ABSTRACT
This study evaluated the inhibitory effects of samples extracted by different solvents from bayberry on eight common foodborne pathogens and investigated the main bacteriostatic components of bayberry extracts using ultraviolet and ultraperformance liquid chromatographic-electrospray mass spectrometry. Among the different solvent extracts, the water-extracted sample (BE1) had the greatest average inhibition zone diameter for the tested foodborne pathogens, which reached 22.1 mm. Among them, Salmonella paratyphi, Listeria innocua, and Listeria monocytogenes were most sensitive to BE1, and the lowest minimal inhibitory concentration value was 2.07 mg/mL. The polyphenol content in BE1 was the highest at 24.11 mg/mL. In addition, the main bacteriostatic component in BE1 was identified as cyanidin 3-O-glucoside. As natural antimicrobial agents, the antimicrobial compounds in berries are likely to have many important applications in food and medical industries in the future.
Introduction
Chinese bayberry fruit (Myrica rubra Sieb. et Zucc.) is native to eastern Asia, mainly China, and it is very popular with the local consumers because of its sweet/sour taste, flavor, and color.[Citation1,Citation2] All parts of the bayberry plant are used in Chinese traditional medicines.[Citation3] Bayberries are an important source of phytochemicals, such as phenolic acids, anthocyanins, and flavonol glycosides. Fruit-derived phytochemicals have received attention owing to their potent anti-inflammatory,[Citation4] antioxidant,[Citation1,Citation5,Citation6] antidiabetic,[Citation7,Citation8] anticancer,[Citation9] antiobesity,[Citation10] antihyperlipidemia,[Citation11,Citation12] antiatherosclerosis,[Citation13,Citation14] and antibacterial properties.[Citation15]
Bayberry extract can prevent the metabolic disorders of C57BL/6 mice induced by a high-fat diet.[Citation16] The flavonoid extract from Chinese bayberry has a protective role against ethanol-induced oxidative damage in mice livers.[Citation17] In addition, the relevant researchers have proved that Myricitrin protects against peroxynitrite-mediated DNA damage and cytotoxicity in astrocytes.[Citation18] However, there are many active components in bayberry extract, and the synergistic effects of functional components are complex. Thus, there are few studies on the effective functional components in bayberry extract.
At present, most of the commonly used food preservatives are chemicals, such as benzoic acid, sorbic acid, and nitrite.[Citation19] In addition, antibiotic substances are often used in the preservation of livestock products. However, the potential harmful effects on human health cannot be ignored. Consumer demand for natural antibacterial and antiseptic substances has grown as knowledge of the abuse of toxic synthetic food preservatives and antibiotics has become more widespread. Thus, in recent years, the study of natural antibacterial substances has become a hot topic principally because of its safety. Puupponen-Pimiä[Citation20] studied the action of berry phenolics against human intestinal pathogens. Yildirim et al.[Citation21] reported cinnamon oil nanoemulsions by spontaneous emulsification. Andriy et al.[Citation22] studied the antimicrobial activities of crude extracts prepared from fungal mycelia. In addition, there are many studies on the antibacterial activities of tea polyphenols, garlic, and honeysuckle.[Citation19,Citation23,Citation24] So far, many studies on natural antimicrobial substances have focused on antimicrobial activities. However, only a few studies exist on the effective antimicrobial components, and there are no systematic and comparative in vitro studies on the antibacterial and phytochemical components of bayberry extract. Thus, there is a demand to find a single or a group of effective antibacterial components in bayberry that acts as a natural antibacterial agent, which would likely have important applications in the food and medical industries.
Thus, in this study, we aimed to systematically evaluate the antibacterial properties and phytochemicals of bayberry extract. Further, the effective bacteriostatic components of bayberry extract were determined using ultraviolet-visible (UV-Vis) absorption spectroscopy and ultraperformance liquid chromatographic-electrospray ionization-mass spectrometry (UPLC-ESI-MS).
