ABSTRACT
The oils obtained from three Indonesian avocado (Persea americana) cultivars namely Merah bundar, Ijo bundar and Ijo panjang, were compared to the oils obtained from imported Fuerte and Shepard avocado varieties in terms of lipid characteristics and tocopherol content. The oils of all avocado varieties existed in a semisolid form, except that of the Fuerte variety. Free fatty acids and peroxide levels in the oils obtained from locally grown avocado were lower than the levels in the oils obtained from imported avocados, implying that local avocado oils have a better oxidative stability. Interestingly, the tocopherol (alpha, beta, gamma and delta) content of local avocado oils was also much higher than that of imported avocado oils. Meanwhile, differences in the FTIR spectrum of avocado oils were found at frequencies of 1034 and 968 cm−1. There were also some differences in the fatty acid and triacylglycerol composition of avocado oils. Due to these differences, the physicochemical characteristics and the solidification and thermal profiles of the oils obtained from local avocado cultivars were completely different from those of the imported avocado varieties (Fuerte and Shepard).
Introduction
Avocado (Persea americana Mill.) belongs to the Lauraceae family. According to the Food and Agriculture Organization of the United Nations [Citation1], Mexico (1,107,140 tons), Chile (300,000 tons), the Dominican Republic (288,684 tons), and Indonesia (224,278 tons) are the leading countries in the production of avocado fruits. However, countries such as Brazil, Indonesia, and the Dominican Republic do not export the avocados. The major production area of the avocado fruit in Indonesia is the Java Island. West Java is the highest producer, followed by East Java, while Central Java produces much smaller quantities. The avocado fruit became popular after the inauguration of the “Es Teller 77” food stall in several big cities in Indonesia. In this stall, avocado is prepared together with jackfruit as a fruit cocktail. In addition, avocado is also used in the preparation of milkshake (which is locally known as “jus avokad”), where the pulp is blended with milk and sugar. In addition to its use in food preparation, avocado is also used as a medicine for stomachache by the local people.[Citation2]
There are three known ecological avocado races namely Mexican race (Persea americana var. drymifolia), Guatemalan race (Persea americana var. guatemalensis) and West Indian race (Persea americana var. americana). Indonesian avocado fruits have been acknowledged to be interracial, and are generally round-, oval- and pear-shaped. The trees are primarily grown in home gardens with several preferred varieties such as Ijo bundar and Ijo panjang. They have “A” flower type with respect to the dual opening flower cycle behavior. These cultivars have been endorsed by the government because of their large production, availability throughout the year, thicker fruit pulp, higher yield, and resistance to pests and diseases.
Avocado is a rich source of oil that is primarily derived from the fruit pulp. Avocado oil has been recognized for its uses in food [Citation3] and also for its potential use in cosmetics and skin care products.[Citation4] Avocado oil has been proposed as a new functional food ingredient because of its high concentration of oleic acid and bioactive compounds. Mono-unsaturated (oleic acid) oils have been known for its thermal oxidative stability and excellent characteristic, which is highly regarded in food application. These oils are suitable for sizzling, sautéing or deep fat frying to maintain product quality and enhance palatability.[Citation5] A higher intake of products with high oleic acid content may improve the public health particularly by decreasing the risk of coronary heart disease.[Citation6]
Some researchers have investigated the effect of different extraction methods on the physicochemical properties of avocado oils.[Citation3,Citation7–Citation10] Moreover, some researchers have also analyzed the biochemical and antioxidant properties of avocado oils.[Citation11–Citation15] In addition, the physicochemical characteristics of avocado oils have been evaluated in several countries.[Citation14,Citation16–Citation22] Indriyani [Citation23] analyzed the physicochemical properties of different varieties of Indonesian avocado oils (Bantul, Purwokerto, and Garut). However, the Indonesian government does not highlight the production of these avocado varieties. Till date, there are no reports on the TAG composition, the tocopherol content, and the solid fat profile of oils obtained from Indonesian avocados. Moreover, there is a scarcity of data on the physicochemical properties and the thermal behavior of oils obtained from the Indonesian avocado cultivars namely Merah bundar, Ijo bundar and Ijo panjang. Analyzing the characteristics of these oils may lead to either formulation of new products or production of new ingredients which adds value to the food industry. Therefore, the objective of this study was to obtain information on the physicochemical properties, the thermal behavior, the solidification profile and the tocopherol content of the oils obtained from the three major avocado cultivars grown in Indonesia in comparison with the oils obtained from imported Fuerte and Shepard varieties, and the prediction of the product application.
