ABSTRACT
A new technique for the rapid detection and analysis of triterpenic compounds in apple extracts using HPLC was developed and validated. The main advantage of this technique is the short duration of the analysis – this makes this technique superior to others currently applied for the routine HPLC analysis of triterpenic compounds. The developed, optimized, and validated technique was used for the evaluation of triterpenic compounds in samples of different cultivars of apples, their peels, and flesh. In total, four triterpenic compounds were isolated and identified. Ursolic acid was the dominant compound in all the tested apple samples. The highest amounts of triterpenic compounds were detected in the peels of the ‘Lodel’ apple cultivar, and thus apples of this cultivar may be potentially useful for the isolation of individual compounds and the production of functional food and dietary supplements.
Introduction
Apples are among the most commonly consumed fruit in the world.[Citation1] They are widely used in food industry for the manufacturing of various products and drinks (e.g. juice, wine, or cider), and are also used unprocessed.[Citation2] In human diet, apples are an important source of different groups of biological active compounds that can positively contribute to the prevention of various diseases.[Citation3] Triterpenic compounds are the biologically active compounds found in apples and are among the most promising and most significant compounds for human health.[Citation4–Citation7]
A reliable analytical instrument is required in order to obtain detailed results in the analysis of the phytochemical composition of apples. The chromatography techniques that are most commonly used in the evaluation of the quantitative and qualitative composition of triterpenic compounds include high-performance liquid chromatography (HPLC), gas chromatography, and thin layer chromatography combined with tandem mass spectrometry.[Citation8,Citation9] Capillary electrophoresis is a less commonly used technique of electrochemical analysis.[Citation10,Citation11] The popularity of the HPLC technique in the analysis of triterpenic compounds is due to its high effectiveness and sensitivity, as well as the excellent reproducibility of its results.[Citation12,Citation13] The reversed-phase HPLC techniques presented by different researchers have several similarities: in all cases, isocratic elution is applied, and columns with particle size of 5 μm are used.[Citation14–Citation16] Plante et al. tried to improve HPLC peak separation in the analysis of triterpenic compounds using triacontane (C30) groups[Citation17], yet in their HPLC analysis, they most commonly used C18 particle-packed columns.[Citation18–Citation20] In the analysis of triterpenic compounds in apple samples, the following eluents are most commonly used: acetonitrile, methanol, and water, which could be acidified with vinegar or formic or phosphatidic acids.[Citation15,Citation16,Citation19] Analyte detection is usually performed using ultraviolet or ultraviolet/visible light, photodiode array, or mass spectrometry detectors, while evaporative light scattering detectors, fluorescence, and charged aerosol detectors are less commonly employed.[Citation14,Citation15,Citation17]
The improvement in the chromatographic separation and analyte detection equipment and the search for new compounds and various metabolites resulted in the use of complex systems with various detection techniques (ultra-HPLC-DAD-MS, ultra-HPLC-DAD-NMR, etc.) for the identification of the synthesis pathways of the bioactive components accumulated in plants.[Citation21,Citation22] The acquisition and maintenance of such equipment are associated with high costs and the need for highly qualified specialists. Meanwhile, the routine analysis of botanical extracts still widely employs classic HPLC with a diode array detector, which can be easily performed and is relatively inexpensive.
In some of the HPLC techniques described in literature, the duration of the partitioning of triterpenic compounds is relatively long (25–30 min), which may increase the time consumption and costs of the analysis.[Citation14,Citation19,Citation20] The techniques of the quantitative and qualitative analyses of the content of triterpenic compounds in apples described in literature sources may not always be suitable for implementation in other laboratories. Because of possible differences in the phytochemical composition of the apples, these techniques may be unsuitable for the analysis of local cultivars. Because of this, a detailed scientific analysis requires the redevelopment of sample preparation and mobile phase gradients of the techniques designed in other laboratories. The need for the development of a novel technique was confirmed by our preliminary attempts to adapt the techniques found in scientific literature sources: these attempts did not yield the desired results. Thus, we decided to develop, optimize, and validate a new reversed-phase HPLC-DAD technique with a short duration of the analysis, which would be suitable for the routine quantitative and qualitative evaluation of triterpenic compounds in apple sample extracts.
Materials and methods
Plant material
The following apple cultivars were included in the study: ‘Aldas’, ‘Auksis’, ‘Connel Red’, ‘Ligol’, ‘Lodel’, and ‘Rajka’. The study was conducted in 2016–2017. The trees were trained as slender spindles. Pest and disease management was carried out according to the rules of the integrated plant protection. The experimental orchard was not irrigated. Tree fertilization was performed based on the results of soil and leaf analysis. Nitrogen was applied before flowering at the rate of 80 kg/ha, and potassium was applied after harvest at the rate of 90 kg/ha. Soil conditions of the experimental orchard were the following: clay loam, pH - 7.3, humus - 2.8%, P2O5 - 255 mg/kg, and K2O - 230 mg/kg. The apple cultivars ‘Auksis’ (early winter cv., bred in Lithuania), and ‘Ligol’ (winter cv., bred in Poland) are the main cultivars in Lithuanian commercial orchards. The cv. ‘Aldas’ (early winter cv., bred in Lithuania), ‘Lodel’ (winter cv., bred in Poland), and ‘Rajka’ (winter cv., bred in the Czech Republic) are scab-immune cultivars recommended for ecological orchards.
