 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Coconut sugar is traditionally produced by evaporating sap until reaching its saturated liquid and formed a crystalline structure. This study investigated the comparison of coconut sugar made by traditional method (crystalline structure) and dried coconut sugar (predominantly amorphous structure) to its characteristics. Two different formulation of coconut sap : maltodextrin (7 : 3) and (6 : 4) (weight/weight) were dried using vacuum oven (70℃, 6 hours) and spray dried (Tinlet 120℃.) Coconut sugar was characterized for moisture content, crystallinity, water sorption isotherm, hygroscopic rate, color, dissolving time, and powder recovery. Initial moisture content was examined and in range of 1.33% - 3.44% (wb). The highest monolayer water content was obtained by using spray drying (6 : 4) and lowest was obtained by traditional method. X-ray diffraction showed that dried coconut sugar powder had dominant amorphous structure (70.9 – 71.4%) while traditional one was dominated with crystalline structure (90.5%). Traditional coconut sugar was the least hygroscopic (1.21 × 10-4 g water/g solid/minutes), followed by vacuum dried coconut sugar (1.48 × 10-4 g water/g solid/minutes) and spray dried ones (1.56 – 1.67 × 10-4 g water/g solid/minutes). Spray dried coconut sugar had the brightest and the whitest color, followed by vacuum dried and traditional coconut sugar. Vacuum dried powder was quicker to dissolve (13.33 – 16.67 s), while increasing maltodextrin in spray drying could not decrease the dissolving time. The highest powder recovery of dried sugar was obtained by using vacuum drying and higher maltodextrin concentration (88.70%) while traditional method produced 100% powder recovery.
Introduction
Traditional coconut sugar powder in Indonesia is made by heating coconut sap until reaching its saturated solution and continued to heat into crystallized-structured sugar Citation1 This method is based on co-crystallization principle. There are two types of coconut sugar, firstly granulated coconut sugar which is produced by continued heating the supersaturated coconut sap until crystallized structure appeared. Secondly, the supersaturated coconut sap is put in a special container and cooled to produce a certain shaped form of coconut sugar.
The drying of coconut sap into coconut sugar powder is difficult to accomplish as it is composed of mostly sucrose (12.30–17.40%) Citation2 and corresponding to the problem of stickiness while drying. One of the factors contributing to stickiness is low glass transition temperature (Tg). Tg is a change observed when a glassy state is turning into a super-cooled melt during heating Citation3, while sucrose has Tg of 62°C and considered low when it comes to drying Citation4 To overcome this problem, the glass transition temperature of the coconut sap can be increased by the addition of a coating material having a higher glass transition temperature than sucrose. Maltodextrin (MD) is a carbohydrate derivative mostly used as drying aid of high sugar material Citation5 Glass transition temperature of MD depends on dextrose equivalent (DE) value, often in range of 140–180°CCitation6 MD with DE 10 with Tg of 112°C is considered a better material to maintain stability compared to other materials such as trehalose Citation7 MD may also increase process stability and storage of solid food to reduce caking, stickiness, and increasing ability to flow Citation8 Addition of MD ≥ 50% (solid/solid, w/w)) is needed to create a significant impact on glass transition temperature.[Citation9,Citation10]
Despite the use of high molecular material, in order to successfully dry high sugar material, the rate of drying process should be high Citation5 This can be accomplished by vacuum drying and spray drying method. Vacuum drying is a process in which the material is dried in a low-pressure environment, which lowers the heat required for rapid drying Citation11 Based on ideal gas law, at low absolute pressure (vacuum conditions), water can evaporate at a lower temperature than normal conditions (ambient pressure, 1 atm). This causes drying to occur more rapidly at lower temperatures Citation5 Similar high sugar content food, mango powder and honey powder, was successfully dried by adding MD as drying aid using vacuum oven at 70°C.[Citation12,]
Spray drying is a method to produce dry and fine particles with atomization process of samples into small droplets in a hot air environment. Due to its large surface area and high temperature, the droplets would dry quickly and hence fall to lower part of the dryer.Citation13 Coconut sugar powder was successfully dried using a spray dryer with inlet temperatures of (120°C, 150°C, and 180°C), and the concentrations of MD (DE 9–12) used were 10%, 20%, and 30% (weight/volume). The optimum condition of producing coconut sugar powder with good appearance and solubility parameters is MD concentration of 20% (w/v) and inlet temperature of 120°C.Citation14 The aim of the recent work was to compare the properties of coconut sugar produced from two different methods, traditional (co-crystallization) and drying method. The observed properties were moisture content, water sorption isotherm, amorphous content, hygroscopic rate, colour, dissolving time, and powder recovery. This work also compared the coconut sugar powder properties produced by vacuum drying and spray drying method.
Materials and method
Materials
Coconut sap was obtained freshly from Kertamukti village, Pangandaran District, West Java, Indonesia (±170 km from the lab). Coconut sap was collected from coconut flower and lime (mainly content Ca(OH)2) was previously added to maintain the pH (8.8) and then boiled in a cauldron for 1 h to reduce microorganisms and the process was done in a farmer’s house. Pre-boiled coconut sap was put in containers and kept in low temperature (10–15°C) during the travel for 4–5 h using an ice box and taken to the laboratory, Department of Food Industrial Technology, Faculty of Agroindustrial Technology, University of Padjadajaran, Jatinangor, Indonesia. Coconut sap was kept frozen (GEA Chest Freezer, China) at −28°C during preparation and only being thawed in room temperature before being used in further experiments. Maltodextrin DE 13–15 (Lihua Starch, China) was used as drying aid. The chemicals in this study include CH3COOK, MgCl2, K2CO3, Mg(NO3)2, NaNO2, and NaCl (Merck, Germany) for water sorption isotherm experiment. Silica gel and aquades were also used to complete the experiment.
Coconut sugar powder production
Traditional method
Coconut sap was poured in a hot pan and continuous stirring was done in 90–100°C using a spatula. As the coconut sap turned into a viscous liquid, heat was reduced while stirring continued until granules were formed.Citation15 Sample was cooled before grinding. The powder obtained from grinding step then was put in a heat-sealed plastic and then kept in a desiccator before further analysis.
