 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Petroleum ether extract from Abutilon theophrasti Medic. leaves was optimized by response surface methodology, and the optimal extraction conditions were as follows: ratio of solvent to material (20.12 mL/g), extraction time (5.45 h), and Soxhlet extraction temperature (61.32°C). And the yield of petroleum ether extract collected in August, September, and October was (2.05 ± 0.02)%, (2.39 ± 0.01)%, and (2.32 ± 0.02)%, respectively. The September and October extracts exhibited a better antioxidant activity, which was proved by DPPH·scavenging ability (IC50 value of 327.5 and 331.5 μg/mL), ABTS·+ scavenging ability (IC50 value of 170.1 and 182.1 μg/mL), and reducing power (0.31 and 0.28 mmol Fe2+/100 μg/mL). Meanwhile, the gas chromatograph-mass spectrometry analysis revealed that the main antioxidant components contained 9, 12, 15-octadecatrienoic acid and 9, 12, 15-octadecatrienoic acid, ethyl ester (Z,Z,Z) in three petroleum ether extracts. Therefore, petroleum ether extract from Abutilon theophrasti Medic. leaves can be a potential resource of natural antioxidants in pharmaceutical, medicine, food, and chemical industries.
Introduction
As an annual herb plant in the Malvaceae family, Abutilon theophrasti Medic. (A. theophrasti) is widely distributed all over tropics and sub-tropics.[Citation1] The different parts of A. theophrasti, including roots, stems, leaves, flowers, seeds, and episperms, have been used for treating many illnesses, such as swell, ulcer, and inflammation in folk.[Citation2,Citation3] In recent years, many bioactive compounds, including tannin, phenols, and flavonoids, have been detected and isolated from A. theophrasti leaves,[Citation4–Citation6] and these compounds exhibited important pharmacological effects, such as antibacterial, antioxidant, anti-inflammatory, hepatoprotective, and anticancer activities.[Citation7–Citation12]
It is well known that low-polarity constituents are not only a major energy source but also containing many biological activities such as antioxidant, anti-inflammatory, and anti-cancer activities.[Citation13–Citation16] Liu et al.[Citation3] reported that fatty acids from A. theophrasti seeds can be used as antidiuretic, antidote, and antipyretic. However, low-polarity compounds from A. theophrasti leaves have not been well studied.
Response surface methodology (RSM) is a powerful tool for evaluating the effects of each factor and their interactions in the extraction process.[Citation17,Citation18] Zhang et al.[Citation19] employed RSM to optimize the extraction conditions of polysaccharides from lotus (Nelumbo nucifera) leaves, such as liquid/solid ratio, processing pressure, processed times, extraction temperature, and extraction time. RSM was also used by Singh and Pradhan[Citation20] for modelling a second-order response surface to estimate the optimum machining condition.
Now there are more and more assay methods for evaluating the antioxidant activity of low-polarity extract, such as superoxide radical, hydroxyl radical, oxygen radical absorbance capacity, β-carotene/linoleic acid, 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical, 2, 2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt) (ABTS) radical scavenging assay, and ferric-ion reducing antioxidant power (FRAP) methods. As reported by Villa-Rodríguez,[Citation12] there was a positive correlation between DPPH· and ABTS·+ radical scavenging ability and the content of some unsaturated fatty acids. Aktumsek et al.[Citation7] focused on the assessment of antioxidant property and fatty acid composition of four Centaurea species, and evaluated their antioxidant activity with phosphomolybedum assay, DPPH·assay, β-carotene/linoleic acid, and ferric and cupric reducing power. Pumpkin seed oil exhibited stronger antioxidant activities with the IC50 values 123.93 and 152.84 mg/mL for DPPH·radical scavenging assay and β-carotene/linoleic acid bleaching test.[Citation21] The above two methods were also adopted for evaluating the antioxidant activities of Isatis indigotica seeds oil with IC50 values 9.58 and 15.03 mg/mL, respectively.[Citation13]
In this work, we firstly optimized the Soxhlet extraction parameters for petroleum ether extract (PEE) of A. theophrasti leaves by a 17-run Box–Behnken design (BBD) with a quadratic regression model set up by RSM. Moreover, the chemical components and antioxidant activities (DPPH·and ABTS·+ radical scavenging ability, FRAP) of PEE obtained in August, September, and October were compared and studied.