Preparation of samples
The whole apple fruit slices, apple peel, and apple flesh were immediately frozen in a freezer (–35°C) with air circulation; subsequently, these frozen samples were lyophilized with a ZIRBUS sublimator 3 × 4 × 5/20 (ZIRBUS technology, Bad Grund, Germany) at a pressure of 0.01 mbar (condenser temperature: −85°C). The lyophilized samples were ground to fine powder by using a Retsch 200 mill (Haan, Germany).
Extraction
During the analysis, 1 g of lyophilizate powder (exact weight) was weighed, added to 10 mL of acetone, and extracted in a Sonorex Digital 10 P ultrasonic bath (Bandelin Electronic GmbH & Co. KG, Berlin, Germany) at room temperature for 10 minutes. The conditions of the extraction were chosen based on the results of the tests for setting the extraction conditions. The extract obtained was filtered through a paper filter, and the residue on the filter was washed with acetone in a 10 mL flask until the exact volume was reached.
Instrumentation and chromatographic conditions
A chromatograph equipped with a Waters 2998 PDA detector (Waters, Milford, USA) was used for HPLC analysis. Chromatographic separations were carried out by using an ACE (5 μm, C18, 250 × 4.6 mm i.d.) column. The column was operated at a constant temperature of 25°C. The volume of the analyzed extract was 10 µL. The flow rate was 1 mL/min. The mobile phase consisted of acetonitrile (solvent A) and water (solvent B). We applied isocratic elution, the eluent ratio being 88% (solvent A) and 12% (solvent B). For quantitative analysis, the calibration curve was obtained by injecting known concentrations of different standard compounds. All the identified triterpenic compounds were quantified at 205 nm wavelength.
Statistical data analysis
All the experiments were carried out in triplicate. Means and standard deviations were calculated using computer software Microsoft Office Excel 2013 (Microsoft, Redmond, Washington, USA) and SPSS 20.0 (Chicago, Illinois, USA). A single factor analysis of variance (ANOVA) along with the post-hoc Tukey test was employed for statistical analysis. Differences were considered to be significant at p < 0.05.
Results and discussions
Selection of the conditions for the extraction of triterpenic compounds from apple samples
Selection of the conditions of extraction is one of the most significant analytical steps in the development of the technique for qualitative and quantitative evaluation of biologically active compounds in botanical raw material.[Citation23] The extractant used in the extraction is an important factor that determines the outcome of the extraction of the studied compounds and allows for the prognostication of the qualitative and quantitative composition of individual compounds in botanical extracts.[Citation24] The extraction of triterpenic compounds from botanical raw material is most commonly carried out with the help of non-polar extractants, such as acetone, alcohols (ethanol, methanol or (less frequently) other lower alcohols), hexane, ethyl acetate, diethyl ether, or liquid chlorinated hydrocarbons (chloroform or dichloromethane) or their mixtures.[Citation24–Citation26]
The analysis of scientific literature revealed a number of publications on the phytochemical composition of apples and its variation in whole apples and apple peel and flesh samples. Most commonly, phenolic compounds were analyzed, whereas other equally important biologically active compounds of other groups (such as pentacyclic triterpenic compounds) received much less attention. For these reasons, it is highly relevant to evaluate the variation in the phytochemical composition of triterpenic compounds in apple samples using modern analysis techniques.
According to scientific literature, sonication is one of the most suitable techniques for the extraction of triterpenic compounds from apple samples. This technique is especially suitable for the extraction of compounds that are sensitive to high temperatures (during microwave extraction, high temperature is used, which may destroy biologically active compounds). This technique is easily applicable in routine analysis (extraction with supercritical fluids or compressed fluids requires expensive equipment, which is not always available in routine analysis), and the duration of the extraction is sufficiently short (the application of maceration and extraction in the Soxhlet extractor requires a lot of time).[Citation27–Citation29] Based on the previous studies of other researchers, we selected the sonication (extraction in an ultrasonic bath) technique for the extraction of triterpenic compounds from whole apples and apple peels and flesh.
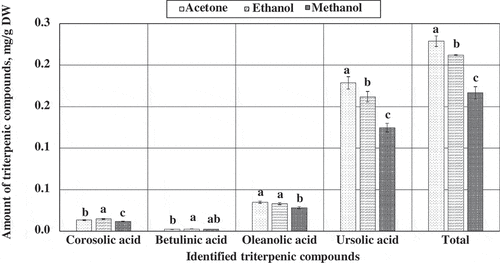
During the first stage of the study on the selection of the conditions of extraction, we evaluated the influence of the extractant on the outcome of the extraction of triterpenic compounds. Based on the research findings published by researchers worldwide[Citation16,Citation30], we selected the following extractants for the investigation of the conditions for the extraction of triterpenic compounds from apples: 100% methanol, 96% ethanol, and 100% acetone. The greatest total amount of the identified triterpenic compounds (2.29 ± 0.06 mg/g, p < 0.05) was detected after the extraction of apple samples with 100% acetone (), and thus this extractant was selected for further study on the determination of the conditions of extraction.
