ABSTRACT
Rambutan seed is discarded during fruit processing. However, the seed contains a considerable amount of crude fat. Hence, the objective of this study was to determine two anti-nutritional constituents, namely saponin and tannin, and to characterize the fat of the seeds of 11 varieties of rambutan fruit. Results showed that the range of crude fat content is fairly narrow (36.13–39.13 g/100 g dried seeds). The iodine value and free fatty acid content of the fat were 38.50–50.61 g I2/100 g fat and 0.99–2.18% as oleic acid, respectively. Oleic (33.35–46.64%) and arachidic (26.03–33.27%) acids were the main fatty acids in the fat. HPLC analysis showed that the fat comprised mainly five unknown triacylglycerols (83.94–95.33%). The melting and crystallization curves showed that the fat exhibited four to nine non-distinct peaks. The complete melting and crystallization onset temperatures of the fat were 24.8–50.6°C and 24.1–39.4°C, respectively, while the melting and crystallization enthalpies of the fat ranged from 71.2 to 141.7 J/g and from 60.4 to 88.9 J/g, respectively. At 0°C, the solid fat index of the fat ranged between 87.4% and 91.6% and the fats of some varieties melted completely at human body temperature. The saponin and tannin contents of the seed were 14.27–18.96 mg soya saponin/100 g and 4.40–26.68 mg catechin equivalent/100 g, respectively. Findings showed that rambutan seed fat has potential to be used in various sectors of food industry.
Introduction
Rambutan (Nephelium lappaceum L.) is a popular tropical fruit from the family Sapindaceae.[Citation1] It is believed that this fruit originates from Malaysia,[Citation2] and is grown in Southeast Asia, Australia, South America, and Africa, but is exported only from Malaysia, Indonesia, and Thailand.[Citation3,Citation4] Thailand, Indonesia, and Malaysia account for approximately 80% of the total world production of rambutan and only these countries export this fruit among the association of southeast asian nations (ASEAN) member countries.[Citation3,Citation4] When rambutan fruit is processed, the fruits are deseeded first and the seeds (~4–9 g/100 g) become a waste by-product.[Citation5] The disposal of this agricultural waste can lead to a serious environmental issue. A more in-depth knowledge of the fruit seed will encourage its utilization and, thus, wastage may be reduced.
The seed contains 12.40% crude protein, 38.90% crude fat, 48.10% carbohydrate by difference, 3.31% moisture, and 2.26% ash.[Citation6] The seed is bitter and has been reported to have narcotic effects as there are traces of alkaloids while the testa contains saponin and tannin.[Citation7] Although they are reputedly poisonous in the raw state, roasted seeds are consumed in the Philippines.[Citation7] The seed contains a relatively high fat content, with oleic acid (42.0%) and arachidic acid (34.3%) being the major fatty acids.[Citation8] The presence of arachidic acid, which is a long-chain (20 carbons) saturated fatty acid, in plant seed is unusual as it mostly contains very low levels of the fatty acid.[Citation9,Citation10] Due to the high contents of arachidic acid, rambutan seed fat is semi-solid at room temperature.[Citation8] The fat is edible and some studies showed that the fat can be used for manufacturing of candles, soaps, and fuels.[Citation7,Citation11] Some researchers suggested that the rambutan seed fat could be used in the high premium specialty fats.[Citation8] Blending the rambutan seed fat with a softer oil or harder fat may lead to products with wider applications.
Although there are many rambutan clones that have been registered with the Ministry of Agriculture and Agro-Based Industry, Malaysia, the properties and thermal behaviors of their seed fat as well as the anti-nutritional constituents of the seeds, especially the underutilized wild types, remain unknown. Moreover, it is essential to study the anti-nutritional constituents of the rambutan seed when it has been said to be poisonous in the raw state[Citation7] prior to transforming the waste into human food, animal feed, and plant fertilizer, although the seeds are no longer toxic and edible after roasting or boiling.[Citation5,Citation12] While the rambutan seed fat may be suitable as a cocoa butter substitute, however to date, there has been no such application in the food industry. Although there have been some reports on the seed fat, more study is needed as there is a huge potential for the fat to be used in various sectors of the food industry. Due to fairly limited information in these areas, the aim of this study was, therefore, to determine the properties and thermal profiles of fat extracted from the seed of 11 varieties of rambutan, including two wild-type varieties, grown in Malaysia and the anti-nutritional constituents of the seeds.
Materials and methods
Fruit
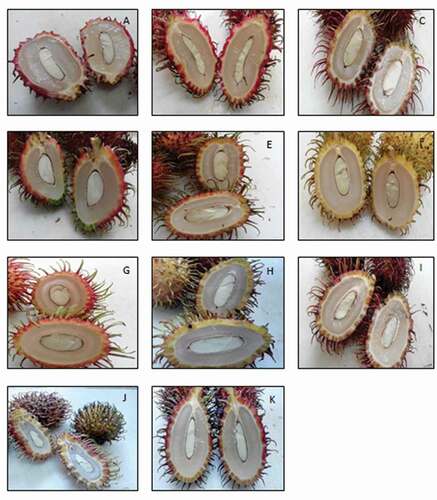
Rambutan fruit samples were obtained from the University Agricultural Park, Universiti Putra Malaysia (UPM). Eleven varieties of ripe rambutan fruits as shown in , namely R3, R4, R7, R10, R153, R156, R169, R191, Sarjan, and two wild types (WT1 and WT2), were sorted so that only those that were of uniform size and color and free from blemishes were used in the study. Some samples from each variety were vacuum-packed and stored at −20°C prior to analysis of saponin and tannin. The remaining fruits were deseeded and the seeds were washed under running tap water before they were dried in an oven at 60°C for 48 h prior to analysis. Two batches of samples (obtained from two different fruiting seasons which were July–September 2015 and December 2015–February 2016) were used in this study. Each batch was analyzed in triplicate.
