 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
To investigate the effects of pre-oxidized myofibrillar protein (MP) on stability of heat-induced MP gelation throughout 28 days of refrigeration, a hydroxyl radical generating system (10 μM FeCl3, 0.1 mM ascorbic acid, with 10 mM H2O2) was employed. Results demonstrated that an increase of carbonyl content followed a similar trend in both pre-oxidized and non-oxidized protein gels. Unexpectedly, pre-oxidized protein gels yielded increased (P < 0.05) hardness at 14 days of storage compared to the initial day of storage. The water holding capacity (WHC) had a significant positive correlation with gel hardness, percentage of immobile water, and T22, while having a negative correlation with carbonyl group content and T23 (P < 0.01). These results suggest that the pre-oxidation treatment increases the susceptibility of MP gel matrices to undergo oxidation and thus provides a better comprehension of the consequences that in vitro pre-oxidation treatments have on protein matrix systems.
Introduction
Meat product qualities, such as textural characteristics and product yields, are largely dependent on the molecular interactions from components such as myofibrillar proteins (MP), which biophysical and biochemical interactions can be influenced by numerous disturbances.[Citation1] Food processing can play a major role in this disturbance of MP integrity. Some of these changes can be traced by to the oxidation of the amino acid moieties constituting MP.[Citation2] The accumulation of such changes can impact on the surrounding microstructural environments. Consequently, isolated MP provides useful models for investigating the mechanisms of altered functionalities, especially the water holding capacity (WHC) of meat products during storage.[Citation3]
Protein oxidation can be determined by the modification of protein structure through a decrease of tryptophan fluorescence, peptide scission, and the formation of intra- and inter- molecular crosslinks, which result in the loss of protein functionality.[Citation4–Citation7] The loss of such functionalities can result in increased water losses, protein fragmentation or aggregation, decreased solubility, and weaker protein gel formation.[Citation8] Debatably, several studies have indicated that mild-to-moderate oxidation can actually promote the gelation of MP.[Citation9] Carbonyl formation has been emphasized as one of the most significant modifications in oxidized proteins.[Citation6] Protein carbonylation refers to the formation of carbonyl moieties from alkaline amino acids (lysine, arginine and/or proline) due to reactions resulting from oxidative stress. This process is irreversible and non-enzymatic.[Citation10]
The preservation of foods through cold refrigeration has historically been the most common method for the maintenance of meat products, but unfortunately it eventually leads to undesirable biochemical changes. It has been previously reported that the Fenton-system, which is normally employed as a means to generate reactive oxygen species (ROS) to induce carbonylation and other covalent chain links, can produce modifications similar to what one could expect from refrigerated muscle.[Citation11] This system accomplishes the generation of ROS through a hydroxyl radical-generating system (HRGS), which utilizes a combination of metal ions with ascorbic acid and hydrogen peroxide.[Citation12–Citation14] These generated ROS, such as hydroxyl radicals, perpetuate oxidation reactions by abstracting a hydrogen atom from adjacent amino acid side chains present in the given system.[Citation15] After the amino acid residues have been subjected to oxidative reactions, their positions within protein structures are markedly affected.[Citation16] Hence, we hypothesize that the degradation of functionality of pre-oxidized and non-oxidized MP gels as a result of refrigeration will be different.
Many studies have provided information on the oxidative modifications occurring in meat proteins during the storage of meat.[Citation17,Citation18] However, although the oxidative-stress has been widely used for the modification of protein functionality, the oxidative stability of the protein gels prepared by such modified protein has not been widely investigated. Therefore, the objectives of this study were to examine how oxidative modifications could 1) change the oxidative stability of MP gels, and 2) affect the gelation and water distribution properties of MP gels during refrigeration storage.
Materials and methods
Chemicals and materials
Eight Wuhua three-yellow chicken (Gallus gallus domesticus, male) were purchased from a local farm (Wenshi Livestock and Poultry Co. Ltd., Nanjing, China) and subsequently slaughtered in a humane manner according to the guidelines of care and animal use from the Jiangsu Provincial Academy of Agricultural Sciences and the Laboratory of Animals at Nanjing Agriculture University (Nanjing, China) to minimize pain and the number of birds utilized. The license number for the farm was SCXK (Su) 2002–0029. After slaughter, the breast muscle (musculus pectoralis major) was immediately removed and cooled on ice. The tissue was then sealed in plastic bags and stored at 0 ~ 4°C for 24 h until further processing. Bovine serum albumin (BSA) was purchased from Sigma Chemical Co. (St. Louis, MO, USA), while other chemicals were of analytical grade.