Figure 1. Variation in the quantitative composition of triterpenic compounds in extracts of apple samples depending on the extractant used.
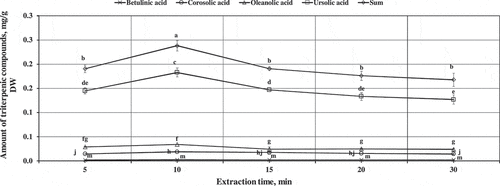
During the next stage of the study on the selection of the conditions of extraction, we evaluated the effect of the duration of the extraction on the outcome of the extraction of triterpenic compounds from apples. The statistically significantly greatest total amount of the identified triterpenic compounds (2.38 ± 0.01 mg/g, p < 0.05) was detected after the extraction of apple samples at room temperature for 10 min (), and therefore this duration was selected for the comparative studies on triterpenic compounds in whole apples, apple peels, and apple flesh of various cultivars.
Figure 2. Variation in the quantitative composition of triterpenic compounds in extracts of apple samples depending on the extraction time. Different letters indicate statistically significant differences in the amounts of triterpenic compounds in extracts of apple samples, according to Tukey, at p > 0.05.
Thus, summing up, the investigation of the effectiveness of the extraction of triterpenic compounds showed that the greatest total amount of the identified triterpenic compounds was detected after the extraction of apple samples with 100% acetone in an ultrasonic bath at room temperature for 10 min. For this reason, these conditions of extraction were selected for further investigation.
Validation of the technique
To evaluate the suitability of the applied HPLC technique for quantitative and qualitative analysis of triterpenic compounds in extracts of apple samples, validation of the technique was conducted. The validated technique ensures that the results obtained during the study are reliable and reproducible and reflect the actual quantitative and qualitative composition of triterpenic compounds in the extracts of the studied apple samples. The validation of the technique was performed according to the ICH recommendations.[Citation31] The following characteristics of validation were evaluated: the selectivity of the method (specificity), precision, the detection and quantitation limits of the analytes, and linearity. The evaluation of the selectivity of the method for peak identification and purity was based on the comparison of the retention times and UV spectra of the analytes with those of the standard compounds. The limit of detection (LOD) and the limit of quantitation (LOQ) of the analytes were assessed by comparing the peak height to baseline noise. The ratio of the peak height to baseline noise (signal-to-noise ratio) used for the estimation of the LOD was 3:1, and for the LOQ – 10:1. The determined LOD ranged from 0.15 µg/mL (betulinic acid) to 0.20 µg/mL (oleanolic acid). The determined LOQ ranged from 0.45 µg/mL (betulinic acid) to 0.60 µg/mL (oleanolic acid). The estimated determination coefficients (R2) of the calibration curves were greater than 0.999, and this proves the linearity of the quantitative determination technique (). The obtained results confirm that this method can be used for quantitative and qualitative analysis of triterpenic compounds.
Table 1. Characteristics of the quantitative evaluation of triterpenic compounds.
The precision of the HPLC method was assessed based on two parameters - repeatability and intermediate precision. The evaluation of repeatability was based on the results of consecutive analyses performed within the same day (6 consecutive analyses of a mixture of standard compounds at 3 different concentrations performed within the same day). The percent relative standard deviation (%RSD) of the repeatability of the method, determined according to the peak area, ranged from 0.28% (corosolic acid) to 1.61% (oleanolic acid). The intermediate precision of the results was assessed based on the results of analyses performed within 3 different days (6 consecutive analyses of a mixture of standard compounds at the mean concentration performed within the same day; 18 analyses in total). All the obtained results describing the precision of the developed HPLC technique are summarized in .
Table 2. Precision of the HPLC technique for the quantitative evaluation of triterpenic compounds. Concentrations of reference compounds used in the assessment of precision are provided in the Supplementary material (Table S1).
The results of the validation of the HPLC technique indicate that the developed technique is suitable and may be applied in studies on the qualitative and quantitative composition of triterpenic compounds in apple samples and their products. The evaluation scores of the validation parameters of the HPLC technique confirmed the suitability of this HPLC technique, and thus it was applied in the analysis of the composition of triterpenic compounds in the extracts of fruit, peels, and flesh of apples of various cultivars grown in Lithuanian climate conditions.
