ABSTRACT
In the present scenario, the literature is available regarding different challenging matters; however, some horizons still need to be elucidated and “Intermittent Fasting” is one of such examples. For millennia, several scientific interventions were carried out to probe the effect of fasting on human metabolic activities. There are three common strategies of fasting like caloric restriction (CR), dietary restriction (DR) and Intermittent fasting (IF) but IF has emerged as an avenue of potential benefit and wellbeing of the consumer. Intermittent fasting is the prehistoric surreptitious of human health as this powerful habit has been virtually forgotten. Nowadays, numerous researchers are reviving this dietary intervention. It carries enormous consequences such as increased energy, weight loss, and reversal of type-II diabetes. Moreover, it encloses the important evidence validating the health claims of IF with special reference to cancer, coronary heart diseases, biomarkers of oxidative stress and insulin sensitivity. Furthermore, the impact of intermittent fasting on human health based upon metabolic case studies are the limelight of the current manuscript. Conclusively, Intermittent fasting (IF) is the most appropriate tactic for its capability to ameliorate different lifestyle related disorders for instance; diabetes, cardiovascular disorder, cancer, antioxidant stress, and renal diseases.
Introduction
Fasting is partial or total refrain from all foods or avoiding prohibited foods. Fasting has remained a centre point owing to the potential non-pharmacological strategy to improve health and increasing longevity in various scientific interventions.[Citation1] Generally, there are three most commonly studied fasting strategies; they are a caloric restriction (CR), dietary restriction (DR) and intermittent fasting (IF).[Citation2] The research outcomes of these strategies in different in vivo and vitro studies are given below.
In calorie restriction approach reduction in kilocalorie intake to about 20–40% of ad libitum consumption is practised.[Citation3] This approach has been extensively investigated in many experimental models including the dog, fruit fly, rodents and non-human primates. CR has a potential to reduce the initiation of certain disease like atherosclerosis, cardiomyopathies, cancer, diabetes, renal diseases, neurodegenerative diseases and respiratory diseases.[Citation4] In case of cardiovascular health, CR is known to reduce resting heart rate (HR) and blood pressure (BP), elevation in heart rate variability, enhancing the left ventricular function and improving flow-mediated vasodilation.[Citation5] Calorie restricted strategy also reduces the blood glucose level thereby decreasing the plasma insulin level and thus favouring the process of lipolysis.[Citation6] In the calorie-restricted mode, the body uses its stored reserves thereby decreasing the body fat percentage and decreases the incidence of diabetes.However, extreme long-term practising CR regimen for a period of 6 months result in excessive fat and muscle loss that may cause a variety of physiological abnormalities.[Citation7]
In dietary restriction approach, one or more food components (macronutrients) are reduced with nominal or no decrease in total caloric intake. The outcomes of extensive scientific research in different experimental subjects have proved that both carbohydrates and fats have no effect on lifespan, however, protein restriction increases maximum lifespan up to 20% and this may be due to a decrease in amino acid methionine.[Citation8] Reduced methionine intake reduces the generation of mitochondrial reactive oxygen species that damage the mitochondrial DNA and ultimately affecting the functioning of mitochondria. Before approaching any solid decision about the outcomes of DR for humans it is necessary to carry out intensive human trials as the previously provided data has been generated through animal studies.[Citation9,Citation10]
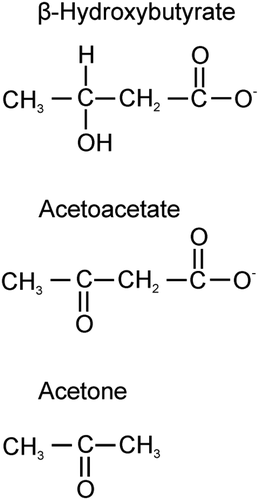
The IF is another emerging avenue of research with superior outcomes than other fasting regimens, it comprises calorie restriction for several hours a day, alternating days or several days a week including feast period in which fasters are allowed to consume food ad libitum while during the fast period the faster refrains from food consumption, however faster is allowed to take water ad libitum all the time.[Citation11] Many scientific studies were carried out to assess the impact of IF on possible health outcomes and it was found that IF resulted in prolonging lifespan and prevention of an array of discrepancies including CVDs, renal diseases, different forms of cancers and diabetes[Citation12] IF was observed to provoke the beneficial outcomes in cardiovascular health including lower heart rate and blood pressure, increased post-exercise heart rate variability.[Citation13] Moreover, gender-specific effects were observed for glucoregulatory health, IF was observed to mend insulin sensitivity in male subjects but no such effect was noticed in females, however glucose tolerance was observed to be impaired in female subjects while no change was observed in male subjects.[Citation14] IF is not a recommended approach for children and teenager on account of rapid growth and development phase and having elevated nutritional and caloric requirements.[Citation15] However, this methodology has proved to be effective in adults with BMI 25 and above with negligible side effects. IF has varied effect in different age groups owing to transitions in metabolic status.[Citation16] IF results in ketone bodies (acetoacetate, β-hydroxybutyrate and acetone) generation, recent studies have shown that ketone bodies enhanced the expression of gene encoded for mitochondrial enzyme and energy metabolism in hippocampus (learning and memory part of brain). Among the ketone bodies β-hydroxybutyrate behaves as even more efficient energy source than glucose by delivering more energy per unit oxygen used. Ketone bodies in contrast to glucose do not release any reactive oxygen species (ROS) but directly inhibit the production of these violent molecules and momentously increase the destruction of these vicious molecules by enhancing the functioning of glutathione peroxidase thus safeguarding body against brain degenerative malady like Parkinson’s disease.[Citation17] The review article is a limelight of health benefits of IF and is an inspiration for future studies on this avenue of pronounced potential.