Myofibrillar protein extraction
Myofibrillar proteins (MP) were extracted according to the method described before,[Citation19] with slight modifications. Briefly, the raw tissue was chopped in a pre-cooled meat-mincer (Waring blender, GM 200, Retsch, Germany) at a speed of 4,000 rpm for 20 s. The breast mince was washed thrice with 6 x (w/v) of phosphate buffer solution (PBS) buffer (0.1 M NaCl, 2 mM MgCl2, 1 mM EGTA, 10 mM Na2HPO4/NaH2PO4, pH 7.0), and then homogenized (T 25 digital, IKA Ltd, Germany) for 30 s at 10,000 rpm before centrifugation (Beckman Coulter model Avanti J-26SXP, Beckman Instruments Inc., USA) at 3,000 × g for 15 min. The pellet was then washed three times with 6 × 0.1 M NaCl using the aforementioned procedure. Before the last centrifugation, the suspension was filtered through four layers of cheesecloth and the pH was adjusted to 6.0 with aliquots of 0.1 M HCl. The final MP pellets were kept in tightly capped bottles and stored at 4°C before further use (within 5 h). The protein concentration of the extracted MP was measured using the Biuret method[Citation20] with BSA as standard.
Protein oxidation
The obtained MP was diluted with 15 mM PIPES buffer (0.6 M NaCl, pH 6.0) to attain a final protein concentration of 20 mg/mL. Then, MP suspensions were pre-oxidized with HRGS (0.01 mM FeCl3, 0.1 mM ascorbic acid, 10 mM H2O2) at 4°C, for 24 h. To stop the reaction, 1 mM (final concentration) of EDTA was added into the system. After 20 min, 4 x (V/V) 20 mM PBS (0.1 M NaCl, pH 7.0) was added prior to centrifugation (Beckman Coulter model Avanti J-26SXP, Beckman Instruments Inc., USA) at 2,000 × g for 15 min. The PBS washing and centrifugation procedure was replicated and the precipitate was collected as the pre-oxidized protein groups. A non-oxidized MP suspension was treated without the addition of the HRGS but only EDTA as the control. Protein concentrations were measured by the Biuret method and the final content of NaCl was adjusted to 0.6 M. All proteins were used within 8 h. Each measurement was performed in triplicate.
Experimental design and gelation preparation
The MP samples of 9 mL (for determination of texture and carbonyl content), 4 mL (for determination of microstructure), 1.5 mL (for determination of WHC) and 2 mL (for determination of Low-field NMR) were transferred to 10 mL beakers, 5 mL beakers, 2 mL capped plastic centrifuge tubes and NMR tubes (40 mm × 13 mm, height × diameter), respectively. Except that the samples of 5 mL beakers had fifteen, others were all thirty. The beakers and tubes were then incubated in a water bath (ZKSY-600, Keer Co.Ltd, Nanjing, Jiangsu Province, China) for 20 min at 20°C. These samples were then heated from 20°C to 90°C with a heating rate set at 1.5 °C/min and was subsequently kept at 90°C for 20 min. Samples were cooled at room temperature (25°C), then randomly assigned to 5 groups and stored at 4°C for future use. All assessments were conducted at specific sampling times in order to determine stability of MP gels during refrigeration at 0, 7, 14, 21 and 28 days.
Carbonyl content
The carbonyl contents of heat-induced gels were measured using the procedure described by Levine et al. and Xing et al.,[Citation21,Citation22] with slight modifications. Diluted gels (10 mg/mL protein concentration) with 20 mM PBS (0.6 M NaCl, pH 6.5) were homogenized (T 18 digital, IKA Ltd, Germany) and reacted with 10 mM DNPH at room temperature for 1 h (about 25°C), and that with 2 M HCl and 10 mM DNPH served as controls. The carbonyl content was measured by a microplate reader (Shimadzu, Japan) and calculated at the peak absorbance of 370 nm using an absorption coefficient of 22,000 M−1 cm−1. The protein content was determined by reading the absorbance at 280 nm with BSA as standard. The carbonyl contents were expressed as μmol/g protein.
Water holding capacity (WHC)
The WHC was measured according to a previously established procedure.[Citation23] Heat-induced MP gels were centrifuged at 10,000 × g for 10 min at 4°C. The following formula was used to determine WHC:
Note: ML is the amount (g) of moisture lost from the gels after centrifugation while CG is the initial weight (g) of gels.
Instrumental textural analyses
Gels were penetrated with a flat-faced stainless steel probe (5 mm diameter) attached to a Model TAXT plus texture analyzer (Stable Micro Systems Ltd., TA .XT .plus, U.K.) at a test speed of 0.3 mm/s. Pre- and post-test speeds were controlled at 1.00 mm/s. The strain of the compression was set at 50% with a trigger force of 4 g.
