 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The purpose of this study was to compare the essential oil composition of Inula viscosa leaves by hydrodistillation (HDE), ultrasonic (UDE) and solvent (SE) extractions followed by hydrodistillation. The total polyphenol and flavonoid contents and their antioxidant effects were studied by different solvent of extraction: ethanol (ET), ethyl acetate (EA), methanol (ME) and aqueous (AE). The principal compounds for HDE were: 2-hexenal (3.70%), caryophyllene oxide (3.11%), γ-selinene (3.09%), 3-hexen-1-ol (2.00%), eugenol (1.70%) and trans-caryophyllene (1.34%), while for UDE were: γ-selinene (5.68%), caryophyllene oxide (4.87%), trans-caryophyllene (1.99%) and nerolidol (1.74%). The oil obtained by SE was shown to contain tridecane (3.89%), dodecane (3.08%), trans-caryophyllene (2.94%), caryophyllene oxide (2.56%) and nerolidol (2.53%). Significant changes on phenolic contents were found between the different solvent of extraction. ME and AE extracts led to the highest total polyphenol (PHL) and flavonoid (FL) amounts. The anti-radical activity and reducing power were maximal in AE and ME extract. HPLC examination established that the ferulic acid as major phenolic acid in ME and AE fractions, whereas luteolin was the main compound of EA and ET fractions.
Introduction
Recently, the research focused on natural molecules having the antioxidants potential, particularly originating from plants. Among, the medicinal and culinary plants, a few endemic species are of special importance owing to they perhaps used for generating raw materials having phytochemicals with antioxidant potential.[1]In other hand, the antioxidant inhibition of plant could be principally attributed to polyphenolic compounds, such as flavonoids and phenolic diterpenes.[Citation2] Phenolics have a main function in individual health by reason of their anti-inflammatory, anti-allergic, antimicrobial, anticarcinogenic and anti-viral capacities.[Citation3] Confer to Pinelo et al.[Citation4] various solvent are developed for the isolation of polyphenols from plant, but the production is inclined by the type of the solvent and the processes of extraction.[Citation5] Bestow to Akowuah et al.,[Citation6] depending on the solvent used for extracting phenolic compounds, the fractions resulted from the same plant may vary widely their antioxidant concentration and activities. In tea, it can be mentioned that the fraction obtained by aqueous mixed with ethanol were most powerful than methanol and acetone for separation of flavonoids.[Citation7] The importance of aromatic and medicinal plants (PAM) comes from a big fraction of the plant biodiversity where each species brings its procession of benefits and assets. In Tunisia, climatic, pedological, and plant genetic diversity has favored the production of a flora (estimated at about 2200 species) containing 149 medicinal species and 38 aromatic species.[Citation8] Indeed, these species are exploited in human food and traditional medicine.
Inula species are the principal of the important PAM from the Asteraceae family. In Tunisia, the genus Inula is represented by four species: Inula viscosa (L.) Ait, Inula graveolens (L.) Desf, Inula crithmoïdes (L.) and Inula montana (L.) var. calycina (Pres l) Batt.[Citation9] I. viscosa is traditionally exploited as an antiseptic, hypoglycemic agent and to treat tuberculosis, anemia, and inflammation.[Citation10–Citation12] Many researches are signaled that the some Inula species presented various functions in individual fitness by reason of their biological capacities.[Citation13–Citation15] In this essay, we have three objectives (i) to compare different extraction methods of EO from I. viscosa, (ii) to estimate the antioxidant potentialities according to the solvent of extraction, and (iii) to mark the phenolic compounds in these species by RP-HPLC.
Materials and methods
Vegetal collection
The leaves of I. viscosa used in this work are native of City Chaker of region Rawed-Ariana. The sampling was collected in February. Leaves were dried in the dark, and then they are finely crushed and kept in a dessiccateur until the moment of the analysis. Authentication was confirmed by Pr. A. Samoui, (Centre of Biotechnology of Borj-Cedria and bestowing to “Fore de la Tunisie”.[Citation9]
Preparation of extracts
The dried powdered were successively extracted to Soxhlet apparatus using: Ethyl acetate (EA), ethanol (ET), methanol (ME) and aqueous (AE). Between each step, solvent extracts are filtrated with a Whatman filter paper (N°4), concentrated under rotary vaccum evaporator (Rotovapor-El, LabortechnikAG, Büchi, Switzerland) at 40°C and conserved at 4°C for the various analyses.