Materials and methods
Materials
Three local avocado cultivars namely Merah bundar (oval, dark-red color), Ijo bundar (oval, green color) and Ijo panjang (pyriform, green color) were collected during fruiting season in December at their physiological maturity stage. The fruit gardens are located on middle-altitude land at around 200–1,000 m above sea level (AMSL) in Java Island, Indonesia. The imported varieties of Fuerte and Shepard fruits were purchased from a local hypermarket. The avocado fruit was cut open, and the flesh was minced manually into fine pieces after removing the seeds. Then, it was dried in an oven at 60°C for 24 h before ground into powder using a 32BL80 Warring blender (Dynamic Corporation of America, New Hartford, USA). All the chemicals used in this study were of analytical grade, unless otherwise specified.
Oil extraction
One hundred and fifty grams of avocado ground fleshes were put into a cellulose paper cone and transferred into a 5 L Soxhlet extractor, and the oil was extracted with petroleum ether (40–60°C) for 8 h as described by AOAC.[Citation24] The fat was recovered using a rotary evaporator (Model A-3S, Eyela, Tokyo Rakakikal Co., Ltd., Tokyo, Japan). The extracted oil was placed in an oven at 60°C for 1 h and transferred into dark bottles, flashed with nitrogen gas, and stored in a freezer at −20°C.
Fat content
Using petroleum ether, 15 g of sample was extracted at 40–60°C as a solvent for 8 h.[Citation25] To remove residual petroleum ether, the oil was dried in an oven at 60°C for 30 min. Oil content was expressed as the percent based on the weight of dry avocado pulp powder.
Physicochemical properties
The slip melting point (SMP), the iodine value (IV), the saponification value (SV), the free fatty acid (FFA) content and the peroxide value (PV) of the oil were determined according to the AOCS method.[Citation26]
Color determination
The oil color was determined using a Lovibond tintometer Model E (Lovibond, Salisbury, England) at 30°C. The oil was put into a 2.3 cm cell and compared to the best possible match with the standard color indices [yellow (Y) and red (R)].
FTIR spectroscopic analysis
The avocado oil was scanned using an FTIR spectrometer Nicolet 6700 (Thermo Electron, Madison, USA) equipped with a horizontal attenuated total reflectance (HATR) element (ZnSe crystal). The OMNIC software version 7.0 (Thermo Nicolet Corp.) was used for data analysis. The oil was placed in a water bath at 60°C until it became completely molten before the sample was placed on the sampling compartment (25°C). The sampling compartment used was a Smart attenuated total reflectance kit (Smart ARK, Thermo Electron) (10 × 60 mm). The penetration depth (IR beam) was 0.2 μm producing 12 internal reflections. The Smart ARK was composed of zinc selenide (ZnSe) crystals with a refractive index of 2.4 at 1000 cm−1 using an aperture angle of 45°. The FTIR spectra were then collected at the mid-infrared region (4000–650 cm−1) using 32 scans and at a resolution of 4 cm−1. All recorded spectra were scanned in the absorbance measurements in triplicates. After each sampling, a new background air spectra sampling was obtained as a reference point.[Citation27]
Fatty acid methyl ester (FAME) compositional analysis
The FA composition of the oil was determined by gas chromatography on a Thermo focus GC (Interscience, Louvain-la-Neuve, Belgium), equipped with a flame ionization detector (FID), after conversion to FAME using 2 N sodium methoxide solution. The polar capillary column RTX-5 (0.32 mm internal diameter, 30 m length and 0.25 µm film thickness; Restex Corp., Bellefonte, PA) was used. FAMEs were identified by comparing their retention time with FAME standards (Sigma-Aldrich). The relative percentage of individual fatty acids was reported as the relative proportion of the total fatty acids.[Citation28]
Triacylglycerol analysis by HPLC
