ones (cyanidin and peonidin), being more abundant malvidin derivatives as expected in these wines. With regard to pyranoanthocyanins, vitisins A and B were detected in low concentration, two pyranoanthocyanins derived from the reaction of malvidin-3-glucoside with two yeast metabolites (pyruvic acid and acetaldehyde, respectively). All wines, regardless of maceration/fermentation temperature used for their elaboration, showed a greater amount of non-acylated anthocyanins than acylated ones. Anthocyanin profile and concentration of wines are largely dependent on grape variety and climatic and agronomic conditions, but also on the technology applied during winemaking and the reactions that take place during maturation and ageing in wood.[Citation5,Citation22] The results showed a lower concentration of total anthocyanins, acylated anthocyanins, and pyranoanthocyanis in wines produced at 17°C, which seems to indicate lower extraction of anthocyanins at lower temperatures. Total anthocyanins content of wines under the three treatments were lower (380–419 mg/L) than those reported for Petit Verdot wines made at 24°C (542 mg/L).[Citation19]
Table 2. Concentration of anthocyanins (mg/L as malvidin-3-O-glucoside) at the end of alcoholic fermentation in Petit Verdot wines elaborated at different temperatures of maceration and fermentation.
Maceration and fermentation at 21°C produced wines with higher total anthocyanin concentration (non-acylated and acylated). On the other hand, wines produced at 25°C showed similar concentration of non-acylated anthocyanins than those made at 17°C. With respect to the anthocyanidins, except cyanidin and delphinidin types whose concentrations are not modified by maceration/fermentation temperature, the rest of the anthocyanins were higher in wines elaborated at 21°C. With respect to pyranoanthocyanins, the contents of vitisin A were higher in wines macerated at 21°C and 25°C. These results showed that the wines obtained at higher maceration/fermentation temperature did not contain higher concentrations of anthocyanins, at least with statistically significant differences, except those obtained at an intermediate temperature of 21°C.
Flavonols
Flavonols contribute to bitterness and colour[Citation23] and they originate from the berry skins of grapes and are transferred to wine during the process of winemaking.[Citation24] They vary in colour from white to yellow, closely related in structure to the flavones.[Citation25] They also contribute to the colour stabilization of red wines by reinforcing the pigmentation due to anthocyanins, a phenomenon known as copigmentation.[Citation26] In this study, there were 14 flavonols detected in wines in the three treatments.
shows the flavonol content (mg/L) in wines elaborated at 17°C, 21°C, and 25°C, displaying all wine values in the range of other red wines.[Citation27] Regarding the flavonol profile, the 3-glucosides of the six aglycones (quercetin, myricetin, laricitrin, syringetin, isorhamnetin, and kaempferol) were detected and quantified in the studied wines. In addition, myricetin and quercetin 3-glucuronides, and the galactoside forms of myricetin, quercetin, and laricitrin were also detected in these wines. Trisubstituted flavonols (myricetin, laricitrin, and syringetin) predominated over disubstituted (quercetin and isorhamnetin) and monosubstituted ones (kaempferol), being more abundant myricetin derivatives. Myricetin-3-glucoside was the main flavonol found in Petit Verdot wines, being in good agreement with other studies.[Citation13,Citation28] Syringetin-3-glucoside was the second mayor flavonol in these wines, followed by quercetin-3-glucuronide and laricitrin-3-glucoside. Flavonols are present in the grape exclusively in the form of glycosides, and the presence of flavonols aglicones in wine is attributed to hydrolysis processes, being unclear if they are chemical or enzymatic and lactic acid bacteria glycosidase enzymes could have some impact on wine flavonols.[Citation29]
Table 3. Concentration of flavonols (mg/L as quercetin-3-glucoside) at the end of alcoholic fermentation in Petit Verdot wines elaborated at different temperatures of maceration and fermentation.
Maceration and fermentation at 17°C produced wines with lower concentration of total flavonols. Higher concentration of disubstituted flavonols was observed in wines macerated at 25°C. On the other hand, wines made at 21°C and 25°C showed similar total flavonol concentration (non-methoxylated and trisustituted). The monosubstituted and methoxylated flavonols were found in similar concentration in all samples. In this study, the maceration/fermentation temperature caused no important changes, neither the profile nor concentration of flavonols, although some slight statistically significant differences, with lower concentration of the derivatives of quercetin, myricetin, and laricitrin were observed in wines macerated and fermented at 17°C.