Total polyphenol amounts (TPH)
The measurement of TPH is determined by spectrophotometry and are performed as detailed by Dewanto et al.[Citation16] The solution mixture composed 125 μL of extract, 500 μL of H2O and 125 μL of Folin-Ciocalteu reagent. After vigorous stirring of the mixture followed by a rest of 3 min, an intake of 1250 μL of 7% Na2CO3 and 3 mL of H2O. After 90 min of reaction at black, the absorbance is interpreted at 760 nm and the TPH content are given as mg equivalent gallic acid/g extract).
Total flavonoid amounts (FL)
250 µL of samples are combined with 75 µL NaNO2 (5%).[Citation16] After 6 min of reaction at ambient temperature, 150 µL of 10% AlCl3 and 500 µL of NaOH (1 M) are infused in the solution, followed by addition of 2.5 mL of H2O. The lecture of FL is done at 510 nm and the contents of FL are given in mg of catechin equivalent per gram of extract (mg CE/g extract).
Anti-radical activity
A 1000 μL of samples are infused in 500 μL of DPPH (0.2 mM).[Citation17] After vigorous stirring of the mixture, it was kept at rest for 30 min in the dark. The lecture is made at 517 nm. The anti-radical activity is calculated as percent inhibition (PI) of the DPPH:
DOcontrol: the absorbance of the control,
DOextract: the absorbance of the extract.
Reducing power
This method consists of mixing 1000 μL of the fraction at different concentrations with 1250 μL of phosphate buffer (0.2 mol/L, pH 6.6) and 1250 μL of K3Fe(CN)6 (1%).[Citation18] Then, the solution is put in the water bath for 20 min at 50°C. In reason to stop the reaction, we are added 1250 μL of TCA (10%), followed by centrifugation at 650 g for 10 min at 25°C. Finally, at the intake of 1250 μL of the supernatant, 1250 μL of H2O and 250 μL of FeCl3 (0.1%) are added. Absorbance is measured at 700 nm.
Analysis of phenolic profile by RP-HPLC
The analysis phenolic compounds are done by RP–HPLC of the type Agilent Technologies 1100, equipped with a visible UV detector to length variable wave and provided with a C18 Hypersil ODS column (250 x 4.6 mm, 4 μm), at 25°C. The mobile phase is given as follows: solvent A: acetonitrile and solvent B: H2O at 0.2% sulfuric acid. The elution gradient chosen is as follows: 15% A/85% B 0–12 min, 40% A/60% B 12–14 min, 60% A/40%B 14–18 min, 80% A/20% B 18-20 min, 90% A/10% B 20–24 min, 100% A 24–28 min. The flow rate is done at 0.5 mL/min.
Isolation methods
Hydro-distillation extraction (HDE): EO is extracted from leaves of Inula by Clevenger system during 4 h placing 100 g of dried material in a ball containing 1000 mL of water brought to a boil.[Citation19]
Solvent extraction (SE): The method used for the extraction of EO by hexane is that recommended by Pérez-Alonso and Negueruela.[Citation20] The sample is extracted for 8 h at 25°C with hexane (1 l). After filtration, the solution is concentrated under rotary vacuum evaporator and maintained to distillation.
Ultrasonic-distillationextraction (UDE): A 75 g of the Inula leaves were emerged in 500 mL of H2O. The solution is conducted in an ultrasonic cleaner (BRANSON 3210,130 watt, 70% amplitude and a frequency of 20 kHz) with distilled water, using selected extraction time for 30 min, ultrasonic temperature (range from 60 to 80°C). Then, the leaves of I. viscosa were hydrodistilled for 4 h using a Clevenger.[Citation21]
GC–FID analysis
The analysis of the constituents of EO was carried by means of a chromatograph HP6890 provided with a flame ionization detector and equipped with a capillary column of type HP Innowax (30 m; 0,25 mm; 0,25 µm) the polar still phase of which is made by polyethylene glycol. The temperature of the oven was scheduled in the following way: isotherm at 35°C during 10 min, from 35°C to 205°C (2°C/min) and isotherm at 205°C during 10 min. The injector and the detector are carried, respectively, at 250°C and 300°C, the flow of the carrier gas (N2, U) was 1.6 ml/min and the analyses are led in mode Split (60:1).
