ABSTRACT
Red-flesh navel orange “Cara Cara” is attractive for customers with its bright red color and has been widely planted in China. In this study, phenolic compounds in “Cara Cara” harvested from five different regions were identified and quantified by ultra high performance liquid chromatography (UPLC) coupled with Quadrupole-Time of Flight -Electrospray ionization-mass spectrometry (Q-TOF-ESI-MSn). Their antioxidant abilities were evaluated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and oxygen radical absorption capacity (ORAC) assays. Twenty phenolics were detected in crude extraction, with hesperidin (29.36–44.06%) as the dominant component, followed by narirutin (13.44–17.03%) and apigenin-6,8-di-C-glucoside (8.28–12.38%). No free phenolic acid was found in crude extracts and three free phenolic acids (FPA) were determined through alkaline hydrolysis, wherein ferulic acid (86.22–90.49%) existed dominantly. Fujian “Cara Cara” showed the highest total phenolic index (TPI) and total flavonoid index (TFI) values, while the lowest TPI and TFI values were detect in Chongqing “Cara Cara”. DPPH value showed positive correlation with TPC value (r2 = 0.921), while no correlation was found between ORAC and phenolic contents.
Introduction
Phenolic compounds, a large group of secondary metabolitesin plants, have aroused great scientific interest for their significant antioxidant activity. [Citation1,Citation2] Citrus fruits and juices are the common sources of natural phenolic compounds, which mainly include flavanone-O-glycosides, flanvone-O-, or -C-glycosides. [Citation1,Citation3] The composition and antioxidant activity of phenolics have been widely investigated in several citrus varieties such as mandarin, sweet orange, lemon, grapefruit, pummel, and tangerine. [Citation4,Citation5]
“Cara Cara”, a kind of lycopene-accumulated sweet orange mutated from Washington Navel orange, was first introduced to China in the 1990s and widely planted in Hubei, Fujian, Chongqing, Jiangxi, and Hunan provinces of China. Due to the accumulation of lycopene, “Cara Cara” fruits display an attractive bright red color, which makes them appealing to customers. [Citation6] However, previous studies concerning phenolic compounds of “Cara Cara” were only focused on the analysis of the dominated ingredients such as hesperidin, narirutin, and didymin. [Citation7,Citation8] The detailed phenolic profiles of “Cara Cara” fruits have not been adequately studied.
Except for their contribution to human health, phenolics are also beneficial for plant itself, acting as attractants, feeding deterrents, stress-protecting agents, and physiological active compounds. [Citation9] The accumulation of phenolics was influenced by various environmental factors. It has been demonstrated that growing region affected the phenolic concentration and composition in fruit. [Citation10] By comparison of phenolic content harvested from different regions, the regional differences on “Cara Cara” could be analyzed, which might be helpful to planters. However, the phenolic differences of “Cara Cara” fruits have not been reported among the growing regions of China.
Phenolic compounds are usually analyzed by HPLC coupled with MS/MS on a single quadrupole instrument or by ion-trap mass spectroscopy. These approaches needed approximately 50 min for phenolic structure analysis with a relatively low accuracy. UPLC system performs the separation of compounds on smaller column particles with higher working pressure, which may take a shorter analysis time and provide a better peak capacity. UPLC-Q-TOF-ESI-MSn is an novel equipment with high mass accuracy in both full scan and MSn stages, which can provide much structural information of glycoside compounds. [Citation11] The objective of this study is to identify and quantify the phenolics in “Cara Cara” from five typical citrus growing regions of China by UPLC-Q-TOF-ESI-MSn, and compare their antioxidant abilities based on 2,2-diphenyl-1-picrylhydrazyl (DPPH) and oxygen radical absorption capacity (ORAC).