Drying methods
Coconut sap was tested by using a refractometer to observe dissolved solid (°Brix). Maltodextrin (MD) DE 13–15 (5.0% moisture content) was added with ratio of coconut sap (C) : maltodextrin (MD) of 7 : 3 and 6:4 (solid to solid) accordingly. Distilled water was added to adjust the total solid content of the solution to 20°Brix. Then, it was added to coconut sap directly using a magnetic stirrer to create a homogenous solution.
Vacuum drying
Vacuum oven (Binder VD 23, Tuttington, German) was used to perform the drying process. Samples are put into a silicone container with thickness of ±1.5 cm. The temperature of vacuum oven was set to 70°C with absolute pressure of 5 inHg and drying time of 56 h Citation12 After drying was completed, samples were put into desiccator to decrease the temperature into ambient temperature. After grinding, the powder was put in a heat-sealed plastic then kept in a desiccator before further analysis.
Spray drying
Spray drying was performed using a pilot scale spray dryer (Kodi Machinery LPG-5, Jiangsu, China) using co-current flow. Inlet temperature of 120°C and outlet temperature of 65°C and flow rate of 8.0 rpm were maintained during the drying.Citation14 The resultingpowder was then packed in a heat-sealed plastic bag and was kept in a desiccator before further analysis was performed.
Sugar content
Sugar content of fresh coconut sap was determined by using High Performance Liquid Chromatography (HPLC) method by using carbohydrate column (4.6 mm × 250 mm), particle size 4µm, mobile phase: Acetonitril–Aquabidest (80:20) (volume/volume), velocity of mobile phase 2.0 mL/min with refractive index detector, and column temperature of 40°C:Citation16
Moisture content
Moisture content of coconut sugar powder was estimated by using gravimetric method. The gravimetric method, based on water removal by heating, was carried out in a convective oven at 105°C ± 5°C for 6 h.Citation16
Water sorption isotherm
Water sorption isotherm was determined by using gravimetric method. Six saturated salt solutions were prepared (CH3COOK, MgCl2, K2CO3, Mg(NO3)2, NaNO2, and NaCl) in order to provide relative humidity values of 22.6%, 32.73%, 43.80%, 52.86%, 68.9%, and 75.32%, respectively. Triplicate samples of 1 g of coconut sugar powder were weighed into vials and equilibrated over such saturated solutions, in desiccators at 25°C. The equilibrium is reached when the weight of the sample did not exceed 0.001 g change.Citation17 The Guggenheim, Anderson, and de Boer (GAB) (Eq. 2) and Brunauer, Emmet, and Teller (BET) (Eq. 3) models were used to define the relationship of equilibrium moisture content to water activity.
X = Moisture content (g water/g dry solid)
aw = Water activity
Xm = Monolayer water content
Xm, C, and K are constants.
The goodness of fit of the model was described by mean relative deviation (MRD):
where Xe is moisture content obtained from observation; Xp is predicted equilibrium moisture content (dry basis) calculated from different models; n is the number of data points. MRD <5 indicates best fit for the sample, while 5 < MRD < 10 indicates good fit, while MRD >10 indicates unsuitable fit of the model used.Citation18
X-ray diffraction
X-ray diffraction analysis (XRD D8 Advance A25 Bruker, Germany) was conducted to determine the structure of coconut sugar powder, i.e., crystalline or amorphous structure. The amorphous and crystalline structure percentage was also determined by using software provided in the equipment.
Hygroscopic rate measurement
Hygroscopic rate was measured by determining the slope of weight change of sample during storage at Relative Humidity (RH) 75% while collecting samples' weight in certain interval for 4 h. Citation19
Colour analysis
Spectrophotometer (Konica Minolta CM-5 Sensing Singapore Pte Ltd) was used to examine the colour characteristics. The results were expressed as L*, a*, and b*. L* value determines lightness or darkness, a* determine redness or greenness, while b* determines yellowness or blueness.Citation20
Dissolving time
Dissolving time is determined by measuring the time for the powder to completely dissolved in water. One gram of the sample is dissolved by pouring into 50 ml of water. Magnetic stirrer was used to create a homogenous solution.Citation21
Powder recovery
Powder recovery was analysed by comparing powder yield to its original solid content in dry Citation22
Results and discussion
Coconut sap properties
Coconut sap is a nutritious material due to its composition and easily fermented and deteriorated during collection. Traditionally, the farmers usually add preservative (natural or chemical preservative) and lime (Ca(OH)2) to maintain the stability of coconut sap during further processing. Properties of fresh coconut sap are shown in . pH of coconut sap is in neutral range Citation23, while pH of coconut sugar was 6.00–7.78 when lime (Ca(OH)2) was added.Citation24 Similar result is shown by the data above that pH of coconut sugar with added lime (Ca(OH)2) is about 7.47 ± 0.04. Water activity of coconut sap is 1.00, which indicates a high-moisture food. Food that contains water activity of 0.90–0.999 contains more than 50% (w/w) water Citation25
Table 1. Properties of coconut sap.
From , it is known the total soluble solid of coconut sap is about 20.70°Brix. It means that total solid in coconut sap is about 20% of total solution. Solids in coconut sap consist of sugar such as sucrose 85.76% (s/s), fructose 1.5% (s/s), and glucose <0.82% (s/s). The sucrose content was slightly higher () than was reported by Kalaiyarasi et al.Citation26 where fresh coconut sap had 12–15% of sucrose (by weight) and some reducing sugar, such as glucose, fructose, maltose, and raffinose. Sucrose contents of coconut sap varied from 9.40 to 12.24 g/100 ml, from 1.63 to1.84 g/100ml glucose content, while fructose constituted from 1.24 to 1.52 g from 100 ml of sap.Citation27 Study reports that coconut sap had rapid fermentation, unless it is collected under hygiene condition, and sugar still can be transformed into alcohol (5–8%).Citation28 This fact might explain the lower content of glucose compared to fructose of the coconut sap sample ().