GC–MS analysis
EO analysis by GC–MS was performed on an Agilent 7890A GC system, fixed to an Agilent 5972C mass spectroscopy detector with electron impact ionization (70 eV). A HP-5 MS capillary column (30 m x 0.25 mm, coated with 5% phenyl methyl silicone, 95% dimethylpolysiloxane, 0.25 mm film thickness; Hewlett-Packard, CA, USA) was used. The column temperature was programmed to rise from 40°C to 240°C with a 5°C/min rate, the carrier gas was helium N60 with a 0.9 mL/min flow rate; split ratio was 100:1. Scan time and mass range were 1 s and 50–550 m/z, respectively.
Statistical analysis
All tests were possessed in three replications and the averaging is performed by Variance Analysis (ANOVA) using STATISTICA software. Duncan’s multiple range test was used at the significance level of 5%. Data are recorded in tables as means with standard errors.[Citation22]
Results and discussion
Aroma distribution
The EO yield of the leaves of Inula isolated by the HDE, UDE, and SE methods were presented in . The essays showed that this yield varies significantly in relation to the method used for extraction. The yields of extractable of the UDE (0.45%) were considerably superior to those separated by the SE (0.2%). However, the lowest yield (0.12%) was recorded by HDE method. Our results were thus in accordance with those of Alalan et al.[Citation23] who found that the yield extracted by steam distillation from Syrian was 0.17%.Pérez-Alonso and Negueruela[Citation20] reported that the yield from Turkey was 0.2%. In other hand, the yield was detected in higher proportion in leaves extracted by supercritical fluid extraction (0.65%). More recently, Haoui et al.[Citation24] showed the leaves essential oil grow of Algeria was 0.148%. The EO isolated by hydrodistillation contained caryophyllene oxide (3.11%), γ-selinene (3.09%), 2-hexenal (3.70%), 3-hexen-1-ol (2.00%) and eugenol (1.70%) as major constituents (). The oil obtained by hexane extraction followed by hydrodistillation was characterized by tridecane (3.89%), dodecane (3.08%), trans-caryophyllene(2.94%), caryophyllene oxide (2.56%) and nerolidol (2.53%). In USE, the relative proportion of the compounds were different although, γ-selinene was the capital element (5.68%), followed by caryophyllene oxide (4.87%), trans-caryophyllene (1.99%), and nerolidol (1.74%). As a measure of another study, the EO compositions separated by hydrodistillation and ultrasonic were similar with those from Jordan, France, and Turkey especially concerning the amounts of caryophylleneoxide. However, the percentage of γ-selinene showed similarities with those from Jordan, France, and Italy.[Citation25,Citation26] Indeed, phytol was just identified in leaves of I.viscosaof Syria.[Citation23] EO profile of these species showed the main differences were compared from different region and habitats. Perez-Alonso and Velasco-Negueruela[Citation20] reported that the major constitutes in the EO in Turkey were: borneol (25.2%), isobornyl acetate (22.5%) and bornyl acetate (19.5%). While in Spain some other considerable chemical variability were reported as the chief components: fokienol(38.8%) and nerolidol (7.71%).[Citation27]
Figure 1. Essential oil yields (%) obtained by three different extraction methods (SDE, UDE, and HDE). Essential oil yields with different letters (a–c) were significantly different at p < 0.05 (Duncan test).
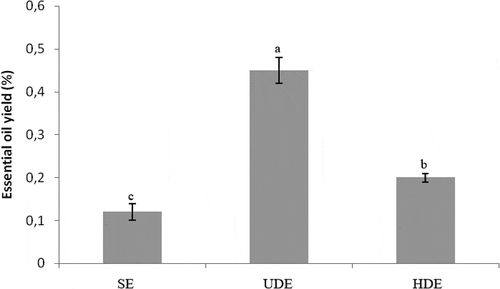
Table 1. Chemical composition of the essential oil of Inula viscosa leaves obtained by three different extraction methods.
TPH amounts
Methanol had the best extractant capacity, resulting in the highest TPH contents (248.87 mg GAE/g), followed by aqueous (243.70 mg GAE/g) and ethanol (96.58 mg GAE/g) (). The TPH separated by methanol fraction in this essay was superior to particular mentioned by the same species[Citation25,Citation28,Citation29] and the closely related species such as I. racemosa[Citation30] and I. crithmoides.[Citation31] However these amounts were lower than other ones reported in case of Inula from Morocco (274.39 ± 6.94) mg GAE/g extract).[Citation32]
Figure 2. Total phenolic and flavonoid contents of different solvent extracts of Inula viscosa leaves. (*) Total phenolics were expressed by mg of GAE/g of DW. Total flavonoid contents were expressed by mg of CE/g of DW. Values are represented as mean ± SD of triplicates. The data marked with the different letters in the histograms of each phenolic category share significant differences at p < 0.05 (Duncan test). GAE, gallic acid equivalents; CE, catechin equivalents.