Materials and methods
Materials and chemicals
“Cara Cara” fruits were collected from Hubei (30.825°N, 110.96°E), Fujian (26.23°N, 117.61°E), Chongqing (29°N, 106°E), Jiangxi (25.83°N, 114.93°E), and Hunan (27.33°N, 109.58°E) provinces of China in December, 2015. The maturity indexes of the fruits ranged from 13.75 to 15.62, which were calculated by the ratio of °Brix/acidity. [Citation12] “Cara Cara” fruits were peeled and milled with a tissue grinder to obtain homogeneous pulp; then, they were lyophilized (−40°C,1 Pa, 48 h) by a freeze dryer (Virtis freeze mobile, Virtis Co., Gardiner, USA). Lyophilized pulp was ground into fine powder, and stored at −80°C for further analysis. In addition, commercial authentic standards of caffeic acid, p-coumaric acid, ferulic acid, rutin, narirutin, hesperidin, didymin and chemicals of ascorbic acid, Folin-Ciocalteu reagent, DPPH, AAPH2,2ʹ-azobis-(2-methylpropionamidine) dihydrochloride, and fluorescein were obtained from Yuanye Bio-Technology Co., Ltd (Shanghai, China). 6-Hydroxy-2,5,7,8-tetramethyl-2-carboxylic acid (Trolox) was purchased from Sigma (St. Louis,USA). HPLC-gradesolvent of acetonitrile was acquired from Thermo Fisher Scientific (Leicestershire, UK). Otheranalytical grade chemicals were purchased from Sinopharm chemical reagent Co., Ltd (Shanghai, China).
Extraction of phenolics
Phenolics in lyophilized pulp of “Cara Cara” were extracted by ultrasonic treatment. Briefly, 0.5 g freeze-dried material was extracted by 20 mL of 84.81% ethanol with 0.1% HCl (v/v) using an ultrasonic cleaner with working frequency at 40 kHz (KQ-500E, Kun Shan Ultrasound Instrument Co., Jiangsu,China) for 5.99 min at 57.37°C, which was repeated twice. The final supernatantvolume was made up to 40 mL and filtered through a 0.22-μm polytetrafluoro ethylene (PTFE) membrane for further analysis.
UPLC analysis of phenolics
The UPLC analysis of phenolics was carried on the Waters Acquity UPLC system (Waters Corp., MA, USA) equipped with an UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm). Solvent-gradient elution was performed with mobile phase consisting of (A) distilled water/formic acid (0.2%, v/v) and (B) acetonitrile/distilled water (40:60, v/v). The linear gradient program was set as follows: 0 min: 95% A; 1.5 min: 90% A; 2 min: 75% A; 6 min: 60% A; 12 min: 45% A; 15 min: 20% A; 16–19 min: 0% A; 20 min: 95% A. The flow rate was controlled at 0.3 mL/min and the UV-Vis spectrum was collected from 210 nm to 600 nm with detection wavelength at 280 nm and 330 nm. Collected supernatant (Section 2.2) was concentrated to 5 mL by a rotary evaporator (40°C), and the column was maintained at 25°C with 3 μL sample injected for UPLC analysis.
For UPLC-Q-TOF-ESI-MSn analysis, a MS system (Synapt G2) equipped with an ESI source and a Q-TOF analyzer (Waters Corp., MA, USA) was used. The HPLC conditions were the same as those described earlier. Parameters for MS were as follows: negative mode (ESI–); source temperature, 120°C; cone gas flow, 50 L/h; desolvation temperature, 450°C; desolvation gas flow, 800 L/h. Voltages of sample cone and capillary were set at 30 and 2500 V, respectively.
Determination of free phenolic acids
FPA in the crude extracts were determined by the existing method with minor modifications. [Citation4,Citation13] Briefly, the concentrated crude extracts (5 mL) were alkali hydrolyzed with 5 mL NaOH (4 M) containing EDTA (10 mM) and 1% ascorbic acid (w/v) for 6 h at room temperature. Then, HCl (6 M) was added to acidify the reaction mixture to pH 2, followed by the addition of diethyl ether:ethyl acetate (1:1 v/v) to extract the FPA for three times from the hydrolysate. Next, the extracts were evaporated to dryness using a rotary evaporator (30°C). Finally, the samples were re-dissolved in 2 mL methanoland filtered through a 0.22-μm PTFE membrane for further UPLC analysis.
Quantification of phenolics
For quantification analysis, concentrations of caffeic acid, p-coumaric acid, ferulic acid, narirutin, hesperidin, and didymin were calculated from the external calibration curve of their corresponding commercial standards in the concentration ranges of 10–100 μg/mL, 10–100 μg/mL, 20–200 μg/mL, 5–500 μg/mL, 100–1000 μg/mL, and 50–200 μg/mL, respectively. Without authentic commercial standards, flavanones and components with maximal absorbance at about 284 nm () were quantified as narirutin equivalents. Other compounds with the maximal absorbance at 310–330 nm () were quantified as rutin equivalents within the concentration range of 5–50 μg/mL.
Table 1. Phenolic compounds identified by UPLC-Q-TOF-ESI-MSn in red-fleshed navel orange “Cara Cara”.
