?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study was to carry out phytochemical screening, to determine the total phenolic and flavonoid content as well as to evaluate the antioxidant properties of methanolic extract of root of Asparagus racemosus. Antioxidant properties were determined by 1,1- diphenyl,2-picrylhydrazyl (DPPH), hydroxyl radical, superoxide radical, hydrogen peroxide assay and total antioxidant activity by the phosphomolybdenum assay. The result showed that the powdered roots of A. racemosus were extracted with methanol and the percentage of yield was 29.2%. The total phenolic and flavonoid contents of the extracts were 12.90 ± 0.002 mg/g and 0.80 ± 0.001 mg/g dry weight respectively. The plant sample possesses high free radical scavenging activity and phytochemical constituents which might be useful for further studies to fight against oxidative stress.
Introduction
Natural products, especially those of wild origin, have always been an important source of therapeutic agents. About 25%-30% of drugs available for the treatment of disease are derived from natural products.[Citation1] Natural product research is frequently based on ethnobotanical information, as of now many of the drugs used are developed from medicinal plants employed in indigenous societies.[Citation2] A major part of ethno pharmaceutical research in recent years has been directed towards a better understanding of the pharmacological effects of individual medicinal plants.[Citation3] Many studies carried out in this field show that plants used in traditional medicine have been tested to be effective models for pharmacological studies.[Citation4] Thus, the medicinal plants and natural product extracts have been considered as an alternative therapy against various diseases.[Citation5]
Asparagus racemosus Linn. belongs to the family Asparagaceae and locally called as Shatavari in Odia. Its medicinal value has been reported in Indian traditional medicine such as Ayurveda, Unani, and Siddha. It is widely used as antioxidant and anti-stress effect,[Citation6] antiulcer[Citation7] and wound healing property.[Citation8] The objective of this study was to perform the phytochemical screening, to determine total phenolic and flavonoid content as well as antioxidant activities of A. racemosus root extracts.
Materials and methods
Plant collection and extractions
The roots of A. racemosus were collected from Phulbani (Kandhamal district of Odisha, India) in December of 2016 (coordinates 20.47° N and 84.23° E). The plant was authenticated by the renowned taxonomist. The sample was placed at the herbarium house of Botany department of Berhampur University, Odisha, India.The roots of the plant were dried separately in an oven at 80°C. The dried plant material was pulverized to powder with a mechanic grinder. The powder of roots (11 g) was extracted using solvent methanol (300 mL) through a Soxhlet apparatus.[Citation9,Citation10] After extraction, the filtrate was concentrated by evaporating in a water bath under normal pressure.[Citation11] The dried extracts were weighed to determine the percentage of yield[Citation12] of the soluble constituents using the formula;
The dried extracts were stored at 4°C for further investigation of potential in vitro free radical scavenging activity.
Qualitative phytochemical screening
Qualitative analysis of methanolic extract was extract carried out to determine the presence of various bioactive compounds using the standard qualitative procedures.[Citation13–Citation15]
Estimation of total phenol and flavonoid content
Total phenolic content (TPC) was analyzed by the Folin-Ciocalteu method using gallic acid as a standard curve and expressed as mg/g gallic acid equivalent.[Citation16] Total flavonoid content (TFC) was analyzed using rutin as standard and this was expressed as mg/g rutin equivalent.[Citation17]
Determination of antioxidant activity
1, 1 Diphenyl-2-picrylhydrazyl radical (DPPH) scavenging activity
The free radical scavenging activity of the methanolic extracts was determined using DPPH assay.[Citation18] Various concentrations of methanolic extract of the sample (1 mL) were mixed with 1 mL of methanolic solution containing 1, 1 Diphenyl-2-picrylhydrazyl radical (DPPH) radicals resulting in the final concentration of DPPH being 0.2 mM. The mixture was shaken dynamically and left to stand for 30 mins, and the absorbance was measured at 517 nm. Ascorbic acid was used as a standard. The percentage of DPPH decolorization of the sample was calculated using the following formula:
Hydroxyl radical scavenging activity
The reaction mixture (3 mL) containing 1 mL FeSO4 (1.5 mM), 0.7 mL hydrogen peroxide (6 mM), 10% of 0.3 mL sodium salicylate (20 mM) and varying concentrations of the extracts (10–500 µg/mL) were taken. After incubation for 1 hr at 37°C, the absorbance of the hydroxylatedsalicylated complex was measured at 562 nm.[Citation19] Ascorbic acid was used as the standard. The percentage scavenging effect was calculated as:
Where A0 was the absorbance of the control (without extract), A1 was the absorbance in the presence of the extract with sodium salicylate, and A2 was the absorbance without sodium salicylate.