Analysis of the variation in the quantitative and qualitative composition of triterpenic compounds in whole apples
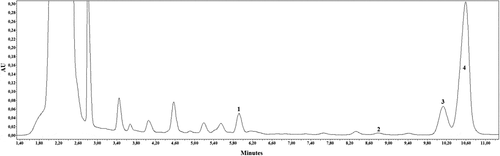
The developed and validated HPLC technique was applied to investigate extracts of lyophilized apple samples of cultivars ‘Aldas’, ‘Auksis’, ‘Connel Red’, ‘Ligol’, ‘Lodel’, and ‘Rajka’. The results of the analysis of triterpenic compounds detected in apples of different cultivars grown in Lithuania will be valuable in the breeding of apple cultivars. This will allow for breeding the cultivars with the greatest amounts of triterpenic compounds and with fruit that may be valuable for the isolation of individual compounds with a specific biological effect, which could first be used in studies in vivo and later – in medical practice. The variation in the quantitative composition of triterpenic compounds in apple samples of different cultivars is presented in . The example of a chromatogram is presented in .
Table 3. Variation in the quantitative composition of triterpenic compounds in apple samples of different cultivars.
Figure 3. HPLC chromatogram of the acetone extract of the apple peel sample (cultivar ‘Lodel’, λ = 205 nm). 1 - corosolic acid, 2 - betulinic acid, 3 - oleanolic acid, and 4 - ursolic acid.
The highest total amount of the identified triterpenic compounds (3.173 ± 0.136 mg/g) was detected in samples of the ‘Lodel’ apple cultivar. Ursolic acid was the predominant compound. It comprised the greatest part (72.1–81.2%) of the total amount of all triterpenic compounds detected in the extracts of the apple samples. The results of the study published by Grigoras et al. confirm our findings – in their study, ursolic acid also predominated among triterpenic compounds detected in extracts of apple samples.[Citation19] This acid may comprise 70% or more of the total amount of all triterpenic compounds detected in extracts of apple samples.[Citation22] All the results of the analysis of individual triterpenic compounds in whole apple samples are presented in . The amounts of oleanolic and corosolic acids detected in the studied extracts of apple samples were similar to those previously detected by other researchers: the amount of oleanolic acid in apple samples may range from 0.16 g/100 g to 1.0 g/100 g of dry raw material[Citation32,Citation33], and the amount of corosolic acid in apple samples reaches up to 0.51 g/100 g.[Citation32] To evaluate the variation in the quantitative composition of triterpenic compounds between apple samples of different cultivars, we calculated variation coefficients reflecting the range of the variation for each compound (). The factor of the cultivar had the greatest effect on the variation in the quantitative composition of corosolic acid (CV = 63.5%). For other detected triterpenic compounds, the coefficients of variation were smaller. Oleanolic acid exhibited the smallest variation in its amount between samples of different apple cultivars (CV = 15.9%). Summing up the obtained results, the identified and quantitatively evaluated triterpenic compounds in extracts of the samples of the studied apple cultivars may be arranged in the following order by decreasing amount: ursolic acid > oleanolic acid > corosolic acid > betulinic acid. These results confirm the findings previously published by Jemmali et al.[Citation32]
Analysis of the variation in the quantitative and qualitative composition of triterpenic compounds in apple peels
A study conducted by Nour et al.[Citation34] showed that the qualitative and quantitative composition of biologically active compounds differed between different parts of the apples. To evaluate the variation in the phytochemical composition of apple cultivars grown in Lithuanian climate conditions, a quantitative and qualitative analysis of triterpenic compounds in different parts of the fruit – i.e. its peels and flesh – is expedient. The obtained knowledge will allow for a broader use of apple flesh and peels in food industry and for health improvement – i.e. to produce dietary supplements, teas, and other preparations.
The analysis of the qualitative and quantitative composition of apple peel extracts showed that the total amount of the identified triterpenic compounds in lyophilized apple peel samples varied from 5.271 mg/g (cultivar ‘Aldas’) to 8.327 mg/g (cultivar ‘Lodel’). The variation in the quantitative composition of triterpenic compounds in apple peel samples of different cultivars is presented in . The amount of ursolic acid detected in apple peel samples was by 2.5–3.4 times greater than that detected in whole apple samples. The greatest amount of ursolic acid was detected in apple peel samples of the ‘Lodel’ cultivar – as was the case with whole apple samples (). Ursolic acid comprised 72.5–79.6% of the total amount of all triterpenic compounds detected in the extracts of the apple peel samples. The percentage of ursolic acid in apple peel samples was very similar to that detected in extracts of whole apple samples. The obtained results confirm the variation in the percentage of ursolic acid detected by other researchers. The percentage of this acid may comprise as much as 98% of the total amount of all the triterpenic compounds identified in extracts of apple peel samples.[Citation35] In the peel samples of all the studied apple cultivars, the detected amount of oleanolic acid was smaller than that of ursolic acid. This pattern was also confirmed by data of studies published by other researchers.[Citation20,Citation30] Compared to ursolic acid, which predominated in apple peel samples, the percentage of oleanolic acid was lower and comprised 14.1–18.8% of the total amount of all the triterpenic compounds identified in extracts of apple peel samples. These results are in line with findings previously published by other researchers. Scientific literature indicates that the amount of oleanolic acid accumulated in apple peels ranges from 7% to 15%.[Citation30,Citation35] The percentage of betulinic acid, compared to that of other identified and quantitatively evaluated triterpenic compounds (ursolic, oleanolic, and corosolic acids) was significantly lower and comprised only 0.7–1.1% of the total amount of all the triterpenic compounds identified in extracts of apple peel samples. The evaluation of the percentage of betulinic acid in extracts of apple peel samples showed that it was similar to the percentage distribution of this acid in extracts of whole apple samples. The obtained results confirmed those previously published in scientific literature – i.e. that the percentage of betulinic acid in extracts of apple peel samples ranges from 1.0 to 2.7%.[Citation36,Citation37]
Table 4. Variation in the quantitative composition of triterpenic compounds in apple peel samples of different cultivars.