Anticancer perspective
Cancer cells are glucose loving as they have more insulin trans-membrane receptor sites to increase the uptake of glucose; however normal body cells are flexible to use other available energy sources like fat and proteins during fasting when glucose is not available.[Citation18] During the fasting phase hepatocytes are capable to carry out a process called ketogenesis that produces ketone bodies (acetoacetate, β-hydroxybutyrate and acetone) that are delivered to peripheral tissues for energy production.[Citation19] In this way cancer cells being inflexible to use an energy source other than glucose result in their death.[Citation20,Citation21] Another approach to initiate ketogenesis is by introducing a ketogenic diet highly recommended for cancer patients, it comprises of a negligible amount of carbohydrates and high-fat content (> 50% of energy intake) ().[Citation22]
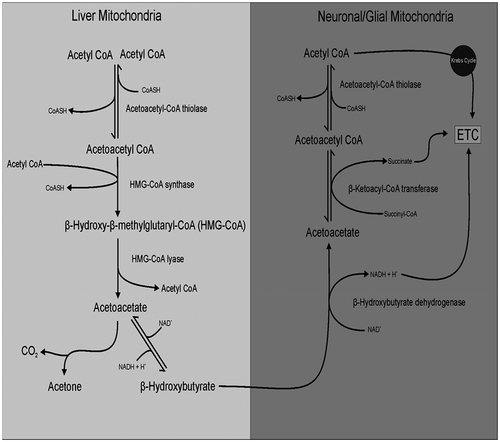
Cancer cells have reduced activity of succinyl-CoA: 3-ketoacid CoA transferase, the rate-controlling step for utilizing β-hydroxybutyrate as a respiratory fuel.[Citation23] Therefore, tumour cells experience high metabolic stress following the gradual replacement of glucose with ketone bodies.[Citation24] The stressed tumour cells being weakly unable to adopt a phenomenon called differential stress sensitization (DSS) that is established on the fact that mutation in cancer cells promote growth under normal conditions but make them unable to harmonize with transitional energy source.[Citation25] In this way cancer in its initial phase can be treated only by fasting, therefore Food and Drug Administration (FDA) recommends IF as necessary therapy to mitigate the atrocious risks of cancer ().[Citation26]
TCA cycle metabolites can also be produced through glutamine in addition to glucose in tumour cells. Glutamine after converting to α-ketoglutarate can provide energy through substrate-level phosphorylation within the Krebs cycle.[Citation27] Substrate level phosphorylation during glycolysis and Krebs cycle can provide sufficient energy for tumour cells having defective oxidative phosphorylation mechanism. Here ketone bodies further reduce the substrate level phosphorylation under hypoxia by reducing the activity of succinyl-CoA synthetase (SCS).[Citation17,Citation28] In this way ketone bodies indirectly play their role in tumour cell death by targeting the ATP production from glutamine metabolism.[Citation29]
Another reason for cancer cell death is due to mitochondrial DNA mutation that results in defective inner mitochondrial membrane altering the proton motive gradient and ATP production even during oxidative metabolism. Moreover, β-hydroxybutyrate dehydrogenase that is involved in the first step of β hydroxyl butyrate to acetoacetate interacts with cardiolipin and other phospholipids in the inner mitochondrial membrane.[Citation30] In a nutshell, the mitochondria of tumour cells are dysfunctional resulting in electrons transfer ineffectively coupled to oxidative energy production thereby generating an insufficient number of ATP through oxidative phosphorylation.[Citation31]
Recently outcomes of rodent modelling experiments on cancer cell lines delineate that leptin has oncogenic, mitogenic, pro-inflammatory, and pro-angiogenic role in cell proliferation and anti-apoptosis in a variety of cells and inducer of cancer stem cells.[Citation32] IF sharply reduces serum insulin-like growth factor – I (IGF-I) and leptin levels, and increases adiponectin that safeguards cell proliferation. IGF-I is a nutrient-responsive growth factor that initiates two main signalling pathways; Ras/MAPK and PI3K/AKT.[Citation33,Citation34] These two cascades are involved in proliferation and cellular growth by favouring the action of transcription factors and succeeding gene expressions. Activation of PI3K/AKT route favours reduced apoptosis by interfering with Bcl-2-associated death promoter complex, enhances the protein production through mTOR initiation, and elevates glucose metabolism by suppressing GSK-3 β supporting the tumour proliferation.
Working antagonistically to IGF-I mediated activation of mTOR, AMP-activated protein kinase (AMPK) under IF regimen behaves as a molecular sensor that enhances catabolism and suppresses anabolism thereby initiating apoptosis in cranial cancer cells lines and safeguarding the normal cells from strain.[Citation35] AMPK triggers SIRT1 metabolism regulator gene expression that results in augmented fatty acid oxidation and glutaminolysis and delivering intermediary substrates for energy production when glucose is unavailable.[Citation36]
Some scientific studies have reported AMPK/mTOR possess autophagy regulatory ability that results in the packing of macronutrients and organelles in doubled- membrane structures and degraded into micro-components that may be utilized in a variety of metabolic cascades.[Citation37] The autophagy is believed to be a tumour oppressive due to abnormalities in autophagy drive oxidation stress, defective mitochondria, damaged DNA, genomic instability and tumour growth.[Citation38] Alternatively, it is thought to be involved in tumour proliferation as tumours can exploit autophagy to decrease oxidative stress and enhance mitochondrial functionality to promote cell survival and overcome stress in low nutrient conditions.[Citation39] Owing to the dual nature of autophagy it has become the subject of mutual interest in cancer controlling therapies.[Citation40]
In a variety of preclinical and human efficacy trials, IF is suggested to suppress the inflammatory response cascades. Under IF regimen amount of adipose tissue the main endocrine organ is reduced that produces pro-inflammatory factors like leptin, adiponectin, monocyte chemoattractant protein-1, tumour necrosis factors, and interleukin-6. These human studies have associated IF with decreased adiposity and low inflammatory adipose secretome together the with reduced systemic pro-inflammatory adipokines levels.[Citation41] Moreover, IF is known to steadily decrease the pro-angiogenic factors vascular endothelial factor expression and plasminogen activator inhibitor-1, these two provide oxygen and glucose to the growing cancer cyst by inducing growth of new blood vessels.[Citation42] IF is also responsible for reducing inflammatory gene expression in cancer cells, including nuclear factor kappa B and peroxisome proliferator-activated receptors mostly expressed in tumour cells playing their role in inflammation regulation, proliferation and glucose and lipid homeostasis.[Citation43,Citation44]
Antihypertensive perspective
In different in vitro studies IF has proved to mitigate the risk factors involved in cardiovascular diseases and stroke. Cardiovascular diseases and stroke have been associated with impaired glucose hemostasis (insulin resistance) resulting in elevated blood glucose level and insulin. In different in vitro studies on rodent modelling IF improved insulin sensitivity. Elevated low-density lipoprotein and low level of high-density lipoprotein cholesterol also increase the risk of atherosclerosis and stroke.[Citation45] Recently fasting studies conducted in human subjects have shown to decrease LDL and the increase HDL levels, as indicated by reduced oxidative modification of proteins and DNA and decreased the level of lipid peroxidation in the heart.[Citation46] Fasting is known to reduce oxidative stress in the cardiovascular system. It also minimizes the inflammatory process that contributes to atherosclerosis, indicated by reduced levels of leukocytes and circulating levels of tumour necrosis factor and other inflammatory cytokines.[Citation47]
Hypertension is another major contributor to coronary heart diseases. Fasting reduces the resting heart rate and blood pressure (both systolic and diastolic) in experimental rodents.[Citation48] It also improves the cardiovascular stress-bearing capacity in the rodents, due to the activation of the stress-responsive hypothalamic pituitary adrenal neuroendocrine system as indicated by an increase in plasma adrenocorticotropin (ACTH) and corticosterone levels.[Citation49] The scientists have discovered two main effects of IF in promoting cardiovascular stability that is a decreased ox radical production and increased cellular stress resistance.[Citation50] In a recent study oxidative damage to protein, lipids and DNA was observed to be reduced in rodents maintained on IF than rodents fed on an ad libitum diet.[Citation51] Oxidative stress is responsible for the ischemic injury to cardiac myocytes that results in atherosclerosis.[Citation42] IF initiates the release of protein chaperones heat-shock protein 70 and glucose-regulated protein 78 these proteins are produced in response to the stress during the IF and involve in the repair of cells by performing chaperone function and stabilizing new proteins to ensure correct folding or by helping to refolding proteins that were damaged by the cell stress.[Citation52] IF imparts antihypertensive effect by autonomic modulation of vascular smooth muscles and cardiac myocytes.[Citation53]
IF decreases the blood pressure by reducing the activity of the sympathetic nervous system. The blood pressure and heart rate are also affected by the decreased production of catecholamines (norepinephrine and epinephrine) as IF decrease the level of dopamine beta-hydroxylase (the enzyme required for the production of catecholamines.[Citation54] Blood pressure may also be modulated by the effect of IF on hypothalamic-pituitary neuroendocrine pathways. The ACTH and corticosterone levels are increased by the stress during IF and improve the cardiovascular system.[Citation55]
In a recent study IF has shown to be effective against cardiac hypertrophy by recovering the bio-markers like cardiac sarcomeric α-actin, β-MHC and elevated brain natriuretic peptide (BNP) responsible for cardiac adenomegaly.[Citation56] Furthermore, the age-linked increase in left ventricle weight and cross-sectional area of individual cardiomyocytes are improved by IF. The ameliorative role of IF is achieved by restoring over activated ERK1/2 and PI3K γ signalling, these two are responsible for pathological cardiac hypertrophy.[Citation57] Moreover, the oxidative stress, fibrosis and inflammation in the ageing heart are reported to be attenuated by IF.
