?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study evaluated the preservation effectiveness of selected antistaling agents on lotus seeds (Nelumbo nucifera Gaertn) status and the alteration of phenolic acids during their storage. Ascorbic acid (AA), benzoic acid (BA), sodium hydrogen sulfite (SHS), BA+ AA and SHS+ AA were identified as effective to keep lotus seeds fresh with the order of SHS+ AA > BA+ AA > AA > SHS > BA in terms of general performance on not-browning degree, microorganism contamination status, sensory evaluation, and the protection of polyphenols against being oxidized. The polyphenols determined by HPLC and HPLC-MS in lotus seeds were mainly gallic, chlorogenic, gentisic, caffeic, cinnamic, p-coumaric, ferulic, rosmarinic, and salicylic acids. Chlorogenic, gentisic, and caffeic acids were the main phenolic acids correlated with oxidative browning. SHS+ AA was found on inhibiting chlorogenic acid oxidized (p < 0.05) as well as SHS or AA did. BA+ AA also had protective effect on all these three phenolics. The increment of gallic, caffeic, chlorogenic, and p-coumaric acids at the beginning of storage, which was from the hydrolyzation of glycosides promoted by BA, had accordance with the deteriorate sensory of lotus seeds and led to worse anti-browning effect. Gallic acid was found to be relevant to the degree of microorganism contamination. The stronger antibacterial capacity antistaling agents had, the later regeneration of gallic acid appeared. In conclusion, the selected antistaling agents had different preservation effectiveness, which would synchronously indicate alteration of relevant phenolic acids profiles of lotus seed during storage.
Introduction
Lotus, a member of the family Nelumbo nucifera Gaertn, is native to China but all parts of it, such as seeds, rhizomes, leaves, flowers, and stamens are consumed worldwide.[Citation1,Citation2] Especially, Lotus seeds was widely used for many medical purposes, such as sedative, diuretic, tonic, antiemetic, and hemostatics[Citation3], and also an excellent material of health-care food for its abundance in proteins, amino acids, active polysaccharides, and various kinds of minerals and so on.[Citation4] What’s more, lotus seeds even might be applied as a potential drug to resist hyperplasia and kidney cancer.[Citation5]
In recent year, with the consumers’ increased consumption of fresh-cut fruits and vegetables due to their healthy benefits,[Citation6] there is a growing demand for fresh lotus seeds. However, fresh lotus seeds are so easily corrupted and brown that make their quality decreased quickly even during the period of freezing storage, which limits the long-distance transport of lotus seeds. So the development of fresh-keeping technologies of fresh lotus seeds is crucial but there are rare relevant studies.
Until now, the major fresh-keeping methods in food industry include modified packaging[Citation7], ultraviolet light,[Citation8] irradiation,[Citation9] and antistaling agent treatment,[Citation10] among which antistaling agent is most widely used because of its convenience and low cost. Usually, benzoic acid, ascorbic acid, and sodium hydrogen sulfite are common antistaling agents. Landi et al.[Citation11] reported that ascorbic acid could inhibit polyphenol oxidase (PPO) and peroxidase activity in browning-sensitive Lactuca sativa var. capitata (L.) and Eruca sativa (Mill.) thereby maintain them quality during storage. As early as 1984, the effect of sodium hydrogen sulfite on inhibiting PPO had been controversial but now it has been recognized as one of the most commonly agents used form in food preservation.[Citation12,Citation13] Benzoic acid is commonly used as antibacterial and antifungal preservatives and as flavouring agents in food, cosmetic, hygiene, and pharmaceutical products.[Citation14] However, these common antistaling agents’ effect on keeping lotus seeds fresh remain unknown.
Browning,[Citation10] deteriorated sensory,[Citation15] and microorganism contamination[Citation16] are three main degradative points of fruits and vegetables, specially oxidative browning, so the selection of available antistaling agents is usually on account of their effectiveness on these three indexes. However, the influence of antistaling agents on the alternation of bioactive substances accompanying with the deteriorative progress attracted less researchers’ attentions, which was more essential to understand the function of agents. Polyphenol is secondary metabolites existing in plants widely and could play an important role in the prevention of chronic diseases including cardio-protection, anti-cancers, anti-diabetes, anti-aging because of their significant antioxidant activities,[Citation17–Citation19] Previous researches have reported that the degree of the oxidative browning in the processing and storage of fresh-cut plants depends on the polyphenol concentration,[Citation20,Citation21] Through being oxidized in the catalysis of PPO to product quinone which finally aggregates into dark pigment, polyphenol could promote the oxidative browning,[Citation22,Citation23] So it’s certain that the antistaling agents could influence polyphenol content when performing anti-browning property theoretically but such theory lacks of ample support of experiments. Besides, it remains unknown that whether the antistaling agents would affect polyphenol content when it exercises a strong influence towards microorganism contamination and sensory.
Therefore, the aims of this study were: (i) to evaluate the effects of several common antistaling agents on not-browning degree, microorganism contamination status, sensory evaluation of lotus seeds in order to prove these reagents effective; (ii) to investigate the different influence of antistaling agents on the relevant alteration of the main phenolic compounds when agents exert different fresh-keeping effects. The results could help to establish a framework of fresh-keeping technologies for lotus seeds.
