?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Mulberry leaves had been used as material of medicine, drink, and functional foods in many countries, but the variety suitability for different applications is still not clear. In this study, the nutritional and phytochemical components of mulberry tender shoots from 19 varieties in China were investigated. Obvious genotypic diversity was observed in all the assessed components. The contents of crude protein (CP), total soluble sugars (TSS), crude fiber (CF), 1-deoxynojirimycin (DNJ), total phenolic (TP), and total flavonoid content (TF) were 27.63–37.36 g/100 g dry weight (DW), 58.71–150.31 mg/g DW, 9.90–13.85 g/100 g DW, 0.08–1.12 mg/g DW, 8.76–20.26 mg gallic acid equivalents (GAE)/g DW, and 21.36–56.41 mg rutin equivalents (RE)/g DW, respectively. Chlorogenic acid, rutin, isoquercitrin, and astragalin were considered to be mainly potential antioxidant compounds in mulberry leaves. According to the result of correlational analysis, principal component analysis (PCA), and hierarchical cluster analysis (HCA), the NS14 variety had high comprehensive healthy properties. GS8, D10, XS, TP2, and DHS could be appropriate materials for functional food or drink because of high content of phenolics or DNJ. Some varieties may have a potential application as protein-rich vegetables. The study suggests that some variety of mulberry could be selected and utilized rationally for their dietary properties.
Introduction
Mulberry (Morus spp.) is widely cultivated in China, India, Japan, and other countries. Mulberry (Morus alba L.) leaves, commonly used as feed of silkworm, have also been used in traditional Chinese medicine (TCM) for over 3000 years. They have long been used to treat cold, diabetes, and other diseases.[Citation1–Citation3] Currently, mulberry leaves were authorized to be pharmaceutical/food resources by the Ministry of Public Health of China. In recent years, mulberry leaves had been commercially available as a kind of special tea or drink in China, Japan, Korea, Thailand, and many other Asian countries.[Citation4,Citation5] Tender shoots or young leaves of mulberry are also consumed as special vegetables in some areas in China.
It is known that the protein, carbohydrate, vitamins, microelements, and dietary fiber are the main factors influencing nutritional quality of vegetables. However, bioactive compounds and their bioactivity were considered high concerned factors when they were used as special tea or materials of functional foods. Both the nutrients and bioactive compounds could have synergistically beneficial effects on human health. Mulberry leaves have been identified as an excellent food resource with high content of protein, carbohydrate, vitamins, microelements, and dietary fiber.[Citation6,Citation7] Some reports indicated that mulberry leaves had high content of bioactive compounds, including phenolic acids, flavonoids, alkaloids, and γ-aminobutyric acid (GABA).[Citation6,Citation8] These compounds had been confirmed to involve in antioxidation,[Citation3,Citation9] decreasing glycemia,[Citation1,Citation10] antihypertensive,[Citation11] preventing atherosclerosis,[Citation12] and anti-inflammation.[Citation13] 1-deoxynojirimycin (DNJ), as one of the main bioactive compounds in mulberry, is a potent inhibitor of α-glycosidases and has shown potential therapeutic effects on many diseases, particularly type II diabetes.[Citation4,Citation14]
The cultivar, harvesting time, and the degree of maturity of mulberry leaves affected the content of nutritional and functional components significantly.[Citation15–Citation18] Top leaves contained more active substances than mature leaves.[Citation4] Li et al.[Citation19] investigated the contents of ascorbic acid, carotenoid, total soluble protein, total soluble sugar, fructose, and sucrose in the tender shoots of eight mulberry cultivars and found that there were obvious differences of some nutrients and flavor components among the different mulberry cultivars. Hu et al.[Citation4] found that DNJ concentrations varied from 0.1341 to 1.472 mg/g dry weight in mature leaves of mulberry varieties. The phenolic content of mulberry leaves also varied with cultivars and harvesting time. Mulberry leaves collected in May were considered to be good sources of phenolic compounds.[Citation16,Citation18]
There are at least 15 species of Morus with more than 3000 mulberry germplasm resources in China.[Citation17,Citation20] Traditionally, mulberry varieties were bred and cultivated to make the need of sericulture, and a large amount of mulberry leaves had been wasted or irrationally utilized. Many studies had focused on developing the medicinal or feeding value of mulberry leaves. Could mulberry leaves from any variety be suitable materials for vegetables, drink, or other functional food? However, until now, there is not a comprehensive quality evaluation or clear description about suitability of different varieties for special applications. Therefore, the objective of this study is to assess and select special variety of mulberry as suitable raw material for different purposes. This assessment was made on a basis of investigating nutritional properties, phytochemical compounds, and antioxidant activity of mulberry tender shoots from 19 typical clones in China.