Materials and methods
Material
To ensure the accuracy of our experimental results, the same variety of bayberry fruit (cultivar Biqi) was purchased from a supermarket in Wuxi City, Jiangsu Province, China. Portions of 200 g were packed in polyethylene bags and subsequently frozen at −18°C. Experiments were performed in quadruplicate.
Bayberry extract preparation
The sample of 200 g bayberry was first extracted with water, ethanol, acetone, and ethyl acetate, respectively, by heating reflux extraction method (50 °C, 8 h). And 10 min of ultrasonic treatment was carried out for every 2 h. The extract was then centrifuged with 4000 xg for 10 min, and the supernatant was freeze-dried to powder. Powdered samples extracted with water, ethanol, acetone, and ethyl acetate were labeled as BE1, BE2, BE3, and BE4, respectively.
Bacteria
Salmonella paratyphi A CMCC 50093 (−), Shigella dysenteriae CMCC 51573–10 (−), Pseudomonas aeruginosa ATCC 14149 (−), Escherichia coli ATCC 8099 (−), β-hemolytic streptococus CMCC 32210 (+), Staphylococcus aureus ATCC 6538 (+), Listeria innocua ATCC 33090 (+), and Listeria monocytogenes ATCC 35152 (+) (CMCC: National Center for Medical Culture Collection, China, American Type Culture Collection (United States)) were grown in nutrient agar (QingdaoHope Bio-Technology Co., Ltd., China). The microbial strains are listed in . Single colonies of the selected bacterial strains were preinoculated in 10 mL of sterile nutrient broth and incubated at 37 °C for 8 h. Then, 1 mL of the culture was added to 100 mL of broth and incubated at 37 °C for 24 h. The culture was diluted to a suitable concentration and used for the antibacterial tests.
Table 1. MIC results of bayberry extract.
Determination of antibacterial activity
The cylinder diffusion method was used to detect the inhibition zone diameter (IZD). The eight bacteria were diluted to obtain concentrations of 106 CFU/mL in the medium and cultured at 48 °C. Then, 15 mL of each bacterial suspension was independently poured into a Petri dish. Three evenly placed wells (6 mm in diameter) were made in the surface of the solidified medium, and 200 µL of the bayberry extract was added to the wells (Finnpipette, France). The IZD was measured to an accuracy of 0.1 mm, and the mean values of six tests were calculated. Water and methanol were used as negative and positive controls, respectively.
The twofold dilution method was used to determine the minimal inhibitory concentration (MIC). A twofold dilution series of each BE (1–4) in nutrient broth was prepared to obtain final concentrations of 8.28, 4.14, 2.07, 1.04, and 0.52 mg/mL. Approximately, 106 CFU/mL of each bacterial culture was added to the broth, and the lowest concentration without any colony growth was recorded as the MIC value. The experiments were conducted in quintuplicate.
Determination of active substances
Determination of total flavonoids: The total flavonoid content was determined in accordance with previously described methods.[Citation25,Citation26] The absorbance levels were quantitatively determined with a UV spectrometer (Hitachi U-2900) at λ 510 nm. The regression equation y = 0.8912x + 0.0036 (R2 = 0.9992) for determining total flavonoid content was established by taking the Rutin mass concentration (mg/mL) as the abscissa and the absorbance value as the ordinate.
Determination of total polyphenols: The total phenolic content was determined in accordance with previously described methods.[Citation27] The absorbance levels were quantitatively determined with a UV spectrometer (Hitachi U-2900) at λ 765 nm. The regression equation y = 1.7848x + 0.1012 (R2 = 0.9969) for determining the total phenol content was established by taking the gallic acid mass concentration (mg/mL) as the abscissa and the absorbance value as the ordinate.