Materials and methods
Plant material
A. theophrasti leaves were gathered from Jilin province of China in August, September, and October 2014, and authenticated by professor Shaofan Du of Shenyang Agricultural University according to Compendium of Materia Medica. The voucher specimens KT/JL/CH/AT/08/14, KT/JL/CH/AT/09/14, and KT/JL/CH/AT/10/14 were deposited at college of Animal Science and Veterinary Medicine, Shenyang Agricultural University for future reference. Plant materials were washed and dried at room temperature.
Apparatus and reagents
The antioxidant assay was determined by Tecan Infinite M200 Pro NanoQuant (Tecan Inc., Switzerland). DPPH, ABTS, and 2, 4, 6-tri (2-pyridyl)-s-triazine (TPTZ) were purchased from Sigma-Aldrich Chemie (Steinheim, Germany). All other chemicals and reagents used in this study were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Preparation of petroleum ether extract
Three grams of A. theophrasti leaves was extracted with petroleum ether (bp, 60–90°C). The Soxhlet extraction conditions were as follows: ratio of solvent to material 20.12 mL/g, extraction time 5.45 h, and extraction temperature 61.32°C. Then, PEE was concentrated by rotary evaporator, and stored in the dark glass bottle at 4°C.
Experimental design
In the present study, three major influence factors, including ratio of solvent to material, extraction time, and extraction temperature, were investigated for response surface experiments with the yield of PEE as index. The range of levels for the three factors was as follows: ratio of solvent to material 12, 14, 16, 18, 20, 22 mL/g; extraction time 2, 4, 6, 8, 10 h; and extraction temperature 40°C, 45°C, 50°C, 55°C, 60°C, 65°C. When evaluating one factor, the levels of the other two were set in the middle.
According to the results of the single factor experiments, three levels of each factor were selected. In the response surface experiment, BBD (Design-Expert software, Trial Version 8.0.6, State, Inc., Minneapolis, USA) with three variables (X1, ratio of solvent to material; X2, extraction time; X3, extraction temperature) and three levels was introduced to optimize the extraction process of PEE with the yield of PEE as dependent variable. The BBD consisted of 12 factorial points and five replicates of central points (). Each experimental run was assayed in triplicate except the five centre points, and the averages of yield of PEE were taken as response.
Table 1. Box–Behnken experimental design and the results for extraction yield of PEE from A. theophrasti leaves (n = 3).
Antioxidant activity assay
Measurement of DPPH radical scavenging activity
DPPH radical scavenging assay was performed according to the method of Brand-Williams et al.[Citation22] with a few modifications. One hundred microlitres of different concentrations of PEE was mixed with 900 μL of 0.1 mmol/L DPPH·solution, and incubated for 30 min at 37°C in the dark. Then the absorbance was measured at 517 nm, and IC50 value was determined for evaluating the antioxidant activity. Each assay was measured in triplicate, and the percentage of inhibition effect was calculated as follows: Inhibition (%) = (1–ODtest/ODcontrol) × 100.
Measurement of ABTS·+ radical scavenging activity
The ABTS·+ radical scavenging ability of PEE was assayed as the method of Ali[Citation23] with minor modifications. The ABTS cation radical was prepared with 2.45 mmol/L potassium persulphate and 7 mmol/L ABTS, and incubated at room temperature in the dark for 12–16 h, and then diluted by phosphate buffer solution to obtain an absorbance of 0.700 ± 0.02 at 734 nm. One hundred microlitres of PEE of A. theophrasti leaves was mixed with 900 μL of ABTS·+ solution. After 30 min incubation at room temperature in the dark, the absorbance of the resulting solution was measured at 734 nm. The values of IC50 were determined as reported above.