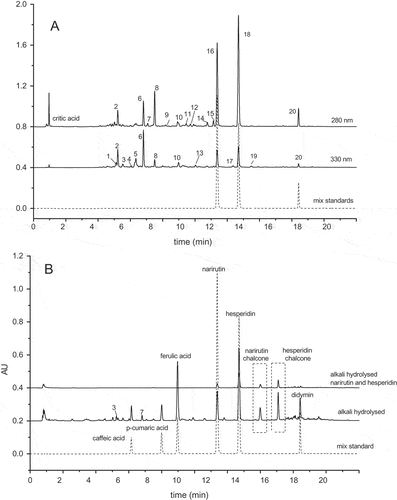
Table 1. HPLC chromatograms of phenolic compounds extracted from Fujian “Cara Cara” as recorded at 280 nm and 330 nm, and the standard mixture of narirutin, hesperidin, and didymin recorded at 280 nm (panel A); phenolic extracts and a mixture standard of narirutin and hesperidin were alkali hydrolyzed and recorded at 280 nm, with a standard mixture of caffeic acid, p-coumaric acid, ferulic acid narirutin, hesperidin, and didymin also recorded at 280 nm (panel B).
Determination of total phenolic content (TPC)
TPC in crude extracts was determined based on Folin-Ciocalteu method with modifications. [Citation14] Briefly, 125-μL Folin-Ciocalteu reagent (0.2 M) was mixed with 25-μL phenolic extracts or gallic acid standard (25–250 μg/mL) in a 96-well plate. After 10 min, 125 μL of 10% Na2CO3 was gently added, and the mixture was incubated for 2 h before the absorbance was recorded at 765 nm on a UV-Vis plate reader (Multiskan GO, Thermo Fisher Scientific, Inc., Waltham, MA, USA). The TPC was expressed as mg gallic acid equivalent (GAE) per 100 g dry weight of “Cara Cara” pulp (mg GAE/100 g DW).
2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
The DPPH-free radical-scavenging activity of the phenolic extracts was evaluated as previously reported. [Citation15] Briefly, 20-μL phenolic extract or standard ascorbic acid (25–300 μM) was mixed with 280 μL of DPPH (65 μM) in a 96-well plate. The extract solvent was used as blank control. The mixture was measured by a UV-Vis plate reader at 540 nm (Multiskan GO, Thermo Fisher Scientific, Inc., Waltham, MA, USA). The final results were expressed as μmol ascorbic acid equivalent (AAE) per gram dry weight of “Cara Cara” pulp (μmol AAE/g DW).
Oxygen radical absorption capacity (ORAC) assay
The ORAC assay was conducted based on the existing protocols with minor modifications.[Citation16] Briefly, 200-μL fluorescein reagent (0.0868 nM) was mixed with 25-μL blank, phenolic extracts and Trolox standard (12.5–125 μM) in a 96-well plate. The mixture was incubated at 37°C for 30 min, followed by the quick addition of 25-μL AAPH (153 mM) to generate peroxyl radical reaction. The fluorescence was monitored every minute at an excited wavelength of 485 nm and an emission wavelength of 528 nm, and the total measurement was kept for 2 h at 37°C on a multi-mode plate reader (Synergy HTX, Bio Tek instrument, Winooski, VT, USA). The area under the fluorescence decay curve (AUC) was calculated, and the net AUC along with the different values of AUC between blank and phenolic extracts or Trolox standard were used for data analysis. The final results were expressed as μmol Trolox equivalent (TE) per gram dry weight of “Cara Cara” pulp (μmol TE/g DW). Phenolic extracts were diluted 40 times for ORAC assay to ensure thefluorescence can decay in 120 min.
Statistical analysis
All the experiments were conducted in triplicate. The data were expressed as mean ± SD of triplicate independent experiments. One way analysis of variance (ANOVA) was used to compare the means, and significant differences were considered at p < 0.05 (Duncan’s post-hoc test). All statistical analyses were performed with IBM SPSS Statistics version 20.0.
Results and discussion
Identification of phenolics compounds
shows the UPLC chromatograms recorded at 280 and 330 nm for phenolic compounds of extracts from “Cara Cara”, along with mixed standards detected at 280 nm. There were 20 phenolic compounds (peaks 1–20) obviously detected in extracts with 19 of them confirmed and tentatively identified. Their retention time, UV-Vis spectra, and MS information are presented in . Narirutin, hesperdin, and didymin (peak 16, 18, and 20) were positively proven based on their corresponding authentic standards and MS information ().