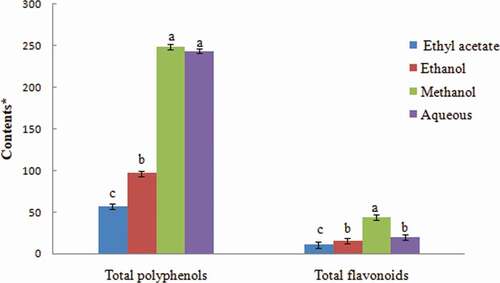
The lowest extracting powers were recorded by ethyl acetate. In this way, it can be classified as the four solvent used of extraction in decreasing order: ME, AE, ET, and EA. In contrast, Kenny et al.[Citation32] in the other Asteraceae species TPH amount dropped from 15.98 to 227.93 mg GAE/g of dry weight. Indeed, the TPH amount showed significant differences due to solvent of extraction and to varieties. In this context, Gökbulutet al.[Citation33] found that the TPH contents of three Inula from Turkey were varied from 21.1 to 190.9 mg GAE/g extract.
The importance of the solvent exploited in the separation, as demonstrated by Sun and Ho[Citation34] which noted that the ME of buckwheat gave a higher yield than the ET while these extracts expressed identical total phenol contents. Indeed, methanol was the best solvent of extracting phenolic acid and catechin, whereas flavonoids and their glycosides, catechols, and tannins were better extracted with ethanol.
TFL amounts
As for polyphenols, the leave extracts of Inula showed a variation of flavonoid contents which also bank on the nature of the solvent (). Methanol and aqueous fractions have the efficient flavonoid extracting powers whose contents were, respectively, 44.06 mg CE/g and 19.81 mg CE/g. However, the lowest contents were recorded in the ET and EA fractions (15.29 mg CE/g and 10.56 mg CE/g, respectively).TFL separated by ME from Turkey leaf was 77.5 mg QE/g extract.[Citation25] While in Morocco region, these level of flavonoid was detected of 44 µg/mg extract.[Citation35] In contrast, other studies were found that the TFL dropped from 1.13 to 182.56 mg CE/g dw.[Citation36] The polyphenol and flavonoid contents increased when the polarity of the solvent of extraction increased. In this context, our result was similar with those Li et al.[Citation37] which found that the nature of the extractant to be crucial to the polyphenol and flavonoid contents: a solvent with a higher polarity yielded upper TPH and TFL amounts.
Antioxidant activity
ME and AE fractions exhibited the upper most anti-radical capacities with the concentration corresponding to 50% of inhibition reaching 21 and 30 μg/mL, respectively (). The lowest antiradical capacity was obtained in ethyl acetate and ethanol fractions (IC50 values were 116 and 132 μg/mL, respectively). Our results were identical to those found by Mahmoudi et al.[Citation29] which found that the methanol fraction exhibited the high activity with 23.33 µg/mL and lower (0.26 mg/mL) than those reported by Trimech et al.[Citation28] In fact, obtained IC50 value were lower than described for other Asteraceae species such as Cichorium sp. (IC50 = 86 μg/mL), Sonchus sp. (IC50 = 72 μg/mL),[Citation38] but these values were varied of the concentration from 12.8 to 36 μg/mL ofInulacrithmoides.[Citation31] However, Gökbulut et al.[Citation33] reported that the ethyl acetate fraction had low antioxidant activity, with high IC50 values, compared to the residue prepared by AE and ME extracts of the Inula species. It was mentioned by numerous works the difference in the amount of polyphenol were related by the nature of the solvent considered of the extraction.[Citation39] In this context, Hayder et al.[Citation40] showed that polar fractions, for example, AE and ME exhibited a higher anti-radical power than a polar extracts (hexane).
Table 3. Phenolic compounds of different solvent extracts of Inula viscosa leaves.