Materials and methods
Chemicals and reagents
Chlorogenic acid (98%), rutin (95%), isoquercitrin (98%), astragalin (97%), quercetin-3-β-D-glucoside (90%), kaempferol (95%), L-ascorbic acid (98%), Trolox (98%), Folin-Ciocalteu’s phenol reagent, 9-fluorenylmethyl chloroformate (FMOC-Cl), 2,2-azinobis-(3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS), 1,1-diphenyl-2-picryl-hydrazl (DPPH), and 2,4,6,-tripyridyl-striazine (TPTZ) were purchased from Sigma (St. Louis, USA). 1-deoxynojirimycin standard (95%) was purchased from Aladdin (Shanghai, China). HPLC-grade acetonitrile and formic acid were purchased from Merck (Darmstadt, Germany). All reagents were of analytical grade.
Mulberry sample preparation
The tender shoots (top three leaves) of mulberry from 19 varieties were collected from the mulberry fields of Jiangxi Sericulture and Tea Research Institute on May 25, 2016. The leaves were identified and authenticated by Professor Xiaohong Peng from Jiangxi Sericulture and Tea Research Institute, where a voucher specimen number MLL-16525 was deposited. Among the 19 varieties, NS14 (Nong Sang Number 14, M. alba L.), DHS (Dahua Sang, M. alba L.), HYS (Heiyou Sang, M. alba L.), TS (Tian Sang, M. alba L.), XGS (Xiaoguan Sang, M. alba L.), XYZL (Xinyizhilai, M. alba L.), Y237 (Yu 237, M. alba L.), QS1 (Qiang Sang Number 1, M. multicaulis Perr.), X7920 (Xiang 7920, M. multicaulis Perr.), JTHY (Jiantouheyebai, M. multicaulis Perr.), LYD (Liyeda, M. multicaulis Perr.), D10 (YueShen Da10, M.atropurpurea Roxb.), LJ504 (Lunjiao 504, M.atropurpurea Roxb.), ZS5801 (Zhong Sang 5801, M.atropurpurea Roxb.) are representative and popular varieties in China, while TP2 (Taiping Number 2, M. multicaulis Perr.), HM (Huangma Sang, M. multicaulis Perr.), XG (Xinguo Sang, M. multicaulis Perr.), XS (XiushuiSang, M. multicaulis Perr.), GS8 (Gansang 8, M. multicaulis Perr.) are native varieties in Jiangxi province, China. The leaves were washed with distilled water and then dried at 60°C. The dried materials were ground into fine powder with a pulverizer (FW135, Taisite Co., Tianjin, China). The powders were stored in airtight container at 4°C for further analysis.
Determination of crude protein, crude fiber, and total soluble sugars content
Crude protein content was calculated as N × 6.25 by Kjeldahl Methods.[Citation21] Crude fiber content was determined in accordance with ceramic fiber filter method.[Citation21] The crude protein and crude fiber contents were expressed as g/100 g dry weight (DW). The total soluble sugar was extracted according to Carnal and Black[Citation22] with the following modifications: 0.2 g mulberry powder was extracted with 40 mL distilled water, followed by sonication in a water bath at 85°C for 60 min, then centrifuged at 8000 rpm for 10 min. This extraction process was repeated once, and the supernatants were collected and combined. The total soluble sugars were estimated according to the phenol–sulfuric acid method described by Fei et al.[Citation23]
Determination of 1-deoxynojirimycin (DNJ) content
Determination of DNJ was followed as descriptions by Kim et al.[Citation24] with slight modifications. Approximately 100 mg of mulberry powder samples was transferred into 10 mL centrifuge tubes containing 3.0 mL of 0.05 M HCl and shaken with a Vortexer Mixer for 30 s, incubated in a ultrasonic bath for 10 min, and then centrifuged for 15 min at 12,000 rpm. The residues were re-extracted as described above. Both supernatants were collected and diluted to 10 mL with 0.05 M HCl. Then, the extract was derivatized with 9-fluorenylmethyl chloroformate (FMOC-Cl) following previous protocols.[Citation24]
Agilent 1100 HPLC system with a G1321A fluorescence detector (Agilent, USA) was used to determine DNJ. The column was Eclipse XDB-C18 column (4.6 × 250 mm, 5 μm) (Agilent, USA). The column temperature was 25°C. Acetonitrile and 0.1% of acetic acid aqueous solution (45:55, v/v) were used as mobile phases. 10 μL of the derivatized samples was injected into the system, and then eluted at a flow rate of 0.3 mL min−1 for 30 min.[Citation4,Citation24] The concentration of DNJ in mulberry was calculated from the calibration curve for standard DNJ at excitation wavelength of 254 nm and emission wavelength of 322 nm.
Determination of phenolics and flavonoids
Preparation of phenolic extraction: Mulberry powder samples (500 mg) were mixed with 20 mL of 80% methanol in 50 mL separate tubes; then, the tubes were immersed in an ultrasonic cleaner (SB5200DT, SCIENTZ, Co., Ningbo, China) at constant power (300 W) for 45 min. The mixture was centrifuged at 10,000 rpm for 5 min, and the supernatant was collected. The procedure was repeated twice. The supernatants were combined and made up to 50 mL with 80% methanol and filtered through a 0.2-mm PTFE membrane filter. The filtrate was used for determination of total phenolics content (TPC), total flavonoids content (TFC) and antioxidant activity.