The earliest eluted peak belonged to critic acid because its molecular ion [M-H]– was shown at m/z 191 with fragment ion [M-H-2H2O-CO2]– at m/z 111. Peak 1 could not be identified based on the available data. Peak 2 showed a UV-Vis spectrum similar to that of ferulic acid, with a fragment ion at m/z 209 and 191, corresponding to a galactaric residue and [galactaric residue-H2O]–, respectively. This component was tentatively identified as feruloyl-galactaric. [Citation17] Molecular ions of [M-H]– at m/z 385 and 355 were observed in peak 5, with fragment ions [M-H-162]– at m/z 223 and [M-H-162]– at m/z 194, indicating that it was a mixture of sinapic acid glucoside and ferulic acid glucoside. [Citation13]
The fragment ions at m/z [M-H-18]–, [M-H-90]–, [M-H-120]–, [A + 83]–, and [A + 113]– are the typical fragmentation patterns for di-C-glycosyl flavones. [Citation18] The precursor ionsof peaks 3, 6, and 13 were, respectively, at m/z 609, 593, and 597, and their UV-Vis characteristics and fragment information () were in agreement with those of di-C-glycosyl flavones. Therefore, peaks 3, 6, and 13 were tentatively identified as luteolin-6,8-di-C-glucoside, apigenin-6,8-di-C-glucoside, and phloretin-3ʹ5’-di-C-glucoside, respectively. [Citation14,Citation19] Peak 10 showed precursor ions at m/z 563, with UV-Vis spectrum and fragment ions at m/z 443 [M-H-120]–, 413 [M-H-150]–, and 293 [M-H-120–150]–, indicating that it was a mono-C-glycosyl flavone [Citation19]; thus, it was tentatively identified as apigenin-8-C-glucoside-2ʹ’-O-xyloside. [Citation20]
It is reported that rutinosides only produced fragment ion at m/z [M-H-308]– [Citation21], and no neohesperidosides was found in “Cara Cara”. [Citation7] As expected, the molecular ions of peaks 16, 18, and 20 were, respectively, detectedat m/z 579, 609, and 593, with their fragments at m/z 271 [M-H-308]–, 301 [M-H-308]–, and 285 [M-H-308]–, which confirmed the structure of naringenin-7-O-rutinoside, hesperetin-7-O-rutinoside, and isosakuranetin-7-O-rutinoside. As shown in the MS spectrum, the molecular ion of peak 4 was detected at m/z 785, with fragments at 623 [M-H-162]– and 315 [M-H-162–308]– due to the successive loss of a molecule of glucose and rutinose. Fragment ion at m/z 315 is the isorhamnetin aglycone; thus, peak 4 was tentatively identified as isorhamnetin-3-O-rutinoside-7-O-glucoside. Likewise, peaks 8, 9, and 14 were tentatively identified as narirutin-4ʹ-O-glucoside, quercetin-3-O-rutinoside-7-O-glucoside and hesperetin derivatives based on their UV-Vis and mass spectral properties (). [Citation21,Citation22]
According to MS information, peaks 7 and 15 showed molecular ions [M-H]– at m/z 625 and 623, with fragment ions of myricetin aglycone at m/z 317 [M-H-308]– and isorhamnetin aglycone at m/z 315 [M-H-308]–, respectively (). They might be identified as myricetin-O-rutinoside and isorhamnetin-O-rutinoside. However, peaks 7 and 15 exhibited a λmax at about 284 nm and typical UV-Vis spectra of flavonones. Therefore, peaks 7 and 15 belonged to flavonone-O-rutinoside, and they were assigned as flavonone-O-rutinoside 1 and flavonone-O-rutinoside 2. The detailed flavonone structure in peaks 7 and 15 cannot be identified based on available information. The molecular ion of dihydroxy dimethoxy flavanone was reported to be at m/z 315 [M-H]– [Citation23], and the flavanone aglycone structures of peaks 7 and 15 need to be further investigated.
