?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Coupled effects of sodium carbonate (SC) pre-treatment and hybrid drying methods (freeze-drying (FD)-instant controlled pressure drop drying (DIC), hot air drying (HAD)-DIC) on the microstructure, physicochemical, nutritional, and antioxidant properties of goji berries were investigated. Dewaxing pre-treatment by SC could decrease drying time and improve quality. A substantial increase in pore size of goji was found after SC pre-treatment coupled with hybrid drying. Although the best colour was found in FD products, goji dried by hybrid methods (especially FD-DIC) showed better overall quality than that dried by HAD or FD alone. FD-DIC products exhibited the lowest moisture content (127 g/kg), the best crispness (21), the highest glass transition temperature (27.82ºC), higher contents of total Lycium barbarum polysaccharide (139.8 g/kg), total carotenoids (2.43 g/kg) as well as ABTS+ radical scavenging activity (57.55 μmol TE/g). FD-DIC could be an alternative drying method for processing valuable agro-products.
Introduction
Goji (Lycium barbarum) is in the Solanaceae family that mainly grows in the north-west of China and other parts in Asia. It has been used as a functional food and traditional herb in Asian countries. [Citation1,Citation2] The interest in the components of goji has enhanced due to the increased awareness of its possible health benefits. Goji contains many active compounds including phenols, carotenoids, and polysaccharides possessing a range of biological activates such as anti-aging, antioxidant properties, immunomodulation, anti-fatigue/endurance, anti-tumour activity, neuroprotection, and cytoprotection. [Citation3,Citation4]Drying is an effective method of preventing microbial spoilage and chemical reactions to prolong the shelf-life of fresh fruits and vegetables. [Citation5] Over 95% of fresh goji was dried after harvest. [Citation6] Several drying methods, such as sun drying, hot air drying, vacuum drying, and freeze drying, have been conducted on goji. [Citation7,Citation8] Among them, hot air drying and sun drying are still the most frequently used techniques for processing goji. However, sun drying has several drawbacks, as low drying efficiency, microbial contamination and insect infestation. Lower quality products were always obtained in hot air drying, such as losses of colour and texture, shrinkage and “case hardening”. [Citation7,Citation9] In terms of freeze drying, owing to the low temperature and the deficiency of water required for the process, many microbiological reactions are mostly inhibited which extends to an excellent quality of the final product. [Citation10] However, its operation cost is much higher than other drying methods. Every drying technique has advantages and restrictions. Intermittent and combination drying process could integrate the strengths of different drying methods and avoid shortcomings of one single drying process so as to preserve the quality of final products and (or) reduce energy consumption. [Citation11] Instant controlled pressure drop drying (DIC), also called explosion puffing drying (EPD), [Citation12,Citation13] is an emerging drying technology which always in conjunction with other drying methods like hot air and freeze drying. [Citation14] The products have appealing crispy taste, porous structure, and better quality. Fruits including carrot, apple, mulberry, and jackfruit were investigated in the literature by DIC combined drying methods, [Citation15,Citation16] however, there is little information on combined drying of goji.
Drying of fresh goji is difficult owing to the wax layer on the outer surface which acts as a barrier to block moisture transport. In the previous study, a wax abrasive pre-treatment, which was implemented inside a motorized drum with sandpaper, was used to remove the wax layer of goji. [Citation17] Instead of abrasive ones, sodium carbonate (SC) was used as a dewaxing agent to break the wax layer of goji in China, new water molecule transport pathway will be formed, so as to accelerate the drying rate. But there is a little report about the effect of SC pre-treatment coupled with combined drying especially on DIC of goji. Hence, to enhance drying efficiency and improve the overall quality, coupled effects of sodium carbonate pre-treatment and hybrid drying methods (FD-DIC and HAD-DIC) on the physicochemical, microstructure, nutritional, and antioxidant properties of goji was evaluated. Freeze drying and hot air drying were used as comparative study.
Materials and methods
Chemicals
DPPH (2,2-Diphenyl-1-picrylhydrazyl radical), TPTZ (2,4,6-tris(2-pyridyl)-s-triazine), ABTS (2,2ʹ-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt), trolox ((±)-6-hydrox-2,5,7,8-tetramethylchromane-2-carboxylic acid), Folin-Ciocalteu phenol reagent, rutin, gallic acid were purchased from Sigma–Aldrich (Shanghai, China). All other analytical grade chemicals and reagents were obtained from Sinopharm Group Co. Ltd. (Beijing, China).
Sample preparation
Raw material
Goji was grown in Yinchuan, Ningxia Hui Autonomous Region, China with initial water content was about 804 ± 2 g/kg dry base (d.b.). Goji berries were collected at the stage of commercial maturity which were transferred to Beijing by aeroplane within one day. Those without mechanical damage and decay were used, and the fruit pedicles were removed before the experiment.