Superoxide anion radical scavenging activity
This assay was based on the reduction of nitro blue tetrazolium (NBT) in the presence of nicotinamide adenine dinucleotide (NADH) and phenazinemethosulfate (PMS) under aerobic condition.[Citation20] TrisHCl buffer (3 mL, 16 mM, pH 8.0) containing 1 mL NBT (50 µM) solution, 1 mL NADH (78 µM) solution and a sample solution of extract (10–500 µg/mL) in distilled water mixed. The reaction was started when 1 mL of PMS solution (10 µM) was added to the mixture. The reaction mixture was incubated at 25°C for 5 min, and the absorbance was read at 560 nm against the corresponding blank samples. Ascorbic acid was used as a standard. The decreased absorbance of the reaction mixture indicated increased superoxide anion scavenging activity.
Hydrogen peroxide radical scavenging activity
The capability of the extract to scavenge hydrogen peroxide (H2O2) was estimated according to the method of.[Citation21] A solution of hydrogen peroxide (40 mM) was prepared in phosphate buffer, pH 7.4. The concentration of hydrogen peroxide was determined by absorption at 230 nm using a UV- visible spectrophotometer. The extracts (10–500 µg/mL) in distilled water were added to a hydrogen peroxide solution at 230 nm was determined after 10 mins against the blank solution containing phosphate buffer without hydrogen peroxide. Ascorbic acid was used as a standard.
Total antioxidant activity by phosphomolybdenum method
The total antioxidant capacity of the methanol extract was determined by the phosphomolybdenum method.[Citation22] The assay is based on the reduction of Mo (VI) to Mo (V) by the extract and subsequent formation of green phosphate complex at acid PH. A 0.3 ml extract was combined with 3 ml of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes containing the reaction solution were incubated at 90°C for 90 mins. Then, after cooling the absorbance of the solution was estimated at 695 nm using a spectrophotometer against the blank. Methanol (0.3 mL) in the place of the extract was used as the blank. The total antioxidant activity is expressed as the number of gram equivalent of ascorbic acid. The calibration curve was prepared by mixing ascorbic acid (10–500 µg/mL) with methanol.
Statistical analysis
All the experiments were carried out in triplicate. Experimental results are expressed as mean ± standard deviation (SD) of three parallel measurements. Linear regression analysis was used to calculate the IC50 value.
Results
After the plant sample was extracted, the yield percentage of the methanolic extract of A. racemosus was 29.2%.
Phytochemical screening
The analysis of phytochemical screening may be useful in the detection of the bioactive compounds and subsequently may lead to the drug discovery and pharmacological formulation. Phytochemical analysis of A. racemosus was carried out in methanolic extract and results are shown in .
Table 1. Phytochemical screening of methanolic extract of Asparagus racemosus.
Total phenolic and flavonoid content
The total phenolic content of methanolic extract of A. racemosus measured by Folin-ciocalteu reagent in terms of gallic acid equivalent (the standard curve equation: y = 0.0006x + 0.0367, R2 = 0.9317). The value obtained for the concentration of total phenols is 12.90 ± 0.002 mg/g dry weight. The flavonoid content was expressed in terms of rutin equivalent (the standard curve equation: y = 0.0013 x + 0.0021, R2 = 0.9877). The concentration of flavonoid in plant extract is 0.80 ± 0.001 mg/g dry weight (). It has been recognized that flavonoid shows antioxidant activity and their effects on human nutrition and health are considerable. The result strongly shows that the phenol is important components of this plant and some of the pharmacological effects could be attributed to the presence of this invaluable component.
Table 2. Total Phenolic and Flavonoid Content and Total Antioxidant activity of methanolic extract of A. racemosus.