Determination of total acidity: The total acidity determination was performed by titration with a 0.1 mol/L sodium hydroxide and phenolphthalein solution using 10 mL of sample and 50 mL of distilled water as recommended by the official compendium of food analysis.[Citation28] All determinations were performed in triplicate. The solutions were standardized as recommended in the official compendium.[Citation29]
Identification of the main bacteriostatic components
UPLC-ESI-MS and UV-Vis absorption spectral analyses: BE1 was further purified to determine its complex active ingredients and the purified extracts were termed BEP, which was used in subsequent experiments. The UPLC-ESI-MS and UV-Vis absorption spectral analyses were obtained from Yao et al.[Citation30]; analyses were performed on phenol compounds having antibacterial activities using a UPLC-ESI-MS (Waters Corp., USA) equipped with a photodiode array detector. Before analysis, the samples were scanned at 190–700 nm (Unico UV-4802, Unico, NJ, USA) to ensure the measurement of the phenolic compounds. Data acquisition was performed using Waters MassLynx_V4_RC9 software. The column was a BEH C-18 (1.7 µm particles, 2.1 mm × 50 mm), and the mobile phase (A) consisted of 0.1% formic acid, 5% acetonitrile and 95% H2O (V/V) and acetonitrile containing 0.1% formic acid (B). A stepwise linear gradient was programmed at 99:1 (A/B, V/V) for 2 min, changed to 98:2 over 5 min, changed to 60:40 over 1 min, and followed by 10:90 over 5 min to wash the column. The injection volume was 5 µL, and the detection wavelength was 254 nm (each fraction had an absorbance at this wavelength). The column temperature was 40 °C.
The mass spectra were achieved by ESI in the positive mode. The following ion optics were used: capillary, 3.50 kV; cone, 25 V; and extractor, 5 V. The source block temperature was 120°C, and the desolvation temperature was 300 °C. The electrospray probe flow was adjusted to 70 mL/min. Continuous mass spectra were recorded over the range m/z 100–800 with a 1 s scan time and an interscan delay of 0.1 s.
Statistical analyses
Experiments were performed in triplicate. Data were analyzed with OriginLab-8s (OriginLab Corporation, Hampton, MA, USA). Means were compared by Duncan’s new multiple range test. Statistically significant differences were set at P < 0.05.
Results and discussion
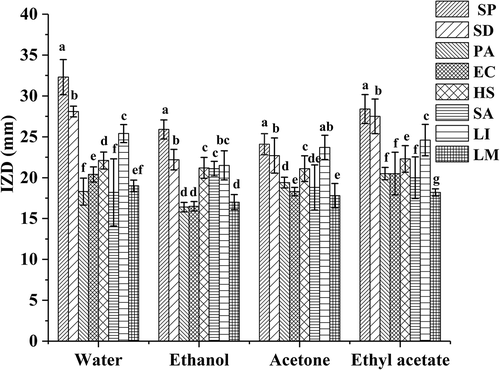
Antibacterial activities of different solvent extracts from bayberry
BE1, BE2, BE3, and BE4 have satisfactory antibacterial activities on all of the tested foodborne pathogens, including Gram-positive bacteria and Gram-negative bacteria, and the inhibitory effects of BE1, BE2, BE3, and BE4 on S. paratyphi were greatest, and the IZD reached 32.33 mm, 25.92 mm, 28.42 mm, and 24.17 mm, respectively (). Moreover, the average IZD of BE1 was the greatest at 22.1 mm for the eight common foodborne pathogens. Thus, the antibacterial effect of the BE1 was significantly (P < 0.05) greater than those of the other solvent extracts. This may be due to the high content of polyphenols in BE1, and the result was consistent with the active components. In view of the bacteriostatic action of bayberry extract, researchers in previous years had explored the preservation effect of bayberry extract on surimi. The results showed that the bayberry extract had a significant inhibitory effect on Serratia marcescens and Pseudomonas aeruginosa, and the addition of plum extract could prolong the shelf life of the surimi products storage at room temperature.[Citation31] Some researchers also studied the effect of the extract of bayberry fruit residue on fresh pork. The results showed that the extract of bayberry fruit residue as a natural antioxidant could be used to improve the color and lustre of cold fresh pork.[Citation32] Some researchers explored the preservation effect of bayberry leaf extract on the large yellow croaker, and the results also showed that the bayberry leaf extract could prolong the shelf life of the yellow croaker.[Citation33] The results of previous studies show that bayberry extract not only has good antibacterial effect, but also has great application value.