Triacylglycerol (TAG) distribution in the oil was determined by a nonaqueous reverse-phase HPLC using Shimadzu Liquid Chromatograph LC-10AD (Shimadzu, Kyoto, Japan), equipped with a system controller (SLC-10Avp), an auto injector, a 0.05 mL sample loop, a Shimadzu CR4AX-integrator, and a refractive index detector (Model RID-6A, Shimadzu). A commercially packed RP-18 column (250 × 4 mm, particle size 5 µm, Merck, Darmstadt, Germany) was used, and the temperature of the column oven was set at 30°C. A mixture of acetone:acetonitrile (63.5:36.5, v/v) was used as the mobile phase with a flow rate of 1 mL/min, and the total run time was 1 h. The individual peaks were identified by comparing the retention times with those of common vegetable oils of known TAG composition, and the peak areas produced by the data integrator were used to quantify the components based on relative percentages.[Citation17,Citation29]
Tocopherol analysis by HPLC
The tocopherol content was determined according to the method described by ChiewWei.[Citation30] Avocado oil (0.1 g) was weighed and dissolved in 25 mL of n-hexane. A Shimadzu Liquid Chromatograph LC-10AD (Shimadzu, Kyoto, Japan) equipped with a fluorescence detector (Shimadzu) and a Purospher STAR NH2 column (250 × 4 mm i.d., 5 mm, Merck, Darmstadt, Germany) was used to separate the tocopherol compounds. The mobile phase was a mixture of n-hexane:THF:2-propanol (500:30:2), and the flow rate was set at 1.0 mL/min. The fluorescence detector was set to detect an emission wavelength of 326 nm and an excitation wavelength of 292 nm. An external standard of tocopherol set (alpha, beta, gamma, and delta) (Merck) was used for the identification of the sample peaks. The quantitation was achieved based on peak area of each tocopherol. Calibration curve of analyst was constructed by plotting peak areas versus various concentrations of each tocopherol. The analysis was done in triplicates, and the resulting data were presented as mean values with standard deviation of the percentage area.
Solid fat content analysis by NMR
A Bruker Minispec (Model Mq 20) pulse nuclear magnetic resonance (pNMR) spectrometer (Bruker, Karlsruhe, Germany) was used to measure the solid fat content (SFC) according to PORIM.[Citation31] NMR tubes (Bruker) were used for the direct SFC measurement. The sample in the NMR tube was melted at 70°C for 15 min to eliminate the thermal history of the samples, followed by chilling at 0°C for 60 min, and then held at each measuring temperature for 30 min prior to measurement. Melting, chilling, and storing of the samples were carried out in pre-equilibrated thermostated glycol-containing baths, accurate to 0.1°C. SFC measurements were taken at 5°C intervals over the range of 0–60°C.
Melting and cooling profile measurements by DSC
The melting and cooling profiles of the oil were evaluated using TA Q1000 differential scanning calorimeter (TA Instruments, New Castle, USA) equipped with a refrigerated cooling system. The DSC system was purged using nitrogen gas. Avocado oil (5–15 mg) was hermetically sealed in aluminum pans. An empty pan was used as a reference during the measurements. The time–temperature program of the measurements was as follows: held at 70°C isotherm for 1 min, then cooled at 5°C/min to −90°C, and held for 1 min. Then, the sample was heated from −90 to 70°C at the same rate. The TA Universal Analysis 2000 software (TA Instruments) [Citation32] was used to evaluate the melting and cooling profiles.
Statistical analysis
In all analyses, three replicates were used and the results were expressed as mean values ± standard deviations. Data were statistically analyzed by the Minitab ver. 15 statistical software (Minitab, Pennsylvania, USA). Differences were considered to be significant at a 0.05 probability level.
Results and discussion
Physicochemical properties
The physicochemical properties of the oils obtained from local avocado cultivars were compared to those of imported varieties as shown in . The oil content of local avocado varieties was found to be lower (ranging from 36.18 to 38.89%) than that of the imported Fuerte and Shepard varieties (47.36 and 47.52%, respectively). Nevertheless, the oil content of local avocado varieties was found to be higher than that of the Malaysian avocado cultivars (30.25–33.45%) [Citation17] and the Mexican avocado cultivars (~16%).[Citation3] The oil content of the Fuerte variety was found to be higher than that reported by previous studies.[Citation21,Citation22] However, the oil content of different avocado varieties (local avocados, Fuerte and Shepard) was found to be higher than that of Hass variety (54.9%).[Citation17]
Table 1. Physicochemical properties of avocado oils.