The plant I. viscosa can be considered rich in phenolic compound which gives it a very important antioxidant capacity. Thereby, these results should reflect the therapeutic usefulness of this species.[Citation41] The consequence of solvent on the antioxidant ability of I. viscosaleaves was also analyzed by the determination of reducing powers (). AE and ME extracts exhibited higher reducing power towards the Fe3+/ferricyanide complex (EC50 = 260 and 270 μg/mL, respectively).EA and ET extracts presented the lower reducing capacity (EC50 = 550 and 500 μg/mL).
Phenolic profiles
The consequence of solvent extraction on the phenolic compound of I. viscosa leaves were presented in . Phenolic acids-p-coumaric, ferulic, and sinapic acids- represent the major fraction of the polyphenols analyzed in methanol (70.07%) and water (43.54%) extracts, while the flavonoid dominated in ethyl acetate (93.9%) and ethanol fractions (85.41%). The flavonoids were detected also in higher proportions with the aqueous extracts (56.46%). Indeed, the flavonoids fraction were characterized by catechin hydrate as a major compound in aqueous and methanol extracts and accounting with 39.59% and 16.49%, respectively.
Table 2. Antioxidant activity of Inula viscosa leaves.
Phenolic acid had an important presence in polarity solvent (methanol and aqueous). In addition, ferulic acid was the major predominant phenolic compounds in polar solvent of extraction, contributing for about 62.33% in methanol and 32.71% in aqueous extracts. As for flavonoids content, luteolin was the principal composite where extracted by EA (44.93%) and ET (39.54%). Gökbulut et al.[Citation33] were detected three flavonoids (quercetin, luteolin, and rutin) from I. viscosa leaves and dropped from 0.02% to 0.10% (w/w).
PCA was used out in raison to establish the rapport between the solvent of extraction related to their phenolic composition (). Obtained results noted the identification of two well-defined groups. The initial cluster was constituted by the extracts extracted by ME and AE implying identical constitution. EA and ET, which found as second group, were significantly discriminated by the later groups.
Figure 3. A three-dimensional visualization of PCA results of solvent extraction of Inula viscosa leaves based on their percentage of phenolic composition ().
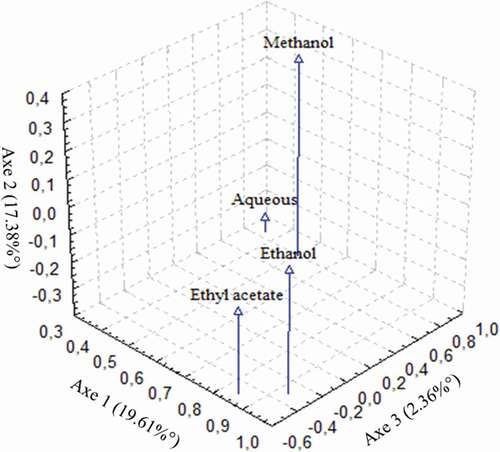
These variations in phenolic composition between the four solvent can be attribute of the polarity and thus extractability of the bioactive substances.[Citation42] Indeed, polarity of solvent produces significantly various extraction capacities for phenolic compounds in plants.[Citation37,Citation43] The result displayed that the TPH using HPLC indicated that ME and AE extracts contained more total phenolics compared to other extracts. However, the phenolic compositions selected by HPLC were different than those prepared by the Folin–Ciocalteu process. This variation observed by the Folin–Ciocalteu method was the procedure for estimating total polyphenol contents, characterized by a sensitivity and speed, but it was often coupled with an overestimation of the results, with a low accuracy.[Citation44]
Conclusion
The results obtained in this study revealed that the chemical composition of I. viscosa was considerably affected by the method of extraction. The UDE offers a net advantage compared the other methods for essential oil extraction which presented the higher yield. Indeed, our findings showed that the ME and AE fractions have higher contents of polyphenol and flavonoid. The same solvents showed the highest antiradical activity. In fact, AE was considered also the solvent suitable for the separation of I. viscosa leaves. Phenolic acids presented the major fraction identified in ME and AE, while the flavonoids were detected in higher amounts in EA and ET.
References
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.P.; Troganis, A.; Boskou, D. Antioxidant Activities and Phenolic Composition of Extracts from Greek Oregano, Greek Sage and Summer Savory. Journal of Food Science and Technology 2002, 50, 5294–5299.
- Pietta, P.G.;. Flavonoids as Antioxidants. Journal of Natural Products 2000, 63, 1035–1042. DOI: 10.1021/np9904509.