Antioxidant activity
DPPH free radical scavenging activity
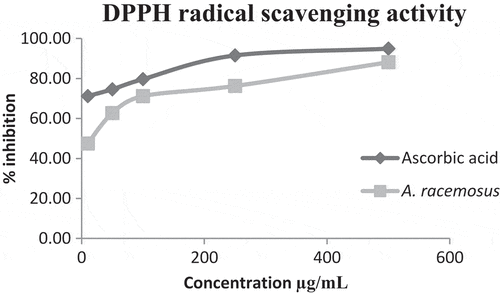
This assay is based on scavenging of the DPPH radical from the antioxidants, which produces a decrease in absorbance at 517 nm. The antioxidant activities of methanolic extract of A. racemosus and the standard ascorbic acid were 47.45% to 88.13% and 71.19% to 94.92% respectively at concentrations of 10 to 500 µg/mL ().
Hydroxyl radical scavenging activity
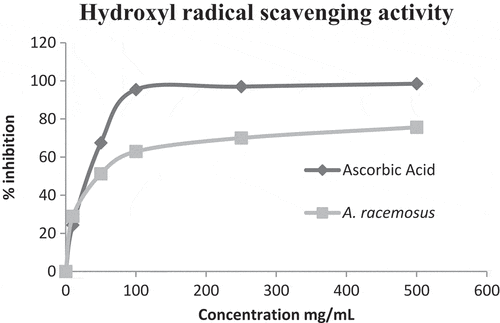
The methanolic extract of A. racemosusshowed the potential inhibitory effect of hydroxyl radical scavenging activity. The plant extract exhibited the minimum activity of 28.93% at 10 µg/mL and the maximum activity of 75.63% at 500 µg/mL ().
Superoxide radical scavenging activity
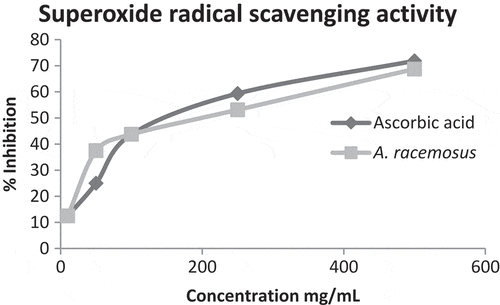
The superoxide radical reduced NBT to blue colored formazan that can be measured at 560 nm. At 10–500 µg/mL, the superoxide scavenging activity of methanolic extract of A. racemosuswas 12.5% to 68.75%, and then the standard ascorbic acid value was 12.5% to 87.5%. The result shows the concentration-dependent radical scavenging activity is increased with sample concentration ().
Hydrogen peroxide radical scavenging activity
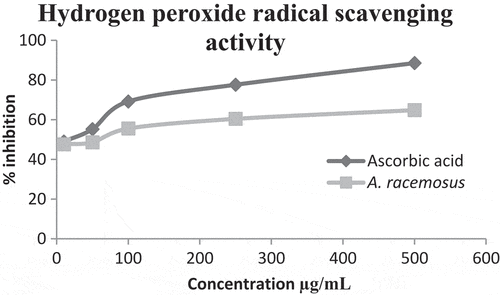
The free radical scavenging activity of A. racemosus was evaluated by hydrogen peroxide (H2O2) scavenging method. From the results, the methanolic plant extract showed concentration-dependent activity and the H2O2 scavenging effect was 47.62% to 64.83% at concentrations of 10 to 500 µg/mL. This was comparable to the scavenging effect of ascorbic acid (49.01% to 88.54%) ().
Total antioxidant activity by phosphomolybdenum method
The total antioxidant activity of A. racemosus was evaluated by based on the reduction of Mo (VI) to Mo (V) by the extract and subsequent formation of green phosphate complex at acid PH. From the results, the total antioxidant activity of the methanolic plant extract was prepared as ascorbic acid equivalents (AAE) per gram. The plant extract showed high antioxidant capacity (132.53 ± 0.12) ().
showed the inhibited concentration (IC50) values of the methanolic extract of A. racemosus. It should be noted that the lowest value of IC50 indicates the strongest activity against free radicals. The results obtained showed that DPPH was the maximum trapping of the free radical with IC50 value was 9.85 ± 0.11 µg/mL followed by hydrogen peroxide, hydroxyl, and superoxide, with IC50 values were 27.64 ± 0.46 µg/mL, 169.27 ± 0.31 µg/mL and 258.26 ± 0.10 µg/mL respectively.