Materials and methods
Experimental materials and chemical reagents
The fresh seeds of No. 36 space-breeding lotus (Nelumbo nucifera Gaertn) with receptaculumloti at full ripening stage were harvested at farm in the morning in Guangchang County, Jiangxi Province, China and stored at 4°C for using within 6 h. Polyphenols reference standards of HPLC grade, including gallic acid, chlorogenic acid, caffeic acid, gentisic acid, cinnamic acid, p-coumaric acid, ferulic acid, rosmarinic acid, salicylic acid, naringin, nuciferine, and phloridzin were purchased from Sigma company. All other chemical reagents used were of analytical grade, mainly including benzoic acid, ascorbic acid, sodium hydrosulfite, etc.
Preparation of lotus seeds
The peeled off lotus seeds were distributed randomly and evenly into five tested groups and one control group. The five tested groups were soaked in 80°C water dissolved the antistaling agents 0.5 g/100mL benzoic acid (BA), 0.5 g/100mL ascorbic acid (AA), 0.5 g/100mL sodium hydrogen sulfite (SHS), 0.25 g/100mL sodium hydrogen sulfite + 0.25 g/100mL ascorbic acid (SHS+ AA), and 0.25 g/100mL benzoic acid + 0.25 g/100mL ascorbic acid (BA+ AA) for 3 min, respectively, while the control group were soaked in 80°C water for 3 min only. Then lotus seeds were air-dried to remove water on the surface in a germfree operating environment, stored in sterile valve bags at 4°C and prepared for further analysis.
Observation of lotus seeds status during storage
The prepared lotus seeds in six groups were stored at 4°C in 0 day, 2, 5, 7, and 10 days, and the not-browning degree, microorganism contamination status, and sensory evaluation used to evaluate the status of lotus seeds during storage were recorded (i) Not-browning degree was evaluated by the chromaticity method through Photoshop Software. Specifically, lotus seeds in different storage period were photographed under the same condition, then Photoshop Software randomly recorded the CMYK chroma values of 20 pixel points of lotus seeds pictures. The CMYK chroma average values were calculated to be equivalent to the colour of lotus seeds. Not-browning degree was scored using a scale ranging from 0 (very disgusting black colour) to 10 (exhilarating white or bright yellow) according to the following equation (1):
where c, m, y, and k were the detected and calculated CMYK chroma average values; C1, M1, Y1 and K1 referred to the chroma average values of the lotus seeds in control group at initial (got 10), which were 2, 0, 47, and 0, respectively; C2, M2, Y2, and K2 referred to the chroma average values of the lotus seeds in control group stored for 20 days (got 0), which were 33, 27, 37, and 0, respectively. (ii) Sensory evaluation was done by 20 healthy volunteers and scored using a scale ranging from 1 (very wet surface, very pulpy touching feeling, very bad taste) to 10 (dry surface, hard touching feeling, very good taste). (iii) Microorganism contamination status was determined according to standard procedures[Citation24] and scored by counting the number of yeasts and mould counts in the degree of no colony for 10 score, 1–2 for 8–9 score, 3–5 for 6–7; more than 8 for less than 6 score. Total scores were calculated using the following equation (2):
Total score was used to evaluate the protecting effect of antistaling agents. The higher total scores, the better the protecting effect of antistaling agents.
Determination of glycosides of lotus seeds during storage
Extraction of glycosides
The extraction was determined using solvent extraction method according to reported literatures,[Citation25,Citation26] Briefly, the prepared lotus seeds were stored at 4°C in 0 day, 2, 5, 7, and 10 days and accurately weighted 10 g, respectively. Then the lotus seeds were ground to be milk-like and mixed evenly with 10 mL de-ionized water. The mixture was extracted by 50 mL petroleum ether for 2 h to remove fats. The aqueous layer was reserved and extracted by 25 mL absolute ethyl alcohol for 12 h. After the extraction, the supernatant was obtained through centrifugation, vacuum dried to be cream-like and extracted by 20 mL trichloromethane and 30 mL ethyl acetate for 1 h. The organic liquid was collected and dried in vacuum to obtain powdery substance. Finally, the substance was reconstituted in methanol for UV scanning.
Observation of glycosides content
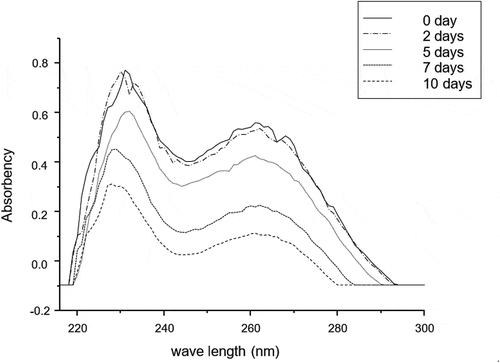
UV scanning was used to observe content change of glycosides of lotus seeds referring to the assay reported by Kanchanapoom et al.[Citation25] and Kato et al.[Citation26]. The section of 216–245 nm is the absorption band of saponin glycosides, and 245–300 nm is the absorption band of flavonoid glycoside and phenolic glycoside. Through scanning absorption of the tested solutions from 216 nm to 300 nm and comparing the peak height and area, the content change of glycosides can be observed in some degree.
Phenolics investigation of lotus seeds during the storage
Extraction of phenolic compounds
The extraction was determined using an ultrasonic-assisted solvent extraction method according to reported literatures,[Citation27] The prepared lotus seeds were stored at 4°C in 0, 2, 5, 7, and 10 days and accurately weighted 7.5 g, respectively. After grinded and removed fats by petroleum ether, phenolic compounds of lotus seeds were extracted by 57 mL 90% ethanol with ultrasound-assistance at 100W in 30°C for 45 min. The supernatant was collected through centrifuging for 10 min at 4200 r/min and dried in vacuum at 45°C to obtain powdery substance. The substance was reconstituted in 30 mL methanol and filtered using 0.45 µm membrane prior to HPLC and HPLC-MS analysis.