Pre-treatments
Whole goji fruits were immersed into the SC solution (20 g/kg) for 1 min at ambient temperature with the liquid to solid ratio of 10:1 (ml:g). Control samples were treated by distilled water (DW) under the same condition. Each treatment was conducted in triplicate. Fresh goji fruits were used for HAD and HAD-DIC. For FD and FD-DIC processing, samples were frozen at −80ºC before used.
Equipment and drying processes
Freeze dryer (Alphal 1-4Lplus, MARIN CHRIST Co. Ltd Osterode, Germany), hot air dryer (HDS-D120B-D, Haiti Sheng Machinery Co. Ltd., Liaoning, China) and instant controlled pressure drop dryer (Tianjin Qin-de New Material Technology Co. Ltd., Tianjin, China) were used. The drying conditions of four different drying methods are exhibited in . Each drying experiment conducted three repeats.
Table 1. Drying conditions of four different drying methods.
Sample extraction
Methanol-water solution was applied for sample extraction. The dried goji products were ground into powder and pass through a 60-mesh sieve, 1 g of goji powder was mixed with 100 mL methanol-water solution (80:20, mL:mL) and then extracted with ultrasonic (40 kHz, 100 W) for 2 h at ambient temperature. [Citation18] Samples were then filtered by 0.45 μm microporous membrane for the analysis of total phenolic content, total flavonoid content, and antioxidant activity. The UV-visible spectrophotometer (UV1800, Shimadzu Co. Ltd., Kyoto, Japan) was used for all absorbance measurements.
Moisture content and glass transition temperature (Tg)
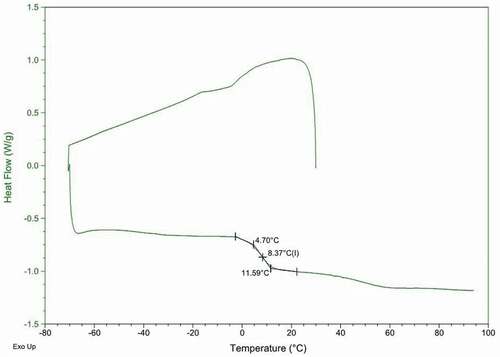
Moisture content was measured by drying 5 g samples at 105ºC until a constant weight was gained. [Citation12] And the results were expressed as g/kg (dry basis, d.b.). Tg was measured by differential scanning calorimetry (DSC). About 7 mg of the sample was enclosed in a sealed aluminium pan, meanwhile, an empty crucible used as reference. Liquid nitrogen (purity >99.99%) was used for sample cooling before DSC runs. The procedure was as same as Chen et al. (2017). [Citation14] A typical DSC thermogram of Goji powder is shown in and the midpoint was chosen as the characteristic Tg. The measurements were conducted in triplicate.
Instrumental texture
Texture profiles analysis were measured using TA-XT2i/50 Texture Analyser (Stable Micro Systems Ltd., Surrey, UK) fitted with a P36R probe. The force-time curve was recorded to analyse the number of peaks and maximum compression force which represent crispness and hardness of sample, respectively. [Citation19] Fifteen analyses were performed for each processing.
X-ray micro-tomography (µCT)
X-ray computed tomography is based on plenties of two-dimensional projections of a sample at different angles to reconstruct a three-dimensional volumetric image. [Citation20] The inner porous structures of the dried goji were visualized through the SkyScan 1272 desktop µCT system with a tungsten anode X-ray tube set to 50 kV and 200 µA. The total 1245 sections count (1408 × 1408 pixels) was obtained under different rotations over 360° with the step size of 0.40°. The following settings were also used in tomographic reconstruction: smoothing = 3, ring artefact correction = 3, and beam hardening correction (%) = 50. The reconstruction work used was the NRecon Program (Version 1.7.0.4). For each treatment, a representative sample was chosen.
Colour
The colour was measured by a Hunter Lab colorimeter D25L (Hunter Co. Ltd, VA, USA). The colour was presented as L (darkness-lightness), b (yellowness-blueness) and a (redness-greenness). ∆E was calculated according to Eq. 1 which represents total colour change. [Citation16]
where L, b, a, were the colour parameters of dried goji, and L*, b*, a*were the colour parameters of fresh goji, respectively. The measurement and analysis for the colour were performed in triplicate.
Nutritional properties
Total carotenoids content
Total carotenoids content (TCC) was measured according to Knockaert et al. (2012). [Citation21] One gram of goji powder was added to 50 mL of hexane-ethanol-acetone solution (2:1:1, mL: mL: mL; with 0.1% BHT). The mixture solution was then stirred (600 rpm) for 30 min at ambient temperature. After adding 15 mL distilled water, the mixture solution was stirred for another 10 min. A separating funnel was used to separate the organic phase from the water phase. The absorbance of the filtrate was measured at 450 nm using hexane with 0.1% BHT as a blank. For calculating TCC, the following Eq. 2. was used:
where A = absorbance at 450 nm, volume = total volume of extract, = extinction coefficient = 2560 for β-carotene in hexane. Analyses were performed in triplicate.