Extraction and determination of crude fat content
The fat of the seed was extracted and the content was determined according to the official method AOAC 945.16.[Citation13] Briefly, 150 g of dried and ground rambutan seeds were placed in a Whatman No. 1 filter paper cone and covered lightly with cotton wool before it was transferred into a 5 L Soxhlet extractor. The fat was extracted with petroleum ether (40–60°C) for 8 h and recovered by evaporating off the solvent using a rotary evaporator (Model N-1, Eyela, Tokyo Rakakikal Co. Ltd., Tokyo, Japan). The fat was then placed in an oven at 60°C for 1 h to evaporate residual petroleum ether. The crude fat content was expressed as g/100 g of dried seeds.
Determination of iodine value (IV) and free fatty acid (FFA) contents
The IV of the seed fat was determined using the standard analytical AOCS Cd 1d-92 method.[Citation14] Briefly, 0.5 g of the extracted fat was mixed with 20 mL glacial acetic/cyclohexane, 1:1, v/v, and 25 mL of Wijs iodine solution and allowed to stand in the dark for 1 h. Thereafter, 20 mL of 10% potassium iodide solution and 100 mL of newly boiled and cooled distilled water were added. Excess iodine was titrated with 0.1 N sodium thiosulfate to neutrality in the presence of 1 mL of 1% starch solution. The control was similarly prepared but without the fat. IV was then calculated as stated in the AOCS Cd 1d-92 method.[Citation14] The FFA content of the seed fat was quantified according to the IOCCC 42–1993 official method.[Citation15] Briefly, 5 g of fat was dissolved in 50 mL of petroleum ether/absolute ethanol mixture (1:1, v/v) and titrated to a phenolphthalein end point with 0.1 N alcoholic KOH. FFA content was expressed as percent oleic acid.
Determination of fatty acid composition
To determine the fatty acid composition of the seed fat, the fat was first converted into methyl esters (FAME) according to the method of Cocks and van Rede[Citation16] by reacting 50 mg of fat with 0.05 mL of 1 mol/L sodium methoxide in 0.95 mL of n-hexane. The mixture was vortexed for 5 s and allowed to settle for 5 min. The top layer (5 μL) was injected into a gas chromatography (GC) (6890N Network GC System, Agilent Technologies, USA) fitted with a flame ionization detector and an automatic injector (7683B Series, Agilent Technologies, USA). A cross-linked, fused-silica capillary column BPX70 (30 m × 0.32 mm id × 0.25 μm) (SGE Analytical Science, Australia) (equivalent to 70% cyanopropyl siloxane) was used at a column pressure of 10 psi. The initial temperature of the column was 100°C and held for 2 min. Then, it was increased at the rate of 5°C/min to 230°C and held for 10 min. The carrier gas was helium. The temperature of the injector and detector was maintained at 250°C. Fourteen fatty acid methyl esters were identified by comparing their retention times with those of standards (Supelco, Bellefonte, PA), namely octanoate (C8:0), decanoate (C10:0), dodecanoate (C12:0), myristate (C14:0), palmitate (C16:0), palmitoleate (C16:1), stearate (C18:0), oleate (C18:1), linoleate (18:2), arachidate (C20:0), linolenate (C18:3), behenate (C22:0), cis-1,3-docosenoate (C22:1), and tetracosanoate (C24:0). The peak areas produced by data integrator were used to quantify the components based on relative percentages.
Determination of triacylglycerol (TAG) profile
The TAG profile of the fat was determined using a Waters 2695 Alliance HPLC (Waters Corp., Milford, MA, USA) connected to a Waters 2414 refractive index (RI) detector, two Waters 515 HPLC pumps, an auto-sampler, and an online degasser without first removing FFAs from the fat samples.[Citation17] Separation of TAG was achieved using an RP-18 column (Purovspher® Star, 250 mm × 4.6 mm I.D., 5 μm particle size, from Merck, Darmstadt, Germany) that was placed in a column oven at 40°C. TAGs were eluted with a mixture of HPLC-grade acetone/acetonitrile (63.5:36.5, v/v) as the mobile phase at a flow rate of 1 mL/min.[Citation18] The total run time was 1 h. Where possible, the TAG peaks were identified by comparing peaks in the TAG profile of palm olein[Citation16] using the same conditions as were used for rambutan seed fat TAG as reported by Manaf et al.[Citation8] The integrated peak areas were used to quantify the components based on relative percentages.
Determination of thermal behavior
Determination of thermal properties of the rambutan seed fats was carried out using a Perkin-Elmer Diamond Differential Scanning Calorimeter (DSC) (Shelton, CT, USA) with PYRIS data processing software.[Citation19] Prior to analysis, the fat samples were warmed at 60°C to ensure complete melting and to remove all crystal memory. Indium and zinc were used to calibrate the DSC. The purge gas was 99.99% nitrogen administered at 100 mL/min and 20 psi. A sealed empty aluminum sample pan (Perkin Elmer, Shelton, Washington, USA) was used as the reference. Approximately 3–5 mg of fat was crimp-sealed in a sample pan with a crimper (Perkin Elmer) and placed in the DSC’s sample furnace. All samples were subjected to the following temperature program: cooling to −60°C at the rate of 5°C/min and held for 2 min; heating from −60°C to 60°C at the rate of 5°C/min and holding at 60°C isothermally for 2 min; then, cooling from 60°C to −60°C also at the rate of 5°C/min. The heating and cooling thermograms provided information on the temperature at which the melting process began, the temperature at which most of the TAG have melted, and the complete melting temperature of the fat.
