 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Liposomes are used as effective nanodelivery devices to improve the physicochemical stability and biological efficacy of the encapsulated peptides and proteins. In this study, nanoliposome composite of lipoid S75-entrapped angiotensin I-converting enzyme (ACE)-inhibitory biopeptides was prepared by conventional (BLS75-CM) and direct heating (BLS75-DHM) methods. The nanoliposomes (BLS75-CM and BLS75-DHM) were stored at 4°C for 8 weeks and evaluated for physicochemical stability in terms of particle size, polydispersity index (pdi), zeta potential, and encapsulation efficiency (EE). These were also studied for residual ACE-inhibitory efficacy following their digestion under simulated gastrointestinal tract condition. The BLS75-CM was found to maintain higher physicochemical stability in terms of particle size, pdi, and zeta potential compared to BLS75-DHM. However, the BLS75-DHM indicated higher EE and efficacy with greater residual ACE-inhibitory activity of 47.37% compared to 44.18% and 36.84% that were obtained for the digested BLS75-CM and digested biopeptides without encapsulation, respectively. In vitro release study showed a cumulative biopeptides release of 66.41% and 69.00% from BLS75-CM and BLS75-DHM, respectively. The results of transmission electron microscopy showed spherical appearance of the nanoliposome capsules while Fourier Transform Infrared spectroscopy indicated the presence of ionic complexation and hydrogen bonds between the biopeptides and their phospholipid matrix.
Introduction
Enzymatic hydrolysis of food protein generates biologically active peptides of short amino acids sequences with diverse physiological benefits. [Citation1–Citation3] Bioactive peptides with strong antioxidants and angiotensin I-converting enzyme (ACE)-inhibitory activity have been produced from different food sources, [Citation4–Citation6] and can be incorporated into different food products to reduce the risk of certain life-threatening diseases like hypertension and diabetes or to supplement demand for patients diagnosed with specific clinical conditions including acute and chronic liver disease, pancreatitis, phenylketonuria, and ulcerative colitis. [Citation7] However, orally administered peptides exhibit low stability in the gastrointestinal tract (GIT) and can be easily degraded. This together with their low intestinal permeability may render them ineffective with low bioavailability and biological efficacy. [Citation8,Citation9] In addition, certain undesirable properties including bitter taste, reactivity with food matrix, and hygroscopicity may limit the incorporation of the biopeptides into functional foods for health benefit and commercial purposes.[Citation10,Citation11] Consequently, encapsulation using lipid-based delivery systems such as liposomes is employed as one of the alternative to overcome these challenges. [Citation11–Citation13] Liposome vesicles have been reported to serve as biocompatible and biodegradable carriers to enhance the biostability, functionality, cellular uptake, pharmacokinetic profile, bioaccessibility, bioavailability, efficacy, reduced toxicity, and controlled release of the encapsulated bioactive compound. [Citation14–Citation20]
Nano- and submicron-sized liposome vehicles have been reported to effectively protect encapsulated peptides against inactivation during gastrointestinal digestion and enhance their intestinal permeation and absorption for safe delivery to the target site. [Citation9] Consequently, nanoliposomes have been used to encapsulate protease-generated biopeptides.[Citation21–Citation25] The liposomes are suitably produced via conventional method (CM) and direct heating method (DHM) using food-based phospholipids (PLPs) that can potentially encapsulate both hydrophilic and hydrophobic or amphiphilic peptides.[Citation26,Citation27]
The biopeptides generated from stone fish protein were proven to be potent antioxidant and ACE-inhibitory molecules [Citation28,Citation29] and can be used as a source of soluble peptides for protein enrichment of food and beverages. However, their low stability against digestive enzymes and during storage together with an unpleasant odor may limit the direct use of stone fish biopeptides as bioingredients for food formulation. Although encapsulation of biopeptides in nanoliposomes can effectively improve their physicochemical stability, gastrointestinal stability, and biological efficacy, the nanoliposomes may be degraded upon storage for an extended period. The degradation of the nanoliposomes may vary with their preparation method and involve an increase in particle size, decrease in entrapment efficiency, and low colloidal stability as a result of their swelling, leakage of the entrapped bioactive agent, and aggregation. Hence, it is important to examine the nanoliposomes prepared by conventional and DHMs for physicochemical storage stability and for efficacy against simulated GIT digestion as measures of their ability to stabilize the loaded biopeptides. Thus, the present study was aimed to evaluate and compare the physicochemical stability and efficacy of stone fish-derived ACE-inhibitory biopeptides encapsulated in nanoliposomes produced by conventional and DHMs.
Materials and methods
Materials
The lipoid S75 used in this study was a kind gift from lipoid (GmbH, Germany); ACE from rabbit lung and hippuryl-histidyl-leucine (HHL) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Monobasic and dibasic potassium salts, bromelain, trypsin, pepsin, sodium hydroxide, and sodium chloride were purchased from Merck (Darmstadt, Germany). All other chemicals used were analytical grade and obtained from Fisher Scientific (Loughborough, UK) and Merck (Darmstadt, Germany).
Preparation of ACE-inhibitory biopeptides from stone fish
This was carried out as described by the method of Auwal et al.[Citation30] The freeze-dried and ground stone fish tissue was suspended in phosphate buffer (50 mM, pH 7) and dialyzed in a 12–14 kDa molecular mass cutoff dialysis tube for a total of 24 h (first for 4 h using deionized water followed by 20 h against 50 mM phosphate buffer pH 7). The mixture was incubated at 40°C and the hydrolysis was carried out using bromelain at 2% enzyme/substrate ratio and 150 rpm for 240 min in a water bath shaker. The reaction mixture was then boiled to 100°C for 10 min to inactivate the enzyme. The supernatant containing the ACE-inhibitory biopeptides with molecular weight (MW<10 kDa) was collected after centrifugation for 20 min at 4°C and 10,000 ×g. This was then freeze-dried and kept at −40°C until use.
Encapsulation of stone fish-derived ACE-inhibitory biopeptides
The lipoid S75-biopeptides nanocomposites were produced through conventional and DHMs.
Conventional method
The nanoliposomes were formed from lipoid S75 through CM as described by the method of da Rosa Zavareze et al.[Citation21] and da Silva Malheiros et al.[Citation31] with minor modifications. Briefly, PLPs were completely dissolved in a mixture of methanol:chloroform (1:9, v/v). The organic solvent was then removed by evaporation at 65°C in a rotary evaporator at 90 rpm to obtain a thin layer of dry lipid film in a round bottom flask. The methanol:chloroform traces were removed by placing the glass flask in a vacuum desiccator for 16 h at room temperature. The resulting residual lipid-film was dispersed in 200 mM phosphate buffer pH 7, containing 50 mg of freeze-dried biopeptides (MW <10 kDa) with (PLP:biopeptides mass ratio of 3:0.5) under agitation (150 rpm) at 60°C for 60 min. The dispersion was then homogenized at 8000 rpm for 30 min and sonicated at 40 Hz in an ice bath for another 10 min. A control capsule without the biopeptides sample was produced under the same encapsulation condition. The nanoliposomes were filtered through a 0.45-µm ministart® RC-15 syringe filter and allowed to stand for 60 min at room temperature for annealing under atmospheric nitrogen before storage at 4°C.