It has been validated through rodent modelling experiments that IF has the capacity to mitigate the risk factors for coronary heart disease like blood pressure abnormalities, variation in heart rate and reduction in insulin levels. The beneficial role of IF against coronary heart diseases is attributed to modulations of adipokines. In many animal studies IF has successfully modified many prominent risk factors associated with CVD and stroke. The experimental subjects with the compromised glucose controlling mechanism, that is solely associated with increased plasma glucose and insulin are more prone to CVDS and stroke.[Citation58] In all the experimental trials IF decreased the threat of diabetes and CVDs by enhancing the insulin sensitivity. The occurrence of CVD and stroke are directly linked with the elevated level of low-density lipoprotein (LDL) cholesterol and decreased levels of high-density lipoprotein (HDL) cholesterol. Some studies on human subjects elucidated that IF can significantly lower down LDL cholesterol, whilst elevating the HDL cholesterol levels.[Citation59] These studies also showed a decrease in plasma reactive oxygen species (ROS) levels during IF regime, as depicted by reduced oxidative modification of proteins and DNA and reduced lipid peroxidation in heart tissues.[Citation60] IF decreases the level of leukocytes and circulating tumour necrosis factor and other inflammatory cytokines resulting in promoting inflammatory responses that probably contribute to atherosclerosis.[Citation61] IF by reducing the progression of atherosclerosis IF plays a major role in reducing the risk of CVD and stroke. Hypertension is the major contributor in CVDs and stroke.[Citation62] IF momentously decreases the resting blood pressure (BP) (both systolic and diastolic) and resting heart rate (HR). It recovers cardiovascular stress adaptation. IF dietary regime elevates the plasma ACTH and corticosterone levels by activating stress responsive hypothalamic-pituitary neuroendocrine system during immobilization stress.[Citation63]
Biomarkers of oxidative stress
The human body is equipped with a natural defence mechanism against the hurtful effects of ROS, the disparities between ROS and antioxidant production is known as oxidative stress. It is a major culprit in a wide range of human maladies like cancer, CVDs, neurological disorders, hepatic necrosis and even the physiological ageing process.[Citation64]
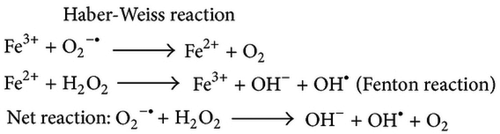
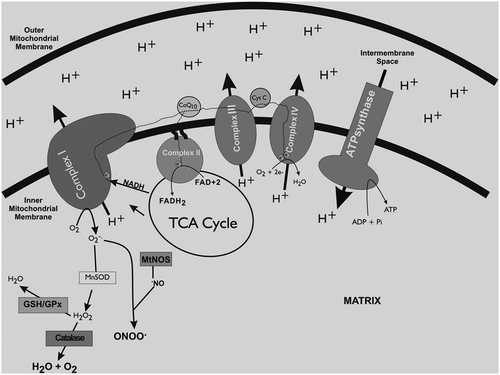
ROS are produced as byproducts during energy generation within the mitochondria through electron transport chain (ETC). The free electrons escape from complex I or II of the ETC reduces oxygen (O2) to superoxide (O2-).[Citation65] Normally it is converted by copper-zinc superoxide dismutase localized in cytosol and manganese superoxide dismutase found in mitochondria to hydrogen peroxide (H2O2). This H2O2 produced may follow two paths depending upon the cellular environment firstly being cytotoxic it is rapidly changed into water through catalase and glutathione peroxidase secondly it can be converted to highly reactive ·OH through iron-catalyzed Fenton reaction.[Citation66] This highly reactive ·OH produces 4-hydroxynonenal (HNE) by the lipid peroxidation of unsaturated fatty acids that binds with the membrane proteins and impairing the cellular function ().[Citation67]
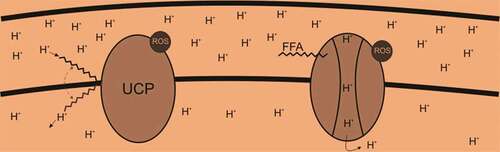
The production of ROS in mitochondria is directly linked with mitochondrial potential (Δψ), hyperpolarization facilitates the ROS synthesis. The background cascade of this phenomenon is the changed redox potential of ETC carriers (reduced) and an enhanced semiquinone anion half-life.[Citation68] Alternatively, ETC is unable to shift protons out of the mitochondrial matrix (against the proton concentration gradient) when the mitochondrion is hyperpolarized (high Δψ), this results in intermediates remain reduced longer and enhancing the probability that the electrons outflow from these intermediates, reducing oxygen and increasing ROS.[Citation69] As the amount of ROS generation primarily depends upon mitochondrial polarization, therefore even a slight decrease through proton permeability across the mitochondrial inner membrane (uncoupling) can reduce ROS.[Citation70] This proton permeability can be increased by endogenous mitochondrial uncoupling mediated by uncoupling proteins (UCPs). UCPs are sensitive to ROS and ATP levels as they are inhibited by purine nucleotides and activated by FFAs and O2-. Recent scientific outcomes have justified that functions and expressions of UCPs are upregulated by fasting during which fatty acids are produced via beta-oxidation within the mitochondrial matrix ().[Citation71]
There are two possible pathways for uncoupling the ETC from ATP production through UCP assisted proton translocation. The first suggested pathways elucidate that protonated free fatty acids flip-flop across the inner membrane (left side of ) by using UCPs as a platform. The second possible mechanisms state that free fatty acids activate UCPs that form pore through which protons can then flow (right side of ). Both of these ROS dependent pathways serve as an endogenous anti-ROS mechanism.