MIC analysis
The antimicrobial activity of BE1 was determined against a series of selected foodborne pathogens. The MIC values obtained are shown in . BE1 was active against all bacteria tested but in the distinct MIC range of 0.52–8.28 mg/mL. Salmonella paratyphi, Listeria innocua, and Listeria monocytogenes were most sensitive to BE1, having the lowest MIC value of 2.07 mg/mL. Liu et al.[Citation34] studied the inhibitory effects of cinnamon essential oils on S. paratyphi, L. innocua, and S. aureus in a gas-phase diffusion experiment, with MIC values of 18, 54, and 36 mg/mL, respectively. Bai et al.[Citation35] also studied the inhibitory effects of peony seeds extract on Salmonella, Rhizopus, and Aspergillus and found MIC values of 0.50% (V/V), 0.10% (V/V), and 0.50% (V/V), respectively. BE1 showed obvious bacteriostasis at lower concentrations.
Active components
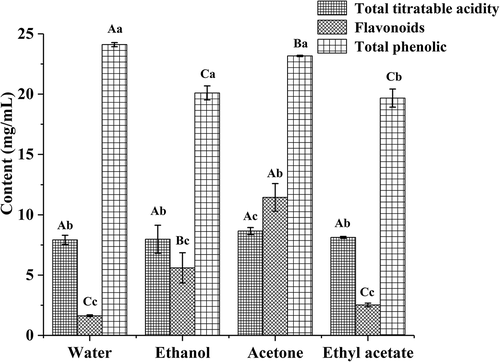
The total titratable acids and flavonoids in BE2 were significantly (P < 0.05) greater than those in the other three extracts (). However, the total phenolic content was the greatest in the BE1, which was consistent with the IZD results. Ju et al.[Citation36] studied the antibacterial activity of corn silk extract in which the main antibacterial components were flavonoids. However, Chen et al.[Citation37] showed that phenolics in blueberries had significant antibacterial activities. Thus, the major inhibitory components of different extracts varied and may have a synergistic bacteriostatic effect. These results were in agreement with those described by Gyawali et al.[Citation38] and Dorman et al.[Citation39]
UV-Vis absorption and UPLC-UV spectral analyses
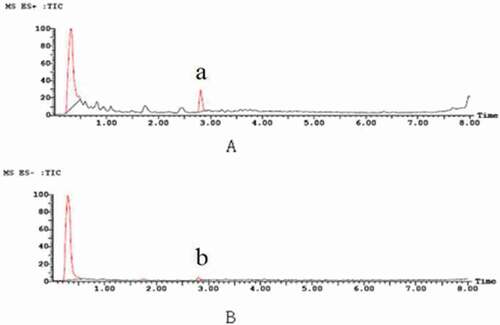
Anthocyanins are flavonoid compounds that have characteristic absorption peaks at wavelengths of 280 nm and 520 nm.[Citation40] There were two characteristic absorption peaks, A and B, in the chromatograms of bayberry extract (a). Thus, it can be inferred that the two peaks represent the anthocyanins, and A is consistent with the maximum absorption wavelength of cyanidin 3-O-glucoside.[Citation30] Therefore, A was preliminarily identified as cyanidin 3-O-glucoside. In addition, the UV-Vis absorption spectrum of the anthocyanins can be used to demonstrate the presence of acyl groups in BEP, and its maximum absorption wavelength range was 310–340 nm. However, the chromatograms showed no absorption peak at 310–340 nm. This indicated that there was no acyl group in the molecule, corroborating the foregoing conclusion.[Citation41]
Figure 3. The UV-Vis absorption spectrum and UPLC-UV spectrum of BEP.
Blue represents diluted once; green represents diluted twice; red represents dilution three times.