Direct heating method
The nanoliposomes were prepared by DHM according to the method of Thompson et al.[Citation32] using lipoid S75 with minor modifications. Lipoid S75 was directly hydrated with 10 mL of 3% (v/v) glycerol in 200 mM phosphate buffer, pH 7 containing biopeptides at a concentration of 5 mg/mL with PLP:biopeptides mass ratio of 3:0.5. The hydration was performed by heating the mixture in a six baffled glass vessel on a hot plate at 60°C and stirred at 150 rpm for 1 h. The suspension was then homogenized at 8000 rpm for 30 min followed by ice bath sonication at 40 Hz for 10 min. An empty nanoliposome was produced without the biopeptides sample under the same encapsulation condition to serve as control. After preparation, the nanoliposomes were filtered through a 0.45-µm ministart® RC-15 syringe filter and allowed to stand at room temperature under nitrogen atmosphere for 60 min to anneal and stabilize before storage at 4°C.
Physicochemical stability study
The nanoliposomes were stored at 4°C and their ability to retain the biopeptides was evaluated by studying the changes in their physicochemical stability (encapsulation efficiency (EE), particle size, polydispersity index (pdi), and zeta potential) once weekly for 8 weeks.
Encapsulation efficiency
The EE of the nanoliposomes was determined by an indirect method as previously described by da Rosa Zavareze et al.[Citation21] with minor modifications. Nanoliposome sample (500 µL) was initially transferred into a centrifuge tube and mixed with 1 mL of acetone. The mixture was centrifuged at 10,000 ×g for 20 min at 4°C, which separates into two phases since phosphatidylcholine is insoluble in the solvent. The supernatant containing the nonencapsulated biopeptides was collected and stored in an oven at 60°C until the solvent was completely evaporated. The dried precipitate of the nonencapsulated biopeptides was then dispersed in 5 mL of deionized water, and the biopeptides concentration was determined following modified Lowry method using Bicinchoninic Acid microprotein Assay kit, thus indirectly estimating the amount of nonencapsulated biopeptides which was dissolved in acetone. A 1 mL of 0.06% (v/v) Triton X-100 was then added to the precipitate of lipoid S75-biopeptides nanoliposome composite and homogenized by vortexing until the phosphatidylcholine was completely demulsified. The total biopeptides in the sample were then estimated as previously described. The amount of encapsulated biopeptides was determined as the difference between the weight of total and nonencapsulated biopeptides. The EE was calculated as the percentage encapsulated biopeptides in relation to their total weight.
EE (%) of the nanoliposome was calculated as follows:
where nonencapsulated biopeptides is the concentration of free biopeptides in the supernatant while total biopeptides is the concentration of biopeptides used in the formulation (nonencapsulated biopeptides + encapsulated biopeptides).
Particle size, polydispersity index, and zeta potential
Dynamic light scattering (DLS) with a Zetasizer Nano ZS Model (Malvern Instruments, Derbyshire, UK) was used to determine the mean particle size and pdi, a measure of degree of homogeneity of the nanoliposome suspension. The same Zetasizer Nano ZS instrument was used to measure the zeta potential of the nanoliposome based on the electrophoretic mobility of individual particles determined by laser Doppler velocimetry. Five replicate measurements were taken at 20°C and 90° angle after autocorrelation for 120 s.
In vitro release studies
The in vitro release of the biopeptides from nanoliposome was evaluated as previously described according to the method of Wang et al.[Citation33] with minor modifications. Approximately 20 mL of the nanoliposome containing the biopeptides was transferred in to a 12–14 kDa MW cutoff dialysis tube which was then immersed into a flask of suspending medium containing 200 mL of phosphate buffered saline pH 7.4, and 1% (v/v) tween 80 to maintain a sink condition for the dissolution of the biopeptides. The experiment was conducted in a water bath at 37°C and 250 rpm for 12 h. At a defined period, 1 mL of nanoliposome was withdrawn and the released biopeptides content was determined as the ratio of change in EE at a given time during the release study (EEt) to that at zero time (EE0) according to the following equation:
where EE0 is the initial EE of the biopeptides-loaded nanoliposomes (BLS75-CM and BLS75-DHM), EEt is the EE of the biopeptides-loaded nanoliposomes (BLS75-CM and BLS75-DHM) at a time “t” during sample withdrawal.
In vitro gastrointestinal digestion and residual ACE-inhibitory activity
The in vitro gastrointestinal digestion of the BLS75-CM, BLS75-DHM, and unencapsulated biopeptides was evaluated under simulated-gastric fluid (SGF) and simulated-intestinal fluid (SIF). Briefly, Both empty and biopeptides containing nanoliposomes were incubated under SGF and SIF as previously described by [Citation22,Citation34,Citation35] with minor modifications. The SGF consisted of 0.32 g of pepsin in a solution of 10 mL deionized water and 0.70 mL of 6 M HCl. Then, 0.20 g of NaCl was added and the final volume was made up to 100 mL with deionized water. Thereafter, the pH was adjusted to 1.20 with 6 M HCl. The SIF was prepared by dispersing 0.68 g of monobasic potassium phosphate salt in 20 mL of deionized water. Then, 7.7 mL of 0.20 M NaOH and 1 g of pancreatin were added, and the final volume was made to 100 mL with deionized water, and the pH to 6.80 with 0.50 M NaOH. A 6 mL acetone was added to 3 mL samples of BLS75-CM or BLS75-DHM suspensions. After centrifugation at 10,000 ×g and 4°C for 20 min, the supernatant layer of acetone containing free biopeptides was removed, and the precipitate was washed twice with deionized water and dried. This was then reacted with 1 mL of SGF and incubated at 37°C for 60 min with continuous shaking at 250 rpm in a water bath. Thereafter, the pH was adjusted to pH 6.80 and re-incubated for another 60 min with 1 mL SIF under the same condition. The mixture was boiled at 100°C for 10 min to inactivate the enzyme and cooled to room temperature. The residual ACE-inhibitory activity of the digested BLS75-CM or BLS75-DHM was then determined after demulsification and centrifugation of the reaction mixture. The unencapsulated biopeptides were also digested under the same simulated gastric and intestinal fluids.