In a recent animal study, it has been proposed that ROS generation is facilitated by elevated blood glucose levels as increased plasma glucose level contribute in glycation of proteins and lipids peroxidation, resulting in the formation of advanced glycation end products (AGEs).[Citation72] Different tissues and cell types including endothelial cells, vascular smooth muscle cells and macrophages contain receptors for AGEs (RAGE). AGEs on binding with RAGE result in ROS production that is further involved in complex cytotoxic cascades.[Citation73]
IF results in a momentous increase in the superoxide dismutase (SOD) levels and malondialdehyde (MDA) levels in hyperglycemic subjects. Recently silent information regulator 2 (Sir2)-family proteins (sirtuins) are reported to affect the apoptosis in response to DNA damage and oxidative stress. Sirtuins are Class III protein deacetylases conserved from prokaryotes to mammals.[Citation71] In vitro and in vivo studies sirtuins have shown to bind and deacetylate p53, and they target many proteins that are not histones. P53 activation results in cell cycle arrest, and the initiation of apoptosis and autophagy.[Citation74] In response to DNA damage and oxidative stress, sirtuins are overexpressed and have shown to inhibit p53 dependent apoptosis. In a recent study conducted in diabetic subjects overexpression of p53gene resulted in apoptosis.[Citation75] However, the expression of p53 was momentously decreased in the diabetic subject on IF retro. It is assumed that the activation, as well as expression of the p53 gene, is mediated by Sir2 dependent deacetylation. Under IF regime the Sir2 expression is reduced and at the same time, p53 is up-regulated. Alterations in p53 expression in diabetic subjects kept on IF regimen elucidates the contribution of anti-apoptotic pathways.[Citation76] Caspases are a family of protease enzymes playing essential roles in programmed cell death and inflammation, caspases when activated initiate a chain reaction of events resulting in cleavage of PARP and self-autolysis producing 20 kDa fragment indicating apoptosis. The cleavage of 20 kDa fragment is distinctively decreased in diabetic subjects following IF regime. It is still unclear that how IF exerts anti-apoptotic effect but a decrease in caspase-3 cleavage and suppression and activation of p53 and Sir2 respectively propose a cross-link between Sir2, p53 and caspase-3. Another stress-activated protein kinase p38 was also identified in diabetic subjects; it is also known to up-regulate the apoptotic cell death.[Citation77] Interestingly, IF decreases the expression of p38 in diabetic subjects with elevated p38 expression. P38 Mitogen-activated protein kinase (MAPK) cascade induces phosphorylation of histone H3. The diabetic subjects on IF regime showed decreased in phosphorylation of histone H3.
Insulin sensitivity
A great deal of available scientific data on animal studies proposes that IF improves the insulin sensitivity. The extended duration of fasting or exercise shifts liver, cardiac tissues and skeletal muscles to fat oxidation and amino acid catabolism, while well-fed state facilitates the metabolic pathways of glucose uptake and oxidation.[Citation78] The two hormones glucagon and insulin along with transitions in cytological levels of metabolites like fatty acids, pyruvate, citrate and malonyl CoA (regulates mitochondrial enzymes) regulate the mutual fat and glucose oxidation systematically this systematic fat and glucose switching is commonly known as metabolic flexibility.[Citation79] During the period of physiological stress and nutrient availability if the body successfully shifts between fat and glucose oxidation, then the body energy metabolism is considered to be optimum.[Citation80] This switching in energy metabolism is responsible for sustaining metabolic well-being and ideal cellular activities.[Citation81] Overfed individuals cannot easily switch between fat and glucose oxidation and therefore experience metabolic inflexibility.[Citation82] The mitochondrial functioning is disturbed by the production of ROS, ceramides, diacylglycerols and acylation of mitochondrial proteins due to simultaneous oxidation of fat, glucose and amino acids. The irregularities in metabolic flexibilities are thought to be the main reason for insulin resistance.[Citation83] IF up-regulates the genes responsible both for lipid storage (PPARγ 2 and Fsp27) and fat oxidation (MCPT1) elucidating optimal metabolic flexibility with enhanced fat oxidation during fasting period and lipogenesis on non-restricted retro of IF.[Citation84] The adipocyte variation and expression of adipocyte regulatory genes is controlled by a transcription factor peroxisome proliferator-activated receptor- γ (PPARγ).[Citation85] This transcription factor has two isoforms i.e PPARγ1 and γ2 resulting from alternate merging and having ligand-dependent and independent initiation sites.[Citation86] PPARγ2 contains ligand-independent activation domain owing to the presence of additional 28 amino acids at its amino end making it more effectively foldable than that of PPARγ1.[Citation87] The ligand-independent activation of PPARγ1 and γ2 is stimulated by insulin, conversely, obesity and nutritional factors only influence the PPARγ2 expression in human adipocytes.[Citation88,Citation89] Martinez et al.[Citation90] reported that a common Pro12Ala replacement in PPARγ2 is associated with lower body mass index (BMI) and improved insulin sensitivity among middle-aged and elderly subjects.
Insulin resistance and type 2 diabetes may be initiated due to irregularities in adipose tissue metabolism.[Citation91] Fatty acids and eicosanoids may initiate the stimulation of target genes responsible for adipocyte differentiation and glucose homeostasis by binding to PPARγ.[Citation92] PPARG gene is responsible for encoding PPARγ it is 100 kb long and is composed of 9 exons. A C®G variant (creating an Hgal site) envisages the exchange of Ala for Pro at location 12 in the PPAR γ2 specific exon B, and a synonymous C®T replacement in exon 6. Previously an association between the synonymous polymorphism and leptin levels was observed among the obese individuals.[Citation93]
Conclusion
Intermittent fasting is a promising strategy among different approaches of fasting such as caloric restriction, dietary restriction and intermittent fasting. Intermittent fasting has proved the most fruitful approach for its ability to cope up with different diseases such as cancer, diabetes, antioxidant stress, cardiovascular diseases, renal diseases and blood pressure through various in-vivo and in-vitro studies. Moreover, intermittent fasting resulted in prolong lifespan. However, further research is needed with respect to health claims of intermittent fasting in humans and animals.
References
- Fontana, L.; Partridge, L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell. 2015, 161, 106e118. DOI: 10.1016/j.cell.2015.02.020.
- Johnstone, A. Fasting for Weight Loss: An Effective Strategy or Latest Dieting Trend? Int. J. Obes. 2015, 39, 727–733. DOI: 10.1038/ijo.2014.214.
- Abreu-Vieira, G.; Xiao, C.; Gavrilova, O.; Reitman, M.-L. Integration of Body Temperature into the Analysis of Energy Expenditure in the Mouse. Mol. Metab. 2015, 4, 461e470. DOI: 10.1016/j.molmet.2015.03.001.
- Patterson, R.-E.; Laughlin, G.-A.; LaCroix, A.-Z.; Hartman, S.-J.; Natarajan, L.; Senger, C.-M.; Martínez, M. E.; Villaseñor, A.; Sears, D. D.; Marinac, C. R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203e1212. DOI: 10.1016/j.jand.2015.02.018.
- Most, J.; Tosti, V.; Redman, L.-M.; Fontana, L. Calorie Restriction in Humans: An Update. Ageing Res. Rev. 2016. DOI: 10.1016/j.arr2016.08.005.
- Anastasiou, C.-A.; Karfopoulou, E.; Yannakoulia, M. Weight Regaining: From Statistics and Behaviors to Physiology and Metabolism. Metabolism. 2015, 64, 1395–1407. DOI: 10.1016/j.metabol.2015.08.006.
- Brandhorst, S.; Choi, I.-Y.; Wei, M.; Cheng, C.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22(1), 86–99. DOI: 10.1016/j.cmet.2015.05.012.
- Mundstock, E.; Sarria, -E.-E.; Zatti, H.; Mattos Louzada, F.; Kich Grun, L.; Herbert Jones, M.; Guma, F. T.; Mazzola In Memoriam, J.; Epifanio, M.; Stein, R.-T.; et al. Effect of Obesity on Telomere Length: Systematic Review and Meta-Analysis. Obesity (Silver Spring). 2015, 23, 2165–2174. DOI: 10.1002/oby.21183.