- Medina, K.L.; Hanson, K.L.; Schweinsburg, A.D.; Cohenzion, M.V.; Nagel, B.J.; Tapert, S.F. Neuropsychological Functioning in Adolescent Marijuana Users: Subtle Deficits Detectable after a Month of Abstinence. Journal of the International Neuropsychological Society 2007, 13, 807–820. DOI: 10.1017/S1355617707071032.
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Núñez, M.J. Extraction of Antioxidant Phenolics from Almond Hulls (Prunusamygdalus) and Pine Sawdust (Pinuspinaster). Food Chemistry 2004, 85, 267–273. DOI: 10.1016/j.foodchem.2003.06.020.
- Goli, A.H.; Barzegar, M.; Sahari, M.A. Antioxidant Activity and Total Phenolic Compounds of Pistachio (Pistachiavera) Hull Extracts. Food Chemistry 2004, 92, 521–525. DOI: 10.1016/j.foodchem.2004.08.020.
- Akowuah, G.A.; Ismail, Z.; Norhayati, I.; Sadikun, A. The Effects of Different Extraction Solvents of Varying Polarities on Polyphenols of Orthosiphonstamineus and Evaluation of the Free Radical-Scavenging Activity. Food Chemistry 2005, 93, 311–317. DOI: 10.1016/j.foodchem.2004.09.028.
- Wang, H.; HelliwellK. Determination of flavonols in Green and Black Tea Leaves and Green Tea Infusions by High-Performance Liquid Chromatography. Food Research International 2001, 34, 223–227. DOI: 10.1016/S0963-9969(00)00156-3.
- Heywood, V.H.; Skoula, M. Identification of Wild Food and Non-Food Plants of the Mediterranean Region: Proceedings. CIHEAM-IAMC. 165 p. ( Cahiers Options Méditerranéennes, vol. 23). 1. Regional Workshop of the MEDUSA Network Identification, conservation, and Use of Wild Plants of the Mediterranean Region, July 28-29, 1996, Chania (Grèce). http://om.ciheam.org/option.php?IDOM=734
- Pottier-Alapetite, G.;. Flora of Tunisia Angiosperms-Dicotyledones Gamopetales; Official Printing Office of the Tunisian Republic: Tunis, Tunisia, 1979; pp 1074.
- Al-Dissi, M.N.; Salhab, A.S.; Al-Hajj, H.A. Effect of Inulaviscosa Leaf Extracts on Abortion and Implantation in Rats. Journal of Ethnopharmacology 2001, 77, 117–121.
- Hernández, V.M.; Carmen, R.; Salvador, M.; Rosa, M.G.; José, L.R. Effects of Naturally Occurring Dihydroflavonols from Inulaviscosa on Inflammation and Enzymes Involved in the Arachidonic Acid Metabolism. Life Sciences 2007, 81, 480–488. DOI: 10.1016/j.lfs.2007.06.006.
- Alarcón de la Lastra, C.; López, A.; Motilva, V. Gastroprotection and Prostaglandin E2 Generation in Rats by Flavonoids of Dittrichia Viscosa. Planta Medica 1993, 59, 497–501.
- Stamatis, G.; Kyriazopoulos, P.; Golegou, S.; Basayiannis, A.; Skalts, S.; Skaltsa, H. In Vitro anti-Helicobacter Pylori Activity of Greek Herbal Medicines. Journal of Ethnopharmacology 2003, 88, 175–179.
- Yuji, O.; Bat-Hen, B.; Yigal, C. Nematicidal Activity of Powder and Extracts of Inulaviscosa. Journal of Nematology 2001, 3, 735–742. DOI: 10.1163/156854101753625245.
- Abad, M.J.; Geurra, J.A.; Bermejo, P.; Iruruzum, A.; Carrasco, L. Search for Antiviral Activity in Higher Plant Extracts. Phytotherapy Research 2000, 14, 604–607. DOI: 10.1002/1099-1573(200012)14:8<604::AID-PTR678>3.0.CO;2-L.
- Dewanto, V.; Wu, X.; Adom, K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. Journal of Agricultural and Food Chemistry 2002, 50, 3010–3014. DOI: 10.1021/jf0115589.
- Hatano, T.; Kagawa, H.; Yasahara, H.T.; Okuda, T. The Effect of Extracts on DPPH Radical Was Estimated according to the Methanol. Food Chemistry 1988, 78, 347–354.