Determination of residual ACE-inhibitory activity
ACE-inhibitory activity of the undigested and digested samples (unencapsulated biopeptides, BLS75-CM and BLS75-DHM) was determined according to the method of Jimsheena and Gowda[Citation36] with minor modifications. A 15 µL aliquot of the sample was mixed with 10 µL of ACE and 50 µL of 5 mM HHL solution and incubated at 37°C for 60 min. The reaction was then terminated with 75 µL of 1M HCl. Then, 150 µL of pyridine and 75 µL of benzene sulfonyl chloride were added followed by vortexing for 60 s. After cooling, a 200 µL of the mixture was added per well of a 96-well plate and read at 410 nm in a micro plate reader (Labomed, model UVD-2950, USA Power Wave X 340, Biotek Instruments. Inc.,Winooski, VT, USA).
where As is the hippuric acid absorbance when ACE, substrate, and biopeptides are present; Ac is the hippuric acid absorbance when ACE and substrate (HHL) are present, and Ab is the hippuric acid absorbance when only substrate (HHL) is present. The percentage ACE-inhibitory activity was calculated as the average of triplicate determinations.
Transmission electron microscopy (TEM)
Transmission electron microscopy (TEM) (Hitachi H-7100 TEM, Hitachi Ltd., Chiyoda, Japan) was used to elucidate the physical appearance of the nanoliposomal vesicles. A drop of the nanoliposome suspension prepared by appropriate dilution was added to a copper grid and dried for 3 min at room temperature. Uranyl acetate prepared at 1% concentration was applied to the grid for 90 s as the negative stain, followed by drying at room temperature and then viewed under TEM for the particle size measurement using an assisted electron micrographs built-in scale bar.
Fourier Transform Infrared (FTIR) spectroscopy
Infrared spectra of the capsules were obtained as an average of 16 scans at a spectral resolution of 4 cm−1 using Spectrum One (Fourier Transform Infrared (FTIR) spectroscopy with ATR, Perkin Elmer, Waltham, Massachusetts, USA) in the frequency region from 4000 to 400 cm−1 at room temperature. The frequency and intensity of the spectral peaks were compared to deduce the nature and strength of interaction between phosphatidylcholine and the encapsulated ACE-inhibitory biopeptides.
Statistical analysis
Minitab software version 16.0 was used for statistical analysis. One-way analysis of variance based on Tukey’s post-test was used for data interpretation and comparison between observations at 5% level of significance.
Results and discussion
The nanoliposomes containing stone fish-derived ACE-inhibitory biopeptides were prepared by CM and DHM and evaluated for physicochemical stability and TEM. The nanoliposomes suspension was then lyophilized and characterized using FTIR spectroscopy. The residual ACE-inhibitory activity was also determined to assess the efficacy of the biopeptides being released from the nanoliposome capsules subjected to in vitro gastrointestinal digestion under SGF and SIF, respectively.
Physicochemical stability of the nanoliposomes
The physicochemical stability of the nanoliposomes-entrapped ACE-inhibitory biopeptides was evaluated by measuring the changes in EE, particle size, pdi, and zeta potential once per week during the 8 weeks of storage at 4°C.
The results for the EEs of the nanoliposome-loaded biopeptides prepared by CM (BLS75-CM) and nanoliposome-loaded biopeptides prepared by DHM (BLS75-DHM) are shown in . EE determines the extent to which the biopeptides are incorporated into the liposome capsules. A high EE can lead to improved bioavailability and efficacy, thereby minimizing the cost of the biopeptides as potential therapeutic agents. However, leakages of the active agents due to overload or destruction of the capsule causes decreased EE. In addition, the heterogeneity nature of biopeptides affects their behavior and entrapment efficiency within the encapsulated system.[Citation11,Citation37] Thus, selection of suitable encapsulation technique is important to achieve high EE and to maintain the stability and biological activity of the encapsulated biopeptides. [Citation21]
Figure 1. Changes in the physicochemical properties of the Lipoid S75-biopeptides nanoliposome composite prepared by conventional method (BLS75-CM) and Lipoid S75-biopeptides nanoliposome composite prepared by direct heating method (BLS75-DHM) during the 8 weeks of storage at 4°C. Means that do not share a common letter are significantly different at p < 0.05. (a) Changes in encapsulation efficiencies of BLS75-CM and BLS75-DHM. (b) Changes in particle size of BLS75-CM and BLS75-DHM. (c) Changes in polydispersity index (pdi) of BLS75-CM and BLS75-DHM. (d) Changes in zeta potential of BLS75-DHM and BLS75-DHM.
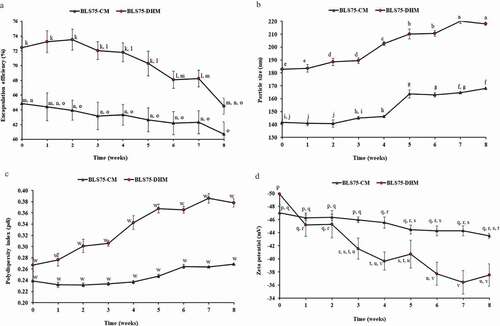
In this study, the EE for the freshly prepared BLS75-DHM and BLS75-CM was 72.45% and 64.86%, respectively. These values were found to be higher compared to the nearly; 28% for nanoliposome containing bioactive peptides from winged bean seeds,[Citation38] 54% for nanoliposome containing nisin antimicrobial peptide,[Citation39] and 40–60% for casein peptides-loaded liposomes.[Citation40,Citation41] The latter was linked to the extent of negative charge repulsion between the anionic residues of phosphopeptides in casein and that of PLPs in the liposomes.[Citation11] The low EE was also attributed to the leakage of the biopeptides as a result of disruption of the liposome membrane that might be due to prolonged homogenization and sonication time.[Citation38] However, high EE > 70% have been achieved for nanoliposome-loaded with peptides produced from collagen of sea bream scales [Citation24] and up to 80% for nanoliposome containing protein hydrolysates generated from whitemouth croaker (Micropogonias) muscle and byproduct.[Citation21] Recently, the EE ˃90% reported for nanoliposome-loaded with different MW whey peptide fractions was related to the surface hydrophobicity of the various peptides present.[Citation25] Although high EE has been observed for some biopeptides-loaded nanoliposomes, few studies were carried out to evaluate the release and residual ACE-inhibitory efficacy of the encapsulated biopeptides. [Citation42]
The higher EE obtained for the BLS75-DHM compared to the BLS75-CM demonstrated that their particle size and interactions with the interior surfaces of the liposome were less affected by the shear rate during the course of their fabrication. However, by the end of the 8 weeks storage period, the EE of both BLS75-DHM and BLS75-CM decreased to 64.51% and 60.72%, respectively, but were still significantly higher compared to the values previously reported.[Citation38–Citation41] The observed decrease in the EE was accompanied by an increase in the particle size and pdi following the storage of the BLS75-DHM and BLS75–CM which might have resulted from their mild aggregation and rupture with the leakage of the encapsulated biopeptides.