Measurement of ferric-ion reducing antioxidant power
According to the method reported by Benzie[Citation24] with some modifications, FRAP reagent was prepared with 100 mL of 10 mmol/L TPTZ in 40 mmol/L HCl, 10 mL of 20 mmol/L FeCl3, and 10 mL acetate buffer (pH 3.6). One hundred microlitres of PEE was added to 400 μL of FRAP reagent, and the absorbance was measured at 593 nm after 30 min of incubation at 37°C. FRAP was expressed as mmol of Fe2+ per 100 μg/mL PEE.
Constituent analysis of PEE
Derivatization reaction
0.1 g of PEE was mixed with 5 mL of petroleum-anhydrous ether (4:3, v/v), 4 mL of 0.5 mol/L potassium hydroxide methanol solution was added, and then mixed for 1 min. After 15 min, the mixture was added 1 mL of distilled water, and the liquid supernatant was analysed.
Conditions of gas chromatography-mass spectrometry
The prepared samples were analysed on Agilent 6890-5973N gas chromatograph-mass spectrometry (GC-MS) by using hydrogen as the carrier gas and a TR-FAME column (30.0 m × 0.25 mm ID × 0.25 μm film thickness, Thermo Fisher, USA). The peaks were identified by using a Database Nist 98.l, and then each constituent was expressed as a percentage of total compounds quantified. The initial temperature of the column was set at 80°C for 1 min, and then raised at 4°C/min to 220°C for 1 min. The temperatures of MS Source and Quad were 230°C and 150°C, respectively. The range of MS scan was m/z 35–520.
Statistical analysis
All determinations were performed in triplicate and processed using Design Expert 8.0.6 software and SPSS 17.0 (SPSS 17.0 for WINDOWS; SPSS Inc., Chicago, IL) with p-values < 0.05 as statistically significant and p-values < 0.01 as very significance.
Results and discussion
Optimization of extraction conditions
Results of single factor experiments
Three factors (ratio of solvent to material, extraction time, and Soxhlet extraction temperature) were evaluated in the single factor experiments (). As shown X1 line in , the ratio of solvent to material was set at 12, 14, 16, 18, 20, and 22 mL/g with the extraction temperature of 50°C and the extraction time of 6 h. X1 line indicated that the yield of PEE increased with increasing ratio of solvent to material, and the yield increased more significantly when the ratio of solvent to material increased from 18 to 20 mL/g, and reached the peak value (1.98%). The positive effect of ratio of solvent to material could be explained by the higher volume of solvent, the higher dissolution rate of PEE in the solvent. However, the yield of PEE started to decrease when the ratio of solvent to material continued to rise; further, it may be that loss of solvent evaporation increased with the increase of solvent volume.
Figure 1. Effect of ratio of solvent to material (X1), extraction time (X2), and extraction temperature (X3) on the extraction yield of PEE.
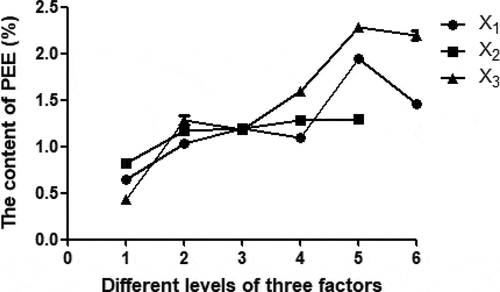
In order to evaluate the influence of Soxhlet extraction time on the yield of PEE, extraction time was set at 2, 4, 6, 8, and 10 h with extraction temperature of 50°C and ratio of solvent to material of 16 mL/g. According to X2 line in , the yield of PEE increased rapidly with the increase of extraction time from 2 to 4 h, and increased slowly from 4 to 10 h. The results indicated that the extraction time would not increase dramatically yield of PEE.
X3 line in represented the effect of Soxhlet extraction temperature on extraction yield of PEE. Temperatures of 40°C, 45°C, 50°C, 55°C, 60°C, and 65°C were investigated with ratio of solvent to material of 16 mL/g and extraction time of 6 h. X3 line described that the yield increased rapidly when the temperature increased from 40°C to 45°C, while yield increased slowly from 45°C to 50°C, and reached the peak value at 60°C. The boiling range of petroleum ether was 60°C to 90°C, so the higher extraction efficiency was achieved at 60°C. However, the possibility of petroleum ether evaporation increased when the temperature was higher than 60°C, and the extraction efficiency decreased.