Peak 12 was tentatively inferred as genistein-7-O-xylosylglucoside malonylated based on previous studies. [Citation24,Citation25] Malonylation of flavonoids widely existed in plants, and the MS spectra at [M-H-44]– and [M-H-44–42]– were derived from the successive loss of carboxyland ketene from malonic acid moiety. [Citation24] Peak 12 had a molecular ion [M-H]– at m/z 649, with fragment ions at m/z 605 [M-H-44]– and 443 [M-H-44–162]– by losing carboxyl and glucose-carboxyl moiety, respectively. Similarly, fragment ions of peak 18 at m/z 565 [M-H-86]–, 403 [M-H-86–162]–, and 271 [M-H-86–162-132]– indicated the successive loss of malonyl, glucosyl, and xylosyl. With molecular ion [M-H]– at m/z 651, peak 17 might be inferred as naringenin-7-O-xylosylglucoside malonylated. Peak 19 without sufficient MS information was tentatively inferred as 1,2-diferuloylgentiobiose by its UV-Vis spectrum and molecular ion.[Citation13]
No free phenolic acid was detected in crude extracts, and alkaline hydrolysis was performed to investigate the FPA present in “Cara Cara” pulps. As shown in , caffeic acid, p-coumaric acid, and ferulic acid appeared after alkaline hydrolysis, and they were positively confirmed by their authentic commercial standards. During alkaline hydrolysis, flavonoids were partly converted into their chalcone forms. In present study, nariutin and hesperidin chalcone were clearly observed in alkali hydrolyzed sample and confirmed by alkali hydrolyzed mixed standards of nariutin and hesperidin (). Phenolic acids, only detectable in alkali hydrolyzed extracts, suggested they might exist in bound forms in “Cara Cara”.
Quantification of phenolic compounds
A total of 20 phenolic compounds were found in crude extraction and three FPA were detected in alkali hydrolyzed extraction, with their individual contents listed in and , respectively. In crude extracts, individual phenolic compounds ranged from 0 to 691 mg/100 g DW, with hesperidin accounting for 29.36–44.06% as the predominant ingredient, followed by narirutin (13.44–17.03%) and apigenin-6,8-di-C-glucoside (8.28–12.38%) (). Total phenolic index (TPI), i.e., sum of individual phenolic contents, ranged from 1177 to 1732 mg/100 g DW, with total flavonoid index (TFI), i.e., sum of individual flavonoid contents, rainging from 962 to 1500 mg/100 g DW, indicated that TFI contributed to the most of TPI. In the detected 20 phenolic compounds (), nine of them were conformed as flavanones, which took up to 64.14–73.23% of TPI. In alkali hydrolyzed samples, ferulic acid accounting for 86.22–90.49% was dominated for FPA, followed by caffeic acid and p-coumaric acid (). Similar trends were also found in other citrus species of Ponkan and Huyou. [Citation26]
Table 2. Contents of individual phenolic compounds (mg/100 g DW) of five “Cara Cara” pulps a.
Table 3. Free phenolic acid contents (mg/100 g DW) in alkali hydrolyzed samples of five “Cara Cara” pulps a.
Due to the same genotype, phenolic profiles in “Cara Cara” harvested from five regions were identical, and the differences of phenolic contents in those fruits might be attributed to environmental influence, including fertilization, irrigation, temperature, light, and so on. [Citation9,Citation27,Citation28] In our study, Fujian “Cara Cara” had the highest TPI and TFI, while the lowest TPI and TFI were detected in Chongqing “Cara Cara”. The geographical origins and morphologic characteristics of “Cara Cara” were described previously [Citation29], and Fujian region had relatively high temperature, moderate rainfall, and lighting time. It has been demonstrated that most of flavonoid genes were responsive to mineral nutrient and light intensity, especially UV-B [Citation30], and flavonoid in citrus was biosynthesized and accumulated during the early period of young fruit, then decreased in the following grow period. [Citation31] However, the accumulation of phenolics in plant was complicated, and several stressful conditions were also reported to enhance the phenolic concentration in fruits, such as wounding, nutrient deficiency, pathogens, and radiations.[Citation27] To investigate the environmental influence on phenolic accumulation in “Cara Cara” fruits, the planting soils and the garden trails of “Cara Cara” trees should be further studied.