Microstructure
Gel microstructure was examined through scanning electron microscopy (SEM) according to the method of Han et al. with slight modifications.[Citation24] Briefly, gels from the 5 mL glass beakers were fixed in 2.5% glutaraldehyde in 0.1 M PBS (pH 7.3) for 24 h at 4°C. These samples were then cut into 3 mm × 3 mm × 2 mm pieces and further diluted by 0.1 M PBS (pH 7.2) three times. Shortly thereafter, samples were dehydrated using incremental ethanol solutions and then transferred into tertiary butyl. Each sample was subsequently freeze-dried and coated with gold to achieve a 10 nm thickness. Samples were observed on a scanning electron microscope (S-3000N, Hitachi, Tokyo, Japan) with the accelerating voltage of 15 kV and a magnification of 3000 × . Three images were obtained for each sample.
Low-field NMR
A low-field 1H NMR analysis of the relaxation times for protein gels was conducted according to a previously established method.[Citation25] Samples were then equilibrated and kept at room temperature (25°C) before measurements. All sample analyses were conducted on a Niumag Benchtop Pulsed NMR analyzer (MicroMR; Niumag Electric Corporation, Shanghai, China). Transverse relaxations (T2) were conducted using the Carr-Purcell-Meiboom-Gill (CPMG) sequences. During measurements, the operation temperature was at 32°C while the resonance frequency was 22.48 MHz with a repetition-time of 11 ms and echo-time of 400 μs. Eight scans were performed for each measurement and a total of 18,000 echoes were collected and fitted with the program MultiExp Inv Analysis (Niumag Electric Corporation, Shanghai, China). Relaxation times (T2b, T21, T22 and T23) and their corresponding water populations (P2b, P21, P22 and P23) were recorded. Each sample was replicated 6 times.
Statistical analyses
Each experiments was repeated three times (n = 3). The three batches of myofibrillar proteins were prepared on different occasions. Reported results represent an average of each experiment assay. Statistical analyses were conducted using SPSS statistics 20.0 (IBM, Armonk, NY, USA). The effects of storage time on protein gelation properties were studied through a general linear model procedure. The differences between least-squares means were determined using an LSD test (P < 0.05). Student’s t-tests were used to study the effects of oxidation treatment on gelation properties. Relationships between parameters were calculated using Pearson’s correlation coefficients. Significance was inferred when the results were within the 95% confidence level (P < 0.05).
Results and discussion
Carbonyl content
Carbonyl content measurements (DNPH assay) revealed an increase in carbonylation during the storage of MP gels. As shown in , protein gels prepared from the oxidatively stressed MPs had higher susceptibility to oxidation during refrigeration (to 21 storage days), when compared to non-oxidized samples, as seen by the greater rate of carbonyl formation. For the pre-oxidized and non-oxidized groups, the carbonyl contents increased from 2.07 and 1.73 nmol/mg, at 0 days, to 3.73 and 2.69 nmol/mg, at 21 days, respectively. However, from 21 days to 28 days, the carbonyl content of pre-oxidized protein gels exhibited no significant difference (P > 0.05), indicating relatively less carbonylation among protein gels at the final refrigeration storage stage or a balance between protein carbonylation and involvement of pre-formed carbonyls in further reactions.
Figure 1. Effects of in-vitro protein oxidization on carbonyl content of heat-induced protein gels during 28 days refrigeration. Note: Control, without oxidative treated samples; Oxidized, pre-oxidative treated samples. Different letters (a, b) are significantly different (P < 0.05) for the same treatment group among difference time, different letters (A, B) are significantly different (P < 0.05) for the same time; Values were presented as means ± standard deviation (n = 3).
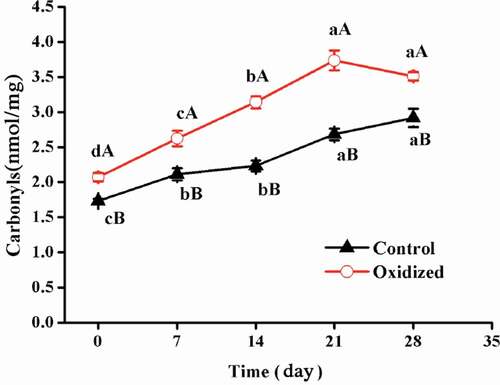
The carbonyls were plausibly formed by: 1) direct oxidation of the side chains of amino acids such as lysine, arginine, and proline; 2) secondary carbonylation intermediates, such as threonine oxidized to amino-3-ketobuyric acid, and 3) oxidative peptide scission events.[Citation26,Citation27] It has previously been reported that the oxidative deamination of amino acid side chains is the primary means of the formation of carbonyl groups in meat proteins, among ferric/ascorbate ROS generating systems.[Citation28] Previous studies report that freezing meat prior to its processing could potentially deteriorate oxidative stability.[Citation29,Citation30] One plausible explanation for this phenomenon relies on the formation of ice crystals during the freezing of meat; the formed ice crystals damage the meat tissues in concurrence with proteolysis. Our results demonstrate that protein pre-oxidation largely impacted the qualities of products during the latter stages of storage. One explanation for these results is that protein carbonyls have the potential to maintain the integrity of highly reactive moieties and might be involved in advanced reactions. However, the specific mechanisms behind this observation require further elucidation.