The calculation of the variation coefficients for individual compounds in apple cultivars showed that corosolic acid (50%) exhibited the greatest variation between the apple peel samples of different studied cultivars, whereas the variation of oleanolic acid was the lowest (10.3%) (). Summing up, the predominant triterpenic compound in apple peel samples was ursolic acid, and the amounts of betulinic acid were the lowest. Such pattern of the variation in the quantitative and qualitative composition of triterpenic compounds was also confirmed by the results of the studies published by Cortina et al.[Citation38]
Analysis of the variation in the quantitative and qualitative composition of triterpenic compounds in apple flesh
The total amount of the identified and quantitatively evaluated triterpenic compounds in lyophilized apple flesh samples ranged from 0.156 mg/g (cultivar ‘Ligol’) to 0.417 mg/g (cultivar ‘Rajka’). It was by 18.4–44.9 times lower than that found in apple peel samples. Like in whole apple and apple peel samples, ursolic acid was the predominant compound among all the determined analytes (). The greatest amount of ursolic acid (0.249 ± 0.012 mg/g, p < 0.05) was detected in apple flesh samples of the ‘Rajka’ cultivar (). The obtained results differ from those discussed above where the greatest amount of ursolic acid was detected in whole apple and apple flesh samples of the ‘Lodel’ cultivar. Scientific literature indicates that the amount of ursolic acid in apple flesh samples varies by up to 1.43 g/100 g.[Citation33] As with whole apple and apple peel samples, in extracts of apple flesh samples, ursolic acid comprised the greatest percentage – 59.8–82.1% of the total amount of all the detected triterpenic compounds. The percentage of ursolic acid in extracts of apple flesh samples was significantly higher than that calculated by Kukina et al. (28.9%).[Citation39]
Table 5. Variation in the quantitative composition of triterpenic compounds in apple flesh samples of different cultivars.
Statistically significantly the greatest amount of oleanolic acid (0.093 ± 0.013 mg/g, p < 0.05) was detected in apple flesh samples of the ‘Rajka’ cultivar (). The data of the evaluation of triterpenic compounds in whole apples and apple flesh samples differed – whole apple samples of the ‘Rajka’ cultivar were found to contain the smallest amounts of oleanolic acid. The obtained results confirm the data published by German researchers Jäger et al., where the amount of oleanolic acid in apple flesh samples could reach up to 0.28 g/100 g.[Citation33] The percentage of oleanolic acid comprised 12.5–29.5% of the total amount of all the triterpenic compounds identified in extracts of apple flesh samples, and was by 2.8–4.8 lower than that of ursolic acid. The obtained results differ from those published by Kukina et al., where the percentage of oleanolic acid in apple flesh samples was only 5.6%.[Citation39]
The detected amount of betulinic acid was the smallest of all the quantitatively evaluated triterpenic compounds in apple flesh samples of all the studied cultivars. Our results are in line with those obtained by Cortina et al.[Citation38] Betulinic acid was detected in extracts of apple flesh samples of only four cultivars - ‘Aldas’, ‘Auksis’, ‘Ligol’, and Rajka’, whereas apple flesh samples of the ‘Connel Red’ and ‘Lodel’ cultivars did not contain this acid (). According to the data of the previously discussed studies, the extracts of whole apple samples of the ‘Lodel’ cultivar and the extracts of apple flesh samples of the ‘Connel Red’ cultivar were found to contain the greatest amounts of betulinic acid. The percentage of this acid in apple flesh samples was the smallest, compared to the percentage of ursolic, oleanolic, and corosolic acids – it comprised only 1.2–7.2% of the total amount of all the detected triterpenic compounds in apple flesh samples. The percentage of betulinic acid in extracts of apple flesh samples was by 1.7–6.6 times greater than that detected in apple peel samples and by 1.3–5.1 times greater than that detected in whole apple samples.