Total phenolic contents and antioxidant abilities
Spectrophotometric calculation of TPC, DPPH, and ORAC values are shown in . In all the extracts, TPC, ranging from 652 to 792 mg GAE/100 g DW as calculated by the Folin-Ciocalteu method, were lower than the TPI, and this phenomenon might be attribute to the fact that some peaks were quantified as using narirutin, not their authentic standards. Previous research demonstrated that TPC were higher than TPI, which might be attributed to the incomplete calculation of all peaks determined by HPLC. [Citation32] However, the calculation of TPC and TPI was based on different quantification methods and their results might be not comparable. The trends that TPC were lower than TPI have also been reported previously. [Citation33]
Table 4. Total phenolic contents (mg/100 g DW) and antioxidant abilities of “Cara Cara” pulps. a
DPPH assay is based on the reception of hydrogenatom from antioxidants; whereas, ORAC assay is used to calculatethe antioxidant ability of eliminating peroxyl radical (ROO·) generated by 2,2ʹ-azobis-(2-methylpropionamidine) dihydrochloride (AAPH). [Citation34] DPPH values of Hubei and Hunan samples were significantly higher than others, while no significant differences were found in ORAC values of tested samples. DPPH value showed positively correlation with TPC value (r2 = 0.921). The strong positive correlations indicated that the phenolics in “Cara Cara” pulp were the major contributors to DPPH value. [Citation33] DPPH radical is only dissolved in organic solvent, and this method is applicable to analyze the antioxidant ability of hydrophobic systems. Hesperidin, a hydrophobic compound, predominantly existed in the phenolic extracts of Cara Cara, and this situation might contribute to the good correlation between DPPH assay and TPC. The lack of correlation was found between ORAC and TFI or TPC, and possible explanations are presented as follows: (1) phenolic compounds were not the only potential antioxidant ingredients of the corresponding samples; (2) in ORAC assay, kinetics action of antioxidants lack essential correlation with phenolics. [Citation16,Citation32]
Conclusion
Phenolic compounds in “Cara Cara” collected from five different regions of China were adequately identified and quantified by UPLC coupled with Q-TOF-ESI-MSn, with 20 phenolics detected in crude extracts. Flavonoids were confirmed to be the main phenolics, especially flavanones. Hesperidin was proved as the predominant ingredient in all samples, followed by narirutin and apigenin-6,8-di-C-glucoside. Phenolic profiles in those “Cara Cara” pulps were similar, while phenolic contents were different, which might be attributed to the environmental differences. Phenolic in “Cara Cara” pulp was the main contributor to DPPH value. These results not only enrich the nutritional knowledge of “Cara Cara”, but also provide valuable guide for “Cara Cara” planting.
Additional information
Funding
References
- Zhang, Y.; Sun, Y.; Xi, W.; Shen, Y.; Qiao, L.; Zhong, L.; Ye, X.; Zhou, Z. Phenolic Compositions and Antioxidant Capacities of Chinese Wild Mandarin (Citrus Reticulata Blanco) Fruits. Food Chemistry 2014, 145, 674–680. DOI: 10.1016/j.foodchem.2013.08.012.
- Kamiloglu, S.; Capanoglu, E.; Yilmaz, O.; Duran, A. F.; Boyacioglu, D. Investigating the Antioxidant Potential of Turkish Herbs and Spices. Quality Assurance and Safety of Crops & Foods 2014, 6, 151–158. DOI: 10.3920/qas2012.0237.
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641.DOI: 10.3390/12081641.
- Ye, X.-Q.; Chen, J.-C.; Liu, D.-H.; Jiang, P.; Shi, J.; Xue, S.; Wu, D.; Xu, J.-G.; Kakuda, Y. Identification of Bioactive Composition and Antioxidant Activity in Young Mandarin Fruits. Food Chemistry 2011, 124, 1561–1566. DOI: 10.1016/j.foodchem.2010.08.013.
- Abad-García, B.; Berrueta, L. A.; Garmón-Lobato, S.; Urkaregi, A.; Gallo, B.; Vicente, F. Chemometric Characterization of Fruit Juices from Spanish Cultivars according to Their Phenolic Compound Contents: I. Citrus Fruits. Journal of Agricultural and Food Chemistry 2012, 60, 3635–3644. DOI: 10.1021/jf300022u.
- Lee, H. Characterization of Carotenoids in Juice of Red Navel Orange (Cara Cara). Journal of Agricultural and Food Chemistry 2001, 49, 2563–2568. DOI: 10.1021/jf001313g.
- Chen, J.; Zhang, H.; Pang, Y.; Cheng, Y.; Deng, X.; Xu, J. Comparative Study of Flavonoid Production in Lycopene-Accumulated and Blonde-Flesh Sweet Oranges (Citrus Sinensis) during Fruit Development. Food Chemistry 2015, 184, 238–246. DOI: 10.1016/j.foodchem.2015.03.087.