Textural profile analyses
The relationships between in vitro oxidation treatment and protein functionality, such as protein solubility and aggregation, have previously been widely investigated.[Citation5,Citation31] However, the exact effects of in vitro oxidation treatments on protein gelation properties remain largely misunderstood with little exploration into the combined effects of storage and oxidation. As shown in ), throughout storage, the non-oxidized gel samples were significantly (P < 0.05) harder than those pre-oxidized. A reason for this increased rigidity might be due to additional intermolecular cross-linking, such as that through disulfide bonds, which could decrease gel strength by interfering with a uniform gel network formation, thus further reducing the integrity of the gel network.[Citation31,Citation32]
Figure 2. Effects of in-vitro protein oxidization on gel hardness (A) and WHC (B) of heat-induced gel during 28 days refrigeration. Note: Control, without oxidative treated samples; Oxidized, pre-oxidative treated samples. Different letters (a, b) are significantly different (P < 0.05) for the same treatment group among difference time, different letters (A, B) are significantly different (P < 0.05) for the same time; Values were presented as means ± standard deviation (n = 3).
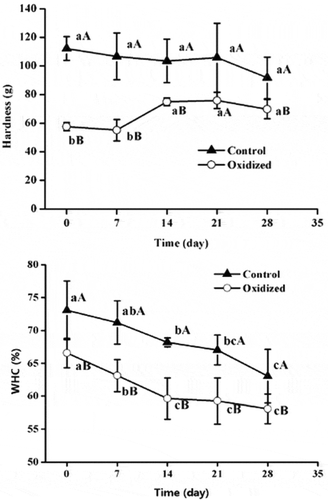
No significant (P > 0.05) effects of storage time on the rigidity of non-oxidized protein gels were found. However, the hardness of pre-oxidized protein gels did increase from 57.6 g, at 0 day of storage, to 75.0 g at 14 days of storage, and then remained constant. Soyer et al. demonstrated [Citation18] that higher protein oxidation resulted in higher hardness values of cooked meat. Improved gelling and emulsifying properties were also observed on mildly oxidized muscle proteins.[Citation33] However, oxidative modifications were considered to lead to polymerization and any massive aggregation might cause deleterious effects in muscle foods.[Citation6] The crosslinks produced by oxidation in meat systems (both Schiff bases and disulphide bonds) have also been considered as a plausible mechanism responsible for the hardening of tough meats.[Citation34]
Water holding capacity (WHC)
The effects of oxidative stress on WHC are presented in ). Oxidation treatment and subsequent refrigeration of MP gels led to significant water purging, which was indicated by the decrease of WHC in MP gel matrices. During the 28-day storage period, the WHC of pre-oxidized proteins and non-oxidized decreased significantly (P < 0.01). Compared to the non-oxidized samples, pre-oxidized samples showed decreased WHC (P < 0.05). These findings were similar to previous reports.[Citation31] It was also noticed that the decreased rate of pre-oxidized samples (decreased 11.64%) was significantly (P < 0.05) higher than the non-oxidized group (decreased 7.16%), which indicates that oxidative stability deteriorated with pre-oxidative stress treatments.
Possible reasons for the impaired WHC are as follows. Firstly, the covalent interactions between and within proteins, such as disulfide and dityrosine bonds, could promote the decrease of WHC.[Citation35] Secondly, oxidative changes could have the capacity to induce carbonylation, carboxylation, and the formation of Schiff bases, which impair the WHC of muscle proteins.[Citation5] It has previously been demonstrated that protein oxidation can adversely impact the WHC of proteins.[Citation36] For protein gels, WHC relies on both protein-water interactions and capillary-force through protein-protein crosslinks. Increased protein crosslinks could reduce water retention interspaces and thus decrease WHC. Recently, intermediary protein oxidation, through compounds like hydrazones, has also been implicated to hamper the WHC of proteins.[Citation5]
Microstructure
Protein gel textures are closely linked to their microstructures. The microstructures of pre-oxidized and non-oxidized MP gels were observed by SEM images and shown in . Compared to pre-oxidized samples, non-oxidized proteins formed a more continuous protein network with more uniform and compact structures. It was clear at 7 days of storage that oxidative treatment had led to porous structures, indicating shrinkage of the gel matrix. With increased storage time, some protein cross-links were broken, resulting in the presence of large pores. At the final stage of storage, larger cracks within the gel networks were evident (: e, f, i and h). These resulting voids then have the potential to form channels that allowed for the expulsion of the previously capillary-stabilized water. This could explain the loss of WHC in pre-oxidized samples ()). Additionally, oxidative stress could promote an increased protein-protein interaction resulting in large protein aggregates with adverse impacts on ordered protein matrices. Feng et al. reached a similar conclusion whereby it was suggested that oxidation treatment leads to severe protein aggregation and a loose gel matrix. [Citation7]
Figure 3. SEM microstructure of heat-induced protein gel subjected to in-vitro protein oxidation during 28 days refrigeration. Note: 0–4 were 0, 7, 14, 21 and 28 days, respectively. C was the scanning electron micrograph of Control samples, T was the scanning electron micrograph of oxidization samples.