Table 3. IC50 values of the free radical scavenging activities of the methanolic extract of A. racemosus.
Discussion
A. racemosus is commonly used to traditionally treat many diseases whose pathogenesis is, among other factors linked to oxidative stress. However, in order to antioxidant potentials of this plant that could be relevant in the treatment of such diseases have not been investigated. In a study, the phytochemical screening of methanolic extracts of A. racemosus found that all the bioactive compounds are detected except anthroquinone and steroid.[Citation23] Phytochemicals are currently receiving the increased attention of interesting new findings regarding their biological activities. These compounds play some metabolic role and control development in a living system.[Citation24] The alkaloids, saponins, and tannins are detected in this extract could implicate these classes of phytochemicals as important bioactive agents of the root parts of this plant and might be involved in the therapeutic action of this plant part.
So far as plant phenolics constitute one of the major groups of compounds acting as primary antioxidants or free radical terminators, it was reasonable to determine their total amount in the selected plant extract. Flavonoids as one of the most diverse and widespread groups of natural compounds are probably the most important natural phenolics. These compounds possess a broad spectrum of chemical and biological activities including radical scavenging properties. Such properties are especially distinct for flavonols.[Citation17] Therefore, the content of both groups of phenolics was also determined in the extract (). In a study, the total phenolic and flavonoid content of methanolic root extracts of A. racemosus were 365 ± 0.45 mg/100 g and 15.94 ± 0.10 mg/100 g respectively.[Citation25] In another study, the total phenolic content was 3.86 ± 0.32 mg/g.[Citation26] Devendra et al. (2013) showed that the total phenolic and flavonoid contents were 18.94 mg/100 g and 2.02 mg/100 g respectively.[Citation27] The amount of total phenolic compounds in the investigated plant extract in most cases correlated with the antiradical activity.
Five different radical scavenging assays have been performed to analyze the antioxidant activity of methanolic extract of A. racemosus. DPPH becomes diamagnetic molecule after gets reduced into its hydrazine form by electron donation by antioxidants.[Citation28] The high antiradical property of extract may be due to the presence of the phenolic compound.[Citation4] In the literature, Karuna et al. (2018) showed that the ethanolic extract of A. racemosus was 468.57 ± 3.002 µg/mL. In the biochemical system, superoxide radical and hydrogen peroxide react together and form different of ROS viz. Hydroxyl radical and singlet oxygen.[Citation28] The total antioxidant capacity (TAC) was based on the reduction of Mo (VI) to Mo (V) by the extract and subsequent formation of green phosphate/Mo (V) complex at acid pH. It evaluates both water soluble and fat soluble antioxidant.[Citation29]
IC50 value is defined as the concentration of substrate that causes 50% loss of the free radicals activity and was calculated by linear regression mentioned of plots of the percentage of antiradical activity against the concentration of the tested compounds.[Citation30] In this study, methanolic extract displayed the strongest inhibition of DPPH activity (IC50 9.85 ± 0.11 µg/mL), showing less potency than the standard ascorbic acid (IC50 4.89 ± 0.38 µg/mL). However, hydrogen peroxide (IC50 27.64 ± 0.46 µg/mL) showing more potency than the standard (IC50 55.84 ± 0.21 µg/mL). This potency may also be related to the high antioxidant activity of the plant’s extract, thereby mopping up free radicals that could be generated under hyperglycaemic condition.[Citation31]
Conclusion
Today, antioxidant properties of this plant have become a vast interest due to their possible uses as natural additives to substitute synthetic ones. Thus, the results obtained in the present study showed that the methanolic extract of root of A. racemosus contains the maximum antioxidant compound which can scavenge different Reactive oxygen species (ROS) and free radicals under in vitro conditions. The present study suggests that it can be used as a good source of natural antioxidants for health benefits and the bioactive compounds are required for identifying the unknown compounds to establish their pharmacological properties.
Acknowledgments
I thank to the Department of Botany, Berhampur University, Odisha, India for providing the necessary facilities and encouragement. The author acknowledges the financial support from UGC, New Delhi in the form of a Senior Research Fellowship (RGNF). I declare that I have no conflict of interest.