According to the method of da Rosa Zavareze et al.[Citation21], liposomes have been classified based on their size into small unilamellar (20–80 nm), large unilamellar (80 nm–1 µm), multilamellar (˃400 nm), and giant unilamellar vesicles (˃1 µm), respectively. The mean particle size of the nanoliposome capsules is dependent on factors such as stirring speed, stirring time, as well as the nature and concentration of the bioactive compound and its coating polymer.[Citation21,Citation43] In this study, the mean particle size obtained for the freshly prepared BLS75-CM (141.62 nm) and BLS75-DHM (182.78 nm) categorized them as large unilamellar with pdi values of 0.24 and 0.27, respectively (). The result agreed closely with the mean particle diameter reported for other nanoliposome-loaded with food protein generated biopeptides.[Citation21,Citation24,Citation25]
As shown in , both BLS75-CM (140.74–168.09 nm) and BLS75-DHM (182.78–218.14 nm) showed similar particle size distribution. A significant (p < 0.05) increase in the particle diameter was observed following the 8 weeks of storage at 4°C. However, the observed increase in particle size was not found to affect the colloidal stability of the nanoliposomes suspensions throughout the storage period as indicated by a high surface charge of ≥±30 mV for both BLS75-CM and BLS75-DHM. Moreover, the low pdi values of <0.5 that was obtained for the BLS75-CM and BLS75–DHM showed that their particles were still discrete and monodispersed with little or no aggregation after the 8 weeks of the storage period.
The zeta potential referred to the surface charge or electric potential and is used to predict the colloidal stability of particle suspension. A high value of zeta potential indicates strong repulsive interactions between anionic groups on the surfaces of liposomes which prevent their aggregation.[Citation44] A zeta potential value of ≥±30 mV indicates a good electrostatic stability of nanocapsules suspension. Similarly, nanoliposomes with low electrostatic stability of <−30 mV, [Citation21,Citation45] and those with high electrostatic stability of >−30 mV [Citation24,Citation25] have been reported. The variation in surface charge was attributed to the nature and purity of the coating material. [Citation25] In the present study, the high values obtained for the zeta potential of BLS75-CM and BLS75-DHM were found to vary in the range of −47.02 to −43.50 mV and −49.22 to −37.52 mV, respectively, during the 8 weeks of storage time (). The decrease in zeta potential corresponded to increase in particle size during the storage period. This observation is in line with the finding of Cui et al. [Citation46] and Hattori and Hashida [Citation47] who reported low zeta potential with increased particle size. Despite a significant change (p < 0.05) in the zeta potential of BLS75-CM and BLS75-DHM, the values of −43.50 and −37.52 mV measured after the eighth week of the storage were still adequate enough to maintain a good colloidal stability of the nanoliposome suspensions. Thus, the high zeta potential values of >−30 mV after the storage indicated high physical stability of both BLS75-CM and BLS75-DHM, respectively.
In vitro release
The results for the in vitro release profile of BLS75-CM and BLS75-DHM are shown in . A biphasic release was observed with an initial burst release of 30.60% and 34.56% for BLS75-CM and BLS75-DHM, respectively, within the first 60 min. This was followed by a sustained release profile up to 12 h of incubation (), with a total biopeptides release of BLS75-CM (66.41%) and BLS75-DHM (69.00%), respectively. The immediate burst release from both BLS75-CM and BLS75-DHM might be due to the biopeptides encapsulated within the outer layer of the nanoliposome capsules, while the slow and sustained release might be ascribed to the release of the biopeptides from the interior of the nanoliposomes.[Citation33,Citation48] The controlled release behavior of the biopeptides from BLS75-CM was not statistically different (p < 0.05) compared to that from BLS75-DHM under the same incubation condition for the 12 h being investigated, indicating similar release pattern and permeability of the biopeptides across the dialysis membrane.
Figure 2. Cumulative release profile of the Lipoid S75-biopeptides nanoliposome composite prepared by conventional method (BLS75-CM) and Lipoid S75-biopeptides nanoliposome composite prepared by direct heating method (BLS75-DHM).
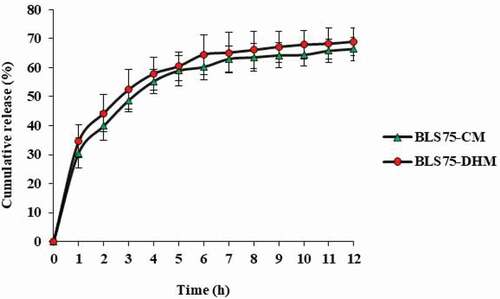
As previously reported, the solubility and release profile of an active agent was found to be depended on factors such as the PLP matrix, surfactant concentration, degree of agitation, and the release temperature. Increased solubility and burst release of itraconazole, an antifungal agent in aqueous phase, was attributed to the increase in temperature and surfactant concentration.[Citation49] Similarly, biphasic releases of active compounds from lipid-based nanocapsules have been reported. [Citation49–Citation51]
Transmission electron microscopy (TEM)
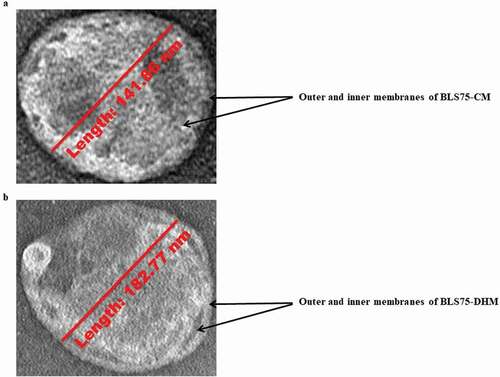
The morphology of the nanoliposomes was studied using TEM imaging which provides highly powerful magnification suitable for nanoparticles examination. As shown in , the TEM images for the nanoliposomes-loaded biopeptides are in form of discrete spherical particles with distinct PLP bilayer membrane, which segregated the biopeptides from the external surrounding and encapsulated them within the nanoliposomes. As previously mentioned, the capsules have been identified as large unilamellar by the DLS measurement with the particle size (80 nm–1 µm). This is supported by the TEM images of the nanoliposomes with the particle size of 141.86 and 182.77 nm for BLS75-CM and BLS75-DHM, respectively, as measured by a built-in scale bar ().
Figure 3. Transmission electron microscopy of the Lipoid S75-biopeptides nanoliposome composite prepared by conventional method (BLS75-CM) and Lipoid S75-biopeptides nanoliposome composite prepared by direct heating method (BLS75-DHM). (a) BLS75-CM nanoliposome with particle size of 141.86 nm measured by a built-in scale bar (b) BLS75-DHM nanoliposome with particle size of 182.77 nm measured by abuilt-in scale bar.
Biopeptides interaction with the Phospholipid (PLP) matrix
The possible mode of interactions between the biopeptides and their surrounding PLP matrix were evaluated by FTIR spectroscopy. The spectral bands of the empty liposomes prepared by CM (EL-CM), empty liposomes prepared by DHM (EL-DHM), nanoliposomes-biopeptides composites (BLS75-CM, BLS75-DHM), and their corresponding functional groups are shown in . The vibrational frequencies obtained depicted the presence of similar functional groups and interaction with PLP in both BLS75-CM and BLS75-DHM ().