Determination of solid fat index (SFI)
The SFI of rambutan seed fat samples was determined according to the method described by Lee et al.[Citation10] and Romain et al.[Citation20] The amounts of solids in rambutan seed fats as a function of the temperature were derived from the heating thermogram of the respective fat sample. By using the PYRIS data processing software, the partial peak areas (%) of the melting peaks against temperature were calculated and correlated with solid percentages, considering that, at 0% of partial area, samples were 100% solids.
Determination of saponin content
Frozen fruit samples were first thawed, peeled, and deseeded. Seeds after washing under running tap water were finely chopped and freeze-dried for 2 days and ground into fine powder. The samples were stored at 4°C in amber glass bottles covered with aluminum foil prior to analysis. The procedure to extract saponin from the samples as described by Lai et al.[Citation21] was followed. Briefly, 0.5 g of ground freeze-dried samples was mixed with 10 mL of petroleum ether (boiling point at 40–60°C) and shaken for 4 h at 1000 rpm and 25 ± 3°C. The solvent was then removed using a vacuum rotary evaporator at 60°C. Saponin was extracted from 20 mg of residues with 5 mL of 80% aqueous methanol and shaken for another 4 h. The extract was filtered and kept at 4°C in the dark until it was subjected to analysis.
Total saponin content was determined using the spectrophotometric method as described by Hiai et al.[Citation22] Briefly, in an ice water bath, 0.1 mL of the extract obtained above was mixed with 0.4 mL of 80% methanol solution, 0.5 mL of freshly prepared 8% (w/v) vanillin solution (prepared in ethanol), and 5.0 mL of 72% sulfuric acid. The mixture was then heated in a water bath at 60°C for 10 min and finally cooled in ice cold water. The reagent blank was prepared using the same procedure, but the extract was replaced with an equivalent volume of 80% methanol. The absorbance at 544 nm was measured against the reagent blank using a UV-Vis spectrophotometer (Shimadzu UV-160A PC, Shimadzu Corporation, Kyoto, Japan). Saponin content was calculated from a standard curve constructed using different concentrations (0, 1000, 2000, and 3000 ppm) of crude soya saponin (containing a minimum of 80% saponin, Waki, Osaki, Japan) in 80% aqueous methanol and expressed as mg soya saponin/100 g sample.
Determination of tannin content
Extraction of tannin from samples was done using the procedure as described by Wijekoon et al.[Citation23] One gram of accurately weighed freeze-dried ground sample obtained for saponin analysis was mixed with 40 mL of absolute methanol in a 100 mL Erlenmeyer flask and stirred using a magnetic stirrer at 1200 rpm (Model FSMQ 143551, Fisher Scientific) for 1 h at room temperature (25 ± 3°C). The mixture was then filtered through a Whatman No. 1 filter paper under suction. The remaining residue on the filter paper was transferred back into the same flask and re-extracted twice following the same procedure. Finally, all filtrates were pooled and collected in reagent bottles covered with aluminum foil.
For the determination of tannins in the sample extracts, the vanillin–HCl method described by Broadhurst and Jones[Citation24] was followed. Briefly, 0.5 mL of the extract was added to 3 mL of vanillin reagent (4%, w/v, vanillin in methanol) and mixed thoroughly. Then, 1.5 mL of concentrated HCl was added to the mixture and mixed by vortex. The content was kept in the dark for 15 min at room temperature. A blank sample was prepared using the same procedure, where the extract was replaced with methanol. The absorbance was read at 500 nm. The content of tannins in the sample was expressed as mg catechin equivalent (CE)/100 g sample. A standard curve based on catechin (Chromadex, Irvine, CA, USA) was plotted in order to quantify the tannin content of the sample.
Statistical analysis
The analytical data were analyzed by one-way analysis of variance followed by Tukey’s test using Minitab v. 16 Statistical Software (Minitab Inc., Coventry, UK). The results were expressed as mean value ± standard deviation. Statistical significance differences were considered at the level of p < 0.05.
Results and discussion
Crude fat content, IV, and FFA content
The crude fat contents of 11 varieties of rambutan seed are shown in . Results indicated that there is a slight but significant difference (p < 0.05) in the crude fat contents. The crude fat content of the rambutan seed ranged between 36.13 and 39.13 g/100 g, where Clones R169 and R4 possessed the lowest and highest crude fat content, respectively. These findings are comparable to the results reported by Augustin and Chua[Citation25] who stated that rambutan Clone R169 and Clone R4 contained 37.9 and 37.1 g/100 g of fat, respectively. On the other hand, researchers in Thailand reported the rambutan seed they analyzed contained 14–41 g/100 g of fat depending on the variety.[Citation26–Citation28] The variability of the tested cultivars, diversity in the maturity of the seeds used as well as the agricultural conditions in the cultivated area may attribute to the differences in the fat content of the seed in this study and that of reported in the literature.[Citation25,Citation29] The higher fat content in the seed of Clone R4 may be favored by the food industry as rambutan seed fat can be used in the formulation of high premium specialty fats like confectionary fat.[Citation8]
Figure 2. Crude fat content (A), IV (B), FFA (C), saponin content (D), tannin content (E), and SFI at 37°C (F) of rambutan seed or seed fat.