Total Lycium barbarum polysaccharide content
The total Lycium barbarum polysaccharide (LBP) content was performed according to the procedure presented by Zhao et al. (2015) [Citation7] with slight modification. Goji powder was extracted by ultrasonic at 40 kHz for 2 h. After centrifugated, the supernatant was condensed to a proper volume. Different from Zhao et al. [Citation7], precipitation was dissolved with ethanol until the concentration of ethanol is over 80%. Then, the solution was placed at 4ºC for 24 h to make the LBP settled. The settling was dissolved with distilled water and set to 100 mL. Solutions were analysed for LBP, by the phenol–sulfuric acid method and concentrations determined against a glucose standard.
Total phenolic content
Briefly, 0.4 mL of the extracts was transferred to the volumetric flask (10 mL) followed by 1 mL of Folin-Ciocalteu’s reagent (10 times dilution) and after 6 min 2 mL of sodium carbonate solution (75 g/kg) was added. Then, the volumetric flask was adjusted to the total volume with the distilled water and mixed thoroughly. The mixture was allowed to stand for 60 min at 30ºC and measured at 765 nm. [Citation22] The TPC was presented as the mg/g dry base (d.b.) of gallic acid equivalents (GAE).
Total flavonoid content
The total flavonoid content (TFC) was using the method described by Sun et al. (2011) [Citation23] with some modification. Exactly 0.3 mL NaNO2 (50 g/kg) was added to 1 mL extracts in a 10 mL volumetric flask and standing for 6 min. Then, 0.3 mL Al(NO3)3 (100 g/kg) and 4 mL NaOH (40 g/kg) were added to the mixture. After adjusted to the total volume, the volumetric flask stood for 15 min at ambient temperature. The absorbance was measured at 510 nm and TFC was expressed as mg/g d.b. of rutin equivalents (RE).
Antioxidant activity measurement
DPPH assay
DPPH scavenging was carried out according to the method described by Wang et al. (2012). [Citation24] The sample extraction (2 mL) was mixed with 4 mL DPPH (100 μmol/L) for 30 min in the dark. Then, the absorbance was read at 510 nm with the methanol-water solution (80:20, mL:mL) as the control blank. The standard curve was linear between 20 to 100 μmol/L Trolox. DPPH scavenging ability was expressed in μmol/g d.b. of trolox equivalents (TE).
FRAP assay
The FRAP assay was measured according to the method of Wang et al. (2012). [Citation24] FRAP reagent was freshly prepared by mixing 250 mL acetate buffer (300 mmol/L, pH = 3.6), 25 mL of 10 mmol/L TPTZ in 40 mmol/L HCl, and 25 mL of 20 mmol/L FeCl3. The mixed solution was incubated at 37ºC for 30 min before used. The sample extraction (0.2 mL) was added to 6 mL FRAP reagent, then held for 30 min at 37ºC. The absorbance was detected at 593 nm. The standard curve was liner between 100 and 800 μmol/L Trolox. Results were expressed in μmol TE/g db.
ABTS assay: A total of 10 mL of 7 mmol/L aqueous ABTS solution was mixed with 10 mL of 2.45 mmol/L potassium persulphate solution and kept for 14 h in the dark under 30ºC to make the ABTS dissolution thoroughly. The ABTS solution was diluted with the methanol-water solution (80:20, mL: mL) to an absorbance of less than 0.70 at 734 nm before analysis. Then, 0.4 mL of sample were mixed with 3.6 mL of the diluted ABTS solution. The absorbance was measured at 734 nm after 1 min. The standard curve was linear between 25 and 150 μmol/L Trolox. [Citation25] The ABTS+ radical scavenging activity were presented in μmol TE/g d.b.
Statistical analysis
Crispness and hardness of dried goji were determined in 15 replicates, while all the other experimental data was conducted in three replicates and the results were expressed as mean ± standard deviation. Analysis of variance (ANOVA) and Duncan’s multiple comparison tests were conducted by SPSS V21.0 Software (IBM Corporation, Chicago, USA). A level of p ≤ 0.05 was considered as statistically significant.