Identification of phenolic compounds by HPLC/HPLC-MS
The identification of phenolic compounds referred to the assay reported by Tarnawski et al.[Citation28] and were carried out by an Agilent 1290 infinity series (Agilent Technologies, Santa Clara, USA) – quadrupole mass selective detector in electrospray ionization (ESI) negative ion mode. Separation was in ODS C18 column (0.46 × 25 cm, 5 µm). The binary mobile phase consisted of 0.5% acetic acid in water (v/v) (solvent A) and acetonitrile (solvent B). The solvent gradient was as follows: 0–20 min, 95% A; 20–25 min, 75% A; 25–30 min, 95% A. Phenolic compounds were monitored at 343 nm which was the suitable absorption wavelength scanned out by UV-vis spectrophotometry scanning. Injection volume was 10 µL and flow rate was kept at 0.8 ml/min for a total run time of 30 min. The mass spectrometer electrospray capillary voltage was maintained at 4.0 kV and the mass to charge ratio (m/z) was scanned across the range 50–700 m/z using a drying gas (N2) temperature of 300°C, a dryness gas flow of 6 L/min and a nebulizer gas (N2) pressure of 15 psi. The compounds were identified by comparing with their corresponding standards and searching PubChem database.
Quantification of phenolic compounds by HPLC: Phenolic compounds were analysed referred to the assay reported by Tarnawski et al.[Citation28] using an Agilent HPLC 1260 infinity series (Agilent Technologies, Santa Clara, USA) system consisting of a binary pump, an auto-sampler, an ultraviolet detector (UVW), and the ChemStation Software. Separation was in ODS C18 column (0.46 × 25 cm, 5 µm). The binary mobile phase consisted of 0.5% acetic acid in water (v/v) (solvent A) and acetonitrile (solvent B). The solvent gradient was as follows: 0–20 min, 95% A; 20–25 min, 75% A; 25–30 min, 95% A. Injection volume was 10 µL and flow rate was kept at 0.8 ml/min for a total run time of 30 min. Phenolic compounds were monitored at 343 nm which was the suitable absorption wavelength scanned out by UV-vis spectrophotometry scanning. Quantification was performed with external standards (gallic, chlorogenic, caffeic, gentisic, cinnamic, p- coumaric, ferulic, rosmarinic, and salicylic acids) using standard curves generated 0.2, 1, 5, 10, 20, 40, 60, 80, and 100 µg/ml.
Statistical analysis
All experiments were carried out in triplicates unless otherwise stated and the results were expressed as mean value ± standard deviations. One-way analysis of variance followed by Duncan’s multiple range tests to determine statistically different values on the level of significance at P < 0.05. The correlation analysis was performed between phenolic contents and browning degree using Pearson’s correlation. All statistical analyses were performed using SPSS statistical software (Version 18.0, SPSS Inc., USA). The HPLC-MS data were acquired and analysed by the software MassHunter Acquisition B.03.01, Qualitative Analysis B.03.01 and Quantitative Analysis B.03.02.
Result and discussion
Determination of phenolic compounds by HPLC and HPLC-MS
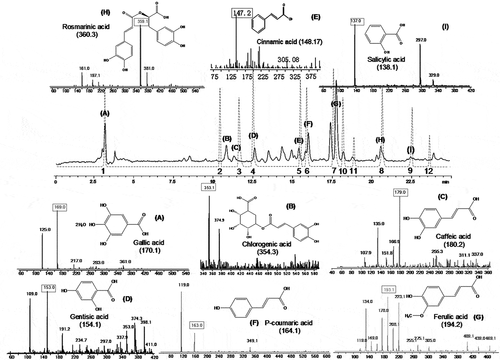
12 phenolic acids and derivatives were detected by comparing with standards using HPLC analysis (Peak 1–12 in ), and finally total nine phenolic acids were confirmed by HPLC-MS (Peak (A)-(I) corresponding to Peak 1–9 in ). They orderly were gallic acid (m/z 169.0), chlorogenic acid (m/z 353.1), gentisic acid (m/z 153.0), caffeic acid (m/z 179.0), cinnamic acid (m/z 147.2), p-coumaric acid (m/z 163.0), ferulic acid (m/z 193.1), rosmarinic acid (m/z 359.1), and salicylic acid (m/z 137.0). Quantification of individual phenolics was performed with external standard method by HPLC and the results were presented in the .
Table 1. Changes of contents of the phenols in different groups (mg/100g).
Figure 1. HPLC-MS spectrogram of phenolics extracted from lotus seeds.
Solid line: fresh lotus seeds extract. (A) Gallic acid; (B) Chlorogenic acid; (C) Caffeic acid; (D) Gentisic acid; (E) Cinnamic acid; (F) P-coumaric acid; (G) Ferulic acid; (H) Rosmarinic acid; (I) Salicylic acidDashed lines: standard reference substances. 1 Gallic acid; 2 Chlorogenic acid; 3 Caffeic acid; 4 Gentisic acid; 5 Cinnamic acid; 6 P- coumaric acid; 7 Ferulic acid; 8 Rosmarinic acid; 9 Salicylic acid; 10 Naringin; 11 Nuciferine; 12 Phloridzin
Change of phenolic acids in control group during storage
had shown the contents of individual phenolics, and of total nine phenolic acids (TNPC) in control group. As shown, TNPC remained stable in first 2 days (from 62.84 to 58.20 mg/100g, P < 0.05) but reduced sharply from 58.20 to 28.09 mg/100g (day 10) (P < 0.05). As shown in , the degree of browning gradually turned into be serious during storage (score from 10 to 6.23), with which the trend of TNPC negatively correlated (r = −0.901, P < 0.05, data not shown). Learning from previous reports[Citation20–Citation23] that polyphenol could promote the oxidative browning and the degree of the oxidative browning depends on the polyphenol concentration, it could be deduced that the browning of lotus seeds was contributed by phenolic acids oxidation under the action of PPO.