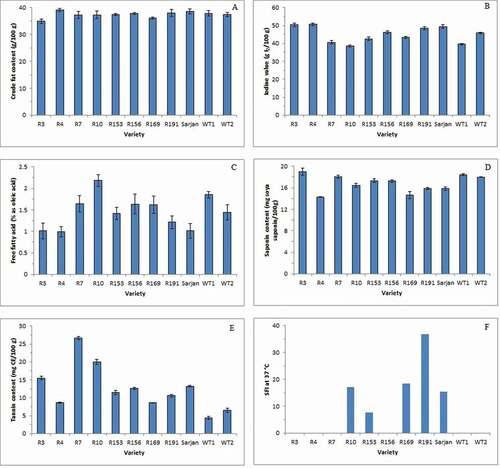
The IV indicates the degree of unsaturation of the fat while the FFA content is one of the parameters to judge the quality of the fat. In this study, there is also a slight but significant difference (p < 0.05) in the IV and FFA contents among the rambutan seed fat of different varieties ( and ). The IV and FFA contents of the seed fat ranged from 38.50%to 50.61 g I2/100 g fat and from 0.99% to 2.18% as oleic acid (0.38–0.81 g/100 g dried seeds), respectively. Rambutan Clone R4 had the highest IV (50.61 g I2/100 g fat) and lowest FFA content (0.99% as oleic acid or 0.38 g/100 g dried seeds), while rambutan Clone R10 had the lowest IV (38.50 g I2/100 g fat) and highest FFA content (2.18% as oleic acid or 0.81 g/100 g dried seeds). The values for IV are consistent with those obtained by other researchers. Manaf et al.[Citation8] found that the IV of rambutan fat was 50.27 g I2/100 g fat, while Kheiri and Mohd Som[Citation30] reported that the IV of 13 varieties of rambutan seed fat in their study ranged from 41.8 to 49.6 g I2/100 g fat. Harahap et al.[Citation6] and Solís-Fuentes et al.[Citation5] discovered that the IVs of their rambutan seed fat samples were 37.64 and 47 g I2/100 g fat, respectively. The FFA values are also in agreement with the values obtained by the researchers such as Solís-Fuentes et al.[Citation5] and Harahap et al.[Citation6]
Fatty acid composition
The fatty acid composition of the rambutan seed fat is presented in . Generally, the fat was composed of 44.19–62.50% unsaturated fatty acids and 37.51–45.18% saturated fatty acids. Among the 11 varieties, Clone Sarjan has the highest percentage of unsaturated fatty acids (62.50%). There are significant differences (p < 0.05) in all fatty acids among the seed fat of different rambutan varieties except for oleic (C18:1) and arachidic (C20:0) acids, which are the major fatty acids in rambutan seed fat, and they made up to 33.35–46.64% and 26.03–33.27%, respectively. The seed fat also contained some gondoic acid (11-eicosenoic acid, C20:1), behenic acid (docosanoic acid, C22:0), and less than 0.5% each of erucic acid (docosenoic acid, C22:1) and lignoceric acid (tetracosanoic acid, C24:0). The result is in agreement with those obtained by other researchers.[Citation5,Citation8,Citation31] However, Augustin and Chua[Citation25] reported a slight difference where arachidic acid was slightly higher (36.14–36.77%). In general, oleic and arachidic fatty acids together comprised 68.11–77.85% of the total fatty acids and this is in agreement with previous studies.[Citation5,Citation6,Citation8,Citation25–Citation27,Citation31] The relatively high concentration of saturated fatty acids causes the rambutan seed fat to be semi-solid at room temperature. This property allows the fat to be used without being subjected to hydrogenation, especially when autoxidation may be a concern.[Citation27] According to Issara et al.,[Citation32] a high concentration of saturated fatty acid, specifically arachidic acid in rambutan seed fat, makes the fat a potential source of cocoa butter substitute for confectionary products. Since rambutan seed fat is abundant in both oleic acid and arachidic acid, it may also be a good source of nutritious low calorie fat suitable for human consumption. Studies have shown that oleic acid from olive oil is responsible for the reduction of blood pressure[Citation33,Citation34] while arachidic acid is less likely to be nutritionally available to the body following hydrolysis by pancreatic lipase due to very high melting temperature (76°C).[Citation35] Hence, chocolate products produced from rambutan seed fat are suitable to those who enjoy the meltdown-in-the-mouth property of chocolate and concern their calorie intake at the same time.
Table 1. Fatty acid composition of rambutan seed fats.