The oil obtained from the local avocado cultivars and the Shepard variety was semisolid, whereas the oil obtained from the Fuerte variety was almost liquid at room temperature. According to Indriyani [Citation23], the avocado oil obtained from the Garut variety also tended to become semisolid in form at room temperature. The SMP value of the avocado oils obtained from local cultivars was found to be higher than that of the oils obtained from imported avocado varieties (). The IVs of the avocado oils obtained from Merah bundar, Ijo bundar, Ijo panjang, Fuerte and Shepard varieties were 82.11, 77.75, 75.49, 88.68 and 77.01 (g I2/100 g), respectively. The slightly higher IVs of the oils of Merah bundar and Fuerte varieties indicated that these oils contained more unsaturated FAs than those of the oils obtained from Ijo bundar, Ijo panjang and Shepard varieties. Other Indonesian avocado oils had IVs between 77.09 and 88.68 (g I2/100 g), which were almost similar to those of imported avocado oils (Fuerte and Shepard varieties).[Citation23] The IVs of all the samples in this investigation were closer to those of other varieties reported by Bora,[Citation33] Moreno,[Citation3] Yanty,[Citation17] and Quinones-Islas.[Citation34] These results were also consistent with some reports stating that the IVs of the avocado oil are in the range of 65–95. The SV is related to the mean value of the molecular weight of the FA in the oil. The SV of local avocado oils ranged from 185 to 198 mg KOH/g oil, whereas those of the Fuerte and Shepard varieties were 188.4 and 196.55 mg KOH/g oil, respectively. These results are consistent with some reports stating that the SVs of the avocado oil are in the range of 170–198 mg KOH/g oil.[Citation35] However, the SV of local oil obtained from the Garut variety was 153.17 mg KOH/g oil, which was lower than that of other local varieties.[Citation23]
The PV and FFA contents are always used as an indicator for monitoring the quality of the oil. A high FFA value can be related to the degree of hydrolysis during oil preparation and storage. In general, good-quality oil has a low FFA value. The FFA of the oils extracted from local and imported avocado varieties was in the range of 1.78–1.89%. PV can be used to estimate the extent of oxidative activity of edible oils. A higher amount of peroxides present in an oil reflects a higher oxidative level at which the oil tends to become rancid. PV is used to quantify hydroperoxide as the primary oxidation product.[Citation36] An edible oil with a PV > 10 meq kg−1 is categorized as being at a high oxidation state.[Citation37] The PVs of local avocado oils (ranging from 5.22 to 5.44 meq kg−1) were found to be lower than those of the oils obtained from the imported Fuerte (6.23 meq kg−1) and Shepard (5.85 meq kg−1) varieties. In the literature, the PVs of avocado oils have been reported to be in the range of 5.1–12.3 meq kg−1.[Citation34] Nevertheless, the PVs of the avocado oils investigated in this study were still in the range of permitted maximum peroxide level of edible oils (≤10 meq of peroxide oxygen/kg of oil) by the Codex Alimentarius Commission.[Citation38] On the other hand, other local varieties such as Bantul (166.10 meq kg−1) and Purwokerto (124.65 meq kg−1) had a higher PV than that of Garut variety (14.85 meq kg−1).[Citation23] In this study, the color of the oils obtained from different avocado cultivars was found to be light or emerald green, which is comparable to the results of Indriyani,[Citation23] Yanty,[Citation17] and Ashton,[Citation12] due to considerable quantities of pigments, including chlorophylls and carotenoids.[Citation12,Citation39]
FTIR spectroscopic analysis
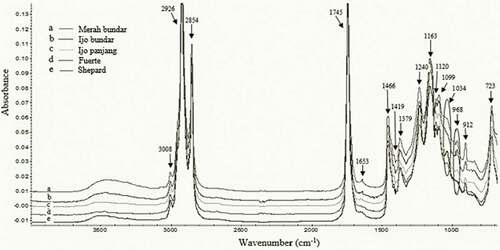
FTIR analysis is important to classify genetic varieties, and the spectrum can be used as a fingerprint. Avocado oil authentication in particular is crucial since the oil has a high value. It thus poses a potential to be adulterated with other edible oils. An overlay of the FTIR spectrum of avocado oils in the region 4000–650 cm−1 is presented in . As shown in the figure, the major differences between them were primarily at two frequencies, namely 1034 and 968 cm−1. A peak at 1034 cm−1 was present in the oils obtained from Merah bundar, Fuerte and Shepard varieties, but it was absent in the spectrum of those of Ijo panjang and Ijo bundar cultivars. This phenomenon could be due to the high amount of triunsaturated and monounsaturated TAGs in Merah bundar, Fuerte and Shepard varieties (). Meanwhile, only the avocado oils obtained from local cultivars had a small peak at 968 cm−1 because they contained a higher amount of total polyunsaturated FAs (linoleic and linolenic acids) than those of the oils obtained from Fuerte and Shepard varieties. These findings are also in agreement with the results of other studies performed on vegetable oils.[Citation34,Citation40,Citation41]
Table 2. Fatty acid composition of oils obtained from different avocado varieties.