Colour of coconut sap is valued by L*, a*, and b*. Positive L* value means coconut sap tends to show a brighter colour, a* and b* values are also positive means coconut sap tends to show red and yellow colours. It is caused by the Maillard reaction. Before coconut sap was taken to the laboratory for the experiment, it had been cooked for several hours until boiled, so that it might cause the Maillard reaction to take place that accentuated the red and yellow colours of the coconut sap.
Moisture content
The moisture content of coconut sugar powder is presented in in the range of 1.33–3.44%. Moisture content of traditional coconut sugar powder is in agreement with ZulianaCitation29, which is 1.37–2.43%. Similar results were found in drying of apple juice using vacuum drying and spray drying with the addition of maltodextrin with moisture content of 1.1–5.6%Citation30, and drying of honey powder.[Citation19,]
Table 2. Moisture content of coconut sugar powder produced by traditional method and drying methods.
Addition of maltodextrin as drying aid to coconut sugar powder leads to an increase in moisture content in both drying methods. This is due to maltodextrin hydrophilic sites, which might bind more water and affect the moisture content overall Citation17 Spray-dried coconut sugar powder showed less moisture content because of rapid drying and higher surface area due to atomization hence less moisture content Citation31
Water sorption isotherm
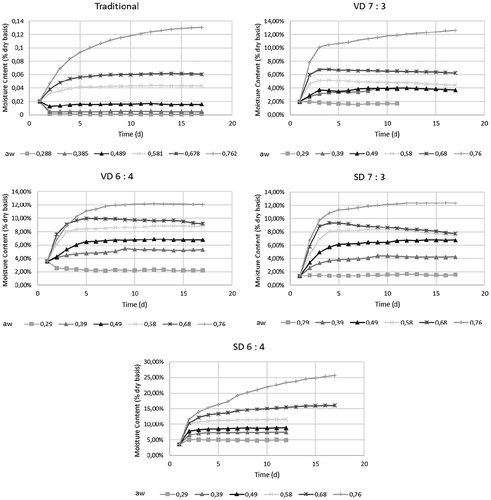
The water sorption of coconut sugar powder produced by traditional method and drying methods is presented in and . The powder which was kept in higher water activity showed longer time to reach equilibrium while the powder which was kept in lower water activity surrounding. A slow increase in moisture content at low water activity and steep rise in high water activity is a typical behaviour for sugar-rich foods, including banana flake.Citation32 Increasing maltodextrin in each drying method impacted on time to reach the equilibrium state. Both spray drying and vacuum drying showed the same behaviour at increased maltodextrin concentrations ().
Figure 1. The increase of coconut sugar powder moisture content exposed to different relative humidities.
The approach to GAB and BET equation to water sorption values of coconut sugar powder was performed by using theleast square difference method. The results of both models are shown in . The BET model analysis is based on an over-simplified assumption; hence, the model is not expected to hold for water sorption in foods for a wide range of moisture, but calculation for the monolayer concept is considered effective for estimating the amount of bound water to specific polar sites in dehydrated food system.Citation33 While the GAB isotherm equation is the extension of the BET model, introducing a third parameter, K, which involves the modified properties of the sorbate in the multilayer region and bulk liquid properties. The GAB model is considered the best fit for food materials with a wide range of water activity 34
Table 3. Estimated GAB and BET parameters of coconut sugar powder produced by traditional method and drying methods.
The fitness of the model was analysed by MRD value, where the less the value the better for the model to fit. MRD <5 indicated best fit for the sample, while 5 < MRD < 10 indicated good fit, while MRD > 10 indicated unsuitable fit of the model used. As given in , based on the smallest value of MRD for each sample, the GAB model gave a better fit to water sorption of dried coconut sugar powder than BET model. The GAB model also fitted the water sorption isotherms of vacuum-dried honey powder compared to BET model Citation10
Xm or the monolayer moisture content indicates the amount of absorbed water to specific sites at the food surface. To ensure that there is a minimal rate of deteriorative reactions, except for oxidation of unsaturated fats, the safest water activity level is the one corresponding with Xm or lower.[Citation35,Citation36] As given in , the BET monolayer water content of coconut sugar powder is in the range of 0.025–0.056 g/g. The highest monolayer moisture content is shown by spray dried coconut sugar powder with the highest maltodextrin content. Similar results were found with vacuum-dried honey powder Citation10, freeze-dried papaya pulp powderCitation37, in which increasing maltodextrin resulted in higher monolayer water content, thus creating a stable honey powder. This is due to the high molecular weight of maltodextrin, which results in a higher glass transition temperature, giving a more stable powder at room temperature.
The coconut sugar powder obtained by traditional method showed the least water sorption at each aw compared to coconut sugar powder from other treatments. This might be due to the predominantly crystalline structure of traditional coconut sugar powder than those of drying methods. Drying method commonly produced amorphous structure Citation38 and semi-crystalline structure. Crystalline material absorb small amount of water until deliquescence occurs, compared to amorphous structure which absorbs more water in low water activity.Citation5 After deliquescence point, equilibrium curve in crystalline will significantly increase.Citation39 Amorphous lactose also absorbed more water in low water activity compared with crystalline lactose.Citation40 Higher monolayer water content correlated to higher amorphous content of semi-crystalline lactose powder.Citation41 From , it is known that the coconut sugar powder obtained by spray drying method showed the highest monolayer water content, meaning higher amorphous content than the coconut sugar powder from other treatments. The traditional coconut sugar which predominantly showed crystalline structure had the least monolayer water content.