Table 2. Evaluation of the effect of antistaling agents and their combination on lotus seed status during storage.
The contents of chlorogenic, gentisic, and caffeic acids all reduced in the first 5 days and the contents were lowest on the last day, which was reversely consistent with the trend of browning. By the analysis of Pearson’s correlation, the correlation coefficients (r) of chlorogenic, gentisic and caffeic acids with the browning degree were −0.952 (P < 0.05), −0.948 (P < 0.05), −0.959 (P < 0.05) (data not shown), respectively, meaning that chlorogenic, gentisic, and caffeic acids were significant indicators to oxidative browning degree of lotus seeds. These phenolic acids have similar changes in the browning process where they were oxidized into corresponding o-quinones or dimmers, then progressively autoxidized into fuscous polymers and finally aggravated the browning or blackening.[Citation29,Citation30]
Salicylic acid was not detected at the beginning but produced 5.21 mg/100g on the 2nd day and up to 15.37 on the 5th day, and the content of gallic acid increased greatly from 3.60 on the 2nd day to 7.67 mg/100g on the 7th day and then kept stable. The variations of cinnamic and p-coumaric acids were reversely similar with that of browning degree and the variation of ferulic acid was not significant (P < 0.05) all the time except on the last day. By the analysis of Pearson’s correlation, the correlation coefficients (r) of cinnamic, ferulic, p-coumaric, salicylic, and gallic acids with the browning degree were −0.857 (P > 0.05), −0.748 (P > 0.05), −0.817 (P > 0.05) −0.475 (P > 0.05), and 0.668 (P > 0.05), respectively, suggesting that the alternation of these phenolic acids might be not only contributed by oxidation and but also another transformative pathway.
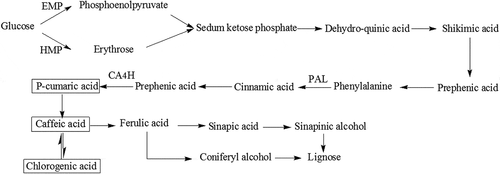
Shikimic acid pathway is one of transformative pathways of phenolic acids widely existing in plants.[Citation31,Citation32] As shown in , cinnamic acid is the front product of shikimic acid pathway, while chlorogenic, caffeic and p-coumaric acids are the end products.[Citation33,Citation34] Considering the interesting results that cinnamic acid as the most abundant phenolics in lotus seeds rapidly descended most from 36.84 (day 0) to 11.21 mg/100g (day 10) whilst the contents of chlorogenic, caffeic, and p-coumaric acids synchronously decreased in the first 5 days, then significantly (P < 0.05) raised from 1.92, 1.97, and 1.91 mg/100g on the 5th day to 3.85, 2.32, and 2.42 mg/100g on the 7th day, respectively, it was reasonably believed that cinnamic acid could not only be oxidized but also transformed to p-coumaric, caffeic, and chlorogenic acids through shikimic acid pathway in lotus seeds. On the other hand, it was well known that glycosides could be broken down into glyco-ligands and corresponding aglycon, one of which was flavanone and phenolic acid micromolecules,[Citation35] so the change of glycosides might contribute to the change of flavanone and phenolic acid. presented that the UV scanning result of glycosides extracts of control group during different storage time, showing the glycosides content did not change significantly in the first 2 days. However, it reduced significantly in the next 8 days, especially fastest from the 5th day to the 7th day (from 245 nm to 300 nm), which was consistent with the change of chlorogenic, caffeic, gentisic, and p-coumaric acids, so it was obviously speculated that these phenolic acids could be replenished from the hydrolyzation of glycosides.
Change of phenolic acids dealt with antistaling agent during storage
Effectiveness of antistaling agents on lotus seeds status
showed the change of lotus seeds status in the form of single scores on not-browning degree, microorganism contamination status and sensory evaluation when with and without the treatment of antistaling agents during storage, and the total scores were calculated according to the equation (1). As shown, the total scores of all experiment groups time-dependently declined whereas total score of control group was significantly lower than those of all tested groups during the whole storage, which were 53.26 (control), 77.55 (BA), 84.25 (AA), 81.76 (SHS), 94.87 (SHS+ AA), and 89.37 (BA+ AA) on the last day, indicating that selected antistaling agents had good fresh-keeping effect on lotus seeds status, as well as the order of fresh-keeping effect was SHS+ AA > BA+ AA > AA > SHS > BA in general.