Table 3. Triacylglycerol (TAG) composition of oils obtained from different avocado varieties.
The differences in the spectral patterns of avocado oils are attributed to the molecular and structural differences, including a degree of unsaturation and chain length, monounsaturated to polyunsaturated acyl group ratio, variations in trans and cis fatty acid content and the presence of minor components in the oils.[Citation41] The stretching of cis C = C was observed at 3008 cm−1 depending on the degree of unsaturation of the oils. The bands at 2926 and 2854 cm−1 were caused due to the bands arising from CH2 stretching vibrations, asymmetric and symmetric, respectively. The major band at 1745 cm−1 was produced from the C = O stretching vibrations of aldehydes, ketones and carboxylic acids. The stretching of unconjugated cis C = C was observed at 1653 cm−1. The CH2 scissoring vibration, the CH rocking vibrations and the CH3 symmetrical bending vibration were observed at 1466, 1419 and 1379 cm−1, respectively. The bands at 1240, 1163, 1120, 1099 and 1034 cm−1 were associated with the C-O stretching vibration. The bands at 968 and 912 cm−1 represented trans double bonds (C = C) and cis double bonds (C = C) in the oils, respectively, while the band at 723 cm−1 corresponded to the CH2 rocking mode.
Fatty acid composition
The fatty acid composition of the different varieties of avocado oils is presented in . The results showed that all the avocado oils contained palmitic, palmitoleic, stearic, oleic, linoleic and linolenic acids. Among these FAs, stearic (ranging from 0.65% in Shepard to 1.04% in Ijo panjang varieties) and linoleic (ranging from 1.10% in Shepard to 2.24% in Ijo bundar varieties) acids were found in small amounts in the avocado oils of all varieties. Meanwhile, the most predominant FAs in the oil obtained from Merah bundar cultivar were oleic (43.44%), followed by palmitic (28.45%), linoleic (16.27%) and palmitoleic (9.81%) acids. The predominant FA contents in the avocado oil of Merah bundar cultivar were found to be comparable to those of Ijo bundar and Fuerte varieties. The Fuerte avocado oil contained 55.64% of oleic acid, followed by 22.13, 14.18 and 6.11% of palmitic, linoleic and palmitoleic acids, respectively. A similar major FA composition has also been reported in the avocado oil of Malaysian cultivars.[Citation17] The avocado oils of Fuerte variety from Iran,[Citation19] Japan [Citation18] and Mexico [Citation3] were also found to contain oleic acid as the predominant FA, followed by palmitic, linoleic and palmitoleic acids. In addition, the avocado oil of Fuerte variety from Cretan was found to contain oleic acid as the predominant FA, whereas linoleic was the second predominant FA, followed by palmitic and palmitoleic acids.[Citation22]
Furthermore, palmitic acid (35.84%) was the predominant FA in the Ijo panjang avocado oil, followed by oleic (21.69%), linoleic (20.64%) and palmitoleic (18.55%) acids as shown in . Similarly, the oil obtained from Shepard variety contained palmitic acid (34.13%) as its major FA, followed by oleic (33.33%), palmitoleic (16.71%) and linoleic (14.10%) acids. Furthermore, Ikhuoria [Citation20] found that African avocado oil contained linolenic acid as the most abundant FA, followed by linoleic and oleic acids. As also shown in , only the avocado oils obtained from Ijo bundar and Ijo panjang cultivars were found to contain margaric acid (0.15 and 0.40%, respectively). Among these oils, the avocado oil of Fuerte variety contained the highest amount of unsaturated FA (77.02%), whereas that of Ijo panjang cultivar contained the highest amount of saturated FA (36.90%). The avocado oils of other local varieties such as Bantul, Purwokerto and Garut contained oleic acid (ranging from 34.79 to 47.99%) as the prominent FA, followed by palmitic acid (ranging from 25.28 to 30.91%). The third most abundant FA of the Purwokerto and Garut varieties was linoleic acid (10.65 and 11.95%, respectively).[Citation23] However, the third most abundant FA of Bantul variety was palmitoleic acid (10.06%). The avocado oils (from Bantul and Purwokerto varieties) contained lower unsaturated FAs (55.73 and 62.84%, respectively) than those of the avocado oils analyzed in this study. The avocado oils (Merah bundar, Ijo bundar, Ijo panjang, Fuerte and Shepard) contained lower amount of oleic acid and unsaturated fatty acids compared to that of Hass variety. According to Yanty,[Citation17] Hass variety contained 63.73% oleic acid, and a total unsaturated fatty acid was ~85%. The differences of fatty acid composition are influenced by the cultivars, maturity stage, anatomical region of the fruit and geographic location for plant growth.[Citation42]