Table 1. Wavenumber (n, cm−1) and functional groups of FTIR spectra for EL-CM, EL–DHM, BLS75-CM and BLS75-DHM.
Figure 4. FTIR spectra of the Lipoid S75-biopeptides nanoliposome composite prepared by conventional method (BLS75-CM) and Lipoid S75-biopeptides nanoliposome composite prepared by direct heating method (BLS75-DHM) and their control capsules. (a) empty/control nanoliposome capsule prepared by conventional method (EL-CM). (b) Lipoid S75-biopeptides nanoliposome composite capsule prepared by conventional method (BLS75-CM). (c) empty/control nanoliposome prepared by direct heating methods (EL-DHM). (d) Lipoid S75-biopeptides nanoliposome composite prepared by direct heating method (BLS75-DHM).
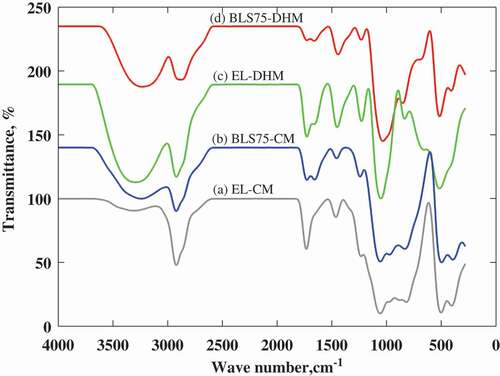
The identical peaks present in EL-CM and BLS75-CM at the wave numbers 2920 and 1057 cm−1 indicated no chemical interaction between biopeptides and the PLP matrix. A similar observation was made at a spectral peak of 515 cm−1 that is common to both BLS75-DHM and EL-DHM. Moreover, the occurrence of band shift with similar wave numbers in both BLS75-CM and BLS75-DHM indicated the existence of common interaction between their biopeptides contents and the surrounding PLP bilayers of liposomes.
The liposome is oriented so that the hydrophobic moieties are sequestered within the interior of the lipid bilayer while the hydrophilic polar groups lie in proximity to the aqueous core and external surface of the capsule. The asymmetric axial stretches of the different functional groups involved are then measured by FTIR.[Citation21]
The shift in the frequency band from 3310.11 cm−1 in EL-CM to 3241.69 cm−1 in BLS75–CM and from 3291.40 cm−1 in EL-DHM to 3232.93 cm−1 in BLS75-DHM represents vibrational stretch of OH, indicating that the biopeptides were oriented within the interior of the capsule and interacted via ionic complexation and hydrogen bond with their PLP matrix. The spectral shift accompanied by the change in wave number from 2920.89 cm−1 in EL-CM to 2921.76 and 1456.58 cm−1 in BLS75-CM and from 2920.70 cm−1 in EL-DHM to 2872.78 and 1443.22 cm−1 in BLS75-DHM was characterized by broadened and shortened peaks representing CH3 stretch. This demonstrated that the encapsulated biopeptides greatly influenced the structural assembly of the liposome capsule via hydrophobic interactions with nonpolar regions of the PLP.
Moreover, the reduced vibrational band due to C= O stretch was demonstrated by the shift from 1732.30 cm−1 in EL-CM to 1727.96 and 1451.26 cm−1 in BLS75-CM and then from 1727.65 and 1656.50 cm−1 in EL-DHM to 1660.12 cm−1 in BLS75-DHM. This showed interaction via hydrogen bond between the biopeptides and the carbonylated group of the PLP within the bilayer of the capsule. The shifted band from 1462.84 cm−1 in EL-CM to 1241.56 cm−1 in BLS75-CM and from 1228.39 cm−1 in EL-DHM to 1231.31 cm−1 in BLS75-DHM indicated C-O stretch, demonstrating more interactions between the polar amino acids side chains of biopeptides and their surrounding PLP bilayer of the liposome matrix. The vibrational stretch of the polar choline (N+CH3) group was observed due to the shift in band from 818.44 cm−1 in EL-CM to 832.74 cm−1 in BLS75-CM and from 834.70 cm−1 in EL-DHM to 851.57 cm−1 in BLS75-DHM, respectively, suggesting more polar interactions with the entrapped biopeptides. As previously reported by Kycia et al.,[Citation52] the symmetric stretch of C–N in [νs(CN+(CH3)3)] showed vibration bend between 860 and 930 cm−1. The νs(N(CH3)3) was characterized by bands at ∼860 and ∼900 cm−1 with gauche conformation for O–C–C–N group of the choline.
Increase in the vibrational frequency of PO2 indicates dehydrated phosphate group due to the absence of hydrogen bond between it and hydrogen atom in water molecule while its decrease commensurate with the presence of the hydrogen bond and hydrated state of the phosphate group.[Citation21,Citation53] A vibrational shift of phosphate band from 1057.67 cm−1 in EL-CM to 1057.07 cm−1 in BLS75-CM and from 1051.60 cm−1 in EL-DHM to 1034.44 cm−1 in BLS75-DHM is accompanied by decrease in the spectral peak intensity and indicated polar interaction between the PO2 group of phosphatidylcholine and the polar side chains of the biopeptides in the interior of the capsule. Thus, the FTIR analysis revealed that the biopeptides were confined to both polar and non-polar region of the nanoliposomes and mainly associated to the PLP matrix through ionic complexation and hydrogen bond with the OH, PO2, and C=O polar groups and hydrophobic interactions with the nonpolar acyl chains of the phosphatidylcholine.
In vitro gastrointestinal digestion and residual ACE-inhibitory efficacy
The unencapsulated/free biopeptides, BLS75-CM, and BLS75-DHM were evaluated for stability against SGF and SIF. The digested nanoliposomal capsules were demulsified to release their biopeptides contents. The residual ACE-inhibitory efficacy of the undigested and digested samples was then determined and compared. shows the ACE-inhibitory activity of undigested samples (unencapsulated biopeptides, BLS75-CM, and BLS75-DHM) to be 69.56%, 32.07%, and 33.45%, respectively. In vitro gastrointestinal digestion was found to decrease the residual ACE-inhibitory activity of the digested unencapsulated biopeptides to 36.84% while the residual ACE-inhibitory activity from the digested BLS75-CM and BLS75-DHM was found to increase to 44.18% and 47.37%, respectively. Thus, the biopeptides released from digested BLS75-DHM showed a higher residual ACE-inhibitory effect of 47.37% compared to 44.18% from the digested BLS75-CM while the digested free biopeptides without encapsulation showed the least residual ACE-inhibitory activity of 36.84%. Hence, encapsulation in nanoliposome greatly improved the stability and efficacy of the ACE-inhibitory biopeptides against digestive enzymes. Similarly, increase in the residual ACE-inhibitory activity of peanut peptides-loaded nanoliposome following simulated gastrointestinal digestion has been observed.[Citation42]
Figure 5. ACE-inhibitory activities of Lipoid S75-biopeptides nanoliposome composite prepared by conventional method (BLS75-CM), Lipoid S75-biopeptides nanoliposome composite prepared by direct heating method (BLS75-DHM) and unencapsulated (free biopeptides) before and after digestion under simulated gastrointestinal fluids (SGF and SIF). Means that do not share a common letter are significantly different (p < 0.05).