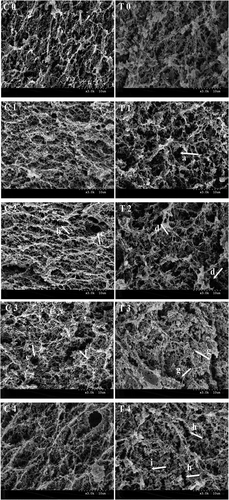
Figure 4. Effects of in-vitro protein oxidization on water distribution of chicken myofibrillar protein heat-induced gel (A: relaxation time; B: proportion of T22, C: proportion of T23) during 28 days refrigeration. Note: Control, without oxidative treated samples; Oxidized, pre-oxidative treated samples, Values were presented as means ± standard deviation (n = 3).
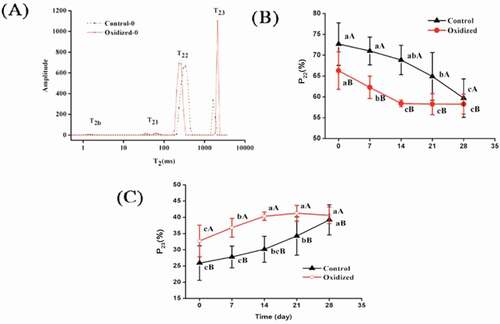
For pre-oxidized protein gels, at 14 days of storage time, some re-established fibrous connections were observed, possibly from newly formed interactions. Such newly formed interactions have previously been described by Lund et al. attributed to dityrosine and disulphide bonds.[Citation37] These covalent bonds formation leaded to protein-protein interaction and shrinkage of the interspace between proteins, which leaded to protein aggregation and thus further extruded immobilized water within protein gels. Hence, it is implied that oxidation treatment could affect water retention ability and water distribution by modifying the stability of protein gel matrices.
Water distribution
Water distribution of gels was measured through the use of low-field NMR, which provides information about diffusive domains in protein gel samples. Curve-fitted distribution results are represented as four water populations: T2b, T21, T22, T23. Among these, T2b (< 10 ms) corresponds to the water components that exist in macromolecular structures; T21 (10–100 ms) is assigned to water tightly associated with protein molecules; T22 (100–500 ms) refers to immobilized water located within the MP network; and T23 (1200–2800 ms) represents free water, located outside myofibrillar networks.[Citation24,Citation38] Typically, slower relaxation times suggest a less limited mobility of protein protons due to tighter gel structure.[Citation24]
As seen in , oxidation treatments and the storage process showed no significant influence for T2b. For T21 of the pre-oxidized protein gel samples, the highest value of 74.33 ms did not occur until 14 days of storage only to then be reduced gradually. It was noticed that T22 of the pre-oxidized groups continuously decreased throughout the whole storage period, and was significant lower (P < 0.05) than the non-oxidized samples at 0–21 days of storage time, thus indicating a more restricted immobilized water population. Compared to the non-oxidized samples, protein gels subjected to the oxidation treatment had higher T23 values throughout the whole storage period, indicating a larger mobility of free water molecules, thus explaining the impaired water retention ability. Noticeably, this adverse impact of oxidative stress decreased with longer storage time.
Table 1. Effect of protein oxidization on stability of heat-induced gel water distribution T2.
The P2 values indicated the water distribution proportions amongst these fractions.[Citation38] The effects of oxidation treatment on dominant water distribution proportions, as represented by P22 and P23, are shown in . During 28 days of storage, the P22 of the pre-oxidized protein gels was significantly lower (P < 0.05) than that of non-oxidized samples, indicating that the oxidation treatment had a strong interference on the interaction between proteins and water, as expected. Oxidative stresses accelerated the decreasing rate of P22 of protein gels within 14 days of storage (decreased 11.86%), while the non-oxidized groups only decreased by 5.27%. Along with P22 reduction, P23 values were all increased significantly (P < 0.05). We therefore conclude that some immobilized water had been transformed into free water and subsequently increased water loss throughout storage, especially in the pre-oxidized groups. Noticeably, for pre-oxidized samples, P22 and P23 both did not change significantly (P > 0.05) after 14 days of storage, implying that weak oxidation induced by refrigeration storage had no obvious effect on water immigration.