Determination of total phenolics and flavonoids content: The total phenolics were determined by Folin–Ciocalteu method as reported.[Citation25] The total phenolics contents in extracts were calculated as gallic acid equivalents (GAE) from a calibration curve and expressed as mg GAE/gDW. The total flavonoids were measured using a colorimetric assay by NaNO2–Al(NO3)3–NaOH method.[Citation26] The total flavonoids content was expressed as mg rutin equivalents on a dry weight basis (mg RE/g DW).
Determination of phenolic compositions with HPLC-MS: Determination of phenolic compositions was performed by an Agilent 1100 system with diode array detector. An Agilent Eclipse XDB-C18 column (4.6 × 250 mm, 5 μm) was used. The column temperature was 30°C. The mobile phases were 0.2% (v/v) aqueous formic acid (phase A) and acetonitrile (phase B). The gradient system was run at a flow rate of 0.8 ml min−1and operated as follows: 0 min, 10% B; 20 min, 15% B; 50 min, 35% B; 60 min, 50% B; 70 min, 100% B. Run time was 70 min followed by a re-equilibration time of 10 min.
The LC-ESI-MS was performed on a triple quadruple mass spectrometer (6430 QqQ LC/MS system, Agilent Technologies, USA) equipped with ESI source.[Citation27] Nitrogen was used as sheath gas and helium was used as collision gas. The optimum values of parameters were as follows: the drying gas temperature was 300°C, and drying gas flow rate was set to 11.0 L min−1. The capillary voltage, collision energy, and fragmentor voltage was 4 kV, 30 eV, and 80 V, respectively. Negative mode was selected for data collection. The mass spectrometer was operated in the scan mode with a m/z range of 100-1000. Individual phenolic contents were quantified against external standards of chlorogenic acid (detected at 320 nm), rutin, isoquercitrin, quercetin-3-β-D-glucoside, astragalin, and kaempferol (detected at 360 nm).
Total antioxidant activity: Total antioxidant activity of the mulberry extract was estimated by antioxidant composite index (ACI) based on three individual antioxidant assays such as DPPH, ABTS, and FRAP. DPPH and ABTS were conducted according to Masek and Chrzescijanska.[Citation28] The capability of mulberry extract on scavenging free radical was expressed as μmol Trolox equivalent (TE) per gram dry weight (μmol TE/g DW). FRAP assay was performed according to Li.[Citation25] FRAP of the samples was calculated on the gradient basis of L-ascorbic acid and expressed as μmol ascorbic acid equivalent (AAE) per gram dry weight (μmol AAE/g DW). Then, the best score for a sample in each of these three assays was considered as an index value of 100, and the corresponding index scores of other samples were then calculated as follows:
The antioxidant potency composite index (ACI) was the average of index scores obtained for the three assays.[Citation29]
Statistical analysis
All assays were conducted in triplicate. Statistical analyses were performed using IBM SPSS Statistics 22.0. Data were expressed as the mean and standard deviation of three independent assays. The statistical analyses were done by one-way ANOVA, followed by Duncan’s multiple range test for mean comparisons. P<0.05 were considered to be significant. Correlation analyses were performed using a Pearson correlation test.
Results and discussion
Crude protein, crude fiber, and total soluble sugars content
The content of crude protein, crude fiber, and total soluble sugars were significantly different in the mulberry tender shoots among all the experimental varieties (). The contents obtained in this study were similar to that of mulberry leaves previously reported.[Citation6] The content of crude protein was found to be 27.63–37.36 g/100 g DW, which was generally higher than that of common leafy vegetables reported in the China Food Composition Tables, such as Chinese cabbage (27.78 g/100 g DW), pakchoi (27.27 g/100 g DW), cabbage (22.06 g/100 g DW), spinach (29.54 g/100 g DW), shepherd’s purse (30.85 g/100 g DW), purple amaranth (25.00 g/100 g DW), and water spinach (30.96 g/100 g DW).[Citation30] The variety HYS had the highest content of crude protein (37.36 g/100 g DW), which was comparable to soybean (38.96 g/100 g DW) and black soybean (39.96 g/100 g DW).[Citation30] The highest total soluble sugars content was found in GS8 (150.31 mg/g DW) and the lowest in XGS (58.71 mg/g DW). The crude fiber content in the tender shoots of mulberry ranged from 11.46 to 16.61 g/100 g DW, which was similar to that of some leafy vegetables such as sweet potato (Ipomoea batatas L.) leaves (9.15−14.26 g/100 g DW).[Citation31] GS8, D10, and XS had higher content of total soluble sugar, but relatively lower level of crude fiber.
Table 1. Crude protein, crude fiber, total soluble sugars, and DNJ content in dried mulberry leaves from 19 varieties.