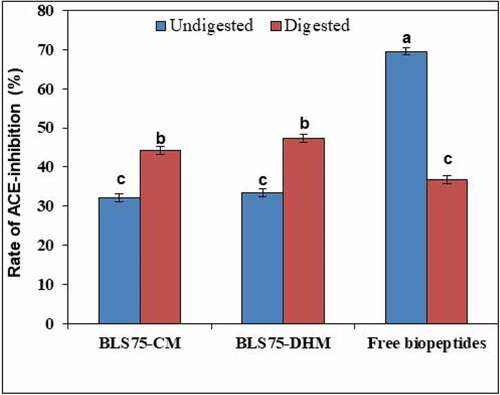
As previously reported, simulated GIT digestion led to decreased ACE-inhibitory activity of susceptible peptides.[Citation54] In contrast, some peptides were shown to be resistant to digestion by gastrointestinal proteases. In some cases, simulated GIT digestion was found to hydrolyze several peptides into shorter sequences with exaggerated activity.[Citation55]
Conclusion
Stone fish biopeptides with strong inhibitory effects on ACE showed susceptibility to degradation and inactivation under gastrointestinal (GIT) digestion. The nanoliposome-entrapped ACE-inhibitory biopeptides prepared by CM (BLS75-CM) showed higher physical stability as measured by the changes in particle size, pdi, and zeta potential following 8 weeks of storage at 4°C compared to the nanoliposome- entrapped ACE-inhibitory biopeptides prepared by DHM (BLS75-DHM). However, BLS75-DHM exhibited higher entrapment efficiency and residual ACE-inhibitory activity while the digested free biopeptides without encapsulation showed the least residual ACE-inhibitory activity after simulated GIT digestion. In vitro release indicated higher cumulative release of the biopeptides from BLS75-DHM (69.00%) compared to BLS75-CM (66.41%). The results of FTIR analysis showed that the biopeptides were entrapped within the nanoliposome capsules and interacted with the PLP matrix via ionic and hydrogen bonds. Thus, liposomes prepared from lipoid S75 can be used as effective carriers to improve the physicochemical stability, bioavailability, and efficacy of food biopeptides as potential therapeutic ingredients for incorporation into functional foods.
Highlights
Nanoliposomes improved the physiochemical stability of the encapsulated biopeptides.
Nanoliposomes formed by CM showed higher physical stability.
Nanoliposomes prepared by direct heating method exerted higher ACE-inhibitory efficacy.
Acknowledgments
The authors are thankful for the funding support under the project No. 02-01-04-SF 2309 by the grant from the Malaysian Ministry of Science, Technology, and Innovation.
Disclosure statement
All the authors declare that they have no conflict of interest.
Additional information
Funding
References
- Hartmann, R.; Meisel, H. Food-Derived Peptides with Biological Activity: From Research to Food Applications. Current Opinion in Biotechnology 2007, 18, 163–169. DOI: 10.1016/j.copbio.2007.01.013.
- Korhonen, H.; Pihlanto, A. Food-Derived Bioactive Peptides–Opportunities for Designing Future Foods. Current Pharmaceutical Design 2003, 9, 1297–1308. DOI: 10.2174/1381612033454892.
- Rutherfurd-Markwick, K. J.;. Food Proteins as a Source of Bioactive Peptides with Diverse Functions. British Journal of Nutrition 2012, 108, S149–157. DOI: 10.1017/S000711451200027X.
- Zarei, M.; Forghani, B.; Ebrahimpour, A.; Abdul-Hamid, A.; Anwar, F.; Saari, N. In Vitro and in Vivo Antihypertensive Activity of Palm Kernel Cake Protein Hydrolysates: Sequencing and Characterization of Potent Bioactive. Industrial Crops and Products 2015, 76, 112–120. DOI: 10.1016/j.indcrop.2015.06.040.
- Samaranayaka, A. G. P.; Li-Chan, E. C. Y. Food-Derived Peptidic Antioxidants: A Review of Their Production, Assessment, and Potential Applications. Journal of Functional Foods 2011, 3, 229–254. DOI: 10.1016/j.jff.2011.05.006.
- Yea, C. S.; Ebrahimpour, A.; Hamid, A. A.; Bakar, J.; Muhammad, K.; Saari, N. Winged Bean [Psophorcarpus tetragonolobus (L.) DC] Seeds as an Underutilised Plant Source of Bifunctional Proteolysate and Biopeptides. Food & Function 2014, 5, 1007–1016. DOI: 10.1039/c3fo60667h.
- Clemente, A.;. Enzymatic Protein Hydrolysates in Human Nutrition Enzymatic Protein Hydrolysates in Human Nutrition. Trends in Food Science & Technology 2000, 11, 254–262. DOI: 10.1016/S0924-2244(01)00007-3.
- Lee, V. H. L.; Yamamoto, A. Penetration and Enzymatic Barriers to Peptide and Protein Absorption. Advanced Drug Delivery Reviews 1989, 4, 171–207. DOI: 10.1016/0169-409X(89)90018-5.
- Renukuntla, J.; Vadlapudi, A. D.; Patel, A.; Boddu, S.; Mitra, A. K. Approaches for Enhancing Oral Bioavailability of Peptides and Proteins. International Journal of Pharmaceutics 2013, 447, 75–93. DOI: 10.1016/j.ijpharm.2013.02.030.
- Molina Ortiz, S. E.; Mauri, A.; Monterrey-Quintero, E. S.; Trindade, M. A.; Santana, A. S.; Favaro-Trindade, C. S. Production and Properties of Casein Hydrolysate Microencapsulated by Spray Drying with Soybean Protein Isolate. LWT - Food Science and Technology 2009, 42, 919–923. DOI: 10.1016/j.lwt.2008.12.004.
- Mohan, A.; Rajendran, S. R. C. K.; He, Q. S.; Bazinet, L.; Udenigwe, C. C. Encapsulation of Food Protein Hydrolysates and Peptides: A Review. RSC Advances 2015, 5, 79270–79278.
- Trevaskis, N. L.; Charman, W. N.; Porter, C. J. H. Lipid-Based Delivery Systems and Intestinal Lymphatic Drug Transport: A Mechanistic Update. Advanced Drug Delivery Reviews 2008, 60, 702–716. DOI: 10.1016/j.addr.2007.09.007.
- Augustin, M. A.; Sanguansri, L. Challenges and Solutions to Incorporation of Nutraceuticals in Foods. Annual Review of Food Science and Technology 2014, 6, 463–477. DOI: 10.1146/annurev-food-022814-015507.