Results and discussion
Moisture content and glass transition temperature (tg)
The moisture contents and glass transition temperature of goji products processed by the different drying conditions are shown in . After drying, the moisture content of gogi was significantly decreased from 804 g/kg to 127 ~ 170 g/kg. Tg values of dried gogi with different treatments varied from 4.6 ~ 27.8 ºC. The definition of Tg is the temperature at which amorphous system changes from a relatively stable glassy state to rubbery state. As the temperature increases above Tg, various changes existed in the system such as decrease in viscosity, loss of crispness and increase in free volume. [Citation26] Goji dried by HAD and FD, the moisture content and Tg had no significant differences between DW and SC treatments. However, SC treatment in HAD could decrease the drying time of 4 h compared with DW treatment (). DW and SC treatments showed significant impact on the moisture content and Tg of goji dried by combined drying, especially in HAD-DIC, with a decreasing moisture content from 160 g/kg to 144 g/kg, the values of Tg increased from 9.56ºC to 20.86ºC. According to Roos [Citation27], for most substances, the lower the moisture content, the higher the Tg. Goji dried by FD-DIC (SC) had the lowest moisture content of 127 g/kg and the highest Tg value of 27.8°C. In contrast, the hot-air dried goji (DW) had the highest moisture content (170 g/kg) and lowest Tg value (4.6°C). The formation of new water channel after SC pre-treatment could lead to higher drying efficiency of goji. Compared with HAD and FD, DIC combined drying induced a significant decrease of drying time and moisture content, indicating DIC technique could increase moisture effective diffusion of food material. The highest Tg was observed in FD-DIC (SC) (27.8ºC) because of the destruction of cytoarchitecture during FD pre-drying process and the fast moisture evaporation in the DIC stage. It indicated that goji dried by FD-DIC showed the best storage stability in all the products.
Table 2. Effect of different drying methods and pretreatments on physicochemical properties of Lycium barbarum.
Texture and x-ray micro-tomography (µCT)
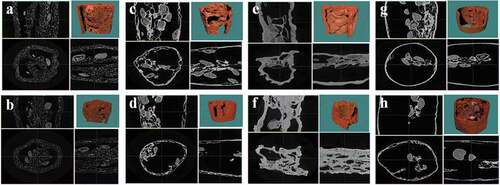
Crispness and hardness of different dried goji samples are shown in . The highest and lowest hardness values were observed in the HAD (DW) and FD (SC) treatments, respectively. It may be related with the microstructure that formed in different treatments. As µCT images shown (), goji dried by HAD had a sharp shrinkage, while the samples dried by FD with SC treatment had a uniform and dense embedded pores and spongy structure. The HAD-dried goji had the lowest crispness value, while the highest crispness value was achieved in the FD-DIC-sample (SC). In DIC process, the pressure drops from atmosphere to vacuum abruptly leading to the formation of porous structure and expansion of products. And a number of micropores in the waxy layer were formed after SC pre-treatment which generated a more anisotropic porous and less connected system clearly (). Thus, higher crispness value was exhibited in the FD-DIC and HAD-DIC dried products. However, due to the hot air pre-drying process, goji dried by HAD-DIC had lower crispness and higher hardness values compared with the FD-DIC ones.
Figure 2. Effect of different drying methods and pretreatments on µCT scanning graphic images of Lycium barbarum. a: FD (DW), b: FD (SC), c: FD-DIC (DW), d: FD-DIC (SC), e: HAD (DW), f: HAD (SC), g: HAD-DIC (DW), h: HAD-DIC (SC). DW, distilled water; SC, sodium carbonate; FD, freeze drying; FD-DIC, freeze drying combined with instant controlled pressure drop drying; HAD, hot air drying; HAD-DIC, hot air drying combined with instant controlled pressure drop drying.
Colour
L, a and b values of fresh gogi were 28.26, 27.8 and 12.3, respectively. The colour of dried goji was obviously influenced by drying process and pre-treatments with L, a and b values in the range of 24.5 ~ 33.5, 12.5 ~ 26.1 and 5.82 ~ 13.0, respectively (). The highest L value of dried goji was obtained in treatment of FD (SC) (33.5), which means it was much lighter than fresh gogi.And FD-products showed the lowest ∆E value of 5.3, which indicates that the colour of the products was the closest to fresh samples. This can be attributed to the comparatively low temperature of freezing drying, thus preventing enzymatic browning reactions and leading to relative stability of the colour values. [Citation28] Significant decrease in L, a and b values were found in thermal processing including FD-DIC, HAD and HAD-DIC (). [Citation13] Correlation analysis results () also showed that significant positive correlations were observed between a, b value, and total carotenoids content (P < 0.01). Carotenoid molecules have characteristic conjugated polyene which is highly susceptible to degradation due to oxidation. [Citation29] Hence, the decrease in a and b values in thermal processing may relate to the degradation of carotenoids. The lowest L, a, b values and the highest ∆E were found in goji dried by HAD (DW), which made it the darkest product among all the samples. It suggested a tendency in enzymatic browning from Maillard reaction and caramelization, [Citation13] which agreed with the results of dried black mulberry. [Citation14] Compared to the DW (HAD), the SC pre-treatment (HAD) could increase L, a, b values and decrease ∆E because of shorter drying time. A similar tendency was also found in the DIC process. This is because vacuum drying stage of DIC after instant decompression can produce thin air and low temperature to decrease oxidation of enzymatic browning, leading to less colour changes of dried goji. [Citation12]
Table 3. Effect of different drying methods and pretreatments on colour values of Lycium barbarum.