According to the results of single factor experiments, three factors with three levels were adopted for the RSM experiments, and experiment parameters were as follows: ratio of solvent to material (18, 20, 22 mL/g), extraction time (4, 6, 8 h), and Soxhelt extraction temperature (55°C, 60°C, 65°C).
RSM experiments
Model fitting and statistical analysis
presents 17 experiments in the design matrix, and the yield of PEE ranged from 1.56% to 2.38%. The experimental data were adopted to calculate the coefficients of the quadratic equation, and the following final equation in terms of coded factors was set up by multiple regression analysis of Design Expert software (version 8.0.6):
In statistical analysis, a larger regression coefficient and a smaller p-value would indicate a more significant effect on the respective response variables.[Citation18] The determined coefficient (R2 = 0.9986), which was presented by the quadratic regression model, indicated that only 0.14% of total variants cannot be explained by the model. A larger value of R2 does not always imply the adequacy of the model, so it is appropriate to adopt an adjusted R2 of over 90% to evaluate the model adequacy.[Citation18] In this research, the adjusted R2 value at 0.9967 suggested a better model adequacy. Meanwhile, the lower p-value (p<0.0001) and coefficient of the variation (C.V., 0.71) all indicated that the model can significantly represent the actual relationship between parameters and response with higher precision, accuracy, reliability, and reproducibility. The results suggest that the model used in this study was able to identify the optimum extraction condition of PEE from A. theophrasti leaves.
ANOVA was applied to determine the statistical significance of regression coefficients in equation, and the significance of each coefficient was evaluated by p-value. The linear coefficients and quadratic coefficients of ratio of solvent to material (X1) and Soxhlet extraction temperature (X3) were very significant (p < 0.01). However, no significant linear and quadratic effect was observed for extraction time (X2, p > 0.05). Meanwhile, a very significant interaction effect was obtained among all interaction factors (p < 0.01). The p-value indicated that Soxhlet extraction temperature (X3) was the most significant factor influenced on PEE, followed by ratio of solvent to material (X1), while extraction time (X2) exhibited insignificant effect.
Response surface analysis of the content of PEE (CPEE)
The graphical interpretation of regression equation was provided by the 3D response surface curves () to illustrate the relationship between dependent variable and experimental levels of each independent variable, and the interaction of any two variables was evaluated with the third factor at zero level. Intensive contour indicated much higher influences of parameters on CPEE, and thin contour indicated weak influences.
Figure 2. (a–c) Response surface plots of ratio of solvent to material (X1, mL/g), extraction time (X2, h), and extraction temperature (X3, oC) on the yield of PEE.
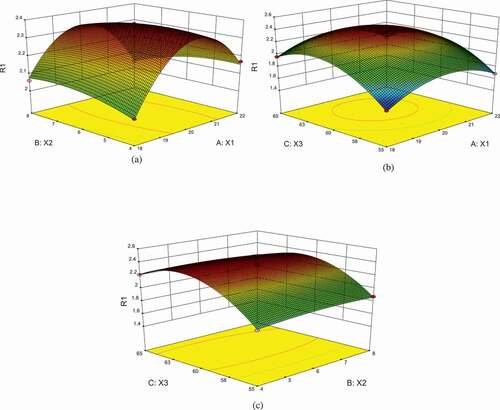
The interactions among ratio of solvent to material (X1), extraction time (X2), and Soxhlet extraction temperature (X3) on the CPEE were described in . Intensive contour and steeper surface indicated that Soxhlet extraction temperature exhibited a higher influence on CPEE than ratio of solvent to material and extraction time. The CPEE improved remarkably with increasing Soxhlet extraction temperature with the ratio of solvent to material at zero level. However, CPEE began to decrease when extraction temperature beyond 61.3°C. This result may be the volatilization loss of extraction solvent under high extraction temperature. CPEE increased slowly with increasing ratio of solvent to material from 18 to 20 mL/g with extraction temperature at zero level, and decreased over 20 mL/g. However, there was no significant effect between extraction time and CPEE. These results suggested that there were quadratic effects between extraction temperature, ratio of solvent to material, and CPEE, and that there was no significant effect for extraction time.