Additional information
Funding
References
- Atanasov, A. G.; Waltenberger, B.; Pferschy-Wenzig, E. M.; Linder, T.; Wawrosch, C.; Ubrin, P.; et al. Discovery and Resupply Of Pharmacologically Active Plant-derived Natural Products: a Review. Biotechnol. Adv. 2015, 33(8),1582–1614
- Heinrich, M.;. Ethnobotany and Natural Products: The Search for New Molecules, New Treatments of Old Diseases or a Better Understanding of Indigenous Cultures? Curr. Topics. Med. Chem. 2003, 3, 141–154.
- Delgado, G.;. Organic Natural Products from Some Mexican Plants. Trends Org. Chem. 1992, 3, 129–140.
- Majouli, K.; Hamdi, A.; Hlila, M. B. Phytochemical Analysis and Biological Activities of Hertiacheirifolia L. Roots Extracts. Asian Pac. J. Trop. Med. 2017, 10, 1134–1139. DOI: 10.1016/j.apjtm.2017.10.020.
- Singh, H.; Bhushan, S.; Arora, R.; Buttar, H. S.; Arora, S.; Singh, B. Alternative Treatment Strategies for Neuropathic Pain: Role of Indian Medicinal Plants and Compounds of Plant origin-A Review. Biomed. Pharmacother. 2017, 92, 634–650. DOI: 10.1016/j.biopha.2017.05.079.
- Kamat, J. P.; Boloor, K. K.; Devasagayam, T. P.; Venkatachalam, S. R. Antioxidant Properties of Asparagusracemosus against Damage Induced by Gamma-Radiation in Rat Liver Mitochondria. J. Ethnopharmacol. 2000, 71, 425–435.
- Sairam, K.; Priyambada, S.; Aryya, N. C.; Goel, R. K. Gastroduodenal Ulcer Protective Activity of Asparagusracemosus: An Experimental, Biochemical and Histological Study. J. Ethnopharmacol. 2003, 86, 1–10. DOI: 10.1016/S0378-8741(02)00342-2.
- Prabhath, K. G.; Satish, K. M. C.; Rajput, R.; Patil, V.; Udupa, A. L. Wound Healing Profile Of Asparagus Racemosus (Liliaceae) Wild. Curr. Pharma. Res. 2011, 1, 111–114
- Handa, S. S.; Khanuja, S. P. S.; Longo, G.; Rakesh, D. D. Extraction Technologies for Medicinal and Aromatic Plants, 1st ed.; United Nations Industrial Development Organization and the International Centre for Science and High Technology: Italy, 2008; Vol. 66.
- Roy, S.; Pradhan, S.; Mandal, S.; Das, K.; Patra, A.; Samanta, A.; Sinha, B.; Kar, S.; Nandi, D. K. Phytochemical Analysis, Antimicrobial Activity and Assessment of Potential Compounds by Thin Layer Chromatography of Ethanol Fraction of Asparagus Racemosusroots. Int. J. Pharm. Pharm. Sci. 2014, 6(8), 367–370.
- Alabri, T. H. A.; Musalami, A. H. S. A.; Hossain, M. A.; Weli, M.; Al-Riyami, Q. Comparative Study of Phytochemical Screening, Antioxidant and Antimicrobial Capacities of Fresh and Dry Leaves Crude Extracts of Daturametel L. J. King Saud Univ. Sci. 2014, 26(3), 237–243. DOI: 10.1016/j.jksus.2013.07.002.
- Patil, S. D.; Ahale, S. V.; Surana, S. J. Antiasthmatic and Antiallergic Activity of Balanitesaegyptiaca (Delile) Balantiaceae. Pharmacology. 2010, 2, 487–495.
- Harborne, J.;. Phytochemical Methods Guide to Modern Technique of Plant Analysis; Chapman and Hall: London, 1998.
- Sofowora, A.;. Medicinal Plants and Traditional Medicine in Africa, 2 ed.; John Wiley and Sons: New York, 1993.
- Trease, G. E.; Evans, W. C. Pharmacognosy; Bailliere Tindall: London, 1989.