Relationship between carbonyl content, gelation properties, and water distribution
In order to assess the relationship between protein oxidation and protein gelation qualities with water distribution properties, Pearson’s correlation coefficients were calculated (). As reported,[Citation3,Citation15] significant and negative correlations have also been found between the amount of protein carbonyls and WHC. Unexpectedly, textural hardness showed no significant correlations with carbonyl content, indicating that protein carbonyl groups could not be used as indicators of textural deterioration of MP gels. Relatively, T2b and P2b were less correlated with gelation properties and carbonyl formation (). Meanwhile, WHC had a significant positive correlation with P22, and a significant negative correlation with P23. These results illustrate that protein gels having higher P22 and lower P23, and could be seen through the dynamic conversion from restrictedly immobilized water to free water. Shao et al. [Citation38] and Han et al. [Citation25] have previously reached similar conclusions about LF-NMR and WHC relationships. We confirmed that protein textural hardness and WHC are significantly correlated, due to the strong and compact gel structure contributing to network uniformity, leading to improved WHC.
Table 2. Correlation among variables in study of the effect of protein oxidization on heat-induced gel storage stability.
Conclusion
The present study demonstrates the significance of in vitro oxidized proteins on the stability of heat-induced MP gels throughout a 28 days refrigeration period at 4°C. We conclude that exposure of MP to an OH- generating system results in an enhanced susceptibility of heat-induced MP gels to undergo increased crosslink formation, leading to undesirable traits in gel-type products such as reduced WHC and impaired gel structures. Evidence from microstructural images implies that oxidation-induced changes to the integrity of matrix porous spaces, along with the restricted water extrusion, reduced the capability of MP gels to hold water. From the water distribution results both T22 and T23, as well as corresponding water distribution proportions P22 and P23, significantly correlated with carbonyl contents and WHC. These results reveal that the water distribution of protein gels subjected to oxidative stress changed during the first 14 days while maintaining consistency for the next 14 days of storage. Thus, it is concluded that gel-type products prepared from oxidatively damaged MP proteins will deteriorate faster than non-oxidized proteins.
Acknowledgments
This research was supported by the Fundamental Research Funds for the Central Universities (KYZ201543), the National Natural Science Foundation of China (No. 31571854, 31501503) and the China Agriculture Research System (CARS-41) funded by the Chinese Ministry of Agriculture and the postgraduate Research & Practice Innovation Program of Jiangsu Province.
References
- Zhang, Z.; Yang, Y.; Zhou, P.; Zhang, X.; Wang, J. Effects of High Pressure Modification on Conformation and Gelation Properties of Myofibrillar Protein. Food Chemistry 2017, 217, 678–686. DOI: 10.1016/j.foodchem.2016.09.040.
- Cao, Y. G.; Xiong, Y. L. Chlorogenic Acid-Mediated Gel Formation of Oxidatively Stressed Myofibrillar Protein. Food Chemistry 2015, 180, 235–243. DOI: 10.1016/j.foodchem.2015.02.036.
- Liu, Z.; Xiong, Y. L.; Chen, J. Protein Oxidation Enhances Hydration but Suppresses Water-Holding Capacity in Porcine Longissimus Muscle. Journal of Agricultural & Food Chemistry 2010, 58(19), 10697–10704. DOI: 10.1021/jf102043k.
- Lund, M. N.; Luxford, C.; Skibsted, L. H.; Davies, M. J. Oxidation of Myosin by Heme Proteins Generates Myosin Radicals and Protein Cross-Links. Biochemical Journal 2008, 410, 565–574. DOI: 10.1042/BJ20071107.
- Utrera, M.; Parra, V.; Estévez, M. Protein Oxidation during Frozen Storage and Subsequent Processing of Different Beef Muscles. Meat Science 2014, 96, 812–820. DOI: 10.1016/j.meatsci.2013.09.006.
- Xiong, Y. L.; Protein Oxidation and Implications for Muscle Foods Quality. In Antioxidants in Muscle Foods; Decker, E. A., Faustman, C., Lopez-Bote, C. J., Eds..; Wiley: New York, 2000. pp 85–111.
- Feng, X.; Li, C.; Ullah, N.; Hackman, R. M.; Chen, L.; Zhou, G. Potential Biomarker of Myofibrillar Protein Oxidation in Raw and Cooked Ham: 3-Nitrotyrosine Formed by Nitrosation. Journal of Agricultural & Food Chemistry 2015, 63(51), 10957–10964. DOI: 10.1021/acs.jafc.5b04107.