Comparison of DNJ content
1-Deoxynojirimycin (DNJ) is an important active substance in mulberry leaves. It showed high α-glucosidase inhibitory, thereby suppressed postprandial blood glucose and prevented diabetes mellitus and decreased lipid accumulation.[Citation32–Citation34] Our results () showed that there were significantly differences on the DNJ content in mulberry leaves from the selected varieties (P < 0.05). The DNJ content varied from 0.08 to 1.12 mg/g DW. The content of TP2, NS14, DHS, XG, QS1, and XYZL were higher than 0.8 mg/g DW, and that of GS8, D10, XS, LJ504, and ZS5801 were lower than 0.3 mg/g DW. Vichasilp et al.[Citation5] demonstrated that the DNJ concentration of young mulberry leaves varied from 0.03 to 1.7 mg/g DW from 35 Thai mulberry varieties. Our results are similar with previously reported data.[Citation4] These findings are particularly helpful for screening raw material or auxiliary material of functional foods or medicines.
Identification and quantification of phenolic compounds
The phenolics and flavonoids content of the investigated mulberry leaves of different varieties are shown in . The phenolics and flavonoids content, expressed on a dry weight (DW) basis, showed statistically significant differences among the mulberry varieties (P < 0.05). The total phenolics content (TPC) of mulberry leaves from GS8, NS14, D10, and XS was higher than 15 mg GAE/g DW; whereas, the total phenolics contents of mulberry leaves from QS1 and TP2 were lower than 10 mg GAE/g DW. Total flavonoids content (TFC) followed a similar trend as TPC. Total flavonoids content reached the highest value in GS8 (56.41 mg RE/g DW), and the lowest in XG variety (21.36 mg RE/g DW). Chlorogenic acid (CHA), rutin, isoquercitrin (IQT), quercetin-malonyl-glucoside (QMG), astragalin, and kaempferol-malonyl-glucoside (KMG) were the primary six polyphenolic compounds. The contents of the monomeric phenols also differed significantly in the mulberry tender shoots of different varieties. Chlorogenic acid (CHA) was the predominant phenolic compound, ranging from 2.45 to 10.24 mg/g DW, and isoquercitrin was the most abundant flavonoid glycoside (0.70–4.83 mg/g DW) in mulberry leaves. GS8 had the highest levels of chlorogenic acid, rutin, isoquercitrin among all the samples. D10 had the highest levels of astragalin (1.32 mg/g DW). Although there were not kaempferol-malonyl-glucoside (KMG) detected in GS8 or D10, and the content of quercetin-malonyl-glucoside (QMG) in these two varieties were also less than the other varieties. The highest level of KMG was found in ZS5801 (1.19 mg/g DW), and the highest level of QMG was found in TS (3.05 mg/g DW). These results were slightly different from reported phenolic profiles of some other varieties in different regions.[Citation35–Citation37]
Table 2. Phenolics and flavonoids content (mg/g DW) in mulberry leaves from 19 varieties.
Total antioxidant activity
The total antioxidant activity in mulberry leaves were determined by three complementary assays: FRAP, ABTS, and DPPH. The results are shown in . DPPH scavenging activities of mulberry leaves ranged from 33.22 to 56.37 μmol TE/g DW. ABTS activity ranged from 51.28 to 70.84 μmol TE/g DW. Reducing capacity (FRAP) ranged from 91.62 to 149.15 μmol AAE/g DW. GS8, ZS5801, D10, NS14, and XS ranked the first to the fifth, respectively, in the order of antioxidant index score (ACI). The rankings reconciled the content of phenolics and flavonoids. The correlation between the total phenolics content/total flavonoids content and antioxidant activities was significantly positive (). These results indicated that total phenolics and flavonoids were most important substances contributing to its antioxidation.[Citation36,Citation38] However, it should be noticed that among the three assays, the correlation coefficient between phenolic content and ABTS scavenging ability was lower than that between phenolic content and the other two antioxidant activities, and those varieties with better DPPH scavenging activity did not always show better reducing capacity when evaluated by ABTS test. For example, the ABTS scavenging activities of JTHY was higher than that of HYS, but the result of FRAP test was reverse for them. Chlorogenic acid, rutin, isoquercitrin, and astragalin had high positive correlation with antioxidant activities, but QMG and KMG had not ().
Table 3. Antioxidant activities determined by FRAP, DPPH, and ABTS methods and resulting antioxidant composite index (ACI) in mulberry leaves from 19 varieties.
Table 4. Analysis of the correlation between total polyphenol content and in vitro antioxidation activity of mulberry leaves.