- Li, T.; Yang, S.; Liu, W.; Liu, C.; Liu, W.; Zheng, H.; Zhou, W.; Tong, G. Preparation and Characterization of Nanoscale Complex Liposomes Containing Medium-Chain Fatty Acids and Vitamin C. International Journal of Food Properties 2015, 18, 113–124. DOI: 10.1080/10942912.2012.685683.
- Toniazzo, T.; Peres, M. S.; Ramos, A. P.; Pinho, S. C. Encapsulation of Quercetin in Liposomes by Ethanol Injection and Physicochemical Characterization of Dispersions and Lyophilized Vesicles. Food Bioscience 2017, 19, 17–25. DOI: 10.1016/j.fbio.2017.05.003.
- Takahashi, M.; Kitamoto, D.; Imura, T.; Oku, H.; Takara, K.; Wada, K. Characterization and Bioavailability of Liposomes Containing a Ukon Extract. Bioscience, Biotechnology and Biochemistry 2008, 72, 1199–1205. DOI: 10.1271/bbb.70659.
- Vauthier, C.; Labarre, D. Modular Biomimetic Drug Delivery Systems. Journal of Drug Delivery Science and Technology 2008, 18, 59–68. DOI: 10.1016/S1773-2247(08)50008-6.
- Solaro, R.; Chiellini, F.; Battisti, A. Targeted Delivery of Protein Drugs by Nanocarriers. Materials 2010, 3, 1928–1980. DOI: 10.3390/ma3031928.
- Thompson, A. K.; Couchoud, A.; Singh, H. Comparison of Hydrophobic and Hydrophilic Encapsulation Using Liposomes Prepared from Milk Fat Globule-Derived Phospholipids and Soya Phospholipids. Dairy Science & Technology 2009, 89, 99–113. DOI: 10.1051/dst/2008036.
- Chen, X.; Zou, L. Q.; Niu, J.; Liu, W.; Peng, S. F.; Liu, C. M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. DOI: 10.3390/molecules200814293.
- Da Rosa Zavareze, E.; Telles, A. C.; Mello El Halal, S. L.; Da Rocha, M.; Colussi, R.; Marques De Assis, L.; Suita De Castro, L. A.; Guerra Dias, A. R.; Prentice-Hernández, C. Production and Characterization of Encapsulated Antioxidative Protein Hydrolysates from Whitemouth Croaker (Micropogonias furnieri) Muscle and Byproduct. LWT - Food Science and Technology 2014, 59, 841–848. DOI: 10.1016/j.lwt.2014.05.013.
- Li, Z.; Paulson, A. T.; Gill, T. A. Encapsulation of Bioactive Salmon Protein Hydrolysates with Chitosan-Coated Liposomes. Journal of Functional Foods 2015, 19, 733–743. DOI: 10.1016/j.jff.2015.09.058.
- Mosquera, M.; Giménez, B.; Montero, P.; Gómez-Guillén, M. C. Incorporation of Liposomes Containing Squid Tunic ACE-inhibitory Peptides into Fish Gelatin. Journal of the Science of Food and Agriculture 2016, 96, 769–776. DOI: 10.1002/jsfa.7145.
- Mosquera, M.; Giménez, B.; Da Silva, I. M.; Boelter, J. F.; Montero, P.; Gómez-Guillén, M. C.; Brandelli, A. Nanoencapsulation of an Active Peptidic Fraction from Sea Bream Scales Collagen. Food Chemistry 2014, 156, 144–150. DOI: 10.1016/j.foodchem.2014.02.011.
- Mohan, A.; McClements, D. J.; Udenigwe, C. C. Encapsulation of Bioactive Whey Peptides in Soy Lecithin-Derived Nanoliposomes: Influence of Peptide Molecular Weight. Food Chemistry 2016, 213, 143–148. DOI: 10.1016/j.foodchem.2016.06.075.
- Mufamadi, M. S.; Pillay, V.; Choonara, Y. E.; Du Toit, L. C.; Modi, G.; Naidoo, D.; Ndesendo, V. M. K., A Review on Composite Liposomal Technologies for Specialized Drug Delivery. Journal of Drug Delivery 2011, 2011, 19 pages. DOI: 10.1155/2011/939851.
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. International Journal of Nanomedicine 2015, 10, 975–999. DOI: 10.2147/IJN.
- Auwal, S. M.; Zarei, M.; Abdul-Hamid, A.; Saari, N., Response Surface Optimisation for the Production of Antioxidant Hydrolysates from Stone Fish Protein Using Bromelain. Evidence-Based Complementary and Alternative Medicine 2017, 2017, 1–10. DOI: 10.1155/2017/4765463.
- Auwal, S. M.; Zarei, M.; Abdul-Hamid, A.; Saari, N. Optimization of Bromelain-Aided Production of Angiotensin I-Converting Enzyme Inhibitory Hydrolysates from Stone Fish Using Response Surface Methodology. Marine Drugs 2017, 15, 104. DOI: 10.3390/md15040104.
- Auwal, S. M.; Zarei, M.; Ping, Tan, C.; Basri, M.; Saari, N. Improved in Vivo Efficacy of Anti-Hypertensive Biopeptides Encapsulated in Chitosan Nanoparticles Fabricated by Ionotropic Gelation on Spontaneously Hypertensive Rats. Nanomaterials 2017, 7, 421. DOI: 10.3390/nano7120458.
- Da Silva Malheiros, P.; Micheletto, Y. M. S.; Da Silveira, N. P.; Brandelli, A. Development and Characterization of Phosphatidylcholine Nanovesicles Containing the Antimicrobial Peptide Nisin. Food Research International 2010, 43, 1198–1203. DOI: 10.1016/j.foodres.2010.02.015.
- Thompson, A. K.; Mozafari, M. R.; Singh, H. The Properties of Liposomes Produced from Milk Fat Globule Membrane Material Using Different Techniques. Le Lait 2007, 87, 349–360. DOI: 10.1051/lait:2007013.
- Wang, M.; Zhao, T.; Liu, Y.; Wang, Q.; Xing, S.; Li, L.; Wang, L.; Liu, L.; Gao, D. Ursolic Acid Liposomes with Chitosan Modification: Promising Antitumor Drug Delivery and Efficacy. Materials Science and Engineering: C 2017, 71, 1231–1240. DOI: 10.1016/j.msec.2016.11.014.
- Agrawal, A. K.; Harde, H.; Thanki, K.; Jain, S. Improved Stability and Antidiabetic Potential of Insulin Containing Folic Acid Functionalized Polymer Stabilized Multilayered Liposomes following Oral Administration. Biomacromolecules 2014, 15, 350–360. DOI: 10.1021/bm401580k.