X-ray diffraction
The structure of powder particle, whether it is a crystalline or amorphous can be determined by means of analysis X-ray diffraction. Each dried sample was compared to coconut sugar powder produced by traditional method. The traditional coconut sugar showed the typical crystalline XRD pattern and had been confirmed being crystalline sucrose structure. In traditional method, coconut sap was heated until reaching its saturated liquid, the heat then lowered and continued to stir until crystallized sugar was formed. The pH of the coconut sap should be neutral (or higher) to avoid hydrolysis of sucrose during heating that resulting in crystallized structure not formed into glucose and fructose. Thus, the farmers usually add preservative and lime into coconut sap to make crystallized sugar in the next process. In drying process (both spray drying and vacuum drying), the evaporation of water is too quick that might be not enough time for sucrose to crystallize during drying and consequently less crystalline structure formed. Some crystalline structure was reported in vacuum-dried honey powder Citation5 and spray-dried sucrose-lactose powder.Citation42 The result is shown in .
Figure 2. The relationship between moisture content and water activity of coconut sugar powder produced by traditional method and drying methods. The insetshows a zoom picture of low aw.
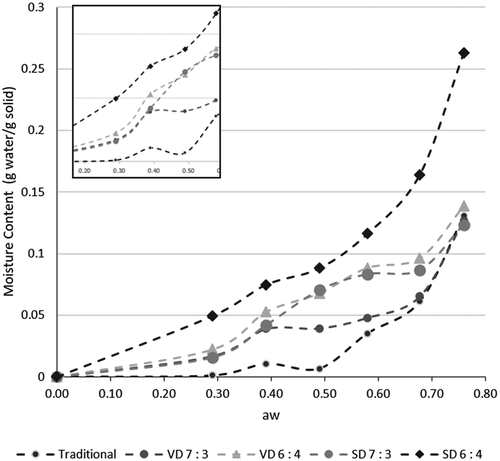
Figure 3. X-ray diffraction poster of coconut sugar powder particles produced by traditional method and drying methods.
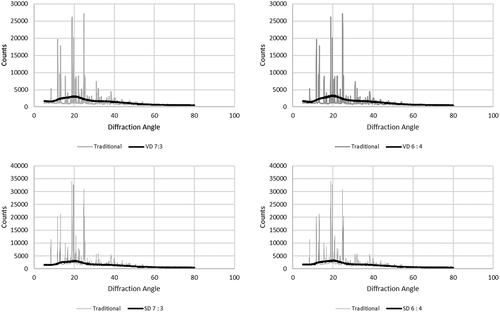
X-ray diffraction profile between traditional and drying methods showed substantial difference. Compared to traditional coconut sugar powder, the presence of sharp and defined peaks in crystalline materials is due to the fact that the molecules are in an orderly manner while amorphous material with its disorderly molecules will produce disperse bands and creating wide peak. Citation43 Maltodextrin incorporated spray-dried mango powder showed similar x-ray diffraction profile Citation44, spray-dried coconut sap Citation45, and spray-dried sucrose powder.Citation46 Amorphous nature of dried coconut sugar powder is due to rapid evaporation and formation of particles; hence, there is no time for the particles to be aligned.
Structural composition of coconut sugar samples is presented in . Traditional method produced 90.5% crystalline and 9.5% amorphous structure. Amorphous structure of dried coconut sugar powder ranged from 70.9% to 71.40% while crystalline state ranged from 28.6% to 30.75%. Increase of maltodextrin concentration showed a slight increase in crystalline composition in coconut sugar powder both in spray drying and in vacuum drying.
Figure 4. Structural composition of coconut sugar powder produced by traditional method and drying methods.
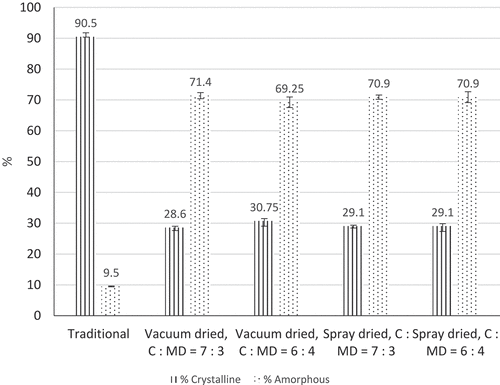
Vacuum drying method seemed to produce coconut sugar with more crystalline structure than spray drying method. This might be due to the lower drying rate that allows crystallization to occur compared to short drying process in spray drying. Vacuu- dried coconut sugar powder was ground to create powder. Grinding or milling crystalline particles might reduce the total crystallinity Citation47
Hygroscopic rate
shows the hygroscopic rate of coconut sugar powder produced by traditional method and drying methods. Increasing maltodextrin concentration reduces the rate of hygroscopicity in spray-dried coconut sugar powder as shown in Figure. Same results were found with spray-dried sugar cane powder Citation48, spray-dried grape syrup Citation49, and spray-dried acai powder Citation17, suggesting that maltodextrin is an efficient drying aid in sugar-rich material spray drying process. This is due to the fact that maltodextrin has the ability to decrease absorbed water by balancing the hydrophilic/hydrophobic sites of powder particles and high glass transition temperature.Citation50
Figure 5. Hygroscopic rate of coconut sugar powder produced by traditional method and drying methods.
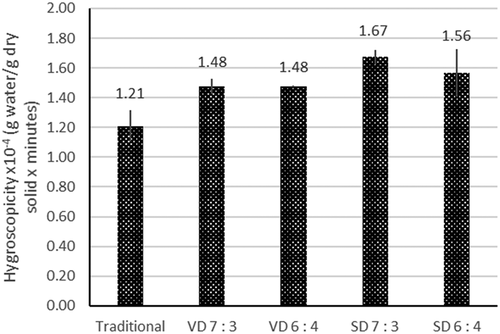
Hygroscopic rate of spray dried coconut sugar powder is higher than vacuum dried coconut sugar powder. Similar result was found in drying of passion fruit pulp powder and honey powder, spray-dried powder resulted in higher water adsorption capacity than vacuum drying due to its spherical particle shape which provides larger surface area.[Citation19,Citation36] Based on XRD analysis, increase in maltodextrin showed a less amount of amorphous structure that might impact its reactivity to surrounding moisture. More amorphous structure in powder is likely to cause more adsorption of water.