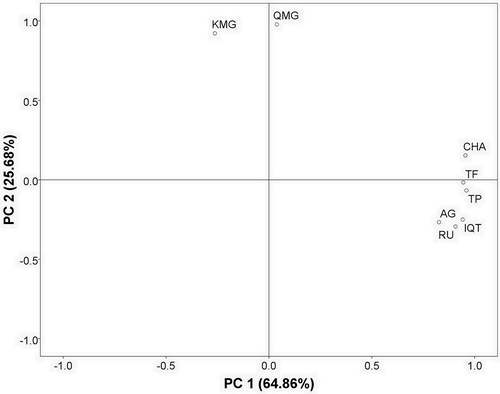
To better understand the correlation of phenolic profiles of different varieties, there applied principal component analysis (PCA). By loading matrix eigenvector and cumulative contribution rate of the factors (), two main principal components (PCs) could explain 90.54% of the total variation (). The TF, TP, and most phenolic acids (chlorogenic acid, rutin, isoquercitrin and astragalin) were positive correlated with PC1, while KMG was negatively correlated with PC1.These results indicated that chlorogenic acid, rutin, isoquercitrin, and astragalin were considered as potential antioxidant compounds in mulberry leaves.
Table 5. Loading matrix eigenvector and cumulative contributionrate of healthcare function evaluation factors of mulberry leaf.
Correlation analysis between the nutrient and phytochemical content
Thousands of organic compounds are produced by plants. Biosynthesis of secondary metabolites starts from basic pathways, some maybe derived from the same precursor, therefore they are closely interlinked (e.g., some alkaloids and flavonoid are derived from the same amino acid phenylalanine).[Citation39] In present study, the correlation coefficient between the compounds of mulberry leaves were calculated (). Total phenolics (TP) content showed significantly negative correlation (P<0.05) with crude protein (CP, r = −0.467), crude fiber (CF, r = −0.513), and DNJ (r = −0.532), which was in agreement with reported data.[Citation40] Positive correlation was found between the TP, total flavonoid (TF), and total soluble sugars (TSS).
Table 6. Analysis of the correlation between the organic compounds.
Classification of the 19 varieties and evaluation of their potential as food
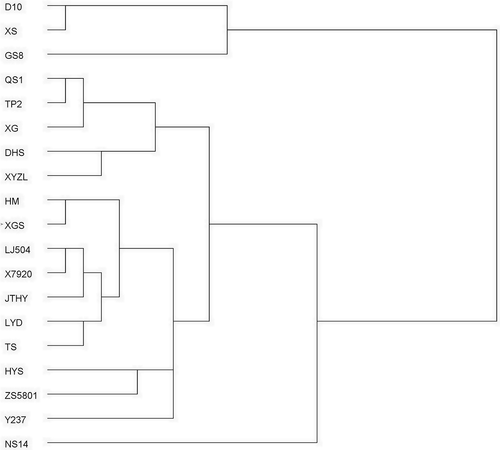
To simplify the chemical pattern recognition and visualize relationships between mulberry varieties, hierarchical cluster analysis (HCA) was used. shows the dendrogram of different mulberry varieties based on the crude protein (CP), total soluble sugars (TSS), crude fiber (CF), total phenolics (TP), total flavonoid (TF), DNJ content, and total antioxidant activity. The 19 mulberry leaves have been classified into three significant clusters. The first cluster was NS14, which had high content of TP, TF, DNJ, and CP. NS14 is one of the most popular mulberry varieties in China at present; therefore, it can be used widely in food. The second cluster was composed of the variety D10, XS, and GS8, with the characteristics of high content of total phenolics, and low content of DNJ and crude protein. Mulberry leaves of these varieties could serve as functional food materials for their high antioxidant activity. The other varieties were classified as the third cluster, in which TP2, DHS, XG, QS1, and XYZL had high content of DNJ. DNJ is a kind of natural alkaloid, one of the crucial components of mulberry leaves.[Citation2] Its chemical structure is similar to that of D-glucose and can help inhibiting intestinal α-glucosidases, control postprandial blood, and prevent diabetes mellitus.[Citation5,Citation32] Because DNJ is water-soluble, these varieties were recommended to be processed as special teas, or raw materials of food product decreasing blood glucose.
Figure 2. Dendrogram of cluster analysis based on the content of crude protein (CP), total soluble sugars (TSS), crude fiber (CF), total phenolics (TP), total flavonoid (TF) and DNJ of mulberry leaves.
Previous study on amino acid composition and nutritional evaluation of mulberry leaves provided evidence that nutritional value of mulberry proteins are comparable to or better than soybean meal, superior to most tropical grasses.[Citation40,Citation41] Considerable nutrients such as calcium, iron, zinc, magnesium, potassium, phosphorus, and vitamin C are present in mulberry leaves.[Citation7] Li et al.[Citation19] found that mulberry tender shoots were rich in nutrient and flavor components. In recent years, many people keep trying to eat some vegetables for their special flavor or functional component in China. Based on this study, mulberry tender shoots (young leaves) of most varieties contain moderate amounts of crude fiber and soluble sugars. Besides, several mulberry leaves such as HYS, NS14 and ZS5801 contain relatively high amounts of protein and specific functional components, therefore, have a potential application as protein-rich vegetables.