Triacylglycerol composition
The TAG composition of the avocado oils obtained from different varieties is presented in . The avocado oil obtained from Merah bundar cultivar was found to contain POO (21.02%), PPO (15.57%), OOO (14.07%) and OOL (12.30%) as its major TAGs. Similarly, palm superolein and red palm olein were also reported to contain POO and PPO as their major TAGs.[Citation43] The avocado oil of Fuerte variety was also found to have POO (22.42%), OOO (18.64%) and OOL (16.73%) as the major TAGs, followed by POL (20.02%). Conversely, the oil of Ijo bundar cultivar was found to have PLL (21.31%) as the predominant TAG, followed by POL (15.68%) and PPO (13.29%).
The avocado oils obtained from Ijo panjang and Shepard varieties contained POL (17.97 and 18.25%, respectively) as the major TAG, but their second and third most abundant TAGs were found to be different. The oil obtained from Ijo panjang cultivar contained POO (15.37%), whereas that of Shepard variety contained OOL (18.06%) as its second most abundant TAG. In addition, the third most abundant TAGs in the avocado oils obtained from Ijo panjang and Shepard varieties were PPO (13.81%) and POO (16.40%), respectively. Among these avocado oils, OOS was not found in the oil of Ijo bundar cultivar. According to Yanty,[Citation17] avocado oil obtained from Hass variety contained OOO, POO and OOL as the predominant TAGs. As a comparison, the major TAGs of Hungarian avocado oils were OOO, POO, PLO, and POP,[Citation44] while the most abundant TAG of Malaysian avocado oils was POO, with their second and third most abundant TAGs varying among the cultivars.[Citation17] The amount of diunsaturated TAG was found to be higher than that of diunsaturated TAG in the oils obtained from Ijo bundar, Ijo panjang and Shepard varieties. However, the diunsaturated and the triunsaturated TAGs of Fuerte avocado oil were found to be almost equal in amount, i.e., 44.92 and 43.22%, respectively. In contrast, the avocado oil obtained from Merah bundar cultivar contained higher triunsaturated TAG (40.41%) than diunsaturated TAG (36.18%).
Tocopherol content of avocado oils
The tocopherol or vitamin E contents of the different varieties of avocado oils are presented in . Tocopherol is an antioxidant compound that acts at the primary level, and it is a peroxyl free radical scavenger. Tocopherols have widely been used for food, feed, pharmaceuticals, cosmetics and resins. In food industry, tocopherols are used as an antioxidant for frying oil, margarine, and so on.[Citation45] Four types of tocopherol have been detected in the avocado oils of Indonesian cultivars, namely alpha, beta, gamma, and delta tocopherols. Interestingly, we found higher concentrations of alpha-tocopherol ranging from 40 to 45 mg/kg, beta-tocopherol ranging from 0.8 to 2.0 mg/kg and gamma-tocopherol ranging from 3.8 to 4.2 mg/kg. Meanwhile, delta-tocopherol was detected at a low level in the range of 0.04–0.08 mg/kg. On the other hand, the avocado oils of the imported Fuerte and Shepard varieties contained lower amounts of alpha-tocopherol (36.72 and 32.42 mg/kg, respectively) and gamma-tocopherol (0.28 and 0.36 mg/kg, respectively). These results could be due to local avocado cultivars are fresher than imported avocados. Therefore, the local avocado cultivars contained more vitamin E compared to imported avocados. However, beta-tocopherol and delta-tocopherol were not detected in the oils of the imported avocado varieties. Jorge [Citation16] also reported that Brazilian avocado oils did not contain beta-tocopherol and delta-tocopherol. The variation of tocopherol composition in avocado oils might depend mainly on the geographical conditions and harvest session of the fruits. [Citation46]
Table 4. Tocopherol content of oils obtained from different avocado varieties.