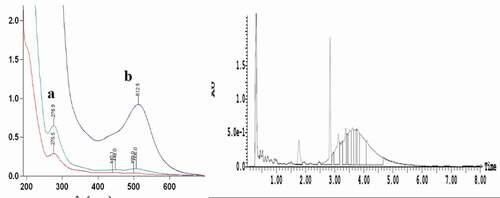
There was a major absorption peak at 520 nm (b). The peak’s area accounted for 13% of the total peak area, and its retention time was 2.84 min. According to a related report,[Citation41] anthocyanins have a characteristic absorption peak at 520 nm. Zhao et al.[Citation40] studied the composition and content of anthocyanins in different peaches and found that the cyanidin-3-O-glucoside in peach mainly contained an anthocyanin peak at 520 nm. Xue[Citation42] also studied the contents of anthocyanidin in Prunus cerasifera leaves at 520 nm, and the extraction rate of anthocyanin was 0.5247%. Thus, b not only showed that anthocyanin was the main antibacterial component in BEP, but also confirmed the inference regarding a.
UPLC-ESI-MS and MS spectral analyses
The UPLC-ESI-MS spectrum showed that there were “a” and “b” peaks in the positive ion mode (ES+) and negative ion mode (ES−), respectively, at 2.84 min (), which was consistent with the retention time of the peaks in b. However, the area of peak “a” was greater than that of peak “b” in the positive ion mode. This may be due to the strong sensitivity of the substance in positive ion mode. This was consistent with previous research.[Citation43]
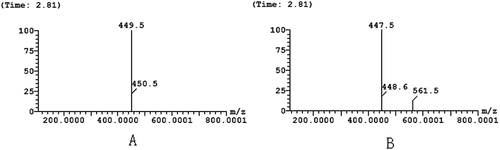
a and b showed the positive ion ESI-MS (a) and negative ion ESI-MS (b) of single-ion chromatograms. A comprehensive elution of the bayberry extracts and their derivatives was achieved within 10 min. However, the mass spectra showed that the m/z in positive ion and negative ion modes were 449.5 and 447.5 m/z, respectively. Thus, it can be speculated that the molecular weight of the BEP was 448 m/z. This corresponds to the molecular weight of cyanidin 3-O-glucoside. Hence, the substance was unambiguously identified as cyanidin 3-O-glucoside by comparing their retention time, UV-Vis spectroscopic data, and the pseudomolecular ion [M+H]+with the authenticated standards. These results were in agreement with the ones described by Zhou[Citation44] and Yao.[Citation30]
Conclusion
The antibacterial effects of samples extracted with different solvents from bayberry were studied and the water-based extraction had the greatest antibacterial effect and the highest total phenolic content. By studying the main antibacterial components of the BEP, we found that its main antibacterial component was cyanidin 3-O-glucoside. The safety of Chinese bayberry extract is indirectly indicated by the daily consumption of Chinese bayberry fruit. To the best of our knowledge, Chinese bayberry extract, which can be obtained from some dietary supplement companies in China, is consumed widely in China. No adverse effects of these extracts have been reported. Therefore, bayberry extract is likely to have many important applications in the future as a natural antimicrobial agent for the food and medical industries.
Declaration of interest
The authors declare no competing interests.
Additional information
Funding
References
- Chen, W.; Zhou, S.; Zheng, X. A New Function of Chinese Bayberry Extract: Protection against Oxidative DNA Damage. LWT-Food Science and Technology 2015, 60(2), 1200–1205. DOI: 10.1016/j.lwt.2014.09.011.
- Xia, Q.; Pan, S.; Zheng, M.; Chen, J.; Fang, Z.; Johnson, S.; Lu, S. Fatty Acid Profile, Oxidative Stability and Toxicological Safety of Bayberry Kernel Oil. Food and Chemical Toxicology 2013, 60, 92–97. DOI: 10.1016/j.fct.2013.06.054.
- Sun, C.; Zheng, Y.; Chen, Q.; Tang, X.; Jiang, M.; Zhang, J.; Chen, K. Purification and Anti-Tumour Activity of Cyanidin-3-O-Glucoside from Chinese Bayberry Fruit. Food Chemistry 2012, 131(4), 1287–1294. DOI: 10.1016/j.foodchem.2011.09.121.
- Farrell, N. J.; Norris, G. H.; Ryan, J.; Porter, C. M.; Jiang, C.; Blesso, C. N. Black Elderberry Extract Attenuates Inflammation and Metabolic Dysfunction in Diet-Induced Obese Mice. The British Journal of Nutrition 2015, 114(8), 1123–1131. DOI: 10.1017/S0007114515002962.