Alteration of phenolic acids and browning degree
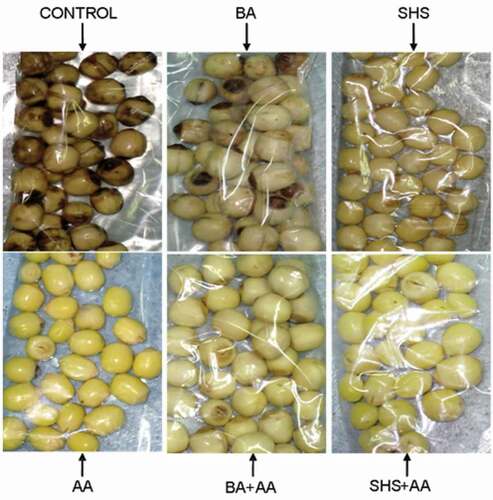
The resulted not-browning degree of Lotus seeds dealt with different agents on the 10th day shown in was visually displayed in . As shown, the control group brown seriously while SHS+ AA group remaining exhilarating white or bright yellow is the best, BA+ SHS the next followed by AA and SHS, and BA the last, so it could be drawn that AA+ SHS had the best protection against browning for lotus seeds.
Figure 4. The lotus seeds appearance between groups stored at 4°C on the 10th day.
BA: 0.5 g/100mL benzoic acid; AA: 0.5 g/100mL ascorbic acid; SHS: 0.5 g/100mL sodium hydrogen sulfite; SH+ AA: 0.25 g/100mL sodium hydrogen sulfite+ 0.25 g/100mL ascorbic acid; BA+ AA: 0.25 g/100mL benzoic acid+ 0.25 g/100mL ascorbic acid
had shown the total contents of chlorogenic, gentisic, and caffeic acids (TTPC) which would be the three contributors to the oxidative browning of lotus seeds. The TTPC in control group declined from 16.24 (day 0) to 3.58 (day 10) mg/100g, except that at the 7th day TTPC increased probably due to the increment of these phenolic acids transformed through shikimic acid pathway and corresponding glycosides. The TTPC in AA group continuously declined from 16.24 (day 0) to 5.18 (day 10) mg/100g but it was still higher than that in control group at all time except on the 7th day, and the TTPC in SHS group remained stable after the 2nd day (P < 0.05) and was higher on the last day (6.27 mg/100g) than that in control group, meaning that AA and SHS could protect phenolic acids against being oxidized. When treated with AA+ SHS, TTPC in lotus seeds gently changed from 10.13 (day 2) to 6.87 (day 10) mg/100g whilst higher than that in AA and SHS group during storage, suggesting that AA+ SHS had a good synergistic effect on the protection of phenolic acids against being oxidized sequentially inhibiting oxidative browning of lotus seeds. The result was consistent with the status of lotus seeds observed in .
To be specific, the protection of AA+ SHS was mainly exerted on chlorogenic acid, but not caffeic acid and gentisic acid. Under the action of AA+ SHS, the contents of chlorogenic acid were gently changed from 6.27 (day 2) to 5.85 (day 10) mg/100g and significantly higher than that in control group (from 3.83 (day 2) to 1.83 (day 10) mg/100g). Just like other studies,[Citation36,Citation37] chlorogenic acid was the main phenolic acid involving in browning and it could be believed that the protection of AA+ SHS on chlorogenic acid against being oxidized contributed large to its action on inhibiting browning of lotus seeds. Such protection of chlorogenic acid by AA + SHS could be explained by not only the inhibition of PPO by AA and SHS,[Citation12,Citation13,Citation38] but also the ability of AA to reduce phenoxyl radicals and quinone forms of phenolics back to colourless diphenols through a coupled oxide-reduction reaction[Citation39] and of SHS to react with the quinones by the redox reaction and addition reaction mechanism.[Citation40,Citation41]
Alteration of phenolic acids and sensory evaluation
showed that AA, SHS, AA+ SHS, and BA+ AA group performed obviously better on maintaining sensory of lotus seeds than control group during the whole storage, whose score was 8.32, 7.96, 9.32, 7.11, and 4.29, respectively, on the last day. However, the while BA got score of sensory evaluation in BA group was only 5.21 on the 2nd day and decreased to 4.20 on the last day in term of sensory evaluation, even lower than that in the control group (from 8.92 at to 4.29 last day), meaning), indicating that BA had the strongest bacteriostaticadverse effect on the sensory of lotus seeds but affected the softness and viscosity negatively.
There was an interesting phenomenon in BA group that when the sensory deteriorated fastest (from 10 to 5.21 in the first two days), the content of gallic, caffeic, chlorogenic, ferulic, and p-coumaric acids increased sharply from 4.2, 3.7, 9.21, 2.85, and 4.2 to 29.83, 6.71, 17.6, 26.89, 29.83 mg/100g, respectively. Considering the facts that phenolic acids could be supplied by the hydrolyzation of glucoside at the same time, the hydrolyzation of glucoside had adverse impact on the quality of plant food through effecting the constitute and function of cell wall and then promoting the degradation of the nutrients, such as protein and lipid,[Citation42] it could be induced that the increment of phenolic acids had the consistence with the decrement of sensory quality, on account of BA accelerating the hydrolyzation of glucoside in short time significantly. Moreover, such transformation from glycosides to corresponding phenolic acids might promote the browning of lotus seeds because there were more phenolic acids available to be oxidized into dark pigments[Citation20–Citation23]. As shown in , there was numerous decrease of phenolic acids from the 2nd day (24.31 mg/100g) to 5th day (9.35 mg/100g), leading to the largest descending score of not-browning degree shown in (from 9.32 to 8.31) sequentially the worst performance of BA on anti-browning among tested agents.
The hydrolyzation of glycosides promoted by BA was restrained by AA where the content of gallic, caffeic, chlorogenic, ferulic, and p-coumaric acids decreased from 16.24 to 14.10 mg/100g in the first 2 days. As a result, BA+ AA group had better performance on the score of sensory evaluation than control group, as well as had better anti-browning effect than BA and AA shown in . Specially, when compared to control group, the contents of chlorogenic and gentisic acids in BA+ AA were higher in last 5 days, and of caffeic acid was higher at last day, meaning that BA+ AA had protective effect on all three phenolic acids.