- Singleton, V. L.; Rossi, J. A. Colorimetry of Total Phenolics with Phosphomolybdicphosphotungsticacid Reagents. Am. J. Enol. Vitic. 1965, 16, 144.
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J. C.; Bailleul, F.; Trotin, F. Phenolic Compounds and Antioxidant Activities of Buckwheat (Fagopyrumesculentummoench) Hulls and Flour. J. Ethnopharmacol. 2000, 72, 35–42.
- Subhashini, N.; Nagarajan, G.; Kavimani, S. In Vitro Antioxidant and Anticholinesterase Activities of Garcinia Combogia. Int. J. Pharm. Pharm. Sci. 2011, 3, 129–132.
- Smirnoff, N.; Cumbes, Q. J. Hydroxyl Radical Scavenging Activity of Compatible Solutes. Phytochemistry. 1989, 28, 1057–1060. DOI: 10.1016/0031-9422(89)80182-7.
- Liu, F.; Ooi, V. E.; Chang, S. T. Free Radical Scavenging Activities of Mushroom Polysaccharide Extracts. Life Sci. 1997, 60, 763–771. DOI: 10.1016/S0024-3205(97)00004-0.
- Nabavi, S.; Ebrahimzadeh, M.; Nabavi, S.; Hamidinia, A.; Bekhradnia, A. Determination of Antioxidant Activity, Phenol and Flavonoids Content of ParrotiapersicaMey. Pharmacologyonline. 2008, 2, 560–567.
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. DOI: 10.1006/abio.1999.4019.
- Jayashree, G.; Rachitha, P.; Krupashree, K.; Hemanth, K.; Farhath, K. Phytochemical Analysis of Methanolic Extract of Roots of Asparagusracemosus (Shatavari). Int. J. Phama. Bio. Sci. 2013, 4, 250–254.
- Ajuru, M.; Williams, L.; Ajuru, G. Qualitative and Quantitative Phytochemical Screening of Some Plants Used in Ethnomedicine in the Niger Delta Region of Nigeria. J. Food Nutr. Sci. 2017, 5, 198–205. DOI: 10.11648/j.jfns.20170505.16.
- Kaur, S.; Mondal, P. Study of Total Phenolic and Flavonoid Content, Antioxidant Activity and Antimicrobial Properties of Medicinal Plants. J. Microbiol. Exp. 2014, 1, 5. DOI: 10.15406/jmen.2014.01.00005.
- Shahin, S.; Ahmed, N. Antioxidant Properties and Total Phenolic Content of Herbs Used in Post Partum Diet Therapy in Patna (Bihar), India. J. Pharm. Biol. Sci. 2014, 9, 17–20. DOI: 10.9790/3008-09231720.
- Devendra, K.; Kiran, D.; Ritesh, V.; Satyaendra, B.; Abhishek, K. To Evaluation of Total Phenolics and Flavonoids in Different Plan of Chhattisgarh. J. Pharmacogn. Phytochem. 2013, 2, 116–118.
- Karuna, D. S.; Dey, P.; Das, S.; Kundu, A.; Bhakta, T. In Vitro Antioxidant Activities of Root Extract of Asparagusracemosus Linn. J. Traditional Complementary Med. 2018, 8, 60–65. DOI: 10.1016/j.jtcme.2017.02.004.
- Aliyu, A. B.; Ibrahim, M. A.; Musa, A. M.; Musa, A. O.; Kiplimo, J. J.; Oyewale, A. O. Free Radical Scavenging and Total Antioxidant Capacity of Root Extracts of Anchomanes Difformis Engl. (Araceae). Acta Poloniae Pharm. 2013, 70, 115–121.
- Nahak, G.; Sahu, R. K. Phytochemical Evaluation and Antioxidant Activity of Piper Cubeba and Piper Nigrum. J. Appl. Pharm. Sci. 2011, 1(8), 153–157.
- Alimi, A. A.; Ashafa, A. O. T. In Vitro Evaluation of the Antioxidant and Antidiabetic Potential of Sutherlandia Montana E. Phillips and R.A. Dyer Leaf Extracts. Asian Pac. J. Trop. Biomed. 2017, 7(9), 765–772. DOI: 10.1016/j.apjtb.2017.08.004.