Table 4. Different drying methods and pretreatments on nutritional components and antioxidant activities of Lycium barbarum.
Table 5. Correlation analysis on the colour, nutritional properties, and antioxidant activity.
Nutritional properties
Total carotenoid content (TCC)
The reddish-orange colour of goji fruits comes from a group of carotenoids, which can be easily oxidized by heat, light, and high oxygen tension. [Citation30] The TCC for fresh goji was 3.64 g/kg extract and the values (1.60 ~ 2.60 g/kg) of dried goji is shown in . DW-products exhibited lower TCC values than SC-ones and the lowest TCC (1.60 g/kg) was obtained in HAD (DW) samples, owing to the effect of coexistence of high temperature, oxygen, and longer drying time which could induce the degradation of carotenoids in thermal processing. The highest retention of TCC (71.4%) was observed in FD (SC) samples (2.60 g/kg), possibly because of the lower temperature and vacuum conditions during FD dehydration. [Citation15] Similarly, less losses of TCC (36%) in FD-DIC (DW) sample (2.34 g/kg) than that of HAD-DIC (DW) (43%) ones (2.07 g/kg).
Total LBP content
LBP was one of the major active components that contributed to the biological activities of goji. [Citation31] There were significant distinctions of the LBP values (90 ~ 139.8 g/kg) between different drying processing. As shown in , the highest retention ratio of LBP (82.7%) was observed in the FD-DIC (SC) products (139.8 g/kg), possibly because of vacuum conditions of most of the drying process. The LBP of FD-DIC-dried goji was 21.3% higher than that of the HAD-DIC-dried one. The lowest content of LBP (90 g/kg) was found in HAD (DW) because of the longer drying time (12 h) at relatively higher drying temperature (60ºC). The content of LBP had no significant difference under the DW and SC pre-treatments of the same drying method except the HAD-DIC.
Total phenolic content (TPC) and total flavonoid content (TFC)
The TPC and TFC of dried goji were significantly influenced by the different pre-treatments and drying methods (P < 0.05). As shown in , TPC and TFC for the fresh samples was 6.73 mg GAE/g and 1.78 mg GAE/g, respectively, which was similar to that had been reported in the literature. [Citation8] The SC pre-treatment exhibited higher TPC and TFC values than that of DW pre-treatment in all the drying methods. For example, the TPC and TFC of FD-DIC dried samples with SC treatment were 7.8% and 7.6% higher than that of DW pre-treatment, respectively. The highest retention of TPC (11.4 mg GAE/g) and TFC (4.8 mg GAE/g) was found in HAD-DIC (SC) method, whereas the FD (DW) samples had the lowest value (8.2 and 2.2 mg GAE/g, respectively). It may be attributed to the thermal treatment that could liberate some phenolics and flavonoids which are mainly found in bound form in plant matrix. [Citation32] On the other hand, the activity of polyphenol oxidase, which is responsible for oxidation of phenolics, tends to deactivation because of exposure to high temperature. [Citation33] Moreover, the phenolic compounds in fruits are more concentrated utside the cell rather than the vacuoles. During thermal treatment, the liberation of phenolics might occur due to the decomposition of cellular components and covalent bonds. [Citation34,Citation35] Hence, the released phenolics tend to be more amenable for extraction. The TPC and TFC contents of FD-DIC (SC) were higher than that of the FD (SC) samples (25.3% and 20.1%, respectively), which indicated that phenolics and flavonoids could be was liberated after DIC treatment.
Antioxidant activity
Antioxidants like phenolic compounds could inhibit oxidation reaction by acting on hydrogen donors or free radical acceptors. [Citation36] Due to the complex composition of oxidative processes, the antioxidant activity could not be evaluated only using one single method. DPPH, FRAP, and ABTS assay were used in this study. As shown in , the antioxidant activities of all dried samples were enhanced compared to the fresh ones. Chang et al. [Citation37], Hamrouni-Sellami et al. [Citation38] showed similar results. And other berries such as strawberry also showed that drying could improve the antioxidant activity as indicated by higher EC50 values. [Citation39] Besides FD dried samples, a significant increase of antioxidant activity of dried goji was observed after SC pre-treatment. The strongest ferrous iron chelating capacity (44 μmol TE/g) and ABTS+ radical scavenging activity (57.6 μmol TE/g) were found in FD-DIC-dried goji, whereas the HAD-DIC-dried sample had the strongest DPPH free radical scavenging activity (15.1 μmol TE/g). The fact that higher TPC and TFC were found in dried samples conducive to higher antioxidant activities compared to the fresh ones (). As presented in , both TPC and TFC showed significant positive correlations with DPPH and FRAP. Similarly, TCC and total LBP content had positive correlations with ABTS+ radical scavenging activity. The DPPH and FRAP values of FD-DIC-dried (SC) samples were 42.7% and 44.5% higher than the FD-dried (SC) samples, respectively, which were in good agreement with the increase of TPC and TFC contents (25.3% and 20.1%, respectively). Therefore, the DIC processing also could enhance the antioxidant activity of dried goji.