Graphical interpretation and optimization of procedure
The PEE final equation obtained in this study was applied for response surface optimization by Design-Expert software, and the optimum conditions were ratio of solvent to material (20.12 mL/g), extraction time (5.45 h), and Soxhlet extraction temperature (61.32°C). The experimental values (2.39 ± 0.01)% were in agreement with the predicted value (2.40%).
Content of PEE in 3-month samples
With the optimal extraction condition, the yields of PEE are (2.05 ± 0.02)%, (2.39 ± 0.01)%, and (2.32 ± 0.02)% for the three samples collected in August, September, and October 2014, respectively (). The results indicated that CPEE varied with the collection month, and the content was highest in September, then October, and last in August.
Table 2. Yield of PEE, IC50 values for evaluated antioxidant assays and EC50 values reducing power of PEE from A. theophrasti leaves.
Antioxidant activity
lists the scavenging DPPH radical, ABTS·+ radical effect, and reducing power of PEE from A. theophrasti leaves collected in August, September, and October. As presented in , the DPPH radical-scavenging capacity of September and October extracts was stronger 2.5 times than August extract with IC50 327.5 and 331.5 μg/mL, respectively.
As described in , three extracts all presented potential ability to scavenge the ABTS·+ radical, and the order of antioxidant activity was August<October<September. The PEE of September and October had a higher antioxidant capacity with IC50 of 170.1 and 182.1 μg/mL compared to August (IC50 278.0 μg/mL).
Contrary to scavenging DPPH radical and ABTS·+ radical assay, the FRAP value is proportional to antioxidant activity. The results of FRAP assay for three extracts were listed in . The September extract exhibited a higher FRAP with 0.31 mmol Fe2+/100 μg/mL, followed by October extract (0.28 mmol Fe2+/100μg/mL). The FRAP of August PEE was 50% lower than other two extracts. The results of the above three antioxidant experiments were consistent, and the antioxidant activity of September and October extracts was similar, and better than August extract. Moreover, the FRAP of September and October extracts was almost the same with BHT.
Chemical component of PEE
The compositions of oils and/or the ratio of saturated-unsaturated fatty acid will affect directly the medicinal and nutritional value.[Citation25] The compositions profiles of PEE from A. theophrasti leaves were analysed by GC-MS, and the results were summarized in . Unsaturated fatty acid (C18:3) 9, 12, 15-octadecatrienoic acid was the most predominant constituent in the PEE collected in August, September, and October, and its content was 43.36%, 50.42%, and 53.14%, respectively. Hexadecanoic acid was the most abundant saturated fatty acid (C16:0) with the content of 21.07%, 17.41%, and 16.39%. 9, 12-Octadecadienoic acid (C18:2) and 9, 12, 15-octadecatrienoic acid, ethyl ester (Z,Z,Z) (C18:3) were two unsaturated fatty acids with relatively high content. In addition, other 21 ingredients were the mainly active constituents with the content lower than 3%. The PEE from A. theophrasti leaves was rich in 9, 12, 15-octadecatrienoic acid, and hence may be viewed as a healthy, nutritive, and cheap alternative to other vegetable oils.
Table 3. Chemical composition of PEE from A. theophrasti leaves.
Pearson correlation analysis
Correlations of DPPH·radical, ABTS·+ radical scavenging activity, and FRAP activity with CPEE
In this study, DPPH·radical, ABTS·+ radical scavenging activity, and FRAP activity assay were used to evaluate antioxidant activity of PEE from A. theophrasti leaves. The correlations parameters were calculated by Pearson correlation analysis test, and the correlation coefficients were listed in . The results indicated that the highest correlation coefficient was found between the CPEE and FRAP activity (1.000, p<0.05), then CPEE and ABTS·+ radical scavenging activity (−0.996), and the last one between the CPEE and DPPH·radical scavenging activity (−0.982). The high correlation between CPEE and DPPH·radical, ABTS·+ radical scavenging activity and FRAP activity assay, suggests that PEE may play an important role in antioxidant activity aspects of A. theophrasti leaves.