TAG profile
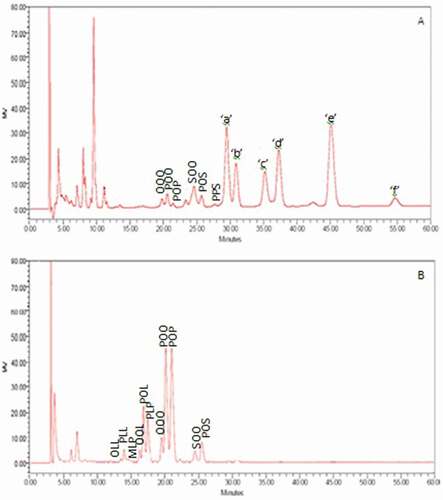
All varieties of rambutan seed fat showed the same trend for their TAG profiles. shows a representative TAG profile of rambutan seed fat and palm olein (for comparison). The distribution of TAG of different varieties of seed fats determined by HPLC is shown in . Six TAGs were successfully identified after comparing with the retention times of known TAGs of palm olein [Citation17] and rambutan seed fat reported by Manaf et al.,[Citation8] namely OOO, POO, PPO, SOO, PSO, and PPS, where O, P, and S denote oleic acid, palmitic acid, and stearic acid, respectively. It also can be seen that there are six TAG peaks that cannot be identified and are labeled as ‘a’, ‘b’, ‘c’, ‘d’, ‘e’, and ‘f’ (), where peaks ‘a’, ‘b’, ‘c’, ‘d’, and ‘e’ are the major TAGs, representing about 83.94–95.33% of the total TAG. Similar to the findings in this study, Kheiri and Mohd Som [Citation30] reported that the major TAGs (C54, C56, and C58) in rambutan seed fat constitute about 90% of the total. Manaf et al.[Citation8] also reported that these five major unknown TAGs of rambutan seed fat represented more than 77% of the total. Manaf et al.[Citation8] also suggested that the presence of a high concentration of arachidic acid in rambutan seed fat causes the fat to possess high molecular weight TAG compared to cocoa butter, but these TAGs could not be identified due to a lack of commercial standards. Kheiri and Mohd Som [Citation30] who studied the TAG composition of eight clones of rambutan seed fat concluded that the TAGs were C50 (0–1.2%), C52 (0–7.0%), C54 (12.5–21.6%), C56 (34.6–39.1%), C58 (28.8–47.2%), and C60 (1.5–6.6%). Examples of C50 TAG are PPS and PPO, C52 are PSO and POO, C54 are LOO and LSO, C56 are AOO and AOS, C58 are AOG and AOA, and C60 are AAA and AAG, where L, A, and G denote linoleic acid, arachidic acid, and gondoic acid, respectively.
Table 2. TAG composition of rambutan seed fats.
Thermal profiles (melting and cooling behaviors)
shows the heating and cooling thermograms of seed fat from 11 varieties of rambutan. The phase transition temperatures, which are represented by the temperature of the peaks in the thermogram and represent the phase change process either from solid to liquid or vice versa, of the fat are summarized in . also shows that the final offset temperature refers to the complete melting temperature of the seed fat. The thermograms of the seed fat of different varieties show relatively simple melting and cooling profiles corresponding to TAGs with similar phase behavior. Regardless of varieties, the melting and crystallization curves show that the rambutan seed fat exhibited four to nine peaks that appeared non-distinct, indicating that heterogeneous types of TAGs were melted and crystallized at different temperatures. The small differences in the melting and crystallization of the fats under study are probably due to slight differences in TAG composition.
Table 3. Temperature of phase transition (T) and crystallization and melting enthalpies (∆H) of rambutan seed fat.
Figure 4. Thermograms of seed fat of R3 (A), R4 (B), R7 (C), R10 (D), R153 (E), R156 (F), R169 (G), R191 (H), Sarjan (I), WT1 (J), and WT2 (K).
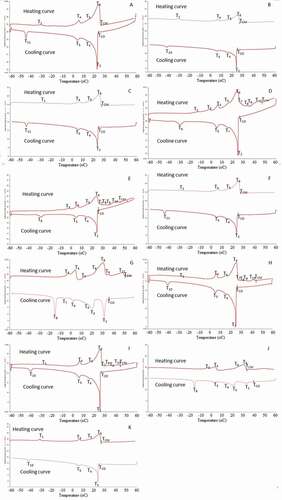
Regardless of variety, the peak T1 of the melting curve of the seed fat was low. This suggests that in this fraction there was a very small percentage of highly unsaturated TAGs such as triolein (OOO) which has a melting point at −32°C.[Citation36] The largest peak is T6 and represents TAG with the highest melting temperature and possible TAG in this group is TAG with more saturated fatty acids such as triarachidic acid (AAA). The complete melting temperature (TCM) of the seed fat was between 24.8°C and 50.6°C, depending on the variety/clone. The ones with lower complete melting temperatures include R3, R4, R7, R156, and WT2, while the varieties/clones with higher complete melting temperatures are those containing some very high melting TAG such as AAA. Romain et al.[Citation20] found that the rambutan seed fat they studied melted completely at below 30°C. However, Manaf et al.[Citation8] and Sonwai and Ponprachanuvut [Citation31] reported that the complete melting point of rambutan seed fat they studied was 40°C and 41.55°C, respectively, while Kheiri and Mohd Som[Citation30] reported that the fat of rambutan Clone R4 and R7 they studied melted completely at 47°C. The melting enthalpy of the 11 varieties of seed fat ranged from 71.2 to 141.7 J/g and this is in agreement to that reported by Solís-Fuentes et al.[Citation5] and Sonwai and Ponprachanuvut.[Citation31]
The liquid-to-solid phase change of a fat corresponds to the crystallization temperature of different groups of TAGs due to the differences in their fatty acid compositions. The crystallization onset temperature (TCO) of the seed fat was relatively high, ranging from 24.1°C to 39.4°C, possibly due to the high arachidic acid content in the fat. Similar observations were made by Solís-Fuentes et al.[Citation5] and Sonwai and Ponprachanuvut.[Citation31] As can be seen in , there is a small peak (T11) at the end of the crystallization curve, indicating that this region has a high content of TAGs with more unsaturated fatty acids such as OOO. The seed fat was completely crystallized at temperatures between −18.1°C and −47.6°C depending on the source, where the fat from Clone R153 had the lowest crystallization point while the fat from WT1 variety had the highest crystallization point. These findings are comparable to that reported by Manaf et al.[Citation8] who stated that the crystallization point of rambutan seed fat that they studied was −43.8°C. The total crystallization enthalpy of the seed fat ranged from 60.4 to 88.9 J/g. Solís-Fuentes et al.[Citation5] and Sonwai and Ponprachanuvut[Citation31] reported that the crystallization enthalpies of rambutan seed fat they studied were 89 and 83.27 J/g, respectively.