Conclusion
Microstructure, physicochemical, nutritional, and antioxidant properties of dried goji were significantly affected by different pre-treatments and drying conditions. Compared to DW-pretreated samples, the SC pre-treatment was more favourable because its improvement in overall qualities of dried goji no matter which drying method was used. In detail, the SC pre-treatment products exhibited shorter drying time, better colour, TPC, TFC, LBP, TCC, and antioxidant activity. Under SC pre-treatment, the FD-DIC was a more beneficial drying method compared with the FD, HAD and HAD-DIC because of the highest retentions of TPC, LBP and antioxidant activities (FRAP and ABTS), especially yielding a superior crispy texture as well as highest Tg. For obtaining high-quality and valuable goji-products, FD-DIC should be the best choice. However, HAD-DIC could be another alternative technique when energy saving was more considered.
Additional information
Funding
References
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Stanisz, E.; Waśkiewicz, A. Potential Health Benefits and Quality of Dried Fruits: Goji Fruits, Cranberries and Raisins. Food Chem. 2017, 221(15), 228–236. DOI: 10.1016/j.foodchem.2016.10.049.
- Kulczyński, B.; Gramzamichałowska, A. Goji Berry (Lycium Barbarum): Composition and Health Effects - a Review. Polish J. Food Nutr. Sci. 2016, 66(2), 67–76. DOI: 10.1515/pjfns-2015-0040.
- Akbulut, M.; Ozcan, M. M. Comparison of Mineral Contents of Mulberry (Morus Spp.) Fruits and Their Pekmez (Boiled Mulberry Juice) Samples. Int J Food Sci Nutr. 2009, 60(3), 231–239. DOI: 10.1080/09637480701695609.
- Potterat, O.;. Goji (Lycium Barbarum & L. Chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Med. 2010, 76(1), 7–19. DOI: 10.1055/s-0029-1186218.
- Cuccurullo, G.; Giordano, L.; Albanese, D.; Cinquanta, L.; Di, M. M. Infrared Thermography Assisted Control for Apples Microwave Drying. J. Food Eng. 2012, 112(4), 319–325. DOI: 10.1016/j.jfoodeng.2012.05.003.
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J. ; Zhang, J.; Li, Y.; Duan, J. A., Lycium ruthenicum studies: Molecular biology, phytochemistry sand pharmacology. Food Chem. 2018, 240, 759–766. DOI: 10.1016/j.foodchem.2017.08.026.
- Zhao, Q.; Dong, B.; Chen, J.; Zhao, B.; Wang, X.; Wang, L.; Zha, S.; Wang, Y.; Zhang, J.; Wang, Y. Effect of Drying Methods on Physicochemical Properties and Antioxidant Activities of Wolfberry (Lycium Barbarum) Polysaccharide. Carbohydr. Polym. 2015, 127(AUG), 176–181. DOI: 10.1016/j.carbpol.2015.03.041.
- Donno, D.; Mellano, M. G.; Raimondo, E.; Cerutti, A. K.; Prgomet, Z.; Beccaro, G. L. Influence of Applied Drying Methods on Phytochemical Composition in Fresh and Dried Goji Fruits by HPLC Fingerprint. Eur. Food Res. Technol. 2016, 242(11), 1–14. DOI: 10.1007/s00217-016-2695-z.
- Saxena, A.; Maity, T.; Raju, P. S.; Bawa, A. S., Degradation Kinetics of Colour and Total Carotenoids in Jackfruit (Artocarpus Heterophyllus) Bulb Slices during Hot Air Drying. Food. Bioprocess. Technol. 2012, 5, 672–679. DOI: 10.1007/s11947-010-0409-2.
- Orphanides, A.; Goulas, V.; Gekas, V. Drying Technologies: Vehicle to High-Quality Herbs. Food Eng. Rev. 2016, 8(2), 164–180. DOI: 10.1007/s12393-015-9128-9.
- Huang, L. L.; Zhang, M. Trends in Development of Dried Vegetable Products as Snacks. Drying Technol. 2012, 30(5), 448–461. DOI: 10.1080/07373937.2011.644648.