Table 4. Correlation coefficients between antioxidant activities and yield of extracts.
Correlations between methods used to evaluate antioxidant activity of PEE
As presented in , the significant correlation (p<0.05) was found between ABTS·+ radical scavenging activity and FRAP activity (−0.997), followed by ABTS·+ and DPPH· (0.996), and then by FRAP and DPPH· (−0.986). In brief, three antioxidant methods used in this research all indicated better correlation, which was the basis of further study antioxidant activity of PEE from A. theophrasti leaves.
Correlations between antioxidant activities and main chemical component of PEE
presents the correlations between antioxidant activities and main chemical component of PEE, including 9, 12, 15-octadecatrienoic acid, hexadecanoic acid, 9, 12-octadecadienoic acid, and 9, 12, 15-octadecatrienoic acid, ethyl ester (Z,Z,Z). As shown in , the contents of 9, 12, 15-octadecatrienoic acid and 9, 12, 15-octadecatrienoic acid, ethyl ester (Z,Z,Z) were negatively correlated with DPPH· (−0.961 and −0.995) and ABTS·+ (−0.931 and −0.981) values, and positively correlated with FRAP (0.900 and 0.964) value. However, the contents of hexadecanoic acid and 9, 12-octadecadienoic acid were positively correlated with DPPH· (0.977 and 0.966) and ABTS·+ (0.952 and 0.938) values, and negatively correlated with FRAP (−0.926 and −0.909) value. The finding was in accordance with the result of antioxidant activities of the PEE collected in different months. Meanwhile, the highest correlation coefficient was found between the content of 9, 12, 15-octadecatrienoic acid and 9, 12-octadecadienoic acid (−1.000, p<0.05), followed by 9, 12-octadecadienoic acid and hexadecanoic acid (0.999, p<0.05), and then by 9, 12, 15-octadecatrienoic acid and hexadecanoic acid(−0.998, p<0.05). Furthermore, the correlation coefficient was much better among the content of 9, 12, 15-octadecatrienoic acid, ethyl ester (Z,Z,Z) and 9, 12, 15-octadecatrienoic acid, hexadecanoic acid, 9, 12-octadecadienoic acid (0.984, −0.993, and −0.987). This further validated the correlations between antioxidant activities and main chemical component of PEE.
Table 5. Correlation coefficients between antioxidant activities and content of four main components.
Conclusion
This study is the first report on the extraction of PEE from A. theophrasti leaves collected in August, September, and October, and optimization of their extraction conditions with Soxhlet extraction method by RSM. The optimized extraction parameters were as follows: ratio of solvent to material (20.12 mL/g), extraction time (5.45 h), and Soxhlet extraction temperature (61.32°C). Also, the yield of PEE was 2.39%. In addition, September and October extracts exhibited stronger antioxidant activity than August extract, which were demonstrated by DPPH·radical, ABTS·+ radical scavenging assay, and FRAP assay. The analysis of chemical component of three PEEs indicated that 9, 12, 15-octadecatrienoic acid and 9, 12, 15-octadecatrienoic acid, ethyl ester (Z,Z,Z) were the major constituents, followed by hexadecanoic acid and 9, 12-octadecadienoic acid. It revealed that the material base of the antioxidant activity of PEE may be 9, 12, 15-octadecatrienoic acid and 9, 12, 15-octadecatrienoic acid, ethyl ester (Z,Z,Z), and PEE from A. theophrasti leaves might be explored as a new resource of natural antioxidants and applied in pharmaceutical, medicine, food, and chemical industries.
Additional information
Funding
References
- Fu, C. D.; Hong, Y. F. Research on Chemical Composition of Abutilon Theophrasti Medic. Foreign Medicine Science 1993, 15, 4–7.
- Gu, G. Y.; Jiang, Y. Research on Chemical Composition and Pharmacological Action of Abutilon Indicum and Abutilon. Modern Pharmacy Clinic 2009, 24, 338–340.