Conclusion
Significant differences of nutritional contents, active constituent content, and antioxidant activity were noted in mulberry young leaves from different varieties. The result suggested that the varieties can be suitable for different food materials. The NS14 leaves had high comprehensive healthy properties. Several varieties had higher content of crude protein than common vegetables. The varieties with high phenolics content such as GS8, NS14, D10, and XS could be used for antioxidant plant materials. TP2, NS14, and DHS were recommended to be functional materials or teas for their high content of DNJ. This study will help further for evaluating nutritional value of mulberry and screening excellent germplasm resources.
Acknowledgments
This work was supported by the State Key Laboratory of Food Science and Technology, Nanchang University, China (Grant number SKLF-ZZA-201610) and the China Agriculture Research System, Ministry of Agriculture of the People’s Republic of China (CARS-18).
Additional information
Funding
References
- Asano, N.; Oseki, K.; Tomioka, E.; Kizu, H.; Matsui, K. N-Containing Sugars fromMorus Alba and Their Glycosidase Inhibitory Activities. Carbohydrate research 1994, 259, 2, 243–255.
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New Potential Phytotherapeutics Obtained from White Mulberry (Morus alba L.). Leaves Biomedical Pharmacotherapy 2016, 84, 628–636. DOI: 10.1016/j.biopha.2016.09.081.
- Arabshahi-Delouee, S.; Urooj, A. Antioxidant Properties of Various Solvent Extracts of Mulberry (Morus indica L.). Leaves Food Chemical 2007, 102(4), 1233–1240. DOI: 10.1016/j.foodchem.2006.07.013.
- Hu, X.-Q.; Jiang, L.; Zhang, J.-G.; Deng, W.; Wang, H.-L.; Wei, Z.-J. Quantitative Determination of 1-Deoxynojirimycin in Mulberry Leaves from 132 Varieties. Industrial Crops and Products 2013, 49, 782–784. DOI: 10.1016/j.indcrop.2013.06.030.
- Vichasilp, C.; Nakagawa, K.; Sookwong, P.; Higuchi, O.; Luemunkong, S.; Miyazawa, T. Development of High 1-Deoxynojirimycin (DNJ) Content Mulberry Tea and Use of Response Surface Methodology to Optimize Tea-Making Conditions for Highest DNJ Extraction. LWT-Food Science and Technology 2012, 45(2), 226–232. DOI: 10.1016/j.lwt.2011.09.008.
- Butt, M. S.; Nazir, A.; Sultan, M. T.; Schroën, K. Morus Alba L. Nature’s Functional Tonic. Trends Food Sciences Technological 2008, 19(10), 505–512. DOI: 10.1016/j.tifs.2008.06.002.
- Srivastava, S.; Kapoor, R.; Thathola, A.; Srivastava, R. P. Nutritional Quality of Leaves of Some Genotypes of Mulberry (Morus alba). International Journal Food Sciences Nutritional 2006, 57(5–6), 305–313. DOI: 10.1080/09637480600801837.
- Devi, B.; Sharma, N.; Kumar, D.; Jeet, K. Morus Alba Linn: A Phytopharmacological Review. Journal Pharmaceutical Pharmaceutical Sciences 2013, 5, 2, 14–18.
- Katsube, T.; Imawaka, N.; Kawano, Y.; Yamazaki, Y.; Shiwaku, K.; Yamane, Y. Antioxidant Flavonol Glycosides in Mulberry (Morus alba L.) Leaves Isolated Based on LDL Antioxidant Activity. Food Chemical 2006, 97(1), 25–31. DOI: 10.1016/j.foodchem.2005.03.019.
- Jeszka-Skowron, M.; Flaczyk, E.; Jeszka, J.; Krejpcio, Z.; Król, E.; Buchowski, M. S. Mulberry Leaf Extract Intake Reduces Hyperglycaemia in Streptozotocin (Stz)-Induced Diabetic Rats Fed High-Fat Diet. Journal Function Foods 2014, 8, 9–17. DOI: 10.1016/j.jff.2014.02.018.
- Yang, N.-C.; Jhou, K.-Y.; Tseng, C.-Y. Antihypertensive Effect of Mulberry Leaf Aqueous Extract Containing γ-aminobutyric Acid in Spontaneously Hypertensive Rats. Food Chemical 2012, 132(4), 1796–1801. DOI: 10.1016/j.foodchem.2011.11.143.
- Harauma, A.; Murayama, T.; Ikeyama, K.; Sano, H.; Arai, H.; Takano, R.; Kita, T.; Hara, S.; Kamei, K.; Yokode, M. Mulberry Leaf Powder Prevents Atherosclerosis in Apolipoprotein E-Deficient Mice. Biochemical and biophysical research communications 2007, 358(3), 751–756. DOI: 10.1016/j.bbrc.2007.04.170.