The traditional coconut sugar powder had the least hygroscopic rate, this might be due to its higher crystalline structure. Properties of crystalline structure are non-hygroscopic, stable, and free-flowing because crystalline structures are tightly packed so that water absorption only takes place on the external surface of the crystal. Amorphous structures are haphazard, tangled, more open, and porous; therefore, an individual molecule possesses more sites for external interaction, so amorphous structure can absorb water easily.Citation38 Some study also states that amorphous structure is more hygroscopic because they have larger porosity and volume.Citation51
Colour
The colour parameters of coconut sugar powder are shown in . Traditional coconut sugar powder showed low L* and high a* and b* values. Spray-dried coconut sugar powder shows a higher value of L* than other methods. Furthermore, higher maltodextrin concentration also contributes to higher L* value. The colour of vacuum-dried coconut sugar powder C : MD (7 : 3) and C : MD (6 : 4) seemed not different visually (). Higher a* values were shown by vacuum-dried coconut sugar powder, while increasing maltodextrin concentration in spray drying will decrease a* value. A slight difference was observed in b* value. The highest value of b* was shown by vacuum-dried C : MD (7 : 3), followed by vacuum-dried C : MD (6 : 4), spray-dried C : MD (7 : 3), and spray-dried C : MD (6 : 4). Increasing maltodextrin ratio may decrease b* value, meaning less yellow in colour.
Figure 6. L*, a*, and b* values of coconut sugar powder produced by traditional method and drying methods.
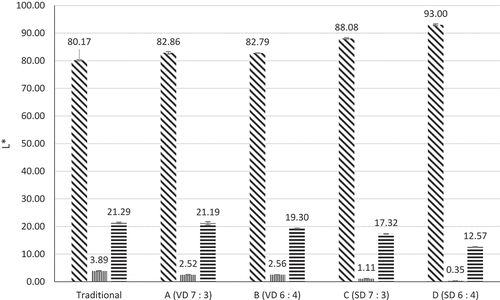
Traditional coconut sugar powder appeared to be more red and yellow, less bright than vacuum drying, while spray-dried sugar was vice versa. Brighter colour was obtained by using spray dryer because the sample was converted into small droplets, hence the quicker the time for the particles to dry.Citation31 Since browning increases with time, it can be easily explained that the thicker samples took more time to dry Citation52, which clarify the darker colours from vacuum-dried samples. Another contributing factor to slightly darker colour is Maillard reaction, which happens between reducing sugars and amino acids in foods which undergo thermal processing.Citation53 Maillard reaction was strongly affected by temperature, where browning reaction can increase alongside with increasing temperature. Traditional coconut sugar powder was produced using high temperature and exposed for a long time, it caused a more dominant browning colour than those produced by drying methods. Additionally, increasing maltodextrin will produce coconut sugar powder with a brighter colour, with a decrease in redness and yellowness. Similar results have been reported in spray-dried coconut sugar powder and foam-mat dried tamarind powder.[Citation14,Citation54]
Dissolving time
Crystalline structure would take more time to dissolve than amorphous structure Citation43 Traditional coconut sugar powder tend to dissolve slowly because dissolution occurs only at the outer surface exposed to the solvent. The particles are impermeable to the solvent because the molecules are bonded tightly and density is high; therefore, the dissolution proceeds from outside to inside. In contrast, amorphous powders particles are porous and hygroscopic and so they can dissolve rapidly.Citation38 Similar dissolving time was observed from vacuum-dried coconut sugar powder and traditional (crystallized) coconut sugar () . This could be explained that coconut sugar both in crystallized and in amorphous forms composed mainly of sucrose having particle density higher than water consequently sink easily when it poured to water and dissolve afterward.
From Figure 8, shorter dissolving time in vacuum drying is due to the different principles of drying between spray dryer and vacuum drying, at which vacuum drying can result in larger particles, more soluble and less dense than those obtained in conventional equipment, which led to particles with lower moisture content and consequently lower water activity.Citation55 The spray-dried coconut sugar powder took the longest time to fully dispersed or completely dissolved in water. This is due to more porous structure of spray-dried coconut sugar powder, hence less density compared to those of vacuum dried. Less density impacted on low sink ability, which would further take effect on longer dissolving time.[Citation56,Citation57] The result also showed that the dissolving time of coconut sugar powder also increases with the increase of maltodextrin concentration in spray drying. Higher maltodextrin concentration can lead to greater bulk density and particle size, which also related to agglomeration process. Powder with high bulk density will be less porous and could be a contributing factor to reduction of water absorption.[Citation14,Citation50]
Powder recovery
Powder recovery of coconut sugar powder is affected by drying methods as shown in . Vacuum-dried coconut sugar powder resulted in higher powder recovery than that of spray drying. Spray drying can produce fine dry particles by atomizing sample into small droplets and blown by hot air, the droplet will dry quickly, and the dried particles will fall into the lower part of the dryer. Citation31 Despite its speed to dry samples, powder can stick onto the drying chamber during spray drying, resulting in smaller powder recovery and operational problems, caused by high adhesiveness and hygroscopicity.[Citation58,Citation59] While the drying rate in vacuum drying was lower compared to spray drying, evaporation of water occurs at low oxygen pressure, resulting in a smaller chance of oxidative degradation, e.g., enzymatic browning caused by the oxidation process in the final product. Vacuum condition can cause water to boil, thus creating a porous structure that helps to remove moisture quickly while drying. Citation10
Figure 8. Dissolving time of coconut sugar powder produced by traditional method and drying methods.
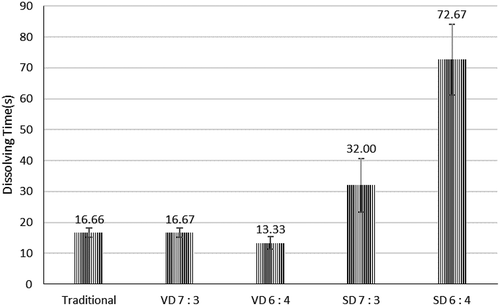
Figure 9. Powder recovery of coconut sugar powder produced by traditional method and drying methods.