- Chen, W.; Zhao, J.; Bao, T. Comparative Study on Phenolics and Antioxidant Property of Some New and Common Bayberry Cultivars in China. Journal of Functional Foods 2016a, 27, 472–482. DOI: 10.1016/j.jff.2016.10.002.
- Huang, H.; Sun, Y.; Lou, S.; Li, H.; Ye, X. In Vitro Digestion Combined with Cellular Assay to Determine the Antioxidant Activity in Chinese Bayberry (Myrica rubra Sieb. Et Zucc.) Fruits: A Comparison with Traditional Methods. Food Chemistry 2014, 146, 363–370. DOI: 10.1016/j.foodchem.2013.09.071.
- Zhang, X.; Lv, Q.; Jia, S.; Chen, Y.; Sun, C.; Li, X.; Chen, K. Effects of Flavonoid-Rich Chinese Bayberry (Morella rubra Sieb.Et Zucc.) Fruit Extract on Regulating Glucose and Lipid Metabolism in Diabetic KK-Ay Mice. Food & Function 2016, 7(7), 3130–3140. DOI: 10.1039/C6FO00397D.
- Sun, C.; Huang, H.; Xu, C.; Li, X.; Chen, K. Biological Activities of Extracts from Chinese Bayberry (Myrica rubra Sieb.Et Zucc.): A Review. Plant Foods for Human Nutrition 2013, 68(2), 97–106. DOI: 10.1007/s11130-013-0349-x.
- Yang, Z.; Cao, S.; Zheng, Y. Chinese Bayberry Fruit Extract Alleviates Oxidative Stress and Prevents 1, 2-Dimethylhydrazine-Induced Aberrant Crypt Foci Development in Rat Colon Carcinogenesis. Food Chemistry 2011, 125(2), 701–705. DOI: 10.1016/j.foodchem.2010.09.070.
- Meireles, M.; Rodriguez-Alcala, L. M.; Marques, C.; Norberto, S.; Freitas, J.; Fernandes, I.; Calhau, C. Effect of Chronic Consumption of Blackberry Extract on High-Fat Induced Obesity in Rats and Its Correlation with Metabolic and Brain Outcomes. Food & Function 2016, 7(1), 127–139. DOI: 10.1039/C5FO00925A.
- Jurgonski, A.; Juskiewicz, J.; Zdunczyk, Z. Ingestion of Black Chokeberry Fruit Extract Leads to Intestinal and Systemic Changes in a Rat Model of Prediabetes and Hyperlipidemia. Plant Foods for Human Nutrition 2008, 63(4), 176–182. DOI: 10.1007/s11130-008-0087-7.
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Mihova, V.; Krasnaliev, I.; Borisova, P.; Belcheva, A. Antihyperlipidemic Effect of Aronia Melanocarpa Fruit Juice in Rats Fed a High-Cholesterol Diet. Plant Foods for Human Nutrition 2007, 62(1), 19–24. DOI: 10.1007/s11130-006-0036-2.
- Chen, Y.; Sun, X. H.; Cao, Y.; Zhao, Y. Progress in Antibacterial Activity of Blueberries. Natural Product Research and Development 2014, 25, 716–721.
- Alarcon, M.; Fuentes, E.; Olate, N.; Navarrete, S.; Carrasco, G.; Palomo, I. Strawberry Extract Presents Antiplatelet Activity by Inhibition of Inflammatory Mediator of Atherosclerosis (Sp-Selectin, sCD40L, RANTES, and IL-1beta) and Thrombus Formation Platelets. 2015, 26(3), 224–229. DOI: 10.3109/09537104.2014.898747.
- Li, J.; Han, Q.; Chen, W.; Ye, L. Antimicrobial Activity of Chinese Bayberry Extract for the Preservation of Surimi. Journal of the Science of Food and Agriculture 2012, 92(11), 2358–2365. DOI: 10.1002/jsfa.v92.11.