- Gotthardt, J.-D.; Verpeut, J.-L.; Yeomans, B.-L.; Yang, J.-A.; Yasrebi, A.; Roepke, T.-A.; Bello, N.-T. Intermittent Fasting Promotes Fat Loss with Lean Mass Retention, Increased Hypothalamic Norepinephrine Content, and Increased Neuropeptide Y Gene Expression in Diet-Induced Obese Male Mice. Endocrinology. 2016, 157, 679–691. DOI: 10.1210/en.2015-1622.
- Gao, Q.; Jia, Y.; Yang, G.; Zhang, X.; Boddu, P.-C.; Petersen, B.; Narsingam, S.; Zhu, Y.-J.; Thimmapaya, B.; Kanwar, Y. S.; et al. PPARalpha-deficient Ob/Ob Obese Mice Become More Obese and Manifest Severe Hepatic Steatosis Due to Decreased Fatty Acid Oxidation. Am. J. Pathol. 2015, 185, 1396e1408. DOI: 10.1016/j.ajpath.2015.01.018.
- Kim, S.-H.; Chun, H.-J.; Choi, H.-S.; Kim, E.-S.; Keum, B.; Jeen, Y.-T. Current Status of Intragastric Balloon for Obesity Treatment. World J. Gastroenterol. 2016, 22, 5495–5504. DOI: 10.3748/wjg.v22.i37.8314.
- Horne, B.-D.; Muhlestein, J.-B.; Anderson, J.-L. Health Effects of Intermittent Fasting: Hormesis or Harm? A Systematic Review. Am. J. Clin. Nutr. 2015, 102(2), 464–470. DOI: 10.3945/ajcn.115.109553.
- Habermann, N.; Makar, K.-W.; Abbenhardt, C.; Xiao, L.; Wang, C.-Y.; Utsugi, H.-K.; Alfano, C.-M.; Campbell, K.-L.; Duggan, C.; Foster-Schubert, K.-E.; et al. No Effect of Caloric Restriction or Exercise on Radiation Repair Capacity. Med. Sci. Sports Exercise. 2015, 47, 896–904. DOI: 10.1249/MSS.0000000000000651.
- Martin, K.; Jackson, C.-F.; Levy, R.-G.; Cooper, P.-N. Ketogenic Diet and Other Dietary Treatments for Epilepsy. Cochrane Database Syst. Rev. 2016, 2, CD001903. DOI: 10.1002/14651858.CD004158.pub3.
- Das, J. K.; Lassi, Z. S.; Hoodbhoy, Z.; Salam, R. A. Nutrition for the Next Generation: Older Children and Adolescents. Ann. Nutr. Metab. 2018, 72(3), 56–64. DOI: 10.1159/000487385.
- Adrienne, R.; Kristin, K.; Terry, G.; Krista, A. Intermittent Fasting Vs Daily Calorie Restriction for Type 2 Diabetes Prevention: A Review of Human Findings. Transl. Res. 2014, 164(4), 302–311. DOI: 10.1016/j.trsl.2014.05.013.
- Tatulli, G.; Mitro, N.; Cannata, M.; Audano, M.; Caruso, D.; D’Arcangelo, G.; Lettieri-Barbato, D.; Aquilano, K. Intermittent Fasting Applied in Combination with Rotenone Treatment Exacerbates Dopamine Neurons Degeneration in Mice. Front. Cell. Neurosci. 2018, 12, 4. DOI: 10.3389/fncel.2018.00191.
- Renehan, A.-G.; Tyson, M.; Egger, M.; Heller, R. F.; Zwahlen, M. Body-Mass Index and Incidence of Cancer: A Systematic Review and Meta-Analysis of Prospective Observational Studies. Lancet. 2008, 371, 569–578. DOI: 10.1016/S0140-6736(08)60269-X.
- Lv, M.; Zhu, X.; Wang, H.; Wang, F.; Guan, W. Roles of Caloric Restriction, Ketogenic Diet and Intermittent Fasting during Initiation, Progression and Metastasis of Cancer in Animal Models: A Systematic Review and Meta-Analysis. PLoS One. 2014, 9(12), e115147. DOI: 10.1371/journal.pone.0115147.
- Lashinger, L.-M.; O’Flanagan, C.-H.; Dunlap, S.-M.; Rasmussen, A.-J.; Sweeney, S.; Guo, J.-Y.; Lodi, A.; Tiziani, S.; White, E.; Hursting, S.-D. Starving Cancer from the outside and Inside: Separate and Combined Effects of Calorie Restriction and Autophagy Inhibition on Ras-Driven Tumors. Cancer Metab. 2016, 4, 18. DOI: 10.1186/s40170-016-0158-4.
- Armitage, C.-J.; Wright, C.-L.; Pegington, M.; McKee, M.; Knai, C. Self- Efficacy for Temptations Is a Better Predictor of Weight Loss than Motivation and Global Self-Efficacy: Evidence from Two Prospective Studies among Overweight/Obese Women at High Risk of Breast Cancer. Patient Educ. Couns. 2014, 95, 254–258. DOI: 10.1016/j.pec.2013.12.013.
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.-M.; Amaravadi, R.-K.; Baehrecke, E.-H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.-A.; Karantza, V.; et al. Autophagy in Malignant Transformation and Cancer Progression. Embo J. 2015, 34(7), 856–880. DOI: 10.15252/embj.201490784.
- Bowers, L.-W.; Rossi, E.-L.; O’Flanagan, C.-H.; de Graffenried, L.-A.; Hursting, S.-D. The Role of the insulin/IGF System in Cancer: Lessons Learned from Clinical Trials and the Energy Balance-Cancer Link. Front. Endocrinol. 2015, 6, 77. DOI: 10.3389/fendo.2015.00077.
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. International Agency for Research on Cancer Handbook Working G: Body Fatness and Cancer – Viewpoint of the IARC Working Group. New Engl. J. Med. 2016, 375, 794–798. DOI: 10.1056/NEJMsr1606602.
- De Groot, S.; Vreeswijk, M.-P.; Welters, M.-J.; Gravesteijn, G.; Boei, -J.-J.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, -J.-J.; Nortier, J.-W.;; et al. The Effects of Short-Term Fasting on Tolerance to (Neo) Adjuvant Chemotherapy in HER2-negative Breast Cancer Patients: A Randomized Pilot Study. BMC Cancer. 2015, 15, 652. DOI: 10.1186/s12885-015-1584-3.
- Altman, B.-J.; Stine, Z.-E.; Dang, C.-V. From Krebs to Clinic: Glutamine Metabolism to Cancer Therapy. Nat. Rev. Cancer. 2016, 16(10), 619–634. DOI: 10.1038/nrc.2016.71.
- Cifarelli, V.; Lashinger, L.-M.; Devlin, K.-L.; Dunlap, S.-M.; Huang, J.; Kaaks, R.; Pollak, M.-N.; Hursting, S.-D. Metformin and Rapamycin Reduce Pancreatic Cancer Growth in Obese Prediabetic Mice by Distinct microRNA-regulated Mechanisms. Diabetes. 2015, 64(5), 1632–1642. DOI: 10.2337/db14-1560.
- Harvey, A.-E.; Lashinger, L.-M.; Hays, D.; Harrison, L.-M.; Lewis, K.; Fischer, S.-M.; Hursting, S.-D. Calorie Restriction Decreases Murine and Human Pancreatic Tumor Cell Growth, Nuclear factor-kappaB Activation, and Inflammation-Related Gene Expression in an Insulin-Like Growth Factor-1-Dependent Manner. PLoS One. 2014, 9(5), e94151. DOI: 10.1371/journal.pone.0094151.