SFC of avocado oils
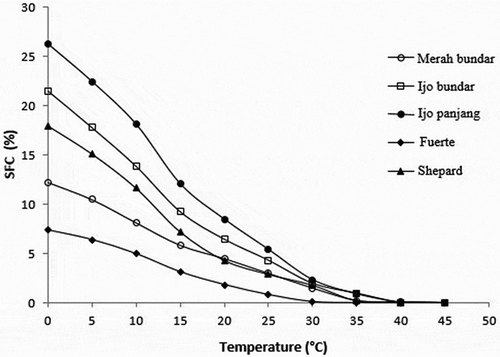
The SFC profile of the avocado oils obtained from local varieties was compared with that of Fuerte and Shepard varieties. The SFC profile is considered as the main parameter for studying the basic physical properties of oils and fats for food applications, particularly for margarines, shortenings and chocolate.[Citation47] As shown in , the SFCs of the oils obtained from Merah bundar, Ijo bundar, Ijo panjang, Fuerte and Shepard varieties at 0°C were 12.26, 21.53, 26.23, 7.40 and 17.28%, respectively. The avocado oils obtained from local cultivars and Shepard variety had higher SFC contents than those of the Fuerte variety throughout the measured temperature range. The avocado oil of Fuerte variety was completely liquid (SFC 0%) at 30°C as the oil was found to have the highest proportion of unsaturated FAs () and triunsaturated TAG molecules ().
However, the SFC of the avocado oil obtained from Shepard variety at 0–25°C was lower than those of Ijo bundar and Ijo panjang varieties. On the other hand, the SFC profile of the avocado oil obtained from Shepard variety at 0–15°C was higher than that of the oil obtained from Merah bundar variety. These observations well correlated with the saturated FAs () and the disaturated and trisaturated TAG () contents. In general, the SFC values of the oils obtained from Shepard and Merah bundar varieties were highly similar at 25°C and above, whereas the SFC values of the oils obtained from all local avocados and Shepard varieties were nearly similar at 30°C and above. The avocado oils obtained from local and Shepard varieties tended to become completely liquid (SFC 0%) at 45°C.
Thermal profile of avocado oils
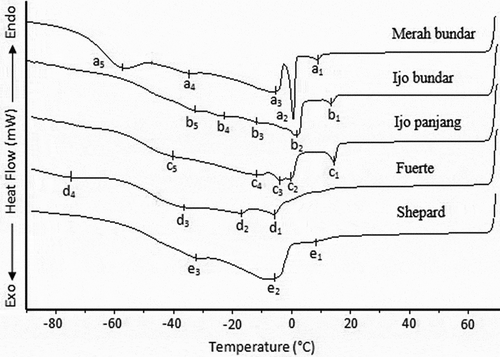
The cooling profiles of the avocado oil obtained from local and imported cultivars are shown in . It can be clearly observed that the cooling profiles of the oils obtained from each local cultivar were different from those of imported Fuerte and Shepard varieties. The initial crystallization points of the oils of Merah bundar, Ijo bundar, Ijo panjang, Fuerte and Shepard varieties were a1 (8.91°C), b1 (13.58°C), c1 (14.77°C), d1 (−5.78°C), and e1 (8.88°C), respectively. These initial crystallization points correlated with FA () and TAG () compositions of the oils. Among these avocado oils, the Fuerte variety oil had the initial crystallization point below 0°C. This observation correlates well with the solidification behavior (), at which it was almost liquid at room temperature. According to Indrayani,[Citation23] the initial crystallization points of the local avocado oils of Bantul, Purwokerto and Garut varieties were 7.82, 12.21 and 23.40°C, respectively. Thus, the initial crystallization points of the avocado oils obtained from Bantul and Purwokerto varieties were found to be lower than those of the oils obtained from Merah bundar, Ijo bundar and Ijo panjang varieties. On the other hand, the avocado oil of Garut variety had a higher initial crystallization point than those of local avocado oils investigated in this study. Among the examined avocado oils, those obtained from Merah bundar and Fuerte varieties had a crystallization peak beyond −50°C. The local avocado oils of Garut variety also showed a crystallization peak beyond −50°C.[Citation23]
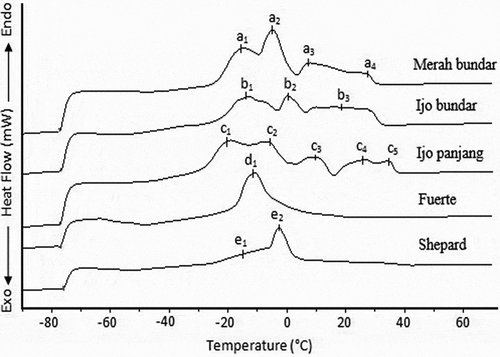