- Liu, H.; Ni, S. F.; Kang, J. H.; Luo, R. F.; Wu, Y. F.; Cui, Y. T.; Li, Z. X. The Pharmaceutical Research Situation of Plants of Abutilon. Northwest Pharmaceutical Journal 2010, 25, 68–69.
- Gaind, K. N.; Chopra, K. S. Phytochemical Investigation of Abutilon Indicum. Planta Medica 1976, 30, 174–185. DOI: 10.1055/s-0028-1097714.
- Sikorska, M.; Matlawska, I. Polyphenolic Compounds from Abutilon Grandiflorum Leaves. Acta Poloniae Pharmaceutica-Drug Research 2008, 65, 467–471.
- Tian, C. L.; Wang, M.; Sheng, C. H.; Zhao, C. J. Accuracy Mass Screening and Identification of Phenolic Compounds from the Five Parts of Abutilon Theophrasti Medic. By Reverse Phase High Performance Liquid Chromatography - Electrospray Ionization - Quadrupoles - Time of flight - Mass Spectrometry. Journal of Separation Science 2012, 35, 763–772. DOI: 10.1002/jssc.201100775.
- Aktumsek, A.; Zengin, G.; Guler, G. O.; Cakmak, Y. S.; Duran, A. Assessment of the Antioxidant Potential and Fatty Acid Composition of Four Centaurea L. Taxa from Turkey. Food Chemistry 2013, 141, 91–97. DOI: 10.1016/j.foodchem.2013.02.092.
- Okoth, D. A.; Chenia, H. Y.; Koorbanally, N. A. Antibacterial and Antioxidant Activities of Flavonoids from Lannea Alata (Engl.) Engl. (Anacardiaceae). Phytochemistry Letters 2013, 6, 476–481. DOI: 10.1016/j.phytol.2013.06.003.
- Lai, D. W.; Odimegwu, D. C.; Esimone, C.; Grunwald, T.; Proksch, P. Phenolic Compounds with in Vitro Activity against Respiratory Syncytial Virus from the Nigerian Lichen Ramalina Farinacea. Planta Medica 2013, 79, 1440–1446. DOI: 10.1055/s-0033-1350711.
- Sivasothy, Y.; Sulaiman, S. F.; Ooi, K. L.; Ibrahim, H.; Awang, K. Antioxidant and Antibacterial Activities of Flavonoids and Curcuminoids from Zingiber Spectabile Griff. Food Control 2013, 30, 714–720. DOI: 10.1016/j.foodcont.2012.09.012.
- Sufiana, A. S.; Ramasamya, K.; Ahmatb, N.; Zakariac, Z. A.; Yusofa, M. I. Isolation and Identification of Antibacterial and Cytotoxic Compounds from the Leaves of Muntingia Calabura L. Journal of Ethnopharmacology 2013, 146, 198–204. DOI: 10.1016/j.jep.2012.12.032.
- Villa-Rodríguez, J. A.; Molina-Corral, F. J.; Ayala-Zavala, J. F.; Olivas, G. I.; González-Aguilar, G. A. Effect of Maturity Stage on the Content of Fatty Acids and Antioxidant Activity Of‘Hass’ Avocado. Food Research International 2011, 44, 1231–1237. DOI: 10.1016/j.foodres.2010.11.012.
- Gai, Q. Y.; Jiao, J.; Mu, P. S.; Wang, W.; Luo, M.; Li, C. Y.; Zu, Y. G.; Wei, F. Y.; Fu, Y. J. Microwave-Assisted Aqueous Enzymatic Extraction of Oil from Isatis Indigotica Seeds and Its Evaluation of Physicochemical Properties, Fatty Acid Compositions and Antioxidant Activities. Industrial Crops and Products 2013, 45, 303–311. DOI: 10.1016/j.indcrop.2012.12.050.