- Park, E.; Lee, S.-M.; Eun Lee, J.; Kim, J.-H. Anti-Inflammatory Activity of Mulberry Leaf Extract through Inhibition of NF-κB. Journal Function Foods 2013, 5(1), 178–186. DOI: 10.1016/j.jff.2012.10.002.
- Hu, X.-Q.; Thakur, K.; Chen, G.-H.; Hu, F.; Zhang, J.-G.; Zhang, H.-B.; Wei, Z.-J. Metabolic Effect of 1-Deoxynojirimycin from Mulberry Leaves on Db/Db Diabetic Mice Using Liquid Chromatography–Mass Spectrometry Based Metabolomics. Journal Agricultural Food Chemical 2017, 65(23), 4658–4667. DOI: 10.1021/acs.jafc.7b01766.
- Iqbal, S.; Younas, U.; Chan, K. W.; Sarfraz, R. A.; Uddin, M. K. Proximate Composition and Antioxidant Potential of Leaves from Three Varieties of Mulberry (Morus Sp.): A Comparative Study. International Journal Molecular Sciences 2012, 13(6), 6651–6664. DOI: 10.3390/ijms13066651.
- Lee, W. J.; Choi, S. W. Quantitative Changes of Polyphenolic Compounds in Mulberry (Morus alba L.) Leaves in Relation to Varieties, Harvest Period, and Heat Processing. Prevention Nutritional Food Sciences 2012, 17(4), 280. DOI: 10.3746/pnf.2012.17.4.280.
- Song, W.; Wang, H.-J.; Bucheli, P.; Zhang, P.-F.; Wei, D.-Z.; Lu, Y.-H. Phytochemical Profiles of Different Mulberry (Morus Sp.) Species from China. Journal Agricultural Food Chemical 2009, 57(19), 9133–9140. DOI: 10.1021/jf9022228.
- Zou, Y.; Liao, S.; Shen, W.; Liu, F.; Tang, C.; Chen, C.-Y. O.; Sun, Y. Phenolics and Antioxidant Activity of Mulberry Leaves Depend on Cultivar and Harvest Month in Southern China. International Journal Molecular Sciences 2012, 13(12), 16544–16553. DOI: 10.3390/ijms131216544.
- Li, L.; Luo, G.; Tang, C.; Wang, Z.; Dai, F.; Wu, F.; Yang, Q.; Liao, S.; Xiao, G. Sensory Evaluations and Analyses of Nutritional and Functional Components in Tender Shoot of Different Mulberry Cultivars for Cooking. Chinese Journal Tropical Crops (In Chinese, with English Abstract) 2012, 33, 8, 1494–1499.
- Weiguo, Z.; Zhihua, Z.; Xuexia, M.; Yong, Z.; Sibao, W.; Jianhua, H.; Hui, X.; Yile, P.; Yongping, H. A Comparison of Genetic Variation among Wild and Cultivated Morus Species (Moraceae: Morus) as Revealed by ISSR and SSR Markers. Biodiversity and conservation 2007, 16(2), 275–290. DOI: 10.1007/s10531-005-6973-5.
- In, A.; Latimer, J. W.; Horwitz, W., editors. Official Methods of Analysis; AOAC International press: Washington DC, 2005.
- Carnal, N. W.; Black, C. C. Soluble Sugars as the Carbohydrate Reserve for CAM in Pineapple Leaves. Plant physiology 1989, 90, 1, 91–100.
- Fei, M. L.; Tong, L.; Wei, L.; De Yang, L. Changes in Antioxidant Capacity, Levels of Soluble Sugar, Total Polyphenol, Organosulfur Compound and Constituents in Garlic Clove during Storage. Industrial Crops Products 2015, 69, 137–142. DOI: 10.1016/j.indcrop.2015.02.021.
- Kim, J.-W.; Kim, S.-U.; Lee, H. S.; Kim, I.; Ahn, M. Y.; Ryu, K. S. Determination of 1-Deoxynojirimycin in Morus Alba L. Leaves by Derivatization with 9-Fluorenylmethyl Chloroformate Followed by Reversed-Phase High-Performance Liquid Chromatography. Journal Chromatographic A 2003, 1002, 1, 93–99.
- Li, H.; Deng, Z.; Liu, R.; Young, J. C.; Zhu, H.; Loewen, S.; Tsao, R. Characterization of Phytochemicals and Antioxidant Activities of a Purple Tomato (Solanum lycopersicum L.). Journal Agricultural Food Chemical 2011, 59(21), 11803–11811. DOI: 10.1021/jf202364v.
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chemical 1999, 64(4), 555–559. DOI: 10.1016/S0308-8146(98)00102-2.
- Li, H.; Deng, Z.; Zhu, H.; Hu, C.; Liu, R.; Young, J. C.; Tsao, R. Highly Pigmented Vegetables: Anthocyanin Compositions and Their Role in Antioxidant Activities. Food research international 2012, 46(1), 250–259. DOI: 10.1016/j.foodres.2011.12.014.