- Farazi, M.; Nguyen, J.; Goldufsky, J.; Linnane, S.; Lukaesko, L.; Weinberg, A.-D.; Ruby, C.-E. Caloric Restriction Maintains OX40 Agonist-Mediated Tumor Immunity and CD4 T Cell Priming during Aging. Cancer Immunol. Immunother. 2014, 63(6), 615–626. DOI: 10.1007/s00262-014-1542-y.
- Marosi, K.; Kim, S.-W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.-P. 3-Hydroxybutyrate Regulates Energy Metabolism and Induces BDNF Expression in Cerebral Cortical Neurons. J. Neurochem. 2016, 139, 769–781. DOI: 10.1111/jnc.13868.
- Houten, S.-M.; Violante, S.; Ventura, F.-V.; Wanders, R.-J. The Biochemistry and Physiology of Mitochondrial Fatty Acid Beta-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23e44. DOI: 10.1146/annurev-physiol-021115-105045.
- Sancar, A.; Lindsey-Boltz, L.-A.; Gaddameedhi, S.; Selby, C.-P.; Ye, R.; Chiou, -Y.-Y.; Kemp, M.-G.; Hu, J.; Lee, J.-H.; Ozturk, N. Circadian Clock, Cancer, and Chemotherapy. Biochemistry. 2015, 54(2), 110–123. DOI: 10.1021/bi5007354.
- Denduluri, S.-K.; Idowu, O.; Wang, Z.; Liao, Z.; Yan, Z.; Mohammed, M.-K.; Ye, J.; Wei, Q.; Wang, J.; Zhao, L.; et al. Insulin-Like Growth Factor (IGF) Signaling in Tumorigenesis and the Development of Cancer Drug Resistance. Genes Dis. 2015, 2(1), 13–25. DOI: 10.1016/j.gendis.2014.10.004.
- Cheng, C.-W.; Adams, G.-B.; Perin, L.; Wei, M.; Zhou, X.; Lam, B.-S.; Da Sacco, S.; Mirisola, M.; Quinn, D.-I.; Dorff, T.-B. Prolonged Fasting Reduces IGF-1/PKA to Promote Hematopoietic-Stem-Cell-Based Regeneration and Reverse Immunosuppression. Cell Stem Cell. 2014, 14(6), 810–823. DOI: 10.1016/j.stem.2014.04.014.
- Wright, C.-E.; Harvie, M.-N.; Howell, A.; Evans, D. G.; Hulbert-Williams, N.; Donnelly, L. S. Beliefs about Weight and Breast Cancer: An Interview Study with High Risk Women following a 12 Month Weight Loss Intervention. Hered. Cancer Clin. Pract. 2015, 13, 1. DOI: 10.1186/s13053-014-0023-9.
- Pietrocola, F.; Pol, J.; Vacchelli, E.; Rao, S.; Enot, D.-P.; Baracco, -E.-E.; Levesque, S.; Castoldi, F.; Jacquelot, N.; Yamazaki, T.; et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell. 2016, 30(1), 147–160. DOI: 10.1016/j.ccell.2016.05.016.
- Schwartz, L.; Buhler, L.; Icard, P.; Lincet, H.; Steyaert, J.-M. Metabolic Treatment of Cancer: Intermediate Results of a Prospective Case Series. Anticancer Res. 2014, 34(2), 973–980.
- Newman, J.-C.; Covarrubias, A.-J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.-P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557. DOI: 10.1016/j.cmet.2017.08.004.
- Klement, R.-J.; Champ, C.-E. Calories, Carbohydrates, and Cancer Therapy with Radiation: Exploiting the Five R’s through Dietary Manipulation. Cancer Metastasis Rev. 2014, 33(1), 217–229. DOI: 10.1007/s10555-014-9495-3.
- Harvie, M.-N.; Sims, A.-H.; Pegington, M.; Spence, K.; Mitchell, A.; Vaughan, -A.-A.; Allwood, J.-W.; Xu, Y.; Rattray, N.-J.; Goodacre, R.; et al. Intermittent Energy Restriction Induces Changes in Breast Gene Expression and Systemic Metabolism. Breast Cancer Res. 2016, 18(1), 57. DOI: 10.1186/s13058-016-0714-4.
- Kelly, A.-S.; Ryder, J.-R.; Marlatt, K.-L.; Rudser, K.-D.; Jenkins, T.; Inge, T.-H. Changes in Inflammation, Oxidative Stress and Adipokines following Bariatric Surgery among Adolescents with Severe Obesity. Int. J. Obes. 2016, 40, 275–280. DOI: 10.1038/ijo.2015.174.
- Toth, C.; Clemens, Z. Halted Progression of Soft Palate Cancer in a Patient Treated with the Paleolithic Ketogenic Diet Alone: A 20-Months Follow-Up. Am. J. Case Rep. 2016, 4(8), 288–292.
- Yang, W.; Cao, M.; Mao, X.; Wei, X.; Li, X.; Chen, G.; Zhang, J.; Wang, Z.; Shi, J.; Huang, H.; et al. Alternate-Day Fasting Protects the Livers of Mice against High-Fat Diet-Induced Inflammation Associated with the Suppression of Toll-Like Receptor 4/Nuclear Factor kappaB Signaling. Nutr. Res. 2016, 36, 586e593. DOI: 10.1016/j.nutres.2016.02.001.
- OFlanagan, C.-H.; Bowers, L.-W.; Hursting, S.-D. A Weighty Problem: Metabolic Perturbations and the Obesity-Cancer Link. Horm. Mol. Biol. Clin. Invest. 2015, 23(2), 47–57.
- Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.-B.; Danaei, G. Metabolic Mediators of the Effects of Body-Mass Index, Overweight, and Obesity on Coronary Heart Disease and Stroke: A Pooled Analysis of 97 Prospective Cohorts with 1·8 Million Participants. Lancet. 2014, 383, 970–983. DOI: 10.1016/S0140-6736(13)61612-8.
- Schwartz, K.; Chang, H.-T.; Nikolai, M.; Pernicone, J.; Rhee, S.; Olson, K.; Kurniali, P.-C.; Hord, N.-G.; Noel, M. Treatment of Glioma Patients with Ketogenic Diets: Report of Two Cases Treated with an IRB-approved Energy-Restricted Ketogenic Diet Protocol and Review of the Literature. Cancer Metab. 2015, 3, 3. DOI: 10.1186/s40170-015-0129-1.
- Eltzschig, H.-K.; Eckle, T. Ischemia and Reperfusion–From Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. DOI: 10.1038/nm.2507.
- Secor, S.-M.; Carey, H.-V. Integrative Physiology of Fasting. Compr. Physiol. 2016, 6, 773e825. DOI: 10.1002/cphy.cv06i02corr.
- Erkanli, K.; Erkanli Senturk, G.; Aydin, U.; Arbak, S.; Ercan, F.; Tuncdemir, M.; Isiksacan, N.; Bakir, I. Oxytocin Protects Rat Skeletal Muscle against Ischemia/Reperfusion Injury. Ann. Vasc. Surg. 2013, 27, 662–670. DOI: 10.1016/j.avsg.2012.10.012.