Solid fat index (SFI)
SFI provides information on the ‘solids’ content of a lipid system as a function of temperature.[Citation37]The SFI of 11 varieties of rambutan seed fat were derived from their heating thermograms and are shown in . A plot of the SFI against temperature gave a sigmoidal curve in which the shape of the curve is similar to that reported by Sonwai and Ponprachanuvut[Citation31] and others.[Citation5,Citation8,Citation20] At 0°C, the SFI of the rambutan seed fat ranged between 87.4% and 91.6%. These values are comparable to the results reported by Romain et al.[Citation20] and Solís-Fuentes et al.[Citation5] who obtained more than 70% of solid fat content (SFC) in their rambutan seed fat samples at 0°C. However, Manaf et al.[Citation8] found that the SFC of rambutan seed fat at 0°C was 53.5%, while Harahap et al.[Citation6] reported that at 0°C the SFC in rambutan seed fat was 30.92%. These differences are most likely due to the varietal differences.
Figure 5. SFI of seed fat of Clones R3, R4, R7, and R10 (A); Clones R153, 156, 169, and 191 (B); and varieties of Sarjan, WT1 and WT2 (C).
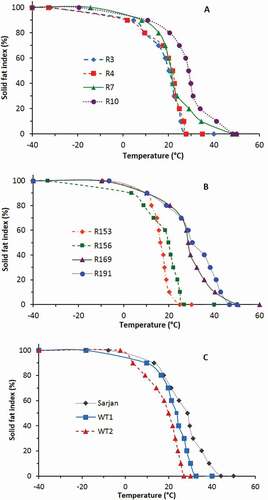
The fat has a sharp melting point between the temperature of 10°C and 30°C (). It can also be noticed that the fat possesses high SFI at supermarket temperature (20–23°C) but complete melting could occur at room temperature (for some varieties). These data support the proposed application of rambutan seed fat to be used as a cocoa butter substitute as the fat has a similar meltdown-in-the-mouth property to that of cocoa butter. The SFI of the seed fat at human body temperature (37°C) is shown in , and it shows that the fat of some varieties melted completely at that temperature.
Saponin and tannin contents
Saponins are high-molecular-weight glycosides with sugar unit(s) linked to a triterpene or steroid aglycone,[Citation38] while tannins are the predominant class of polyphenols that occur widely in food grains and legumes.[Citation39] Rambutan seeds are bitter and have been reported to have narcotic effects as there are traces of alkaloids in the seed and the testa contains saponin and tannin.[Citation7] It has been reported that a high intake of saponin and tannin may cause growth depression.[Citation39] The saponin and tannin contents of the 11 varieties of rambutan seed are shown in and , respectively. There are significant differences (p < 0.05) in the saponin and tannin contents among the rambutan seeds of different varieties. In general, the rambutan seeds contained between 14.27 and 18.96 mg soya saponin/100 g and 4.40–26.68 mg CE/100 g of sample. Both wild-type varieties appeared to have lower tannin contents (4.40 mg CE/100 g for WT1 and 6.51 mg CE/100 g for WT2) compared to that of the registered clones. However, Mehdizadeh et al.[Citation40] found that the seed of the Anak Sekolah rambutan clone contained 0.4 mg/g (40 mg/100 g) of soya saponin and 13.8 mg CE/g (1380 mg/100 g or 1.3 g/100 g) of tannin, while Bullangpoti et al.[Citation41] reported that the rambutan seed they examined contained 5.41% (w/w) (~5000 mg/100 g or 5 g/100 g) saponin. Another group of researchers found that the saponin and tannin contents of fresh rambutan seed were 0.98 mg/100 g of sample and 0.15 mg/100 g of sample, respectively.[Citation42] However, the methods they used to quantify these constituents were not described. The differences in the saponin and tannin contents of the seed in this study and previous findings may be due to the variability of the tested cultivars, diversity in the maturity of the seeds used as well as the agricultural conditions in the cultivated area. In comparison, bitter kola seed and alligator pepper seed are known to contain saponin and tannin with a concentration of 11.48 mg/100 g and 1.24 mg/100 g, respectively, for saponin and 0.26 mg/100 g and 0.38 mg/100 g, respectively, for tannin.[Citation43] Muzquiz et al.[Citation44] found that bitter lupin seed contains 47 mg of total saponin/100 g.
Conclusion
In this study, 11 varieties (including two underutilized wild types) of rambutan seed fats were characterized. Rambutan seeds contain relatively high crude fat content. The seed fat is abundant in saturated fatty acids, especially arachidic fatty acid, making the fat semi-solid at room temperature (30°C). The fat contained several unidentified large molecular weight TAGs that comprised more than 83.94–95.33% of the total TAG. The seed fat phase behavior shows relatively simple profiles of melting and crystallization with four to nine peaks. The fat of some varieties melted completely at 37°C, making the fat suitable to be used as a cocoa butter substitute. Apart from the seed fat, the seed itself contained 14.27–18.96 mg soya saponin/100 g and 4.40–26.68 mg CE/100 g. These findings indicated that it is possible to use rambutan seed fat in various industries, from confectionery to cosmetics. By doing so, rambutan fruit including its seed can be fully utilized and, subsequently, wastage can be reduced.