Alteration of phenolic acids and microorganism contamination status
showed that the order of the bacteriostasis effect of antistaling agents was BA and BA+ AA > AA+ SHS > AA and SHS > control group (10, 10, 9.51, 7.57, 8.03, and 4.51, respectively, on the last day). Both BA and BA+ AA got full scores on microorganism contamination during storage, meaning that BA had the strongest bacteriostatic effect no matter when it worked solely or with others together. It has been known for over 100 years that benzoate inhibits fungal growth[Citation43,Citation44] and the mainly recognized antibacterial mechanism is that antifungal effects are based on the accumulation of benzoate at low external pH which lowers the intracellular pH into the range where phosphofructokinase is sensitive[Citation45].
Gallic acid regeneration was the most common phenomenon and it was observed that the stronger antibacterial capacity of antistaling agents, the later appearance of increasing gallic acid. In control group, the contents of gallic acid were stable in the first 2 days but significantly increased from 3.6 mg/100g on the 2nd day to 7.32 mg/100g on the 5th day (P < 0.05), while in other tested groups the regeneration of gallic acid did not happen as early as that in control group. The gallic acid contents in lotus seeds with AA, SHS, and AA+ SHS increased greatly from 10.64, 14.23, and 14.07 to 38.95, 47.13, and 32.96 mg/100g, in the last 2 days, respectively. When lotus seeds treated with BA and BA+ AA, changes of gallic acid were much gentler than that in AA, SHS, and AA+ SHS groups (). Basing on some precious reports,[Citation46,Citation47] the relationship between bacteriostasis of agents and regeneration of gallic acid might resulted from the pathway where some tannin analogues could be degraded into gallic acids by tannase under the action of microorganism.
Conclusion
In summary, lotus seeds without the treatment of antistaling agents deteriorated severely in aspects of not-browning degree, microorganism contamination status and sensory evaluation during storage, while AA, BA, SHS, AA+ B, and SHS+ AA have the efficient effects on the status of lotus seeds. Meanwhile, it was found that these effects of antistaling agents on keeping fresh were in accordance with those on the phenolic acids. Different phenolics variation could be affected by different agents leading to counterpart appearance of lotus seeds through preventing phenolic oxidation against browning, or promoting the hydrolysis of glycosides into corresponding phenolic acids to accelerate the deterioration of sensory, or changing the microbial environment to influence regeneration of gallic acid. To our knowledge, it is first time to set up the relevance between polyphenol and appearance of lotus seeds under the action of antistaling agents, which would be helpful to find out suitable fresh-keeping technologies for lotus seeds.
Supplemental Material
Download ()Acknowledgements
We thank the financial and technical support of State Key Laboratory of Food Science and Technology of Nanchang University (Grant number SKLF-ZZA-201610) and National Natural Science Foundation of China (Grant number Project No. 31301578). The authors report no conflicts of interest.
Supplementary Material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Bhat, R.; Sridhar, K. R. Nutritional Quality Evaluation of Electron Beam-Irradiated Lotus (Nelumbo Nucifera) Seeds. Food Chemistry 2008, 107(1), 174–184. DOI: 10.1016/j.foodchem.2007.08.002
- Wu, J.-Z.; Zheng, Y.-B.; Chen, T.-Q.; Yi, J.; Qin, L.-P.; Rahman, K.; Lin, W.-X. Evaluation of the Quality of Lotus Seed of Nelumbo Nucifera Gaertn from Outer Space Mutation. Food Chemistry 2007, 105(2), 540–547. DOI: 10.1016/j.foodchem.2007.04.011
- Kredy, H. M.; Huang, D.; Xie, B.; He, H.; Yang, E.; Tian, B.; Xiao, D. Flavonols of Lotus (Nelumbo Nucifera, Gaertn.) Seed Epicarp and Their Antioxidant Potential. European Food Research and Technology 2010, 231(3), 387–394. DOI: 10.1007/s00217-010-1287-6.
- Man, J.; Cai, J.; Cai, C.; Xu, B.; Huai, H.; Wei, C. Comparison of Physicochemical Properties of Starches from Seed and Rhizome of Lotus. Carbohydrate Polymers 2012, 88(2), 676–683. DOI: 10.1016/j.carbpol.2012.01.016.
- Agnihotri, V. K.; ElSohly, H. N.; Khan, S. I.; Jacob, M. R.; Joshi, V. C.; Smillie, T.; Khan, I. A.; Walker, L. A. Constituents of Nelumbo Nucifera Leaves and Their Antimalarial and Antifungal Activity. Phytochemistry Letters 2008, 1(2), 89–93. DOI: 10.1016/j.phytol.2008.03.003
- Fulton, S. L.; McKinley, M. C.; Young, I. S.; Cardwell, C. R.; Woodside, J. V. The Effect of Increasing Fruit and Vegetable Consumption on Overall Diet: A Systematic Review and Meta-Analysis. Critical Reviews in Food Science and Nutrition 2016, 56(5), 802–816. DOI: 10.1080/10408398.2012.727917
- Wei, W.; Lv, P.; Xia, Q.; Tan, F.; Sun, F.; Yu, W.; Jia, L.; Cheng, J. Fresh-Keeping Effects of Three Types of Modified Atmosphere Packaging of Pine-Mushrooms. Postharvest Biology and Technology 2017, 132, 62–70. DOI: 10.1016/j.postharvbio.2017.05.020
- Xu, F.; Wang, S.; Xu, J.; Liu, S.; Li, G. Effects of Combined Aqueous Chlorine Dioxide and UV-C on Shelf-Life Quality of Blueberries. Postharvest Biology and Technology 2016, 117, 125–131. DOI: 10.1016/j.postharvbio.2016.01.012
- Mami, Y.; Peyvast, G.; Ziaie, F.; Ghasemnezhad, M.; Salmanpour, V. Improvement of Shelf-Life and Postharvest Quality of White Button Mushroom by 60co Gama-Ray Irradiation. Plant Knowledge J. 2013, 2, 2, 1.