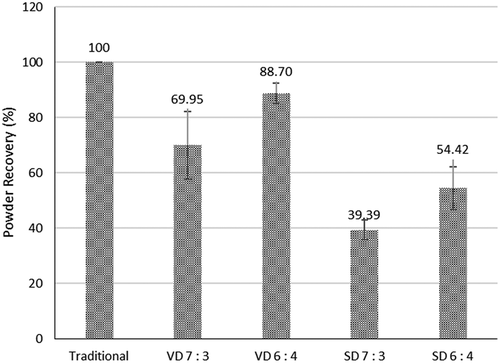
Powder recovery of traditionally produced coconut sugar powder has a higher value than vacuum-dried and spray-dried coconut sugar powder. Traditionally produced coconut sugar powder is made by the slow crystallization of sucrose because of low rate of water evaporation from the sap until it becomes oversaturated.Citation60 This condition caused a co-crystallization in sugar and turns the particles in coconut sugar powder into crystalline form.Citation61 This high amount of crystalline particle in traditionally produced coconut sugar powder makes them more stable. Thus, the instantaneous process of stickiness in the amorphous sugar because of plasticization of particle surfaces which allows a sufficient decrease of surface viscosity for the formation of liquid bridges between particles does not occur.Citation62 Because of it, traditionally produced coconut sugar powder has the highest powder recovery among the others with the value nearly reaching 100%. Sugarcane processing into crystals was made by adding calcium carbonate, yielding 96 to 98% sucrose. Citation38
A higher result of powder recovery is obtained with increasing maltodextrin concentration by using spray drying and vacuum drying. Increasing maltodextrin will also create a higher content of total solids in the solution, it would produce more density, meaning higher viscosity; this condition could lead to lower values of radial speed of spray drying and will reduce the intensity and speed of droplets colliding with the internal wall of the drying chamber, thus more powder is recovered and lesser deposits in the chamber.Citation48 Addition of maltodextrin into coconut sap solution will increase Tg, hence reducing stickiness by balancing hydrophilic and hydrophobic sites of sugar powder. This condition will reduce the amount of absorbed water in which hydrogen bond in the water molecule and hydroxyl in the amorphous and crystalline structure are responsible.Citation59 More powder recovery is also obtained with increasing maltodextrin concentration in spray-dried coconut sap, sucrose, honey, and concentrated fruit juices.[Citation45,Citation58] Substituting maltodextrin with whey protein isolate could also increase powder recovery of spray dried coconut sugar powder from 39.39–54.42% up to 67.82%–72.55% (data not shown).
Conclusion
Coconut sugar powder was produced using traditional and drying methods (spray drying and vacuum drying) by addition of drying aid in the form of maltodextrin. Traditional coconut sugar powder has a dominant crystalline structure while dried ones have amorphous structure. Traditional coconut sugar powder had a dominant crystalline structure (90.5%), with lowest monolayer moisture content, hygroscopic rate, L* and highest a* and b* values compared to drying methods. The drying methods (vacuum and spray drying) produced coconut sugar powder successfully with properties different from sugar obtained by using traditional method. Increasing maltodextrin would increase the stability of coconut sugar powder obtained indicated by higher monolayer moisture content, low higroscopic rate, and low amorphous content. This study offers a better understanding of crystalline and amorphous coconut sugar characteristics which is produced by adifferent method. Such information can be used as an alternative process to produce coconut sugar powder with desired properties.
Additional information
Funding
References
- Karseno, E.; Yanto, T.; Setyowati, R.; Haryanti, P. Effect of pH and Temperature on Browning Intensity of Coconut Sugar and Its Antioxidant Activity, Food Res 2018, 2, 32–38.
- Santoso, H.B.;. Pembuatan Gula Kelapa; Penerbit Kanisius: Yogyakarta, 1993.
- Le Meste, M.; Champion, D.; Roudaut, G.; Blond, G.; Simatos, D. Glass Transition and Food Technology : A Critical Appraisal. Journal of Food Science 2002, 67, 7.
- Nurhadi, B.; Nurhasanah, S. Sifat Fisik Bahan Pangan; Widya Padjadjaran: Bandung, 2010.
- Nurhadi, B.; Roos, Y.H. Dynamic Water Sorption for the Study of Amorphous Content of Vacuum-Dried Honey Powder. Powder Technol [Internet] 2016, 301, 981–988. Available from. doi:10.1016/j.powtec.2016.07.055
- Roos, Y.; Karel, M. Amorphous State and Delayed Ice Formation in Sucrose Solutions. International Journal Food Sciences Technological 1991, 26(6), 553–566.
- Nurhadi, B.; Roos, Y.H.; Maidannyk, V. Physical Properties of Maltodextrin DE 10: Water Sorption, Water Plasticization and Enthalpy Relaxation. Journal of Food Engineering 2016, 174, 68–74.
- Nurhadi, B.; Roos, Y.H. Influence of Anti-Caking Agent on the Water Sorption Isotherm and Flow-Ability Properties of Vacuum Dried Honey Powder. Journal of Food Engineering 2017, 210, 76–82.
- Roos, Y.H.;. Phase Transitions in Foods; Academic Press, Inc: California, 1995.
- Nurhadi, B.; Roos, Y.H. Water Sorption and Water Plasticization Behavior of Vacuum Dried Honey. International Journal Food Prop [Internet] 2015, 2912(September), 150911025551003. Available from http://www.tandfonline.com/doi/full/10.1080/10942912.2015.1081607
- Nurhadi, B.;. Maltodextrin-Incorporated-Vacuum-Dried Honey Powder: Processing and Stability; University College Cork, 2016.
- Jaya, S.; Das, H.; Mani, S. Optimization of Maltodextrin and Tricalcium Phosphate for Producing Vacuum Dried Mango Powder. Int J Food Prop 2006, 9(1), 13–24.