The greatest coefficient of variation between the apple flesh samples of the studied cultivars was observed for corosolic acid (82%), and the lowest – for ursolic acid (44.3%). These data also confirm the importance of the cultivar as a factor affecting the quantitative composition of apples, described in other literature sources.[Citation40] The analysis of the quantitative and qualitative composition of triterpenic compounds in apple flesh samples showed that in extracts of apple flesh samples, ursolic acid was the predominant triterpenic compound, whereas the amounts of betulinic acid were the smallest. A similar variation in the quantitative composition of triterpenic compounds in apple flesh samples was found in a study by Jeong et. al.[Citation41]
The obtained results of the analysis of whole apples, apple peels, and apple flesh and the calculated CV for triterpenic compounds proved the influence of the part of the fruit and the cultivar on the variation in the quantitative content of these compounds. In addition, factors such as genetic variation, growth period, growing season[Citation42], rootstocks[Citation43] and management technologies, geographic location[Citation44], and tree nutrition[Citation45] could affect the concentration of biologically active compounds.
Conclusion
The HPLC-based analytical procedure for the detection and evaluation of triterpenic compounds in apple samples of 6 popular cultivars was developed, optimized, and validated. The specificity, precision, and assay range confirmed the suitability of this method. The distribution of 4 triterpenic compounds was determined in the tested whole apple, apple peel, and apple flesh extracts. Ursolic acid was the major component in the all samples of all the tested cultivars. The greatest total amounts of the identified and quantitatively evaluated triterpenic compounds were detected in apple flesh samples, smaller amounts – in whole apple samples, and the smallest amounts – in apple flesh samples. The greatest influence of the cultivar factor on the variation in the total amounts of triterpenic compounds was detected in apple flesh samples (CV = 43.7%).
The results of the study proved that this developed and validated HPLC technique may be successfully applied in the evaluation of the composition of triterpenic compounds in the extracts of whole apple, apple peel, and apple flesh samples of various cultivars. This technique might also be suitable for the analysis of the extracts of other botanical raw material that accumulates triterpenic compounds.
Supplemental Material
Download ()Supplementary material
Supplemental data can be accessed here
Additional information
Funding
References
- Ran, J.; Sun, H.; Xu, Y.; Wang, T.; Zhao, R. Comparison of Antioxidant Activities and High-Performance Liquid Chromatography Analysis of Polyphenol from Different Apple Varieties. International Journal Food Prop 2016, 19(11), 2396–2407. DOI: 10.1080/10942912.2015.1037958.
- Marks, S. C.; Mullen, W.; Crozier, A. Flavonoid and Chlorogenic Acid Profiles of English Cider Apples. Journal of the Science of Food and Agriculture 2007, 87(4), 719–728. DOI: 10.1002/jsfa.3006.
- Liaudanskas, M.; Viškelis, P.; Kviklys, D.; Raudonis, R.; Janulis, V. A Comparative Study of Phenolic Content in Apple Fruits. International Journal Food Prop 2015, 18(5), 945–953. DOI: 10.1080/10942912.2014.911311.
- Nazaruk, J.; Borzym-Kluczyk, M. The Role of Triterpenes in the Management of Diabetes Mellitus and Its Complications. Phytochem Reviews 2015, 14(4), 675–690. DOI: 10.1007/s11101-014-9369-x.
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid - a Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules (Basel, Switzerland) 2015, 20(11), 20614–20641. DOI: 10.3390/molecules201119721.
- Somova, L. O.; Nadar, A.; Rammanan, P.; Shode, F. O. Cardiovascular, Antihyperlipidemic and Antioxidant Effects of Oleanolic and Ursolic Acids in Experimental Hypertension. Phytomedicine : International Journal of Phytotherapy and Phytopharmacology 2003, 10(2–3), 115–121. DOI: 10.1078/094471103321659807.
- Wolska, K.; Grudniak, A.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial Activity of Oleanolic and Ursolic Acids and Their Derivatives. Open Life Sciences 2010, 5, 5, 543–553.
- Lesellier, E.; Destandau, E.; Grigoras, C.; Fougère, L.; Elfakir, C. Fast Separation of Triterpenoids by Supercritical Fluid Chromatography Evaporative Light Scattering Detector. Journal Chromatographic A 2012, 1268, 157–165. DOI: 10.1016/j.chroma.2012.09.102.
- Naumoska, K.; Vovk, I. Analysis of Triterpenoids and Phytosterols in Vegetables by Thin–Layer Chromatography Coupled to Tandem Mass Spectrometry. Journal Chromatographic A 2015, 1381, 229–238. DOI: 10.1016/j.chroma.2015.01.001.
- Ganzera, M.;. Quality Control of Herbal Medicines by Capillary Electrophoresis: Potential, Requirements and Applications. Electrophoresis 2008, 29, 3489–3503. DOI: 10.1002/elps.v29:17.
- Li, G. L.; You, J. M.; Song, C. H.; Xia, L.; Zheng, J.; Suo, T. S. Development of a New HPLC Method with Precolumn Fluorescent Derivatization for Rapid, Selective and Sensitive Detection of Triterpenoid Acids in Fruits. Journal Agricultural Food Chemical 2011, 59, 2972–2979. DOI: 10.1021/jf104224t.
- Boligon, A. A.; Athayde, M. L. Importance of HPLC in Analysis of Plants Extracts. Austin Chromatographic 2014, 1, 3, 2.