- Krasnova, I.; Misina, I.; Seglina, D.; Aboltins, A.; Karklina, D. Application of Different Anti-Browning Agents in order to Preserve the Quality of Apple Slices, 11th Baltic Conference on Food Science and Technology” Food Science and Technology in a Changing World”. FOODBALT 2017. Latvia University of Agriculture: Jelgava, Latvia:27-28 April 2017; pp 106–111.
- Landi, M.; Degl’Innocenti, E.; Guglielminetti, L.; Guidi, L. Role of Ascorbic Acid in the Inhibition of Polyphenol Oxidase and the Prevention of Browning in Different Browning‐Sensitive Lactuca Sativa Var. Capitata (L.) And Eruca Sativa (Mill.) Stored as Fresh‐Cut Produce. Journal of the Science of Food and Agriculture 2013, 93(8), 1814–1819. DOI: 10.1002/jsfa.5969.
- Golan-Goldhirsh, A.; Whitaker, J. R. Effect of Ascorbic Acid, Sodium Bisulfite, and Thiol Compounds on Mushroom Polyphenol Oxidase. Journal of Agricultural and Food Chemistry 1984, 32(5), 1003–1009. DOI: 10.1021/jf00125a013.
- Perera, C. O.; Smith, B., Technology of processing of horticultural crops. Handbook of farm, dairy, and food machinery engineering, 2nd ed; Kutz, M., Eds.; Academic Press: Cambridge, MA, 2013; pp 259-315.
- Del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic Acid and Its Derivatives as Naturally Occurring Compounds in Foods and as Additives: Uses, Exposure, and Controversy. Critical Reviews in Food Science and Nutrition 2017, 57(14), 3084–3103. DOI: 10.1080/10408398.2015.1087964.
- Ngamchuachit, P.; Sivertsen, H. K.; Mitcham, E. J.; Barrett, D. M. Effectiveness of Calcium Chloride and Calcium Lactate on Maintenance of Textural and Sensory Qualities of Fresh‐Cut Mangos. Journal of Food Science 2014, 79, 5. DOI: 10.1111/1750-3841.12446.
- Raybaudi‐Massilia, R. M.; Mosqueda‐Melgar, J.; Soliva‐Fortuny, R.; Martín‐Belloso, O. Control of pathogenic and spoilage microorganisms in fresh‐cut fruits and fruit juices by traditional and alternative natural antimicrobials. Comprehensive Reviews in Food Science 2009, 8 (3), 157-180.
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial By-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chemistry 2006, 99(1), 191–203. DOI: 10.1016/j.foodchem.2005.07.042.
- Ky, I.; Crozier, A.; Cros, G.; Teissedre, P.-L. Polyphenols Composition of Wine and Grape Sub-Products and Potential Effects on Chronic Diseases. Nutrition 2014, 2, 2, 3, 165–177.
- Quideau, S.; Deffieux, D.; Douat‐Casassus, C.; Pouysegu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angewandte Chemie International Edition 2011, 50(3), 586–621. DOI: 10.1002/anie.201000044.
- Burda, S.; Oleszek, W.; Lee, C. Y. Phenolic Compounds and Their Changes in Apples during Maturation and Cold Storage. Journal of Agricultural and Food Chemistry 1990, 38(4), 945–948. DOI: 10.1021/jf00094a006.
- Coseteng, M.; Lee, C. Changes in Apple Polyphenoloxidase and Polyphenol Concentrations in Relation to Degree of Browning. Journal of Food Science 1987, 52(4), 985–989. DOI: 10.1111/j.1365-2621.1987.tb14257.x.
- Mathew, A.; Parpia, H. Food Browning as a Polyphenol Reaction. Advances Food Science 1971, 19, 75–145.
- Queiroz, C.; Mendes Lopes, M. L.; Fialho, E.; Valente-Mesquita, V. L. Polyphenol Oxidase: Characteristics and Mechanisms of Browning Control. Journal of Food, Agriculture and Environment 2008, 24(4), 361–375. DOI: 10.1080/87559120802089332.
- Downes, F. Compendium of Methods for the Microbiological Examination of Foods; American Public Health Association: Washington DC, 1992.
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Phenolic Glycosides from Barnettia Kerrii. Phytochemistry 2002, 59, 5, 565–570.
- Kato, H.; Li, W.; Koike, M.; Wang, Y.; Koike, K. Phenolic Glycosides from Agrimonia Pilosa. Phytochemistry 2010, 71(16), 1925–1929. DOI: 10.1016/j.phytochem.2010.08.007.
- Xu, G.; Ye, X.; Liu, D.; Ma, Y.; Chen, J. Composition and Distribution of Phenolic Acids in Ponkan (Citrus Poonensis Hort. Ex Tanaka) and Huyou (Citrus Paradisi Macf. Changshanhuyou) during Maturity. Journal of Food Composition and Analysis 2008, 21(5), 382–389. DOI: 10.1016/j.jfca.2008.03.003.