- Lyu, J.; Yi, J. Y.; Bi, J. F.; Gao, H.; Zhou, M.; Liu, X., Impacts of Explosion Puffing Drying Combined with Hot-Air and Freeze Drying on the Quality of Papaya Chips. Int. J. Food Eng. 2017, 13, 2. DOI: 10.1515/ijfe-2016-0250.
- Zou, K.; Teng, J.; Huang, L.; Dai, X. W.; Wei, B. Y., Effect of Osmotic Pretreatment on Quality of Mango Chips by Explosion Puffing Drying. LWT Food Sci. Technol. 2013, 51, 253–259. DOI: 10.1016/j.lwt.2012.11.005.
- Chen, Q.; Li, Z.; Bi, J.; Zhou, L.; Yi, J.; Wu, X., Effect of Hybrid Drying Methods on Physicochemical, Nutritional and Antioxidant Properties of Dried Black Mulberry. LWT Food Sci. Technol. 2017, 80, 178–184. DOI: 10.1016/j.lwt.2017.02.017.
- Cui, Z. W.; Li, C. Y.; Song, C. F.; Song, Y., Combined Microwave-Vacuum and Freeze Drying of Carrot and Apple Chips. Drying Technol. 2008, 26, 1517–1523. DOI: 10.1080/07373930802463960.
- Yi, J. Y.; Wang, P.; Bi, J. F.; Liu, X.; Wu, X. Y.; Zhong, Y. G., Developing Novel Combination Drying Method for Jackfruit Bulb Chips: Instant Controlled Pressure Drop (Dic)-Assisted Freeze Drying. Food. Bioprocess. Technol. 2016, 9, 452–462. DOI: 10.1007/s11947-015-1643-4.
- Adiletta, G.; Alam, M. R.; Cinquanta, L.; Russo, P.; Albanese, D.; Di Matteo, M., Effect of Abrasive Pretreatment on Hot Dried Goji Berry. Chem. Eng. Trans. 2015, 44, 127–132. DOI: 10.3303/CET1544022.
- Istrati, D.; Vizireanu, C.; Iordachescu, G.; Dima, F.; Garnai, M. Physico-Chemical Characteristics and Antioxidant Activity of Goji Fruits Jam and Jelly during Storage. Ann. Univ. Dunarea de Jos Galati Fascicle VI Food Technol. 2013, 37(2), 100–110.
- Prachayawarakorn, S.; Raikham, C.; Soponronnarit, S. Effects of Ripening Stage and Steaming Time on Quality Attributes of Fat Free Banana Snack Obtained from Drying Process Including Fluidized Bed Puffing. J. Food Sci. Technol. 2016, 53(2), 946–955. DOI: 10.1007/s13197-015-2051-5.
- Voda, A.; Homan, N.; Witek, M.; Duijster, A.; Dalen, G. V.; Sman, R. V. D.; Nijsse, J.; Vliet, L. V.; As, H. V.; Duynhoven, J. V. The Impact of Freeze-Drying on Microstructure and Rehydration Properties of Carrot. Food Res. Int. 2012, 49(2), 687–693. DOI: 10.1016/j.foodres.2012.08.019.
- Knockaert, G.; Lemmens, L.; Van, B. S.; Hendrickx, M.; Van, L. A. Changes in β-carotene Bioaccessibility and Concentration during Processing of Carrot Puree. Food Chem. 2012, 133(1), 60–67. DOI: 10.1016/j.foodchem.2011.12.066.
- Chan, E.; Lim, Y. Y.; Wong, S. K.; Lim, K. K.; Tan, S. P.; Lianto, F. S.; Yong, M. Y. Effects of Different Drying Methods on the Antioxidant Properties of Leaves and Tea of Ginger Species. Food Chem. 2009, 113(1), 166–172. DOI: 10.1016/j.foodchem.2008.07.090.
- Sun, L. J.; Zhang, J. B.; Lu, X. Y.; Zhang, L. Y.; Zhang, Y. L. Evaluation to the Antioxidant Activity of Total Flavonoids Extract from Persimmon (Diospyros Kaki L.) Leaves. Food Chem. Toxicol. 2011, 49(10), 2689–2696. DOI: 10.1016/j.fct.2011.07.042.
- Wang, Y. T.; Liu, F. X.; Cao, X. M.; Chen, F.; Hu, X. S.; Liao, X. J. Comparison of High Hydrostatic Pressure and High Temperature Short Time Processing on Quality of Purple Sweet Potato Nectar. Innovative Food Sci. Emerging Technol. 2012, 16(39), 326–334. DOI: 10.1016/j.ifset.2012.07.006.
- Jihyun, J.; Hana, J.; Saerom, L.; Heejae, L.; Keumtaek, H.; Taeyoung, K. Anti-Oxidant, Anti-Proliferative and Anti-Inflammatory Activities of the Extracts from Black Raspberry Fruits and Wine. Food Chem. 2010, 123(2), 338–344. DOI: 10.1016/j.foodchem.2010.04.040.