- Anandharamakrishnan, C.; Ishwarya, P.I. Spray Drying Technique for Food Ingredient Encapsulation. West Sussex, UK: IFT Press; 2015, 294(p).
- A-Sun, K.; Thumthanaruk, B.; Lekhavat, S.; Jumnongpon, R. Effect of Spray Drying Conditions on Physical Characteristics of Coconut Sugar Powder. International Food Researcher Journal 2016, 23(3), 1315–1319.
- Putra, I.N.K.P.;. Upaya Memperbaiki Warna Gula Semut Dengan Pemberian Na-Metabisulfit. Journal Apl Teknol Pangan [Internet] 2016, 5(1), 1–6. Available from http://jatp.ift.or.id/index.php/jatp/article/view/2
- AOAC. Official Methods of Analysis, 18th Editi ed.; AOAC International: AOAC International. USA, 2006.
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe Oleraceae Mart.) Powder Produced by Spray Drying. Journal Food Engineering 2008, 88(3), 411–418.
- Seid, R.M.; Hensel, O. Experimental Evaluation of Sorption Isotherms of Chili Pepper: An Ethiopian Variety, Mareko Fana (Capsicum Annum L.). Agricultural Engineering International CIGR Journal 2012, 14(4), 163–172.
- Nurhadi, B.; Andoyo, R.; Mahani, I.R. Study the Properties of Honey Powder Produced from Spray Drying and Vacuum Drying Method. International Food Researcher Journal 2012, 19(3), 907–912.
- Konica Minolta. Spectrophotometer; Japan: Konica Minolta, 2009.
- GEA Niro Research Laboratory. A 6 A -Powder Dispersibility IDF Method GEA Niro Method No. A 6 A. 2005;(September). Available from: http://www.gea.com/ru/binaries/A 6 a - Powder Dispersibility IDF Method_tcm27-30911.pdf
- Wilhelm, L.R.; Suter, D.A.; Brusewitz, G.H. Food & Process Engineering Technology. Chapter 10: Drying and Dehydration. In American Society of Agricultural Engineers. Michigan: American Society of Agricultural Engineers 2004; 259–284. Available fromhttps://www.asabe.org/media/184966/chapter_10_in_wilhelm_food_proc._eng._tech.pdf
- Xia, Q.; Li, R.; Zhao, S.; Chen, W.; Chen, H.; Xin, B.;, et al. Chemical Composition Changes of Post-Harvest Coconut Inflorescence Sap during Natural Fermentation. African J Biotechnol. 2011, 10(66), 14999–15005.
- Haryanti, P.; Supriyadi, M.D.W.; Santoso, U. Chemical Properties of Coconut Sap Obtained at Different Tapping Time and Addition of Preservatives. International Journal Sciences Technoledge [Internet] 2017, 5(3), 52–59. Available from www.theijst.com
- Roos YH. Water Activity | Effect on Food Stability. In: Encyclopedia of Food Sciences and Nutrition. Second Edi. Academic Press, Inc.; 2003. p. 6094–6101.
- Kalaiyarasi, K.; Sangeetha, K.; Rajarajan, S.; Author, S., Dr; Rajarajan, C. A Comparative Study on the Microbial Flora of the Fresh Sap from Cut Inflorescence and Fermented Sap (Toddy) of Borrassus Flabellifer Linn (Palmyrah Tree) and of Cocos Nucifera Linn (Coconut Tree) to Identify the Microbial Fermenters. International Journal Researcher Pure Applications Microbiologic 2013, 3(3), 43–47.
- Ysidor, K.N.; Rachel, A.R.; Jean-Louis, K.K.; Muriel, O.D.; Prades, A.; Kouassi, A.;; et al. Glucide Factors of the Inflorescence Sap of Four Coconut (Cocos Nucifera L .). Cultivars from Côte D ’ Ivoire 2014, 4(2), 116–127.
- Iwuoha, C.I.; Eke, O.S. Nigerian Indigenous Fermented Foods: Their Traditional Process Operation, Inherent Problems, Improvements and Current Status. Food Researcher International [Internet] 1996, 29(5–6), 527–540. Available from http://linkinghub.elsevier.com/retrieve/pii/0963996995000453
- Zuliana, C.; Widyastuti, E.; Susanto, W.H. Pembuatan Gula Semut Kelapa (Kajian pH Gula Kelapa Dan Konsentrasi Natrium Bikarbonat). J Pangan dan Agroindustri 2016, 4(1), 109–119.
- Michalska, A.; Lech, K. The Effect of Carrier Quantity and Drying Method on the Physical Properties of Apple Juice Powders. Beverages 2018, 4(2), 1–15.
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent Advances on Chitosan-Based Micro- and Nanoparticles in Drug Delivery. Journal of Controlled Release : Official Journal of the Controlled Release Society 2004, 100(1), 5–28.
- Sonthipermpoon, W.; Suwonsichon, T.; Wittaya-Areekul, S.; Wuttijumnong, P. Effect of Maltodextrin on Glass Transition Temperature and Water Activity of Production Banana Flake. Kasetsart Journal - Natural Sciences 2006, 40(3), 708–715.
- Rizvi, S.S.H.;. Thermodynamic Properties of Foods in Dehydration. In Engineering Properties of Foods 3rd edtion; Rao, M.A.; Rizvi, S.S.H.; Datta, A.K. Eds.; CRC Press: Boca Raton, 2005.
- Goula, A.M.; Karapantsios, T.D.; Achilias, D.S.; Adamopoulos, K.G. Water Sorption Isotherms and Glass Transition Temperature of Spray Dried Tomato Pulp. Journal of Food Engineering 2008, 85(1), 73–83.
- Pedro, M.A.M.; Telis-Romero, J.; Telis, V.R.N. Effect of Drying Method on the Adsorption Isotherms and Isosteric Heat of Passion Fruit Pulp Powder. Ciência E Tecnol Aliment [Internet] 2010, 30(4), 993–1000. Available from http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-20612010000400024&lng=en&nrm=iso&tlng=en
- Canuto, H.M.P.; Afonso, M.R.A.; Da Costa, J.M.C. Hygroscopic Behavior of Freeze-Dried Papaya Pulp Powder with Maltodextrin. Acta Sciences - Technological 2014, 36(1), 179–185.