- Masek, A.; Chrzescijanska, E. Effect of UV-A Irradiation and Temperature on the Antioxidant Activity of Quercetin Studied Using ABTS, DPPH and Electrochemistry Methods. International Journal Electrochem Sciences 2015, 10, 5276–5290.
- Šamec, D.; Gruz, J.; Strnad, M.; Kremer, D.; Kosalec, I.; Grubešić, R. J.; Karlović, K.; Lucic, A.; Piljac-Žegarac, J. Antioxidant and Antimicrobial Properties of Teucrium Arduini L. (Lamiaceae) Flower and Leaf Infusions (Teucrium arduini L. Antioxidant Capacity). Food Chemical Toxicogical 2010, 48(1), 113–119. DOI: 10.1016/j.fct.2009.09.026.
- Yang, Y.; Wang, G.; Pan, X. China Food Composition, 2nd ed.; Peking University Medical Press: Beijing, 2009.
- Sun, H.; Mu, T.; Xi, L.; Zhang, M.; Chen, J. Sweet Potato (Ipomoea batatas L.) Leaves as Nutritional and Functional Foods. Food Chemical 2014, 156, 380–389. DOI: 10.1016/j.foodchem.2014.01.079.
- Asai, A.; Nakagawa, K.; Higuchi, O.; Kimura, T.; Kojima, Y.; Kariya, J.; Miyazawa, T.; Oikawa, S. Effect of Mulberry Leaf Extract with Enriched 1‐Deoxynojirimycin Content on Postprandial Glycemic Control in Subjects with Impaired Glucose Metabolism. Journal Diabetes Iinvest 2011, 2(4), 318–323. DOI: 10.1111/j.2040-1124.2011.00101.x.
- Li, Y.-G.; Ji, D.-F.; Zhong, S.; Lin, T.-B.; Lv, Z.-Q.; Hu, G.-Y.; Wang, X. 1-Deoxynojirimycin Inhibits Glucose Absorption and Accelerates Glucose Metabolism in Streptozotocin-Induced Diabetic Mice. Scientific Reports 2013, 3, 1377. DOI: 10.1038/srep01377.
- Tsuduki, T.; Nakamura, Y.; Honma, T.; Nakagawa, K.; Kimura, T.; Ikeda, I.; Miyazawa, T. Intake of 1-Deoxynojirimycin Suppresses Lipid Accumulation through Activation of the β-oxidation System in Rat Liver. Journal Agricultural Food Chemical 2009, 57(22), 11024–11029. DOI: 10.1021/jf903132r.
- Dugo, P.; Donato, P.; Cacciola, F.; Paola Germanò, M.; Rapisarda, A.; Mondello, L. Characterization of the Polyphenolic Fraction of Morus Alba Leaves Extracts by HPLC Coupled to a Hybrid IT‐TOF MS System. Journal of Separation Science 2009, 32(21), 3627–3634. DOI: 10.1002/jssc.200900348.
- Sánchez-Salcedo, E. M.; Mena, P.; García-Viguera, C.; Hernández, F.; Martínez, J. J. (Poly) Phenolic Compounds and Antioxidant Activity of White (Morus alba) and Black (Morus nigra) Mulberry Leaves: Their Potential for New Products Rich in Phytochemicals. Journal Function Foods 2015, 18, 1039–1046. DOI: 10.1016/j.jff.2015.03.053.
- Thabti, I.; Elfalleh, W.; Hannachi, H.; Ferchichi, A.; Campos, M. D. G. Identification and Quantification of Phenolic Acids and Flavonol Glycosides in Tunisian Morus Species by HPLC-DAD and HPLC–MS. Journal Function Foods 2012, 4(1), 367–374. DOI: 10.1016/j.jff.2012.01.006.
- Yen, G.-C.; Wu, S.-C.; Duh, P.-D. Extraction and Identification of Antioxidant Components from the Leaves of Mulberry (Morus alba L.). Journal Agricultural Food Chemical 1996, 44(7), 1687–1690. DOI: 10.1021/jf9503725.
- Patra, B.; Schluttenhofer, C.; Wu, Y.; Pattanaik, S.; Yuan, L. Transcriptional Regulation of Secondary Metabolite Biosynthesis in Plants. BBA Gene Regulations Mechanisms 2013, 1829, 11, 1236–1247.
- Wang, W.; Yang, H.; Bo, Y.; Ding, S.; Cao, B. Nutrient Composition, Polyphenolic Contents, and in Situ Protein Degradation Kinetics of Leaves from Three Mulberry Species. Livest Sciences 2012, 146(2), 203–206. DOI: 10.1016/j.livsci.2012.03.009.
- Chan, E. W.-C.; Phui-Yan, L.; Siu-Kuin, W. Phytochemistry, Pharmacology, and Clinical Trials of Morus alba. Chinese Journal Natural Medica 2016, 14(1), 17–30. DOI: 10.3724/SP.J.1009.2016.00017.