- Lin, J. T.; Liu, S. C.; Hu, C. C.; Shyu, Y. S.; Hsu, C. Y.; Yang, D. J. Effects of Roasting Temperature and Duration on Fatty Acid Composition, Phenolic Composition, Maillard Reaction Degree and Antioxidant Attribute of Almond (Prunus Dulcis) Kernel. Food Chemistry 2016, 190, 520–528. DOI: 10.1016/j.foodchem.2015.06.004.
- Shinohara, H.; Taniguchi, K.; Kumazaki, M.; Yamada, N.; Ito, Y.; Otsuki, Y.; Uno, B.; Hayakawa, F.; Minami, Y.; Naoe, T.; et al. Anti-Cancer Fatty-Acid Derivative Induces Autophagic Cell Death through Modulation of PKM Isoform Expression Profile Mediated by Bcr-Abl in Chronic Myeloid Leukemia. Cancer. Letters 2015, 360, 1, 28–38.
- Yates, C. M.; Calder, P. C.; Rainger, G. Pharmacology and Therapeutics of Omega-3 Polyunsaturated Fatty Acids in Chronic Inflammatory Disease. Pharmacology & Therapeutics 2014, 141(3), 272–282. DOI: 10.1016/j.pharmthera.2013.10.010.
- Bas, D.; Boyaci, I. H. Modelling and Optimization. I. Usability of Response Surface Methodology. Journal of Food Engineering 2007, 78(3), 836–845. DOI: 10.1016/j.jfoodeng.2005.11.024.
- Krishnaiah, D.; Bono, A.; Sarbatly, R.; Nithyanandam, R.; Anisuzzaman, S. M. Optimisation of Spray Drying Operating Conditions of Morinda citrifoliaL. Fruit Extract Using Response Surface Methodology. Journal of King Saud University-Engineering Sciences 2015, 27, 26–36. DOI: 10.1016/j.jksues.2012.10.004.
- Zhang, L.; Tu, Z. C.; Wang, H.; Kou, Y.; Wen, Q. H.; Fu, Z. F.; Chang, H. X. Response Surface Optimization and Physicochemical Properties of Polysaccharides from Nelumbo Nucifera Leaves. International Journal of Biological Macromolecules 2015, 74, 103–110. DOI: 10.1016/j.ijbiomac.2014.11.020.
- Singh, V.; Pradhan, S. K. Optimization of WEDM Parameters Using Taguchi Technique and Response Surface Methodology in Machining of AISI D2 Steel. Procedia Engineering 2014, 97, 1597–1608. DOI: 10.1016/j.proeng.2014.12.310.
- Jiao, J.; Li, Z. G.; Gai, Q. Y.; Li, X. J.; Wei, F. Y.; Fu, Y. J.; Ma, W. Microwave-Assisted Aqueous Enzymatic Extraction of Oil from Pumpkin Seeds and Evaluation of Its Physicochemical Properties, Fatty Acid Compositions and Antioxidant Activities. Food Chemistry 2014, 147, 17–24. DOI: 10.1016/j.foodchem.2013.09.079.
- Brand-Williams, W.; Cuvelier, M. E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Science and Technology 1995, 28, 25–30. DOI: 10.1016/S0023-6438(95)80008-5.
- Ali, I. B. E. H.; Bahri, R.; Chaouachi, M.; Boussaïd, M.; Harzallah-Skhiri, F. Phenolic Content, Antioxidant and Allelopathic Activities of Various Extracts of Thymus Numidicus Poir. Organs. Industrial Crops and Products 2014, 62, 188–195. DOI: 10.1016/j.indcrop.2014.08.021.
- Benzie, I. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Analytical Biochemistry 1996, 239, 70–76. DOI: 10.1006/abio.1996.0292.
- Briones-Labarca, V.; Plaza-Morales, M.; Giovagnoli-Vicuña, C.; Jamett, F. High Hydrostatic Pressu Re and Ultrasound Extractions of Antioxidant Compounds, Sulforaphane and Fatty Acids from Chilean Papaya (Vasconcellea Pubescens) Seeds: Effects of Extraction Conditions and Methods. LWT-Food Science and Technology 2015, 60, 525–534. DOI: 10.1016/j.lwt.2014.07.057.