- Brasili, E.; Chaves, D.; Xavier, A. A. O.; Mercadante, A. Z.; Hassimotto, N. M. A.; Lajolo, F. M. Effect of Pasteurization on Flavonoids and Carotenoids in Citrus Sinensis (L.) Osbeck Cv. ‘Cara Cara’ and ‘Bahia’ Juices. Journal of Agricultural and Food Chemistry 2017, 65, 1371–1377. DOI: 10.1021/acs.jafc.6b05401.
- Treutter, D. Significance of Flavonoids in Plant Resistance: A Review. Environmental Chemistry Letters 2006, 4, 147–157. DOI: 10.1007/s10311-006-0068-8.
- McGhie, T.; Hunt, M.; Barnett, L. Cultivar and Growing Region Determine the Antioxidant Polyphenolic Concentration and Composition of Apples Grown in New Zealand. Journal of Agricultural and Food Chemistry 2005, 53, 3065–3070. DOI: 10.1021/jf047832r.
- Sommella, E.; Pepe, G.; Pagano, F.; Tenore, G.; Dugo, P.; Manfra, M.; Campiglia, P. Ultra High Performance Liquid Chromatography with Ion-Trap TOF-MS for the Fast Characterization of Flavonoids in Citrus Bergamia Juice. Journal of Separation Science 2013, 36, 3351–3355. DOI: 10.1002/jssc.201300591.
- Roussos, P. Phytochemicals and Antioxidant Capacity of Orange (Citrus Sinensis (L.) Osbeck Cv. Salustiana) Juice Produced under Organic and Integrated Farming System in Greece. Scientia Horticulturae 2011, 129, 253–258. DOI: 10.1016/j.scienta.2011.03.040.
- Lin, L.-Z.; Harnly, J. Phenolic Component Profiles of Mustard Greens, Yu Choy, and 15 Other Brassica Vegetables. Journal of Agricultural and Food Chemistry 2010, 58, 6850–6857. DOI: 10.1021/jf1004786.
- Zou, Y.; Chang, S.; Gu, Y.; Qian, S. Antioxidant Activity and Phenolic Compositions of Lentil (Lens Culinaris Var. Morton) Extract and Its Fractions. Journal of Agricultural and Food Chemistry 2011, 59, 2268–2276. DOI: 10.1021/jf104640k.
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - Food Science and Technology 1995, 28, 25–30. DOI: 10.1016/S0023-6438(95)80008-5.
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-Assisted Extraction of Phenolics with Maximal Antioxidant Activities in Tomatoes. Food Chemistry 2012, 130, 928–936. DOI: 10.1016/j.foodchem.2011.08.019.
- Dueñas, M.; Martínez-Villaluenga, C.; Limón, R.; Peñas, E.; Frias, J. Effect of Germination and Elicitation on Phenolic Composition and Bioactivity of Kidney Beans. Food Research International 2015, 70, 55–63. DOI: 10.1016/j.foodres.2015.01.018.
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First Evidence of C- and O-Glycosyl Flavone in Blood Orange (Citrus Sinensis (L.) Osbeck) Juice and Their Influence on Antioxidant Properties. Food Chemistry 2014, 149, 244–252. DOI: 10.1016/j.foodchem.2013.10.096.
- Barreca, D.; Bisignano, C.; Ginestra, G.; Bisignano, G.; Bellocco, E.; Leuzzi, U.; Gattuso, G. Polymethoxylated, C- and O-Glycosyl Flavonoids in Tangelo (Citrus Reticulata × Citrus Paradisi) Juice and Their Influence on Antioxidant Properties. Food Chemistry 2013, 141, 1481–1488. DOI: 10.1016/j.foodchem.2013.03.095.
- Hauck, B.; Gallagher, J.; Morris, S.; Leemans, D.; Winters, A. Soluble Phenolic Compounds in Fresh and Ensiled Orchard Grass (Dactylis Glomerata L.), A Common Species in Permanent Pastures with Potential as a Biomass Feedstock. Journal of Agricultural and Food Chemistry 2014, 62, 468–475. DOI: 10.1021/jf4040749.
- Zhang, M.; Duan, C.; Zang, Y.; Huang, Z.; Liu, G. The Flavonoid Composition of Flavedo and Juice from the Pummelo Cultivar (Citrus Grandis (L.) Osbeck) and the Grapefruit Cultivar (Citrus Paradisi) from China. Food Chemistry 2011, 129, 1530–1536. DOI: 10.1016/j.foodchem.2011.05.136.