- Oyaizu, M.;. Studies on Products of the Browning Reaction Prepared from Glucose Amine. The Japanese Journal of Nutrition and Dietetics 1986, 44, 307–315. DOI: 10.5264/eiyogakuzashi.44.307.
- Da Porto, C.; Decorti, D.; Natolino, A. Ultrasound-Assisted Extraction of Volatile Compounds from Industrial Cannabis Sativa L. Inflorescences. International Journal of Applied Research in Natural Products 2014, 7, 8–14.
- Perez-Alonso, M.J.; Velasco-Negueruela, A. Composition of the Volatile Oil from the Aerial Parts of Inulaviscosa (L) Aiton. Flavour and Fragrance Journal 1996, 11, 349–351. DOI: 10.1002/(SICI)1099-1026(199611)11:6<349::AID-FFJ593>3.0.CO;2-1.
- Kheyar, N.; Meridja, D.; Belhamel, K. Etude De L’activité Antibactérienne Des Huiles Essentielles d’Inula Viscosa, Salvia Officinalis Et Laurus Nobilis De La Région De Bejaia. Algerian Journal of Natural Products 2014, 2, 18–26.
- Statsoft. STATISTICA for Windows (Computer Program Electronic Manual); StatSoftInc: Tulsa, OK, 1998.
- Alalan, L.; AL-Shammaa, I.; Al-Nouri, A.S. Analysis of the Chemical Composition of Essential Oil Extracted from Syrian Inulaviscosa (L). Journal of Chemical and Pharmaceutical Research 2015, 7, 861–864.
- Haoui, I.E.; Derriche, R.; Madani, L.; Oukali, Z. Analysis of the Chemical Composition of Essential Oil from Algerian Inulaviscosa (L.) Aiton. Arabian Journal of Chemistry 2011. DOI: 10.1016/j.arabjc.2011.05.005.
- Orhan, N.; Gökbulut, A.; DeliormanOrhan, D. Antioxidant Potential and Carbohydrate Digestive Enzyme Inhibitory Effects of five Inula Species and Their Major Compounds. South African Journal of Botany 2017, 111, 86–92. DOI: 10.1016/j.sajb.2017.03.040.
- Blanc, M.C.; Bradesi, P.; Goncalves, M.J.; Salgueiro, L.; Casanova, J. Essential Oil of Dittrichia Viscosa Spp. Viscosa: Analysis by 13C-NMR and Antimicrobial Activity. Flavour and Fragrance Journal 2006, 21, 324–332. DOI: 10.1002/ffj.1605.
- Camacho, A.; Fernandez, A.; Fernandez, C.; Altarejos, J.; Laurent, R. Composition of the Essential Oil of Dittrichiaviscosa (L.) W. Greuter. Rivista Italiana 2000, 29, 3–8.
- Trimech, I.; Weiss, E.K.; Chedea, V.S.; Marin, D.; Detsi, A.; Ioannou, E.; Roussis, V.; Kefalas, P. Evaluation of Anti-Oxidant and Acetylcholinesterase Activity and Identification of Polyphenolics of the Invasive Weed Dittrichiaviscosa. Phytochemical Analysis 2014, 25, 421–428. DOI: 10.1002/pca.v25.5.
- Mahmoudi, H.; Hosni, K.; Zaouali, W.; Amri, I.; Zargouni, H.; Ben Hamida, N.; Kaddouri, R.; Hamrouni, L.; Ben Nasri, M.; Ouerghi, Z. Comprehensive Phytochemical Analysis, Antioxidant and Antifungal Activities of InulaviscosaAiton Leaves. Journal of Food Safety 2015, 36, 77–88. DOI: 10.1111/jfs.12215.
- Manghatayaru, K.; Kuruvilia, S.; Balakrishna, K.; Venkhatesh, J. Modulatory Effect of Inularacemosa Hook. (Asteraceae) on Experimental Atherosclerosis in Guinea-Pigs. Journal of Pharmacy and Pharmacology 2009, 61, 1111–1118. DOI: 10.1211/jpp.61.08.0016.
- Jallali, I.; Zaouali, Y.; Missaoui, I.; Smeoui, A.; Abdelly, C.; Ksouri, R. Variability of Antioxidant and Antibacterial Effects of Essential Oils and Acetonic Extracts of Two Edible Halophytes: Crithmummaritimum L. And Inulacrithmoïdes L”. Food Chemistry 2014, 145, 1031–1038. DOI: 10.1016/j.foodchem.2013.09.034.