- Dai, Y.; Lu, Y.; Wu, W.; Lu, X. M.; Han, Z. P.; Liu, Y.; Li, X. M.; Dai, R. T. Changes in Oxidation, Color and Texture Deteriorations during Refrigerated Storage of Ohmically and Water Bath-Cooked Pork Meat. Innovative Food Science & Emerging Technologies 2014, 26, 341–346. DOI: 10.1016/j.ifset.2014.06.009.
- Liu, G.; Xiong, Y. L.; Butterfield, D. A. Chemical, Physical, and Gel-Forming Properties of Oxidized Myofibrils and Whey- and Soy-Protein Isolates. Journal of Food Science 2000, 65(5), 811–818. DOI: 10.1111/jfds.2000.65.issue-5.
- Berlett, B. S.; Stadtman, E. R. Protein Oxidation in Aging, Disease, and Oxidative Stress. Journal of Biological Chemistry 1997, 272, 33, 20313–20316.
- Srinivasan, S.; Hultin, H. O. Chemical, Physical, and Functional Properties of Cod Proteins Modified by a Nonenzymic Free-Radical-Generating System. Journal of Agricultural & Food Chemistry 1997, 45(2), 310–320. DOI: 10.1021/jf960367g.
- Cao, Y. G.; Ai, N.; True, A. D.; Xiong, Y. L. Effects of (–)-Epigallocatechin-3-Gallate Incorporation on the Physicochemical and Oxidative Stability of Myofibrillar Protein–Soybean Oil Emulsions. Food Chemistry 2017. DOI: 10.1016/j.foodchem.2017.10.111.
- Decker, E. A.; Xiong, Y. L.; Calvert, J. T.; Crum, A. D.; Blanchard, S. P. Chemical, Physical, and Functional Properties of Oxidized Turkey White Muscle Myofibrillar Proteins. Journal of Agricultural & Food Chemistry 1993, 41(2), 186–189. DOI: 10.1021/jf00026a007.
- Feng, X.; Chen, L.; Lei, N.; Wang, S.; Xu, X. L.; Zhou, G.; Li, Z. Emulsifying Properties of Oxidatively Stressed Myofibrillar Protein Emulsion Gels Prepared with (-)-Epigallocatechin-3-Gallate and NaCl. Journal of Agricultural & Food Chemistry 2017, 65(13), 2816–2826. DOI: 10.1021/acs.jafc.6b05517.
- Utrera, M.; Estévez, M. Oxidation of Myofibrillar Proteins and Impaired Functionality: Underlying Mechanisms of the Carbonylation Pathway. Journal of Agricultural & Food Chemistry 2012, 60(32), 8002–8011. DOI: 10.1021/jf302111j.
- Estévez, M.;. Protein Carbonyls in Meat Systems: A Review. Meat Science 2011, 89(3), 259–279. DOI: 10.1016/j.meatsci.2011.04.025.
- Eymard, S.; Jacobsen, C.; Baron, C. P. Assessment of Washing with Antioxidant on the Oxidative Stability of Fatty Fish Mince during Processing and Storage. Journal of Agricultural & Food Chemistry 2010, 58(10), 6182–6189. DOI: 10.1021/jf904013k.
- Soyer, A.; ÖZalp, B.; Dalmış, Ü.; Bilgin, V. Effects of Freezing Temperature and Duration of Frozen Storage on Lipid and Protein Oxidation in Chicken Meat. Food Chemistry 2010, 120(4), 1025–1030. DOI: 10.1016/j.foodchem.2009.11.042.
- Thawornchinsombut, S.; Park, J. W. Frozen Stability of Fish Protein Isolate under Various Storage Conditions. Journal of Food Science 2006, 71(3), C227–C232. DOI: 10.1111/j.1365-2621.2006.tb15622.x.
- Gornall, A. G.; Bardawill, C. J.; David, M. M. Determination of Serum Proteins by Means of Biuret Reaction. Journal of Biological Chemistry 1949, 177, 2, 751–766.
- Levine, R. L.; Garland, D.; Oliver, C. N.; Amici, A.; Climent, I.; Lenz, A. G.; Stadtman, E. R. Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods In Enzymology 1990, 186, 464–478.
- Xing, T.; Zhao, X.; Wang, P.; Chen, H.; Xu, X. L.; Zhou, G. H. Different Oxidative Status and Expression of Calcium Channel Components in Stress-Induced Dysfunctional Chicken Muscle. Journal of Animal Science 2017, 95(4), 1565–1573. DOI: 10.2527/jas.2016.0868.
- Li, K.; Kang, Z. L.; Zhao, Y. Y.; Xu, X. L.; Zhou, G. H. Use of High-Intensity Ultrasound to Improve Functional Properties of Batter Suspensions Prepared from PSE-like Chicken Breast Meat. Food & Bioprocess Technology 2014, 7(12), 3466–3477. DOI: 10.1007/s11947-014-1358-y.