- Bresciani, L.; Calani, L.; Cossu, M.; Mena, P.; Sayegh, M.; Ray, S.; Del-Rio, D. (Poly)Phenolic Characterization of Three Food Supplements Containing 36 Different Fruits, Vegetables and Berries. PharmaNutrition 2015, 3, 11–19. DOI: 10.1016/j.phanu.2015.01.001.
- Ameer, B.; Weintraub, R.; Johnson, J.; Yost, R.; Rouseff, R. Flavanone Absorption after Naringin, Hesperidin, and Citrus Administration. Clinical Pharmacology and Therapeutics 1996, 60. DOI:10.1016/s0009-9236(96)90164-2.
- Kachlicki, P.; Einhorn, J.; Muth, D.; Kerhoas, L.; Stobiecki, M. Evaluation of Glycosylation and Malonylation Patterns in Flavonoid Glycosides during LC/MS/MS Metabolite Profiling. Journal of Mass Spectrometry 2008, 43, 572–586. DOI: 10.1002/jms.1344.
- Wojakowska, A.; Piasecka, A.; García-López, P.; Zamora-Natera, F.; Krajewski, P.; Marczak, Ł.; Kachlicki, P.; Stobiecki, M. Structural Analysis and Profiling of Phenolic Secondary Metabolites of Mexican Lupine Species Using LC–MS Techniques. Phytochemistry 2013, 92, 71–86. DOI: 10.1016/j.phytochem.2013.04.006.
- Xu, G.; Ye, X.; Liu, D.; Ma, Y.; Chen, J. Composition and Distribution of Phenolic Acids in Ponkan (Citrus Poonensis Hort. Ex Tanaka) and Huyou (Citrus Paradisi Macf. Changshanhuyou) during Maturity. Journal of Food Composition and Analysis 2008, 21, 382–389. DOI: 10.1016/j.jfca.2008.03.003.
- Slimestad, R.; Verheul, M. Review of Flavonoids and Other Phenolics from Fruits of Different Tomato (Lycopersicon Esculentum Mill.) Cultivars. Journal of the Science of Food and Agriculture 2009, 89, 1255–1270. DOI: 10.1002/jsfa.3605.
- Navarro, J.; Botía, P.; Pérez-Pérez, J. Influence of Deficit Irrigation Timing on the Fruit Quality of Grapefruit (Citrus Paradisi Mac.). Food Chemistry 2015, 175, 329–336. DOI: 10.1016/j.foodchem.2014.11.152.
- Lu, Q.; Huang, X.; Lv, S.; Pan, S. Carotenoid Profiling of Red Navel Orange “Cara Cara” Harvested from Five Regions in China. Food Chemistry 2017, 232, 788–798. DOI: 10.1016/j.foodchem.2017.04.064.
- Lillo, C.; Lea, U. S.; Ruoff, P. Nutrient Depletion as a Key Factor for Manipulating Gene Expression and Product Formation in Different Branches of the Flavonoid Pathway. Plant, Cell & Environment 2008, 31, 587–601. DOI: 10.1111/j.1365-3040.2007.01748.x.
- Moriguchi, T.; Kita, M.; Tomono, Y.; Endo-Inagaki, T.; Omura, M. Gene Expression in Flavonoid Biosynthesis: Correlation with Flavonoid Accumulation in Developing Citrus Fruit. Physiological Plant 2001, 111, 66–74. DOI: 10.1034/j.1399-3054.2001.1110109.x.
- Zhang, B.; Deng, Z.; Ramdath, D.; Tang, Y.; Chen, P.; Liu, R.; Liu, Q.; Tsao, R. Phenolic Profiles of 20 Canadian Lentil Cultivars and Their Contribution to Antioxidant Activity and Inhibitory Effects on α-glucosidase and Pancreatic Lipase. Food Chemistry 2015, 172, 862–872. DOI: 10.1016/j.foodchem.2014.09.144.
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of Phenolics, Betacyanins and Antioxidant Activities of the Seed, Leaf, Sprout, Flower and Stalk Extracts of Three Amaranthus Species. Journal of Food Composition and Analysis 2015, 37, 75–81. DOI: 10.1016/j.jfca.2014.09.003.
- Sánchez-Moreno, C. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Revista De Agaroquimica Y Tecnologia De Alimentos 2002, 8, 121–137. DOI: 10.1106/108201302026770.