- Kenny, O.; Smyth, T.J.; Walch, D.; Kelleher, C.T.; Hewage, C.M.; Brunton, N.P. Investigating the Potential of Under-Utilised Plants from the Asteraceae Family as a Source of Natural Antimicrobial and Antioxidant Extracts. Food Chemistry 2014, 61, 79–86. DOI: 10.1016/j.foodchem.2014.03.126.
- Gökbulut, A.; Özhan, O.; Satılmış, B.; Batçıoğlu, K.; Günal, S.; Şarer, E. Antioxidantand Antimicrobial Activities and Phenolic Compounds of Selected Inula Species from Turkey. Natural Product Communications 2013, 8, 475–478.
- Sun, T.; Ho, C.T. Antioxidant Activities of Buckwheat Extracts. Food Chemistry 2005, 90, 743–749. DOI: 10.1016/j.foodchem.2004.04.035.
- Chahmi, N.; Anissi, J.; Jennan, S.; Farah, A.; Sendide, K.; El Hassouni, M. Antioxidant Activities and Total Phenol Content of Inulaviscosa Extracts Selected from Three Regions of Morocco. Asian Pacific Journal of Tropical Biomedicine 2015, 5, 228–233. DOI: 10.1016/S2221-1691(15)30010-1.
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sanchez-Mata, M.C.; Camara, M.; Fernadez-Ruiz, V.; Pardo-Do-Santayana, M.; Tardio, J. Mediterranean Non-Cultivated Vegetables as Dietary Sources of Compounds with Antioxidant and Biological Activity. LWT - Journal of Food Science and Technology 2014, 55, 389–396. DOI: 10.1016/j.lwt.2013.08.017.
- Li, H.; Zhang, D.; Tan, L.H.; Yu, B.; Zhao, S.P.; Cao, W.G. Comparison of the Antioxidant Properties of Various Solvent Extracts from Dipsacus Asperoides and Identification of Phenolic Compounds by LC-ESI-QTOF-MS–MS. South African Journal of Botany 2017, 109, 1–8. DOI: 10.1016/j.sajb.2016.12.018.
- Conforti, F.; Marrelli, M.; Carmela, C.; Menichini, F.; Valentina, P.; Uzunov, D.; Statti, G.A.; Duez, P.; Menichini, F. Bioactive Phytonutrients (Omega Fatty Acids, Tocopherols, Polyphenols), in Vitro Inhibition of Nitric Oxide Production and Free Radical Scavenging Activity of Non-Cultivated Mediterranean Vegetables. Food Chemistry 2011, 129, 1413–1419. DOI: 10.1016/j.foodchem.2011.05.085.
- Galvez, M.; Cordero, C.M.; Hourghton, P.H.; Ayuso, M.J. Antioxidant Activity of Methanol Extracts Obtained from Plantago Species. Journal of Agricultural and Food Chemistry 2005, 53, 1927–1933. DOI: 10.1021/jf048076s.
- Hayder, N.; Abdelwahed, A.; Kilani, S.; Ammar, R.B.; Mahmoud, A.; Ghedira, K.; Chekir-Ghadira, L. Anti-Genotoxic and Free-Radical Scavenging Activities of Extracts from (Tunisian) Myrtus Communis. Mutation Research 2004, 564, 89–95. DOI: 10.1016/j.mrgentox.2004.08.001.
- Saleem, M.T.; Chetty, M.C.; Kavimani, S. Putative Antioxidant Property of Sesame Oil in an Oxidative Stress Model of Myocardial Injury. Journal of Cardiovascular Disease Research 2013, 4, 177–181. DOI: 10.1016/j.jcdr.2013.07.001.
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D. Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chemistry 2006, 97, 654–660. DOI: 10.1016/j.foodchem.2005.04.028.
- Parida, A.K.; Das, A.B.; Sanada, Y.; Mohanty, P. Effects of Salinity on Biochemical Components of the Mangrove, Aecerascorniculatum. Aquatic Botany 2004, 80, 77–87. DOI: 10.1016/j.aquabot.2004.07.005.
- Escarpa, A.; Gonzalez, M.C. Approach to the Content of Total Extractable Phenolic Compounds from Different Food Samples by Comparison of Chromatographic and Spectrophotometric Methods. Analytica Chimica Acta 2001, 427, 119–127. DOI: 10.1016/S0003-2670(00)01188-0.