- Yu, L.; Cai, W.; Zhang, Y.; Feng, L.; Huang, C. Red Bayberry Extract Prevents High-Fat Diet-Induced Metabolic Disorders in C57BL/6. mice. Journal of Functional Foods 2015, 14, 278–288. DOI: 10.1016/j.jff.2015.02.003.
- Liu, H.; Qi, X.; Cao, S.; Li, P. Protective Effect of Flavonoid Extract from Chinese Bayberry (Myrica rubra Sieb. Et Zucc.) Fruit on Alcoholic Liver Oxidative Injury in Mice. Journal of Natural Medicines 2014, 68(3), 521–529. DOI: 10.1007/s11418-014-0829-9.
- Chen, W.; Zhuang, J.; Li, Y. Myricitrin Protects against Peroxynitrite-Mediated DNA Damage and Cytotoxicity in Astrocytes. Food Chemistry 2013, 141(2), 927–933. DOI: 10.1016/j.foodchem.2013.04.033.
- Ju J, Wang C, Qiao Y, et al. Effects of Tea Polyphenol Combined with Nisin on the Quality of Weever (Lateolabrax japonicus) in the Initial Stage of Fresh-Frozen or Chilled Storage State[J]. Journal of Aquatic Food Product Technology 2017, 26(99), 1-10.
- Puupponen-Pimia, R.; Nohynek, L.; Alakomi, H. L. The Action of Berry Phenolics against Human Intestinal Pathogens. Biofactors 2005, 23(4), 243–251. DOI: 10.1002/biof.5520230410.
- Tutku, S.; Halil, M. Cinnamon Oil Nanoemulsions by Spontaneous Emulsification: Formulation, Characterization and Antimicrobial Activity. LWT- Food Science and Technology 2017, 84, 122-128.
- Andriy, S.; Alla, S.; Roman, B.; Ján, G.; Janka, N.; Radovan, O.; Jana, Č.; Ján, B. Cinnamon Oil Nanoemulsions by Spontaneous Emulsification: Formulation, Characterization and Antimicrobial Activity. Potravinárstvo 2010, 65, 236-244.
- Wang, X. M.; Zhang, M.; Yang, Y. K.; Activities, A. Component Analysis of Solvent Extracts from Aged Garlic Extract. Modern Food Technology 2017, 31, 65–70.
- Oszmiański, J.; Wojdyło, A.; Lachowicz, S. Effect of Dried Powder Preparation Process on Polyphenolic Content and Antioxidant Activity of Blue Honeysuckle Berries. LWT-Food Science and Technology 2016, 67, 214–222. DOI: 10.1016/j.lwt.2015.11.051.
- Jing, E. L.; Song, T. F.; Zeng, H. Q.; Chang, L.; Shao, P. N. Total Flavonoids Content, Antioxidant and Antimicrobial Activities of Extracts from Mosla chinensis Maxim. Cv. Jiangxiangru. LWT-Food Science and Technology 2015, 64, 1022–1027. DOI: 10.1016/j.lwt.2015.07.033.
- Shi, J.; Zou, X.; Zhao, J. Determination of Total Flavonoids Content in Fresh Ginkgo Biloba Leaf with Different Colors Using near Infrared Spectroscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2012, 94, 271–276. DOI: 10.1016/j.saa.2012.03.078.
- Cao, W.; Suo, Z. R. Determination of Total Phenolic Acids in Honey by Folin-Ciocalteu Colorimetric Method. Food and Fermentation Industry 2003, 29, 80–82.
- Tischer B, Oliveira A S, Costa A B, et al. Rapid and simultaneous determination of acidity and salt content of pickled vegetable brine by using thermal infrared enthalpimetry. Journal of Food Composition & Analysis 2017, 63,34-37.
- Tischer, B.; Oliveira, A. S.; Costa, A. B.; Cichoski, A. J.; Barcia, M. T.; Wagner, R.; Barin, J. S. Rapid and Simultaneous Determination of Acidity and Salt Content of Pickled Vegetable Brine by Using Thermal Infrared Enthalpimetry. Journal of Food Composition and Analysis 2017, 63(1), 34–37. DOI: 10.1016/j.jfca.2017.07.029.