- Trepanowski, J.-F.; Kroeger, C.-M.; Barnosky, A.; Klempel, M. C.; Bhutani, S.; Hoddy, K. K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177(7), 930–938. DOI: 10.1001/jamainternmed.2017.0936.
- Seimon, R.-V.; Roekenes, J.-A.; Zibellini, J.; Zhu, B.; Gibson, -A.-A.; Hills, A.-P.; Wood, R.-E.; King, N.-A.; Byrne, N.-M.; Sainsbury, A. Do Intermittent Diets Provide Physiological Benefits over Continuous Diets for Weight Loss? A Systematic Review of Clinical Trials. Mol. Cell. Endocrinol. 2015, 418(2), 153–172. DOI: 10.1016/j.mce.2015.09.014.
- Wang, S.; Zhang, H.; Xu, Y. Crosstalk between Microglia and T Cells Contributes to Brain Damage and Recovery after Ischemic Stroke. Neurol. Res. 2016, 38, 495–503. DOI: 10.1080/01616412.2016.1188473.
- Fann, D.-Y.; Ng, G.-Y.; Poh, L.; Arumugam, T.-V. Positive Effects of Intermittent Fasting in Ischemic Stroke. Exp. Gerontology. 2017, 89, 93–102. DOI: 10.1016/j.exger.2017.01.014.
- Newman, J.-C.; Verdin, E. beta-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. DOI: 10.1146/annurev-nutr-071816-064916.
- Camandola, S.; Mattson, M.-P. Brain Metabolism in Health, Aging, and Neurodegeneration. Embo J. 2017, 36, 1474–1492. DOI: 10.15252/embj.201695810.
- Arumugam, T.-V.; Manzanero, S.; Furtado, M.; Biggins, P.-J.; Hsieh, Y.-H.; Gelderblom, M.; MacDonald, K.-P.; Salimova, E.; Li, Y.-I.; Korn, O.; et al. An Atypical Role for the Myeloid Receptor Mincle in Central Nervous System Injury. J. Cereb. Blood Flow Metab. 2017, 37, 2098–2111. DOI: 10.1177/0271678X16661201.
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.-W.; Budniak, J.; Groshen, S.; Mack, W.-J.; Guen, E.; Di Biase, S.; et al. Fasting-Mimicking Diet and Markers/Risk Factors for Aging, Diabetes, Cancer, and Cardiovascular Disease. J. Transl. Med. 2017, 9(377), eaai870. DOI: 10.1126/scitranslmed.aai8700.
- Moro, T.; Tinsley, G.; Bianco, A. Effects of Eight Weeks of Time-Restricted Feeding (16/8) on Basal Metabolism, Maximal Strength, Body Composition, Inflammation, and Cardiovascular Risk Factors in Resistance-Trained Males. J. Transl. Med. 2016, 14(1), 290. DOI: 10.1186/s12967-016-0867-z.
- Jankowski, M.; Broderick, T.-L.; Gutkowska, J. Oxytocin and Cardioprotection in Diabetes and Obesity. BMC Endocr. Disord. 2016, 16, 34. DOI: 10.1186/s12902-016-0110-1.
- Kaneko, Y.; Pappas, C.; Tajiri, N.; Borlongan, C.-V. Oxytocin Modulates GABAAR Subunits to Confer Neuroprotection in Stroke in Vitro. Sci. Rep. 2016, 6, 35659. DOI: 10.1038/srep35659.
- Longo, V.-D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23(6), 1048–1059. DOI: 10.1016/j.cmet.2015.10.016.
- Stonge, M.-P.; Ard, J.; Baskin, M.-L. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement from the American Heart Association. Circulation. 2017, 135(9), e96–e121. DOI: 10.1161/CIRCULATIONAHA.117.027305.
- Lok, K.-Z.; Basta, M.; Manzanero, S.; Arumugam, T.-V. Intravenous Immunoglobulin (Ivig) Dampens Neuronal Toll-Like Receptor-Mediated Responses in Ischemia. J. Neuroinflammation. 2015, 12, 73. DOI: 10.1186/s12974-015-0294-8.
- Barnes, D.-E.; Yaffe, K. The Projected Effect of Risk Factor Reduction on Alzheimer’s Disease Prevalence. Lancet Neurol. 2011, 10, 819–828. DOI: 10.1016/S1474-4422(11)70072-2.
- Dupuis, N.; Curatolo, N.; Benoist, J.-F.; Auvin, S. Ketogenic Diet Exhibits Anti-Inflammatory Properties. Epilepsia. 2015, 56(7), e95–8. DOI: 10.1111/epi.2015.56.issue-7.
- Marseglia, L.; Mantim, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2015, 16, 378–400. DOI: 10.3390/ijms16010378.
- Duggan, C.; Tapsoba, J.-D.; Wang, C.-Y.; Campbell, K.-L.; Foster-Schubert, K.; Gross, M.-D.; McTiernan, A. Dietary Weight Loss, Exercise, and Oxidative Stress in Postmenopausal Women: A Randomized Controlled Trial. Cancer Prev. Res. (Phila). 2016, 9, 835–843. DOI: 10.1158/1940-6207.CAPR-16-0163.
- Schmatz, R.; Bitencourt, M.-R.; Patias, L.-D.; Beck, M.; Da, C.-A.-G.; Zanini, D.; Gutierres, J.-M.; Diehl, L.-N.; Pereira, L.-B.; Leal, C.-A.; et al. Evaluation of the Biochemical, Inflammatory and Oxidative Profile of Obese Patients Given Clinical Treatment and Bariatric Surgery. Clin. Chim. Acta. 2017, 465, 72–79. DOI: 10.1016/j.cca.2016.12.012.
- Manoogian, E.-N.; Pandam, S. Circadian Rhythms, Time-Restricted Feeding, and Healthy Aging. Ageing Res. Rev. 2017, 39, 59–67. DOI: 10.1016/j.arr.2016.12.006.
- Wegman, M.-P.; Guo, M.-H.; Bennion, D.-M.; Shankar, M.-N.; Chrzanowski, S.-M.; Goldberg, L.-A.; Xu, J.; Williams, T.-A.; Lu, X.; Hsu, S.-I.; et al. Practicality of Intermittent Fasting in Humans and Its Effect on Oxidative Stress and Genes Related to Aging and Metabolism. Rejuvenation Res. 2015, 18, 162–172. DOI: 10.1089/rej.2014.1624.
- Sleiman, S.-F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, -S.-S.; Holson, E.-B.; Ratan, R.-R.; et al. Exercise Promotes the Expression of Brain Derived Neurotrophic Factor (BDNF) through the Action of the Ketone Body Beta-Hydroxybutyrate. ELife. 2016, 5, e15092. DOI: 10.7554/eLife.15092.
- Mattson, M.-P.; Longo, V.-D.; Harvie, M. Impact of Intermittent Fasting on Health and Disease Processes. Ageing Res. Rev. 2017, 39, 46–58. DOI: 10.1016/j.arr.2016.10.005.
- Mattison, J.-A.; Colman, R.-J.; Beasley, T.-M.; Allison, D.-B.; Kemnitz, J.-W.; Roth, G.-S.; Ingram, D.-K.; Weindruch, R.; de Cabo, R.; Anderson, R.-M. Caloric Restriction Improves Health and Survival of Rhesus Monkeys. Nat. Commun. 2017, 8, 14063. DOI: 10.1038/ncomms14063.