- Tarnawski, M.; Depta, K.; Grejciun, D.; Szelepin, B. HPLC Determination of Phenolic Acids and Antioxidant Activity in Concentrated Peat Extract—A Natural Immunomodulator. Journal of Pharmaceutical and Biomedical Analysis 2006, 41(1), 182–188. DOI: 10.1016/j.jpba.2005.11.012.
- Duesterberg, C. K.; Waite, T. D. Kinetic Modeling of the Oxidation of P-Hydroxybenzoic Acid by Fenton’s Reagent: Implications of the Role of Quinones in the Redox Cycling of Iron. Environmental Science and Technology 2007, 41, 11, 4103–4110
- Oliveira, C. M.; Ferreira, A. C. S.; De Freitas, V.; Silva, A. M. Oxidation Mechanisms Occurring in Wines. Food Research International 2011, 44(5), 1115–1126. DOI: 10.1016/j.foodres.2011.03.050.
- Gershenzon, J. Secondary Metabolites and Plant Defense. In Taiz, L., Zeiger, E., Eds.; Plant Physiol., 3rd ed; Sinauer: Sunderland, Ma, 2002; pp 283–308.
- Seigler, D. S.; Shikimic Acid Pathway. In Plant Secondary Metabolism; Springer: Boston, MA, 1998; pp 94–105.
- Cartea, M. E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16(1), 251–280. DOI: 10.3390/molecules16010251.
- Patterson, D.; Effects of Allelopathic Chemicals on Growth and Physiological Responses of Soybean (Glycine Max). Weed Sci. 1981, 29(1), 53–59.
- Brito-Arias, M.;. Hydrolysis of Glycosides. In Synthesis and Characterization of Glycosides; Springer: Boston, MA, 2016; pp. x355–367.
- Luo, L.; Hao, Y.; Jia, L.; Zhu, W. Analysis of Chlorogenic Acid Oxidation Pathway in Simulated Enzymatic Honeysuckle Browning System by High Performance Liquid Chromatography and Mass Spectrometry. Trop. J. Pharm. Res. 2016, 15(2), 405–409. DOI: 10.4314/tjpr.v15i2.25.
- Tomic, D.; Grigorakis, S.; Loupassaki, S.; Makris, D. P. Implementation of Kinetics and Response Surface Methodology Reveals Contrasting Effects of Catechin and Chlorogenic Acid on the Development of Browning in Wine Model Systems Containing either Ascorbic Acid or Sulphite. European Food Research and Technology 2017, 243(4), 565–574. DOI: 10.1007/s00217-016-2766-1.
- Saba, M. K.; Sogvar, O. B. Combination of Carboxymethyl Cellulose-Based Coatings with Calcium and Ascorbic Acid Impacts in Browning and Quality of Fresh-Cut Apples. LWT - Food Science and Technology 2016, 66, 165–171. DOI: 10.1016/j.lwt.2015.10.022.
- Louarme, L.; Billaud, C. Evaluation of Ascorbic Acid and Sugar Degradation Products during Fruit Dessert Processing under Conventional or Ohmic Heating Treatment. LWT - Food Science and Technology 2012, 49(2), 184–187. DOI: 10.1016/j.lwt.2011.12.035.
- Danilewicz, J. C.; Seccombe, J. T.; Whelan, J. Mechanism of Interaction of Polyphenols, Oxygen, and Sulfur Dioxide in Model Wine and Wine. Am. J. Enol. Vitic. 2008, 59, 2, 128–136.
- Youngblood, M. P.;. Kinetics and Mechanism of the Addition of Sulfite to P-Benzoquinone. Journal of Organic Chemistry 1986, 51(11), 1981–1985. DOI: 10.1021/jo00361a008.
- Tanaka, T.; Watarumi, S.; Matsuo, Y.; Kamei, M.; Kouno, I. Production of Theasinensins A and D, Epigallocatechin Gallate Dimers of Black Tea, by Oxidation–Reduction Dismutation of Dehydrotheasinensin A. Tetrahedron 2003, 59(40), 7939–7947. DOI: 10.1016/j.tet.2003.08.025.
- Ricke, S.;. Perspectives on the Use of Organic Acids and Short Chain Fatty Acids as Antimicrobials. Poultry Science 2003, 82(4), 632–639. DOI: 10.1093/ps/82.4.632.
- Zhu, B. K. Synthesis and Properties of a New Bacteriostatic Agent of Ballast Water. In Zhao, J., Iranpour, R.; Li, X.; Jin, B., Eds; Advanced Materials Research; Trans Tech Publ: Switzerland, 2013; pp 2461–2467.
- Krebs, H. A.; Wiggins, D.; Stubbs, M.; Sols, A.; Bedoya, F. Studies on the Mechanism of the Antifungal Action of Benzoate. Biochem. J. 1983, 214(3), 657. DOI: 10.1042/bj2140657.
- Banerjee, R.; Mukherjee, G.; Patra, K. C. Microbial Transformation of Tannin-Rich Substrate to Gallic Acid through Co-Culture Method. Bioresource Technology 2005, 96(8), 949–953. DOI: 10.1016/j.biortech.2004.08.004.
- Sharma, K. P.; John, P.; Goswami, P.; Soni, M. Enzymatic Synthesis of Gallic Acid from Tannic Acid with an Inducible Hydrolase of Enterobacter Spp. Biocatalysis and Biotransformation 2017, 35(3), 177–184. DOI: 10.1080/10242422.2017.1306740.