- Han, M. Y.; Zhang, Y.; Fei, Y.; Xu, X. L.; Zhou, G. H. Effect of Microbial Transglutaminase on NMR Relaxometry and Microstructure of Pork Myofibrillar Protein Gel. European Food Research & Technology 2009, 228(4), 665–670. DOI: 10.1007/s00217-008-0976-x.
- Han, M. Y.; Wang, P.; Xu, X. L.; Zhou, G. H. Low-Field NMR Study of Heat-Induced Gelation of Pork Myofibrillar Proteins and Its Relationship with Microstructural Characteristics. Food Research International 2014, 62, 1175–1182. DOI: 10.1016/j.foodres.2014.05.062.
- Rysman, T.; Hecke, T. V.; Poucke, C., . P.; Smet, S. D.; Royen, G. V. Protein Oxidation and Proteolysis during Storage and in Vitro Digestion of Pork and Beef Patties. Food Chemistry 2016, 209, 177–184. DOI: 10.1016/j.foodchem.2016.04.027.
- Taborsky, G.; Oxidative Modification of Proteins in the Presence of Ferrous Ion and Air. Effect of Ionic Constituents of the Reaction Medium on the Nature of the Oxidation Products. Biochemistry 1973, 12, 7, 1341–1348.
- Xiong, Y. L.; Decker, E. A. Alterations of Muscle Protein Functionality by Oxidative and Antioxidative Processes. Journal of Muscle Foods 1995, 6(2), 139–160. DOI: 10.1111/jmf.1995.6.issue-2.
- Lorido, L.; Ventanas, S.; Akcan, T.; Estévez, M. Effect of Protein Oxidation on the Impaired Quality of Dry-Cured Loins Produced from Frozen Pork Meat. Food Chemistry 2016, 196, 1310. DOI: 10.1016/j.foodchem.2015.10.092.
- Utrera, M.; Morcuende, D.; Estévez, M. Temperature of Frozen Storage Affects the Nature and Consequences of Protein Oxidation in Beef Patties. Meat Science 2014, 96(3), 1250–1257. DOI: 10.1016/j.meatsci.2013.10.032.
- Li, C. Q.; Xiong, Y. L.; Chen, J. Protein Oxidation at Different Salt Concentrations Affects the Cross‐Linking and Gelation of Pork Myofibrillar Protein Catalyzed by Microbial Transglutaminase. Journal of Food Science 2013, 78(6), C823–C831. DOI: 10.1111/1750-3841.12027.
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Prodpran, T.; Tanaka, M. Characterization of Edible Films from Skin Gelatin of Brownstripe Red Snapper and Bigeye Snapper. Food Hydrocolloids 2006, 20(4), 492–501. DOI: 10.1016/j.foodhyd.2005.04.007.
- Srinivasan, S.; Hultin, H. O. Chemical, Physical, and Functional Properties of Cod Proteins Modified by a Nonenzymic Free-Radical-Generating System. Journal Agricultural and Food Chemistry 1997, 45(2), 310–320. DOI: 10.1021/jf960367g.
- Soladoye, O. P.; Juárez, M. L.; Aalhus, J. L.; Shand, P.; Estévez, M. Protein Oxidation in Processed Meat: Mechanisms and Potential Implications on Human Health. Comprehensive Reviews in Food Science & Food Safety 2015, 14(2), 106–122. DOI: 10.1111/crf3.2015.14.issue-2.
- Xiong, Y. L.; Park, D.; Ooizumi, T. Variation in the Cross-Linking Pattern of Porcine Myofibrillar Protein Exposed to Three Oxidative Environments. Journal of Agricultural & Food Chemistry 2009, 57(1), 153–159. DOI: 10.1021/jf8024453.
- Bao, Y.; Boeren, S.; Ertbjerg, P. Myofibrillar Protein Oxidation Affects Filament Charges, Aggregation and Water-Holding. Meat Science 2018, 135, 102–108. DOI: 10.1016/j.meatsci.2017.09.011.
- Lund, M. N.; Heinonen, M.; Baron, C. P.; Estévez, M. Protein Oxidation in Muscle Foods: A Review. Molecular Nutrition & Food Research 2011, 55(1), 83–95. DOI: 10.1002/mnfr.201000453.
- Shao, J. H.; Deng, Y. M.; Jia, N.; Li, R. R.; Cao, J. X.; Liu, D. Y.; Li, J. R. Low-Field NMR Determination of Water Distribution in Meat Batters with NaCl and Polyphosphate Addition. Food Chemistry 2016, 200, 308–314. DOI: 10.1016/j.foodchem.2016.01.013.