- White, E.; Mehnert, J.-M.; Chan, C.-S. Autophagy, Metabolism, and Cancer. Clin. Cancer Res. 2015, 21(22), 5037–5046. DOI: 10.1158/1078-0432.CCR-15-0490.
- Zhou, Y.; Zhao, R.-H.; Tseng, K.-F.; Li, K.-P.; Lu, Z.-G.; Liu, Y.; Han, K.; Gan, Z.-H.; Lin, S.-C.; Hu, H.-Y.; et al. Sirolimus Induces Apoptosis and Reverses Multidrug Resistance in Human Osteosarcoma Cells in Vitro via Increasing microRNA-34b Expression. Acta Pharmacol. Sin. 2016, 37(4), 519–529. DOI: 10.1038/aps.2016.30.
- Tinsley, G.-M.; Bounty, P.-M.-L. Effects of Intermittent Fasting on Body Composition and Clinical Health Markers in Humans. Nutr. Rev. 2015, 73(10), 661–674. DOI: 10.1093/nutrit/nuv041.
- Imai, S.-I.;. SIRT1 and Caloric Restriction: An Insight into Possible Trade-Offs between Robustness and Frailty. Curr. Opin. Clin. Nutr. Metab. Care. 2009, 12(4), 350. DOI: 10.1097/MCO.0b013e32832c932d.
- Abd El-Kader, S.-M.; Saiem Al-Dahr, M.-H. Impact of Weight Loss on Oxidative Stress and Inflammatory Cytokines in Obese Type 2 Diabetic Patients. Afr. Health Sci. 2016, 16, 725–733. DOI: 10.4314/ahs.v16i3.12.
- Baumeier, C.; Kaiser, D.; Heeren, J.; Scheja, L.; John, C.; Weise, C. Caloric Restriction and Intermittent Fasting Alter Hepatic Lipid Droplet Proteome and Diacylglycerol Species and Prevent Diabetes in NZO Mice. Biochim. Biophys. Acta. 2015, 1851, 566e576.
- Park, S.; Yoo, K.-M.; Hyun, J.-S.; Kang, S. Intermittent Fasting Reduces Body Fat but Exacerbates Hepatic Insulin Resistance in Young Rats Regardless of High Protein and Fat Diets. J. Nutr. Biochem. 2017, 40, 14–22. DOI: 10.1016/j.jnutbio.2016.10.003.
- Linden, M.-A.; Lopez, K.-T.; Fletcher, J.-A.; Morris, E.-M.; Meers, G.-M.; Siddique, S.; Laughlin, M.-H.; Sowers, J.-R.; Thyfault, J.-P.; Ibdah, J.-A. Combining Metformin Therapy with Caloric Restriction for the Management of Type 2 Diabetes and Nonalcoholic Fatty Liver Disease in Obese Rats. Appl. Physiol. Nutr. Metab. 2015, 40(10), 1038–1047. DOI: 10.1139/apnm-2015-0236.
- Chung, H.; Chou, W.; Sears, -D.-D.; Patterson, R.-E.; Webster, N.-J.; Ellies, L.-G. Time-Restricted Feeding Improves Insulin Resistance and Hepatic Steatosis in a Mouse Model of Postmenopausal Obesity. Metabolism. 2016, 65, 1743e 1754. DOI: 10.1016/j.metabol.2016.09.006.
- Hodakoski, C.; Hopkins, B.-D.; Barrows, D.; Mense, S.-M.; Keniry, M.; Anderson, K.-E.; Kern, P.-A.; Hawkins, P.-T.; Stephens, L.-R.; Parsons, R. Regulation of PTEN Inhibition by the Pleckstrin Homology Domain of P-REX2 during Insulin Signaling and Glucose Homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 155–160. DOI: 10.1073/pnas.1213773111.
- Montagner, A.; Polizzi, A.; Fouche, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARalpha Is Crucial for Whole-Body Fatty Acid Homeostasis and Is Protective against NAFLD. Gut. 2016, 65, 1202e1214. DOI: 10.1136/gutjnl-2015-310798.
- Contreras, A.-V.; Torres, N.; Tovar, A.-R. PPAR-alpha as a Key Nutritional and Environmental Sensor for Metabolic Adaptation. Adv. Nutr. 2013, 4, 439e452. DOI: 10.3945/an.113.003798.
- Yan, F.; Wang, Q.; Xu, C.; Cao, M.; Zhou, X.; Wang, T.; Yu, C.; Jing, F.; Chen, W.; Gao, L.; et al. Peroxi- Some Proliferator-Activated Receptor Alpha Activation Induces Hepatic Steatosis, Suggesting an Adverse Effect. PLoS One. 2014, 9, e99245. DOI: 10.1371/journal.pone.0099245.
- Ding, J.; Li, M.; Wan, X.; Jin, X.; Chen, S.; Yu, C.; Li, Y. Effect of miR-34a in Regulating Steatosis by Targeting PPARalpha Expression in Nonalcoholic Fatty Liver Disease. Scientific Rep. 2015, 5, 13729. DOI: 10.1038/srep13729.
- Carulli, L.; Anzivino, C.; Baldelli, E.; Zenobii, M.-F.; Rocchi, M.-B.; Bertolotti, M. Telomere Length Elongation after Weight Loss Intervention in Obese Adults. Mol. Genet. Metab. 2016, 118, 138–142. DOI: 10.1016/j.ymgme.2016.04.003.
- Blackburn, G.;. Effect of Degree of Weight Loss on Health Benefits. Obesity Res. 1995, 3, 211S–216S. DOI: 10.1002/j.1550-8528.1995.tb00466.x.
- Martinez de la Escalera, L.; Kyrou, I.; Vrbikova, J.; Hainer, V.; Sramkova, P.; Fried, M.; Piya, M.-K.; Kumar, S.; Tripathi, G.; McTernan, P.-G. Impact of Gut Hormone FGF-19 on Type-2 Diabetes and Mitochondrial Recovery in a Prospective Study of Obese Diabetic Women Undergoing Bariatric Surgery. BMC Med. 2017, 15, 34. DOI: 10.1186/s12916-017-0797-5.
- Thazhath, -S.-S.; Wu, T.; Bound, M.-J.; Checklin, H.-L.; Standfield, S.; Jones, K.-L.; Horowitz, M.; Rayner, C.-K. Effects of Intraduodenal Hydroxycitrate on Glucose Absorption, Incretin Release, and Glycemia in Response to Intraduodenal Glucose Infusion in Health and Type 2 Diabetes: A Randomised Controlled Trial. Nutrition. 2016, 32(5), 553–559. DOI: 10.1016/j.nut.2015.11.004.
- Ratziu, V.; Harrison, S.-A.; Francque, S.; Bedossa, P.; Lehert, P.; Serfaty, L.; Romero-Gomez, M.; Boursier, J.; Abdelmalek, M.; Caldwell, S.; et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-Alpha and -Delta, Induces Resolution of Nonalcoholic Steatohepatitis without fibrosis Worsening. Gastroenterology. 2016, 150, 1147e1159 e1145. DOI: 10.1053/j.gastro.2016.01.038.
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular Mechanism of PPAR- Alpha Action and Its Impact on Lipid Metabolism, Inflammation and fibrosis in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2015, 62, 720e733. DOI: 10.1016/j.jhep.2014.09.033.