Additional information
Funding
References
- Wall, M. M.; Ascorbic Acid and Mineral Composition of Longan (Dimocarpus Longan), Lychee (Litchi Chinensis) and Rambutan (Nephelium Lappaceum) Cultivars Grown in Hawaii. Journal of Food Composition and Analysis. 2006, 19, 655–663.
- Raihana, A. R. N.; Marikkar, J. M. N.; Amin, I.; Shuhaimi, M. A. Review on Food Values of Selected Tropical Fruits’ Seeds. International Journal of Food Properties. 2015, 18, 2380–2392.
- Ahmad, I.; Chua, P. C. In Trends in Production and Trade of Tropical Fruits in ASEAN Countries. In 4th International Symposium on Tropical and Subtropical Fruits, Bogor, Indonesia, Ept; Palupi, E. R., Warrington, I. J., Eds.; International Society for Horticultural Science: Belgium, 2013. pp 559–580.
- Tindall, H. D. Sapindaceous Fruits: Botany and Horticulture. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons: London, 1994. pp 143–196.
- Solís-Fuentes, J. A.; Camey-Ortíz, G.; Hernández-Medel, M. D. R.; Pérez-Mendoza, F.; Durán-de-Bazúa, C. Composition, Phase Behavior and Thermal Stability of Natural Edible Fat from Rambutan (Nephelium Lappaceum L.) Seed. Bioresource Technology. 2010, 101, 799–803.
- Harahap, S. N.; Ramli, N.; Vafaei, N.; Said, M. Physicochemical and Nutritional Composition of Rambutan Anak Sekolah (Nephelium Lappaceum L.) Seed and Seed Oil. Pakistan Journal of Nutrition. 2012, 10, 1073–1077.
- Morton, J. F.; Rambutan. In Fruits of Warm Climates; Morton, J. F., Ed.; Julia F. Morton: Miami, 1987. pp 262–265.
- Manaf, Y. N. A.; Marikkar, J. M. N.; Long, K.; Ghazali, H. M. Physico-Chemical Characterisation of the Fat from Red-Skin Rambutan (Nephellium Lappaceum L.) Seed. Journal of Oleo Science. 2013, 62, 335–343.
- Cheng, W. Y.; Akanda, J. M. H.; Nyam, K. L. Kenaf Seed Oil: A Potential New Source of Edible Oil. Trends in Food Science and Technology. 2016, 52, 57–65.
- Lee, S. T.; Radu, S.; Ariffin, A.; Ghazali, H. M. Physico-Chemical Characterization of Oils Extracted from Noni, Spinach, Lady’s Finger, Bitter Gourd and Mustard Seeds, and Copra. International Journal of Food Properties. 2015, 18, 2508–2527.
- Zee, F. T.; Rambutan and Pili Nuts: Potential Crops for Hawaii. In New Crops; Janick, J., Simon, J. E., Eds.; Wiley and Sons Ins: New York, 1993. pp 461–465.
- Rajasekaran, A.; Ganesan, S.; Kamini, N.; Lavanya, C.; Lee Yoon, L.; Shian Oh, H. Anti-Nociceptive, CNS, Antibacterial and Antifungal Activities of Methanol Seed Extracts of Nephelium Lappaceum L. Oriental Pharmacy and Experimental Medicine. 2013, 13, 149–157.
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Washington, DC, 1984.
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 6th ed.; American Oil Chemists’ Society: Champaign, USA, 1997.
- International Office of Cocoa, Chocolate and Sugar Confectionery (IOCCC). Determination of Free Fatty Acids (FFA) Content of Cocoa Fat as a Measure of Cocoa Nib Acidity. Analytical Method. 1996, 42, 130–136.
- Cocks, L. V.; Van Rede, C. Laboratory Handbook for Oil and Fats Analysts; Academic Press: London, 1966; p. 419.
- Ghazali, H. M.; Hamidah, S.; Man, Y. B. C. Enzymatic Transesterification of Palm Olein with Nonspecific and 1,3-Specific Lipases. Journal of the American Oil Chemists’ Society. 1995, 72, 633–639.
- Swe, P. Z.; Che Man, Y. B.; Ghazali, H. M. Improved NARP-HPLC Method for Separating Triglycerides of Palm Olein and Its Solid Fractions Obtained at Low Temperature Storage. Food Chemistry. 1996, 56, 181–186.
- Abdulkarim, S. M.; Long, K.; Lai, O. M.; Muhammad, S. K. S.; Ghazali, H. M. Some Physico-Chemical Properties of Moringa Oleifera Seed Oil Extracted Using Solvent and Aqueous Enzymatic Methods. Food Chemistry. 2005, 93, 253–263.
- Romain, V.; Ngakegni-Limbili, A. C.; Ouamba, J. Thermal Properties of Monoglycerides from Nephelium Lappaceum L. Oil, as a Natural Source of Saturated and Monounsaturated Fatty Acids. Industrial & Engineering Chemistry Research. 2013, 52, 14089–14098.
- Lai, L. R.; Hsieh, S. C.; Huang, H. Y.; Chou, C. C. Effect of Lactic Fermentation on the Total Phenolic, Saponin and Phytic Acid Contents as Well as Anti-Colon Cancer Cell Proliferation Activity of Soymilk. Journal of Bioscience and Bioengineering. 2013, 115, 552–556.
- Hiai, S.; Oura, H.; Nakajima, T. Color Reaction of Some Sapogenins and Saponins with Vanillin and Sulfuric. Planta Medica. 1976, 29, 116–122.