- Jain, S.; Patil, S. R.; Swarnakar, N. K.; Agrawal, A. K. Oral Delivery of Doxorubicin Using Novel Polyelectrolyte-Stabilized Liposomes (Layersomes). Molecular Pharmaceutics 2012, 9, 2626–2635. DOI: 10.1021/mp300202c.
- Jimsheena, V. K.; Gowda, L. R. Colorimetric, High-Throughput Assay for Screening Angiotensin I-Converting Enzyme Inhibitors. Analytical Chemistry 2009, 81, 9388–9394. DOI: 10.1021/ac901775h.
- McClements, D. J. Nanoparticle- and Microparticle-Based Delivery Systems, 1st; CRC Press Taylor & Francis: New York, 2015; Vol. 1
- Yea, C. S.; Kiat, T. W.; Saari, N. Preparation and Characterisation of Nanoliposomes Containing Winged Bean Seeds Bioactive Peptides. Journal of Microencapsulation 2015, 1–8. DOI:10.3109/02652048.2015.1057250.
- Were, L. M.; Bruce, B.; Davidson, P. M.; Weiss, J. Encapsulation of Nisin and Lysozyme in Liposomes Enhances Efficacy against Listeria Monocytogenes. Journal of Food Protection 2004, 67, 922–927. DOI: 10.4315/0362-028X-67.5.922.
- Morais, H. A.; Barbosa, C.; Delvivo, F. M.; Mansur, H. S.; De Oliveira, M. C.; Silvestre, M. P. C. Comparative Study of Microencapsulation of Casein Hydrolysates M Lipospheres and Liposomes. Journal of Food Biochemistry 2004, 28, 21–41. DOI: 10.1111/j.1745-4514.2004.tb00053.x.
- Yokota, D.; Moraes, M.; Pinho, S. C. Characterization of Lyophilized Liposomes Produced with Non-Purified Soy Lecithin : A Case Study of Casein Hydrolysate Microencapsulation. Brazilian Journal of Chemical Engineering 2012, 29, 325–335. DOI: 10.1590/S0104-66322012000200013.
- Gong, K.-J.; Shi, A.; Liu, H.; Liu, L.; Hu, H.; Yang, Y.; Adhikari, B.; Wang, Q., Preparation of Nanoliposome Loaded with Peanut Peptide Fraction: Stability and Bioavailability. Food & Function 2016, 7, 2034–2042. DOI: 10.1039/C5FO01612F.
- Moinard-Chécot, D.; Chevalier, Y.; Briançon, S.; Beney, L.; Fessi, H. Mechanism of Nanocapsules Formation by the Emulsion-Diffusion Process. Journal of Colloid and Interface Science 2008, 317, 458–468. DOI: 10.1016/j.jcis.2007.09.081.
- Da Silva Malheiros, P.; Sant’Anna, V.; Micheletto, Y. M. S.; Da Silveira, N. P.; Brandelli, A. Nanovesicle Encapsulation of Antimicrobial Peptide P34: Physicochemical Characterization and Mode of Action on Listeria monocytogenes. Journal of Nanoparticle Research 2011, 13, 3545–3552. DOI: 10.1007/s11051-011-0278-2.
- Taylor, T. M.; Gaysinsky, S.; Davidson, P. M.; Bruce, B. D.; Weiss, J. Characterization of Antimicrobial-Bearing Liposomes by ζ-potential, Vesicle Size, and Encapsulation Efficiency. Food Biophysics 2007, 2, 1–9. DOI: 10.1007/s11483-007-9023-x.
- Cui, J.; Li, C.; Guo, W.; Li, Y.; Wang, C.; Zhang, L.; Zhang, L.; Hao, Y.; Wang, Y. Direct Comparison of Two Pegylated Liposomal Doxorubicin Formulations: Is AUC Predictive for Toxicity and Efficacy? Journalof Controlled Release 2007, 118, 204–215. DOI: 10.1016/j.jconrel.2006.12.002.
- Hattori, Y.; Hashida, M. Evaluation of Size and Zeta Potential of DNA/Carrier Complexes. In Non-Viral Gene Therapy: Gene Design and Delivery; Taira, K., Kataoka, K., Niidome, T., Eds.; Springer-Verlag: Tokyo, 2005; p. 293–299.
- Panwar, P.; Pandey, B.; Lakhera, P. C.; Singh, K. P. Preparation, Characterization, and in Vitro Release Study of Albendazole-Encapsulated Nanosize Liposomes. International. Journal of Nanomedicine 2010, 5, 101–108. DOI: 10.2217/nnm.10.2.
- Lim, W. M.; Rajinikanth, P. S.; Mallikarjun, C.; Kang, Y. B. Formulation and Delivery of Itraconazole to the Brain Using a Nanolipid Carrier System. International Journal of Nanomedicine 2014, 9, 2117–2126. DOI: 10.2147/IJN.
- Yoon, H. Y.; Kwak, S. S.; Jang, M. H.; Kang, M. H.; Sung, S. W.; Kim, C. H.; Kim, S. R.; Yeom, D. W.; Kang, M. J.; Choi, Y. W. Docetaxel-Loaded RIPL Peptide (Iplvvplrrrrrrrrc)-Conjugated Liposomes: Drug Release, Cytotoxicity, and Antitumor Efficacy. International Journal of Pharmaceutics 2017, 523, 229–237. DOI: 10.1016/j.ijpharm.2017.03.045.
- Hao, W.; Xia, T.; Shang, Y.; Xu, S.; Liu, H. Characterization and Release Kinetics of Liposomes Inserted by pH-responsive Bola-Polymer. Colloid and Polymer Science 2016, 294, 1107–1116. DOI: 10.1007/s00396-016-3871-1.
- Kycia, A. H.; Su, Z.; Christa, L.; Lipkowski, J.; Brosseau, C. L.; Lipkowski, J. In Situ PM–IRRAS Studies of Biomimetic Membranes Supported at Gold Electrode Surfaces. Vibrational Spectroscopy at Electrified Interfaces 2013, 345–417.
- Toyran, N.; Severcan, F. Competitive Effect of Vitamin D2 and Ca2+ on Phospholipid Model Membranes: An FTIR Study. Chemistry and Physics of Lipids 2003, 123, 165–176. DOI: 10.1016/S0009-3084(02)00194-9.
- Hernández-Ledesma, B.; Miguel, M.; Amigo, L.; Aleixandre, M. A.; Recio, I. Effect of Simulated Gastrointestinal Digestion on the Antihypertensive Properties of Synthetic Beta-Lactoglobulin Peptide Sequences. Journal of Dairy Research 2007, 74, 336–339. DOI: 10.1017/S0022029907002609.
- Jao, C.-L.; Huang, S.-L.; Hsu, K.-C. Angiotensin I-Converting Enzyme Inhibitory Peptides: Inhibition Mode, Bioavailability, and Antihypertensive Effects. Biomedicine / [Publiee Pour l’A.A.I.C.I.G.] 2012, 2, 130–136. DOI: 10.1016/j.biomed.2012.06.005.