Flavan-3-ols and stilbenes
Flavan-3-ols are the main phenolic compounds related to the astringency, bitterness, and structure of wines, and also an important factor in stabilizing the colour of ageing wines as anthocyanin copigments.[Citation23,Citation30,Citation31] Proanthocyanidins are polymers of flavan-3-ols and the primary source of astringency and bitterness.[Citation2,Citation32] Studies showed that overall intensity and persistence are positively correlated with astringency, and therefore to proanthocyanidin content.[Citation4,Citation33]
The results obtained are shown in . Maceration and fermentation at 25°C provided wines with higher concentration of proanthocyanidins, according to increase of these compounds by raising the fermentation temperature,[Citation34,Citation35] although no differences were statistically significant. Wines elaborated at 21°C and 25°C showed lower concentration of flavan-3-ols monomers and dimers than wines macerated at 17°C. However, the mean degree of polymerization (mDP) was similar in all three treatments although there were decreases in macerated and fermented wines at 25°C. For galloylation and prodelphinidin percentages, our results seem to indicate that Petit Verdot wines macerated a higher temperature showing lower percentage of galloylation and higher percentage of prodelphinidins, showing that the wines macerated at 25°C had higher proportion of proanthocyanidins from the grape skins, because of the fact that there are a higher proportion of prodelphinidins in grape skins[Citation36,Citation37] and lower proportion of galloylated procyanidins than seeds.[Citation36,Citation38] Moreover, it has been accepted that the extraction of proanthocyanidin skins needs less maceration time than seeds.[Citation39,Citation40] The increase of proanthocyanindin content at 25°C and lower mDP may indicate that proanthocyanidin skin with low mDP were extracted more easily at higher temperature than those in skins with high mDP.
Table 4. Concentration of flavan-3-ol monomers and dimers, proanthocyanidins, and stilbenes (mg/L) at the end of alcoholic fermentation in Petit Verdot wines elaborated at different temperatures of maceration and fermentation.
Regarding stilbenes, Petit Verdot wines elaborated for this study did not contain resveratrol in detectable levels, and only its glycoside forms (cis and trans isomers of piceid) were quantified, being in good agreement with other studies.[Citation5] The results indicated that the macerated and fermented wines at 21°C and 25°C showed higher concentration of these compounds, wherein differences are statistically significant. Stilbenes are important due to their putative protective effects against cardiovascular diseases and a remarkable inhibitory potential of various stages of tumour development.[Citation41] It was suggested that the concentrations of these compounds in wines vary, depending on many factors such as grape variety, fungal infections, winemaking procedures, and weather conditions.[Citation42]
Multivariate data analysis
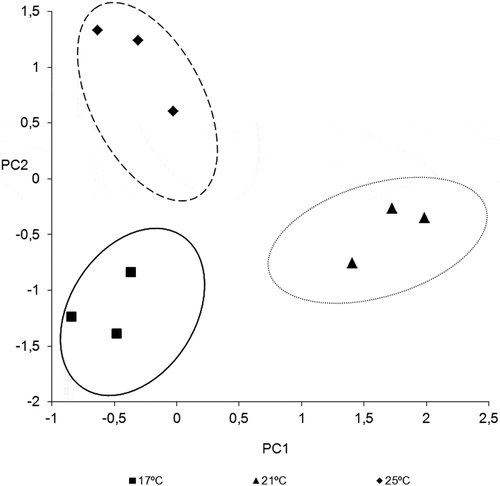
PCA was applied to the results obtained from phenolic compound studied in the wines. shows the variables with the highest correlation with principal component 1 (PC1) and principal component 2 (PC2). A total of 86.00% of the variance was explained by the first two principal components.
Table 5. Principal component analysis (PCA) applied to the data from phenolic compounds.
shows the distribution of the wines on the plane formed by the two principal components PC1 and PC2. For PC1, two different groups were evident; in positive value, the wines elaborated a 21°C had higher concentration of total anthocyanins, specifically derivatives of malvidin, petunidin, and peonidin. On the other hand, CP2 separates the samples again in two other groups, differentiating the wines obtained at 25°C by its composition in gallocatequin and flavonols (mainly quercetin-3-glucoside).