- Onluwata, C.;. Encapsulated and Powdered Foods; Taylor & Francis Group: Boca Raton, 2005.
- Lipasek, R.A.; Taylor, L.S.; Mauer, L.J. Effects of Anticaking Agents and Relative Humidity on the Physical and Chemical Stability of Powdered Vitamin C. Journal of Food Science 2011, 76(7), 1062–1074.
- Bronlund, J.; Paterson, T. Moisture Sorption Isotherms for Crystalline, Amorphous and Predominantly Crystalline Lactose Powders. International Dairy Journal / Published in Association with the International Dairy Federation 2004, 14(3), 247–254.
- Vollenbroek, J.; Hebbink, G.A.; Ziffels, S.; Steckel, H. Determination of Low Levels of Amorphous Content in Inhalation Grade Lactose by Moisture Sorption Isotherms. International Journal of Pharmaceutics 2010, 395(1–2), 62–70.
- Islam, I.U.; Langrish, T.A.G. Modelling Crystallization in Spray Drying for Food Powder Production. In Handbook of Food Powders Process and Properties; Bhandari, B.; Bansal, N.; Zhang, M.; Schuck, P., Eds.; Woodhead Publishing: Oxford, 2013.
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the Carriers on the Microstructure of Mango Powder Obtained by Spray Drying and Its Functional Characterization. Innov Food Sciences Emergency Technological 2005, 6(4), 420–428.
- Jayasundera, M.;. Spray Drying of Unfermented Coconut Sap; Ann Food Sci Technol. 2014, 15(2), 259–264.
- Jayasundera, J.M.M.A.; Kulatunga, A.R. Spray-Drying of Coconut Treacle into an Amorphous Powder. Emirates Journal Food Agricultural 2014, 26(8), 672–678.
- Adhikari, B.; Howes, T.; Bhandari, B.R.; Langrish, T.A.G. Effect of Addition of Proteins on the Production of Amorphous Sucrose Powder through Spray Drying. Journal Food Engineering [Internet] 2009,94(2), 144–153. Available from. doi: 10.1016/j.jfoodeng.2009.01.029.
- Einfal, T.; Planinšek, O.; Hrovat, K. Methods of Amorphization and Investigation of the Amorphous State. Acta Pharmaceutica (Zagreb, Croatia) 2013, 63(3), 305–334.
- Avila, E.L.; Rodríguez, M.C.; Velásquez, H.J.C. Influence of Maltodextrin and Spray Drying Process Conditions on Sugarcane Juice Powder Quality. 2015, 68(1), 7509–7520.
- Sarabandi, K.; Peighambardoust, S.H.; Shirmohammadi, M. Physical Properties of Spray Dried Grape Syrup as Affected by Drying Temperature and Drying Aids. International Journal Agricultural Crop Sciences 2014, 7, 928–934.
- Shi, Q; Fang, ZBhandari, B. Physicochemical properties of spray dried honey powder produced with whey protein isolate and maltodextrin as carrier agents. Shandong University of Technology; 2013. 31 113-1692 doi:10.1080/07373937.2013.783593
- Bhandari, B.R.; Datta, N.; Crooks, R.; Howes, T.; Rigby, S. A Semi-Empirical Approach to Optimise the Quantity of Drying AIDS Required to Spray Dry Sugar-Rich Foods. Dry Technol 1997, 15(10), 2509–2525.
- Mitra, J.; Shrivastava, S.L.; Rao, P.S. Non-Enzymatic Browning and Flavour Kinetics of Vacuum Dried Onion Slices. International Agrophysics 2015, 29(1), 91–100.
- Mottram, D.S.; Elmore, J.S. Control of the Maillard Reaction during the Cooking of Food. ACS Symposium Series 2010, 1042, 143–155.
- Ekpong, A.; Phomkong, W.; Onsaard, E. The Effects of Maltodextrin as a Drying Aid and Drying Temperature on Production of Tamarind Powder and Consumer Acceptance of the Powder. International Food Researcher Journal 2016, 23(1), 300–308.
- Ramos, F.D.M.; Oliveira, C.C.M.D.; Soares, A.S.P.; Silveira Júnior, V. Assessment of Differences between Products Obtained in Conventional and Vacuum Spray Dryer. Food Sciences Technological 2016, 36(4), 724–729.
- Peleg, M.;. Physical Characteristics of Food Powders. In Physical Properties of Food; Peleg, M.; Bagley, E., Eds.; AVI Publishing Company: Connecticut, 1983.
- Barbosa-Canovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Hon, Y. Food Powders: Physical Properties, Processing, and Functionality; Kluwer Academic/Plenum Publisher: New York, 2005.
- Bhandari, B.R.; Senoussi, A.; Dumoulin, E.D.; Lebert, A. Spray Drying of Concentrated Fruit Juices. Dry Technological An International Journal 1993, 11(5), 1081–1092.
- Shi, Q.; Fang, Z.; Bhandari, B. Effect of Addition of Whey Protein Isolate on Spray-Drying Behavior of Honey with Maltodextrin as a Carrier Material. Dry Technol 2013, 31(13–14), 1681–1692.
- Starzak, M.; Mathlouthi, M. Formation of Amorphous Sugar in the Syrup Film – A Key Factor in Modelling of Industrial Sugar Drying. Food Chemistry 2010, 122(2), 349–409.
- Sekhon, B.S.;. Pharmaceutical Co-Crystals – A Review. Ars Pharmaceutical 2009, 50(3), 99–117.
- Palzer, S.; Sommer, K. Caking of Water-Soluble Amorphous and Crystalline Powders - Material Properties, Adhesion Forces, Stickiness and Kinetic of Time Consolidation. Food Engineering Interfaces. Food Engineering Interfaces 2009, 491–514.