- Negi, J. S.; Singh, P.; Pant, G. J. N.; Rawat, M. S. M. High–Performance Liquid Chromatography Analysis of Plant Saponins: An Update 2005–2010. Pharmacogn Reviews 2011, 5(10), 155–158. DOI: 10.4103/0973-7847.91109.
- Andre, C. M.; Legay, S.; Deleruelle, A.; Nieuwenhuizen, N.; Punter, M.; Brendolise, C.; Cooney, J. M.; Lateur, M.; Hausman, J. F.; Larondelle, Y.; et al. Multifunctional Oxidosqualene Cyclases and Cytochrome P450 Involved in the Biosynthesis of Apple Fruit Triterpenoid Acids. The New Phytologist 2016, 211, 1279–1294. DOI: 10.1111/nph.13996.
- He, X.; Liu, R. H. Phytochemicals of Apple Peels: Isolation, Structure Elucidation, and Their Antiproliferative and Antioxidant Activities. Journal of Agricultural and Food Chemistry 2008, 56, 9905–9910. DOI: 10.1021/jf8015255.
- Fan, J. P.; Liao, D. D.; Zhang, X. H. Ultrasonic Assisted Extraction of Ursolic Acid from Apple Pomace: A Novel and Facile Technique. Sep Sciences Technological 2016, 51, 1344–1350. DOI: 10.1080/01496395.2016.1165253.
- Plante, M.; Bailey, B.; Crafts, C.; Acworth, I. N. Sensitive HPLC Method for Triterpenoid Analysis Using Charged Aerosol Detection with Improved Resolution. Thermo Fisher Scientific 2012, 2–6.
- Andre, C. M.; Greenwood, J. M.; Walker, E. G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N. B.; Laing, W. A. Anti–Inflammatory Procyanidins and Triterpenes in 109 Apple Varieties. Journal of Agricultural and Food Chemistry 2012, 60, 10546−10554. DOI: 10.1021/jf302809k.
- Grigoras, C. G.; Destandaua, E.; Fougèrea, L.; Elfakira, C. Evaluation of Apple Pomace Extracts as a Source of Bioactive Compounds. Industrial Crops Products 2013, 49, 794–804. DOI: 10.1016/j.indcrop.2013.06.026.
- Tostes, J. B. D. F.; Nakamura, M. J.; Gomes, C.; Saboya, F.; Mazzei, J. L.; Siani, A. C. Efficient and Selective Method to Separate Triterpene Acids by Direct Treatment of Apple Peels with Alkalin Ethanol. Sep Sciences Technological 2016, 51(12), 2–25. DOI: 10.1080/01496395.2016.1200088.
- Moldoveanu, S. C.; Scott, W. A. Analysis of Four Pentacyclic Triterpenoid Acids in Several Bioactive Botanicals with Gas and Liquid Chromatography and Mass Spectrometry Detection. Journal Sep Sciences 2016, 39, 324–332. DOI: 10.1002/jssc.201501041.
- Poirier, B. C.; Buchanan, D. A.; Mattheis, J.; Rudell, D. Differential Partitioning of Triterpenes and Triterpene Esters in Apple Peel. Journal of Agricultural and Food Chemistry 2018, 66(8), 1800–1806. DOI: 10.1021/acs.jafc.7b04509.
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K. M.; Latha, L. Y. Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. African Journal Tradit Complementary Alternative Medica 2011, 8, 1, 1–10.
- Pandey, A.; Tripathi, S. Concept of Standardization, Extraction and Pre Phytochemical Screening Strategies for Herbal Drug. Journal Pharmacogn Phytochem 2014, 2, 5, 115–119.
- Siani, A. C.; Nakamura, M. J.; Santos, D. S.; Mazzei, J. L.; Nascimento, A. C.; Valente, L. M. M. Efficiency and Selectivity of Triterpene Acid Extraction from Decoctions and Tinctures Prepared from Apple Peels. Pharmacogn. Mag. 2014, 10, 225–231. DOI: 10.4103/0973-1296.133294.
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical Screening and Extraction: A Review. International Pharmaceutical Sciences 2011, 1, 98–106.
- Cheok, C. H.; Salman, H. A. K.; Sulaiman, R. Extraction and Quantification of Saponins: A Review. Food Research International (Ottawa, Ont.) 2014, 59, 16–40. DOI: 10.1016/j.foodres.2014.01.057.
- Handa, S. S.; Khanuja, S. P. S.; Longo, G.; Rakesh, D. D. Extraction Technologies for Medicinal and Aromatic Plants; International centre for science and high technology: Trieste, 2008, p. 25–33.
- Kataoka, H.;. New Trends in Sample Preparation for Analysis of Plant Derived Medicines. Current Organic Chemical 2010, 14, 1698–1713. DOI: 10.2174/138527210792927627.
- Frighetto, R. T. S.; Welendorf, R. M.; Nigro, E. N.; Frighetto, N.; Siani, A. C. Isolation of Ursolic Acid from Apple Peels by High Speed Counter Current Chromatography. Food Chemistry 2008, 106, 767–771. DOI: 10.1016/j.foodchem.2007.06.003.