Conclusion
Phenolic characterization of Petit Verdot wines elaborated at different temperatures of maceration and fermentation (17°C, 21°C and 25°C) was carried out and discussed. It is clear that maceration/fermentation temperature plays a very important role in the phenolic composition of wine. However, according to the results obtained, wines elaborated at the highest temperature (25 °C) did not show the greatest concentration of all phenols families. In the case of anthocyanins, they were found in greater quantity in wines made at 21°C but proanthocyanidins in wines elaborated at 25°C. Therefore, with regard to the phenolic content and composition of the resulting wines, it is important to control maceration/fermentation temperature. This will enable winemakers to choose the most suitable maceration and alcoholic fermentation temperature for Petit Verdot grapes according to the type of wine desired, since phenolic composition will have a great influence on the final organoleptic characteristics of the wine.
Additional information
Funding
References
- Alvarez, I.; Aleixandre, J. L.; García, M. J.; Lizama, V. Impact of Prefermentative Maceration on the Phenolic and Volatile Compounds in Monastrell Red Wines. Analytica Chimica Acta 2006, 563, 109–115. DOI: 10.1016/j.aca.2005.10.068.
- Lerno, L.; Reichwage, M.; Ponangi, R.; Hearne, L.; Block, D. E.; Oberholster, A. Effects of Cap and Overall Fermentation Temperature on Phenolic Extraction in Cabernet Sauvignon Fermentations. American Journal of Enology and Viticulture 2015, 66(4), 444–453. DOI: 10.5344/ajev.2015.14129.
- Soto-Vázquez, E.; Rio-Segade, S.; Orriols-Fernández, I. Effect of the Winemaking Technique on Phenolic Composition and Chromatic Characteristics in Young Red Wines. European Food Research and Technology 2010, 231, 789–802. DOI: 10.1007/s00217-010-1332-5.
- Bautista-Ortín, A. B.; Busse-Valverde, N.; Fernández-Fernández, J. I.; Gómez-Plaza, E.; Gil-Muñoz, R. The Extraction Kinetics of Anthocyanins and Proanthocyanidins from Grape to Wine in Three Different Varieties. Journal International Des Sciences De La Vigne Et Du Vin 2016, 50, 91–100.
- Izquierdo-Cañas, P. M.; García Romero, E.; Mena Morales, A.; Gómez Alonso, S. Effects of Malolactic Fermentation on Colour Stability and Phenolic Composition of Petit Verdot Red Wines. Wine Studies 2016, 5(5795), 1–6. DOI: 10.4081/ws.2016.5795.
- Garrido, J.; Borges, F. Wine and Grape polyphenols—A Chemical Perspective. Food Research International 2011, 44, 3134–3148. DOI: 10.1016/j.foodres.2011.08.010.
- Sacchi, K. L.; Bisson, L. F.; Adams, D. O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. American Journal of Enology and Viticulture 2005, 56, 197–206.
- Girard, B.; Kopp, T. G.; Reynolds, A. G.; Cliff, M. A. Influence of Vinification Treatments on Aroma Constituents and Sensory Descriptors of Pinot Noir Wines. American Journal of Enology and Viticulture 1997, 48, 198–206.
- Reynolds, A.; Cliff, M.; Girad, B.; Kopp, T. G. Influence of Fermentation Temperature on Composition and Sensory Properties of Semillon and Shiraz Wines. American Journal of Enology and Viticulture 2001, 52, 235–240.
- Gerbaux, V.; Vincent, B.; Bertrand, A. Influence of Maceration Temperature and Enzymes on the Content of Volatile Phenols in Pinot Noir Wines. American Journal of Enology and Viticulture 2002, 53, 131–137.
- Sener, H.; Yildirim, H. K. Influence of Different Maceration Time and Temperatures on Total Phenols, Colour and Sensory Properties of Cabernet Sauvignon Wines. Food Science and Technology International 2013, 19, 523–533. DOI: 10.1177/1082013212462229.
- OIV. Compendium of International Methods of Wine and Musts Analysis Edition, 2009 ed.; OIV: Paris, 2014.
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Gómez, M. V.; Velders, A. H.; Hermosín-Gutiérrez, I. Flavonol 3-O-Glycosides Series of Vitis vinifera Cv. Petit Verdot Red Wine Grapes. Journal of Agricultural and Food Chemistry 2009, 57, 209–219. DOI: 10.1021/jf802863g.