- Yao, W. R.; Wang, H. Y.; Wang, S. T.; Sun, S. L.; Zhou, J.; Luan, Y. Y. Assessment of the Antibacterial Activity and the Antidiarrheal Function of Flavonoids from Bayberry Fruit. Journal of Agricultural and Food Chemistry 2011, 59(10), 5312–5317. DOI: 10.1021/jf200211m.
- Li, J.; Han, Q.; Chen, W. Antimicrobial Activity of Chinese Bayberry Extract for the Preservation of Surimi. Journal of the Science of Food and Agriculture 2012, 92(11), 2358–2365. DOI: 10.1002/jsfa.5641.
- Zhang, J. J.; Gu, W. G.; Lu, B. B. The Studies on the Antibacterial and Antioxidation Effects of Extracts from Bayberry Pomace in Fresh and Cooked Pork. Chinese Journal of Food Science 2011, 11, 100–107.
- Su, H.; Chen, W.; Fu, S. Antimicrobial Effect of Bayberry Leaf Extract for the Preservation of Large Yellow Croaker (Pseudosciaena crocea). Journal of the Science of Food and Agriculture 2014, 94(5), 935–942. DOI: 10.1002/jsfa.6338.
- Liu, X. L.; Zhong, S. S.; Wu, K. G.; Yu, H. P.; Chai, X. H. Study on Gas-Phase Antibacterial Activity of Cinnamon Essential Oil. Food and Fermentation Industry 2010, 77, 21–24.
- Bai, X. T.; Zhu, X. W.; Luo, L.; Fan, J. L.; Xiao, H. F. Study on the Antimicrobial Characteristics of Peony Seed Extracts. Chinese Brewing 2010, 28, 59–62.
- Ju., J.; Yu, Q.; Zhou, W. X. Flash Extraction and Antibacterial Effects of Total Flavonoids Fromcorn Silk. Food Science and Technology 2016, 41 (3), 208-212.
- Chen, W.; Su, H.; Xu, Y.; Bao, T.; Zheng, X. Protective Effect of Wild Raspberry (Rubus hirsutus Thunb.) Extract against Acrylamide-Induced Oxidative Damage Is Potentiated after Simulated Gastrointestinal Digestion. Food Chemistry 2016b, 196, 943–952. DOI: 10.1016/j.foodchem.2015.10.024.
- Gyawali, R.; Ibrahim, S. A. Natural Products as Antimicrobial Agents. Food Control 2014, 46, 412–429. DOI: 10.1016/j.foodcont.2014.05.047.
- Dorman, H.; Deans, S. Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile Oils. Journal of Applied Microbiology 2000, 88(2), 308–316. DOI: 10.1046/j.1365-2672.2000.00969.x.
- Zhao, X. L.; Wang, F. R.; Xu, L. Y.; He, H. P.; Tian, Y. C. Determination of Anthocyanin in Different Fruit of Peach by HPLC Method. Food Science 2013, 34, 208–211.
- Hong, V.; Wrolstad, R. E. Use of HPLC Separation/Photodiode Detection for Characterization of Anthocyanins. Journal Agriculture Food Chemical 1990, (38), 708–715. DOI: 10.1021/jf00093a026.
- Xue, X. L.; Determination of the Contents of Anthocyanidin in Prunus Cerasifera Leaves. Hubei Agricultural Sciences 2010, 49, 956–958.
- Fan, Y. H.;. Studies on migration and control of food contaminations in three-piece metal cans ( Master’s thesis, Jiangnan University). 2015.
- Zhou, M.; Chen, Q.; Bi, J.; Wang, Y.; Wu, X. Degradation Kinetics of Cyanidin 3-O-Glucoside and Cyanidin 3-O-Rutinoside during Hot Air and Vacuum Drying in Mulberry (Morus alba L.) Fruit: A Comparative Study Based on Solid Food System. Food Chemistry 2017, 229, 574–579. DOI: 10.1016/j.foodchem.2017.02.131.