- Baber, N.;. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). British Journal Clinical Pharmacology 1994, 37(5), 401–404. DOI: 10.1111/j.1365-2125.1994.tb05705.x.
- Jemmali, Z.; Chartiern, A.; Dufresne, C.; Elfakir, C. Optimization of the Derivatization Protocol of Pentacyclic Triterpenes Prior to Their Gas Chromatography – Mass Spectrometry Analysis in Plant Extracts. Talanta 2016, 147, 35–43. DOI: 10.1016/j.talanta.2015.09.026.
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M. N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants Rich Sources for a New Group of Multi Potent Plant Extracts. Molecules (Basel, Switzerland) 2009, 14, 2016–2031. DOI: 10.3390/molecules14031081.
- Nour, V.; Trandafir, I.; Ionica, M. E. Compositional Characteristics of Fruits of Several Apple (Malus Domestica Borkh.) Cultivars. Not Botanic Hort Agrobot Cluj 2010, 38, 3, 228–233.
- Szakiel, A.; Paczkowski, C.; Pensec, F.; Bertsch, C. Fruit Cuticular Waxes as a Source of Biologically Active Triterpenoids. Phytochemistry Reviews : Proceedings of the Phytochemical Society of Europe 2012, 11(2–3), 263–328. DOI: 10.1007/s11101-012-9241-9.
- Cargnin, S. T.; Gnoatto, S. B. Ursolic Acid from Apple Pomace and Traditional Plants: A Valuable Triterpenoid with Functional Properties. Food Chemistry 2017, 220, 477–489. DOI: 10.1016/j.foodchem.2016.09.202.
- He, Q. Q.; Yang, L.; Zhang, J. Y.; Ma, J. N.; Ma, C. M. Chemical Constituents of Gold–Red Apple and Their α–Glucosidase Inhibitory Activities. Journal of Food Science 2014, 79, 970–1983. DOI: 10.1111/1750-3841.12599.
- Cortina, D. B.; Maci, A.; Iglesias, I.; Romero, M. P.; Motilva, M. J. Phytochemical Profiles of New Red–Fleshed Apple Varieties Compared with Traditional and New White Fleshed Varieties. Journal of Agricultural and Food Chemistry 2016, 5, 8, 1684–1696.
- Kukina, T. P.; Frolova, T. S.; Salnikova, O. I. Lipophilic Constituents from Malus Baccata. Chemical Natural Compd 2014, 6, 1096–1097. DOI: 10.1007/s10600-014-1168-5.
- Ceymann, M.; Arrigoni, E.; Schärer, H.; Bozzi, N. A.; Hurrell, R. F. Identification of Apples Rich in Health-Promoting Flavan-3-Ols and Phenolic Acids by Measuring the Polyphenol Profile. Journal Food Compos Analysis 2012, 26(1), 128–135. DOI: 10.1016/j.jfca.2011.12.002.
- Jeong, J. W.; Shim, J. J.; Choi, I. D.; Kim, S. H.; Ra, J.; Ku, H. K.; Lee, D. E.; Kim, T. Y.; Jeung, W.; Lee, J. H.;; et al. Apple Pomace Extract Improves Endurance in Exercise Performance by Increasing Strength and Weight of Skeletal Muscle. Journal Medica Food 2015, 18(12), 1380–1386.
- Liaudanskas, M.; Brunevičiūtė, R.; Gaivelytė, K.; Viškelis, J.; Viškelis, P.; Kviklys, D.; Janulis, V. Seasonal Variation of Qualitative and Quantitative Composition of Phenolic Compounds and Antioxidant Activity in Apple (Malus Domestica Borkh.) Fruits. International Journal Biochemical Researcher Reviews 2016, 14(3), 1–13. DOI: 10.9734/IJBCRR/2016/28856.
- Kviklys, D.; Liaudanskas, M.; Janulis, V.; Viškelis, P.; Rubinskienė, M.; Lanauskas, J.; Uselis, N. Rootstock Genotype Determines Phenol Content in Apple Fruits. Plant, Soil Environment 2014, 60(5), 234–240. DOI: 10.17221/71/2014-PSE.
- Viškelis, J.; Uselis, N.; Liaudanskas, M.; Janulis, V.; Bielicki, P.; Univer, T.; Lepsis, J.; Kviklys, D. Triterpenoid Acid Content in the Fruit Peel of Malus × Domestica Borkh. Depends on the Growing Technology. Zemdirbyste-Agriculture 2018, 105(1), 71–78. DOI: 10.13080/z-a.2018.105.010.
- Lanauskas, J.; Kviklys, D.; Liaudanskas, M.; Janulis, V.; Uselis, N.; Viškelis, J.; Viškelis, P. Lower Nitrogen Nutrition Determines Higher Phenolic Content of Organic Apples. Hort Sciences 2017, 44, 3, 113–119.