- Lago-Vanzela, E. S.; Da-Silva, R.; Gomes, E.; García-Romero, E.; Hermosín-Gutiérrez, I. Phenolic Composition of the Edible Parts (Flesh and Skin) of Bordô Grape (Vitis labrusca) Using HPLC–DAD–ESI-MS/MS. Journal of Agricultural and Food Chemistry 2011, 59, 13136–13146. DOI: 10.1021/jf203679n.
- Bordiga, M.; Coïsson, J. D.; Locatelli, M.; Arlorio, M.; Travaglia, F. Pyrogallol: An Alternative Trapping Agent in Proanthocyanidins Analysis. Food Analytical Methods 2013, 6, 148–156. DOI: 10.1007/s12161-012-9427-1.
- Torija, M. J.; Rozès, N.; Poblet, M.; Guillamón, J. M.; Mas, A. Effects of Fermentation Temperature on the Strain Population of Saccharomyces cerevisiae.. International Journal of Food Microbiology 2003, 80, 47–53. DOI: 10.1016/S0168-1605(02)00144-7.
- Reddy, L. V. A.; Reddy, O. V. S. Effect of Fermentation Conditions on Yeast Growth and Volatile Composition of Wine Produced from Mango (Mangifera indica L.) Fruit Juice. Food and Bioproducts Processing 2011, 89, 487–491. DOI: 10.1016/j.fbp.2010.11.007.
- Scanes, K.; Hohmann, S.; Prior, B. Glycerol Production by the Yeast Saccharomyces cerevisiae. South African Journal of Enology and Viticulture 1998, 19, 17–24.
- Bellincontro, A.; Catelli, C.; Cotarella, R.; Mencarelli, F. Postharvest Ozone Fumigation of Petit Verdot Grapes to Prevent the Use of Sulfites and to Increase Anthocyaninin Wine. Australian Journal of Grape and Wine Research 2017, 23(2), 200–206. DOI: 10.1111/ajgw.2017.23.issue-2.
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of Oxygenation on Polyphenol Changes Occurring in the Course of Winemaking. Analytica Chimica Acta 2002, 458, 15–27. DOI: 10.1016/S0003-2670(01)01617-8.
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Evolution of Polyphenols in Red Wines from Vitis vinifera L. During Aging in Bottle. I. Anthocyanins and Pyranoanthocyanins. European Food Research and Technology 2005, 220, 607–614.
- Moreno-Arribas, M. V.; Gómez-Cordovés, C.; Martín-Álvarez, P. J. Evolution of Red Wine Anthocyanins during Malolactic Fermentation, Postfermentative Treatments and Ageing with Lees. Food Chemistry 2008, 109, 149–158. DOI: 10.1016/j.foodchem.2007.12.040.
- Obreque-Slier, E. A.; Peña-Neira, A. L.; López-Solís, R.; Zamora-Marín, F. Ricardo Da Silva J.M., Laureano O. Comparative Study of the Phenolic Composition of Seeds and Skins from Carmenere and Cabernet Sauvignon Grape Varieties (Vitis vinifera L.) During Ripening. Journal of Agricultural and Food Chemistry 2010, 58, 3591–3599. DOI: 10.1021/jf904314u.
- He, Y. H.; Ning, P. F.; Yue, T. X.; Zhang, Z. W. Comparing Phenolic Composition of Cabernet Gernischet Wines between Rain-Shelter Cultivation and Open-Field Cultivation. Czech Journal of Food Sciences 2015, 34, 1–11.
- Makris, D. P.; Kallithraka, S.; Kefalas, P. Flavonols in Grapes, Grape Products and Wines: Burden, Profile and Influential Parameters. Journal of Food Composition and Analysis 2006, 19, 396–404. DOI: 10.1016/j.jfca.2005.10.003.
- Boulton, R.;. The Copigmentation of Anthocyanins and Its Role in the Color of Red Wine: A Critical Review. American Journal of Enology and Viticulture 2001, 52, 67–87.
- Hermosín-Gutiérrez, I.; Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E. Chapter 8.Flavonol Profiles for Grapeand Wine Authentication. In Progress in Authentication of Food and Wine; Ebeler, S. E., Takeoka, G. R., Winterhalter, P.; ACSQ11 Symposium Series; American Chemical Society: Washington, DC, 2011, pp. 113–129.
- Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. HPLC Analysis of Diverse Grape and Wine Phenolics Using Direct Injection and Multidetection by DAD and Fluorescence. Journal of Food Composition and Analysis 2007, 20, 618–626. DOI: 10.1016/j.jfca.2007.03.002.
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol Profiles of Vitis vinifera White Grape Cultivars. Journal of Food Composition and Analysis 2010, 23, 699–705. DOI: 10.1016/j.jfca.2010.03.017.
- Hufnagel, J. C.; Hofmann, T. Or Sensory-Directed Identification of Astringent Mouthfeel and Bitter-Tasting Compounds in Red Wine. Journal of Agricultural and Food Chemistry 2008, 56, 1376–1386. DOI: 10.1021/jf073031n.
- Zhu, L.; Zhang, Y.; Deng, J.; Li, H.; Lu, J. Phenolic Concentrations and Antioxidant Properties of Wines Made from North American Grapes Grown in China. Molecules 2012, 17, 3304–3323. DOI: 10.3390/molecules17033304.
- Casassa, L. F.; Harberstson, J. F. Extraction, Evolution, and Sensory Impact of Phenolic Compounds during Red Wine Maceration. Annual Review of Food Science and Technology 2014, 5, 83–109. DOI: 10.1146/annurev-food-030713-092438.
- Mercurio, M. D.; Dambergs, R. G.; Cozzolino, D.; Herderich, M. J.; Smith, P. A. Relationship between Red Wine Grades and Phenolics: 1. Tannin and Total Phenolics Concentrations. Journal of Agricultural and Food Chemistry 2010, 58, 12313–12319. DOI: 10.1021/jf103230b.
- Budić –Leto, I.; Lovrić, T.; Vrhovšek, U. Influence of Different Maceration Techniques and Ageing on Proanthocyanidins and Anthocyanins of Red Vine Cv. Babic (Vitis vinifera L.). Food Technology and Biotechnology 2003, 41, 299–303.
- Pajović, R.; Popović, T.; Krstić, M. Effect of Fermentation Temperature on Polyphenolic Composition and Sensory Properties of Red Vranac Wines. Acta Agriculturae Serbica 2011, 16, 145–154.
- Labarbe, B.; Cheynier, V.; Brossand, F.; Souquet, J.; Moutounet, M. Quantitative Fractionation of Grape Proanthocyanidins according to Their Degree of Polymerization. Journal of Agricultural and Food Chemistry 1999, 47, 2719–2723. DOI: 10.1021/jf990029q.
- Souquet, J.; Cheynier, V.; Broussaud, F.; Moutounet, M. Polimeric Proanthocyanidins from Grape Skins. Phytochemistry 1996, 43, 509–512. DOI: 10.1016/0031-9422(96)00301-9.
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and Polymeric Procyanidins from Grape Seeds. Phytochemistry 1994, 36, 781–784. DOI: 10.1016/S0031-9422(00)89817-9.
- González-Manzano, S.; Rivas-Gonzalo, J. C.; Santos-Buelga, C. Extraction of Flavan-3-Ols from Grape Seed and Skin into Wine Using Simulated Maceration. Analytica Chimica Acta 2004, 513, 283–289. DOI: 10.1016/j.aca.2003.10.019.
- Canals, R.; Llaudy, M. C.; Canals, J. M.; Zamora, F. Influence of the Elimination and Addition of Seeds on the Colour, Phenolic Composition and Astringency of Red Wine. European Food Research and Technology 2008, 226, 1183–1190. DOI: 10.1007/s00217-007-0650-8.
- Saiko, P.; Szakmary, A.; Jaeger, W.; Szekeres, T. Resveratrol and Its Analogs: Defense against Cancer, Coronary Disease and Neurodegenerative Maladies or Just a Fad?. Mutation Research-Reviews in Mutation Research 2008, 658, 68–94. DOI: 10.1016/j.mrrev.2007.08.004.
- Vitrac, X.; Monti, J. P.; Vercauteren, J.; Deffieux, G.; Merillon, J. M. Direct Liquid Chromatographic Analysis of Resveratrol Derivatives and Flavanonols in Wines with Absorbance and Fluorescence Detection. Analytica Chimica Acta 2002, 458, 103–110. DOI: 10.1016/S0003-2670(01)01498-2.