- Wijekoon, M. M. J. O.; Bhat, R.; Karim, A. A. Effect of Extraction Solvents on the Phenolic Compounds and Antioxidant Activities of Bunga Kantan (Etlingera Elatior Jack.) Inflorescence. Journal of Food Composition and Analysis. 2011, 24, 615–619.
- Broadhurst, R. B.; Jones, W. T. Analysis of Condensed Tannins Using Acidified Vanillin. Journal of the Science of Food and Agriculture. 1978, 29, 788–794.
- Augustin, M. A.; Chua, B. C. Composition of Rambutan Seeds. Pertanika. 1988, 11, 211–215.
- Kalayasiri, P.; Jeyashoke, N.; Krisnangkura, K. Survey of Seed Oils for Use as Diesel Fuels. Journal of the American Oil Chemists’ Society. 1996, 73, 471–474.
- Sirisompong, W.; Jirapakkul, W.; Klinkesorn, U. Response Surface Optimization and Characteristics of Rambutan (Nephelium Lappaceum L.) Kernel Fat by Hexane Extraction. LWT - Food Science and Technology. 2011, 44, 1946–1951.
- Winayanuwattikun, P.; Kaewpiboon, C.; Piriyakananon, K.; Tantong, S.; Thakernkarnkit, W.; Chulalaksananukul, W.; Yongvanich, T. Potential Plant Oil Feedstock for Lipase-Catalyzed Biodiesel Production in Thailand. Biomass & Bioenergy. 2008, 32, 1279–1286.
- Cheikh-Rouhou, S.; Besbes, S.; Hentati, B.; Blecker, C.; Deroanne, C.; Attai, H. Nigella Sativa L.: Chemical Composition and Physiochemical Characteristics of Lipid Fraction. Food Chemistry. 2007, 101,673–681.
- Kheiri, M. S. A.; Mohd Som, N. M. Physicochemical Characteristics of Rambutan Kernel Fat. MARDI Report. No. 186; Mardi Publication: Serdang, 1987.
- Sonwai, S.; Ponprachanuvut, P. Characterization of Physicochemical and Thermal Properties and Crystallization Behavior of Krabok (Irvingia Malayana) and Rambutan Seed Fats. Journal of Oleo Science. 2012, 61, 671–679.
- Issara, U.; Zzaman, W.; Yang, T. A. Rambutan Seed Fat as a Potential Source of Cocoa Butter Substitute in Confectionary Product. International Food Research Journal. 2014, 21, 25–31.
- Lv, Z.; Chen, K.; Zeng, Y.; Peng, Y. Nutritional Composition of Canarium Pimela L. Kernels. Food Chemistry. 2011, 125, 692–695.
- Terés, S.; Barceló-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J. E.; Escribá, P. V. Oleic Acid Content Is Responsible for the Reduction in Blood Pressure Induced by Olive Oil. Proceedings of the National Academy of Sciences of the United States of America. 2008, 105, 13811–13816.
- Febrianto, N. A.; Issara, U.; Yang, T. A.; Abdullah, W. N. W. Thermal Behavior, Microstructure, and Texture Properties of Fermented-Roasted Rambutan Seed Fat and Cocoa Butter Mixtures. Pelita Perkebunan. 2014, 30, 65–79.
- Maximo, G. J.; Costa, M. C.; Meirelles, A. J. A. Solid-Liquid Equilibrium of Triolein with Fatty Alcohols. Brazilian Journal of Chemical Engineering. 2013, 73, 33–43.
- Van De Voort, F. R.; Memon, K. P.; Sedman, I.; Ismail, A. A. Determination of Solid Fat Index by Fourier Transform Infrared Spectroscopy. Journal of the American Oil Chemists’ Society. 1996, 30, 411–416.
- Hostettmann, K.; Marston, A. Saponins. Chemistry and Pharmacology of Natural Products; Cambridge University Press: Australia, 1995; p. 584.
- Reddy, N. R.; Pierson, M. D. Reduction in Antinutritional and Toxic Components in Plant Foods by Fermentation. Food Research International. 1994, 1994, 281–290.
- Mehdizadeh, S.; Lasekan, O.; Muhammad, K.; Baharin, B. Variability in the Fermentation Index, Polyphenols and Amino Acids of Seeds of Rambutan (Nephelium Lappaceum L.) During Fermentation. Journal of Food Composition and Analysis. 2015, 37, 128–135.
- Bullangpoti, V.; Visetson, S.; Milne, J.; Pornbanlualap, S. Effects of Mangosteen’s Peels and Rambutan’s Seeds on Toxicity, Esterase and Glutathione-S-Transferase in Rice Weevil (Sitophilus Oryzae L.). Kasetsart Journal (Natural Science). 2004, 38, 84–89.
- Fila, W. O.; Johnson, J. T.; Edem, P. N.; Odey, M. O.; Ekam, V. S.; Ujong, U. P.; Eteng, O. E. Comparative Anti-Nutrients Assessment of Pulp, Seed and Rind of Rambutan (Nephelium Lappaceum). Annals of Biological Research. 2012, 3, 5151–5156.
- Okwu, D. E.;. Phytochemicals, Vitamins and Mineral Contents of Two Nigerian Medicinal Plants. International Journal of Molecular Medicine and Advance Sciences. 2005, 1, 375–381.
- Muzquiz, M.; De Produccion, D.; Ridout, C. L.; Price, K. R.; Fenwick, G. R. The Saponin Content and Composition of Sweet and Bitter Lupin Seed. Journal of the Science of Food and Agriculture. 1993, 63, 47–52.