- Shi, Q. L.; Wang, X. H.; Zhao, Y.; Fang, Z. X. Glass Transition and State Diagram for Freeze-Dried Agaricus Bisporus. J. Food Eng. 2012, 111(4), 667–674. DOI: 10.1016/j.jfoodeng.2012.02.038.
- Roos, Y. H.;. Water Activity and Physical State Effects on Amorphous Food Stability. J. Food Process. Preserv. 1993, 16(6), 433–447. DOI: 10.1111/j.1745-4549.1993.tb00221.x.
- Krokida, M. K.; Maroulis, Z. B.; Saravacos, G. D. The Effect of the Method of Drying on the Colour of Dehydrated Products. Int. J. Food Sci. Technol. 2015, 36(1), 53–59. DOI: 10.1046/j.1365-2621.2001.00426.x.
- Saxena, A.; Bawa, A. S.; Raju, P. S. Phytochemical Changes in Fresh-Cut Jackfruit (Artocarpus Heterophyllus L.) Bulbs during Modified Atmosphere Storage. Food Chem. 2009, 115(4), 1443–1449. DOI: 10.1016/j.foodchem.2009.01.080.
- Dias, M. G.; Camões, M. F.; Oliveira, L. Carotenoid Stability in Fruits, Vegetables and Working Standards - Effect of Storage Temperature and Time. Food Chem. 2014, 156(2), 37–41. DOI: 10.1016/j.foodchem.2014.01.050.
- Zhang, X. R.; Qi, C. H.; Cheng, J. P.; Liu, G.; Huang, L. J.; Wang, Z. F.; Zhou, W. X.; Zhang, Y. X. Lycium Barbarum Polysaccharide LBPF4-OL May Be a New Toll-Like Receptor 4/MD2-MAPK Signaling Pathway Activator and Inducer. Int. Immunopharmacol. 2014, 19(1), 132–141. DOI: 10.1016/j.intimp.2014.01.010.
- Hayat, K.; Zhang, X. M.; Chen, H. Q.; Xia, S. Q.; Jia, C. S.; Zhong, F. Liberation and Separation of Phenolic Compounds from Citrus Mandarin Peels by Microwave Heating and Its Effect on Antioxidant Activity. Sep. Purif. Technol. 2010, 73(3), 371–376. DOI: 10.1016/j.seppur.2010.04.026.
- Krapfenbauer, G.; Kinner, M.; Gössinger, M.; Schönlechner, R.; Berghofer, E. Effect of Thermal Treatment on the Quality of Cloudy Apple Juice. J. Agric. Food Chem. 2006, 54(15), 5453–5460. DOI: 10.1021/jf0606858.
- Papoutsis, K.; Pristijono, P.; Golding, J. B.; Stathopoulos, C. E.; Bowyer, M. C.; Scarlett, C. J.; Vuong, Q. V. Effect of Vacuum‐Drying, Hot Air‐Drying and Freeze‐Drying on Polyphenols and Antioxidant Capacity of Lemon (Citrus Limon) Pomace Aqueous Extracts. Int. J. Food Sci. Technol. 2016, 52(4), 1–8. DOI: 10.1111/ijfs.13351.
- Vashisth, T.; Singh, R. K.; Pegg, R. B. Effects of Drying on the Phenolics Content and Antioxidant Activity of Muscadine Pomace. LWT-Food Sci. Technol. 2011, 44(7), 1649–1657. DOI: 10.1016/j.lwt.2011.02.011.
- Shahidi, F.; Zhong, Y. Lipid Oxidation and Improving the Oxidative Stability. Chem Soc Rev. 2010, 39(11), 4067. DOI: 10.1039/b922183m.
- Chang, C. H.; Lin, H. Y.; Chang, C. Y.; Liu, Y. C. Comparisons on the Antioxidant Properties of Fresh, Freeze-Dried and Hot-Air-Dried Tomatoes. J. Food Eng. 2006, 77(3), 478–485. DOI: 10.1016/j.jfoodeng.2005.06.061.
- Hamrouni-Sellami, I.; Rahali, F. Z.; Rebey, I. B.; Bourgou, S.; Limam, F.; Marzouk, B. Total Phenolics, Flavonoids, and Antioxidant Activity of Sage (Salvia Officinalis L.) Plants as Affected by Different Drying Methods. Food. Bioprocess. Technol. 2013, 6(3), 806–817. DOI: 10.1007/s11947-012-0877-7.
- Adak, N.; Heybeli, N.; Ertekin, C., Infrared Drying of Strawberry. Food Chem. 2017, 219, 109–116. DOI: 10.1016/j.foodchem.2016.09.103.