The melting profiles of the avocado oils obtained from local cultivars were compared to those of Fuerte and Shepard varieties, as shown in . It was obvious that the melting profiles of the oils of local avocado cultivars and imported Fuerte and Shepard varieties were different. The final melting points of Merah bundar, Ijo bundar, Ijo panjang, Fuerte and Shepard varieties were 31.36, 31.75, 36.68, −4.64 and 1.84°C, respectively. The melting profiles of Merah bundar and Ijo bundar varieties were almost similar but different in exothermic peak temperatures. Meanwhile, the oil obtained from Fuerte variety was found to consist only one peak at −11.1°C, whereas the oil of Shepard variety had two peaks at −16.87 and −2.76°C. The thermal profile differences between the local cultivars and the imported Fuerte and Shepard varieties could primarily be due to the differences in their FA and TAG compositions ( and , respectively). The final melting point of the avocado oils obtained from Garut variety (45.29°C) was higher than those of the avocado oils analyzed in this study. Meanwhile, the final melting points of the avocado oils obtained from other local varieties (Bantul and Purwokerto) were 20.32 and 21.45°C, respectively, and were lower than those of the avocado oils obtained from Merah bundar, Ijo bundar and Ijo panjang varieties.[Citation23] However, the DSC cooling and melting profiles of the avocado oils investigated in this study were different from those of local (different varieties) and Malaysian avocado oils as previously reported by Indriyani [Citation23] and Yanty.[Citation17] The differences in the thermal behaviors of oils could be attributed to the differences in their fatty acid and TAG molecular composition.
Conclusion
This study demonstrated that the oils obtained from Indonesian avocado cultivars are distinguishable from the oils obtained from the imported Fuerte and Shepard varieties based on their physicochemical characteristics, tocopherol content, and solidification and thermal profiles. Among the evaluated avocado oils, the oil obtained from Ijo panjang cultivar was found to be the most stable oxidative avocado oil with the lowest PV and FFA content. Furthermore, the FTIR spectrum also showed some differences at frequencies of 1034 and 968 cm−1. The oil extracted from different avocado varieties contained palmitic, oleic and linoleic acids as the major FAs, while the HPLC profile showed that local avocado oils contained high amount of triunsaturated, diunsaturated and disaturated TAGs comparable to those of Shepard variety. The avocado oils obtained from local varieties possessed high content of tocopherol, and thus, they can be utilized as oil-based supplements. Meanwhile, their solidification and thermal profiles suggested that the oils can also be used in the formulation of creams, lotions and lipsticks. In addition, local avocado oils can particularly be used for food applications. Since the oils are semisolid at room (tropical) temperature, they are suitable for bakery and confectionery industries.
References
- FAOSTAT. Production-Crops; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013.
- FAO. Report of the expert consultation on avocado production development in Asia and the Pacific; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2000.
- Moreno, A. O.; Dorantes, L.; Galíndez, J.; Guzmán, R. I. Effect of Different Extraction Methods on Fatty Acids, Volatile Compounds, and Physical and Chemical Properties of Avocado (Persea Americana Mill.) Oil. J. Agric. Food Chem. 2003, 51, 2216–2221. DOI: 10.1021/jf0207934.
- Athar, M.; Nasir, S. M. Taxonomic Perspective of Plant Species Yielding Vegetable Oils Used in Cosmetics and Skin Care Products. Afr. J. Biotechnol. 2005, 4, 36–44.
- Corbett, P.;. It Is Time for an Oil Change! Opportunities for High Oleic Vegetables Oils. Inform. 2003, 14, 480–481.
- Gerhard, G. T.; Ahmann, A.; Meeuws, K.; McMurry, M. P.; Duell, P. B.; Connor, W. E. Effects of a Low-Fat Diet Compared with Those of a High-Monounsaturated Fat Diet on Body Weight, Plasma Lipids and Lipoproteins, and Glycemic Control in Type 2 Diabetes. Am. J. Clin. Nutr. 2004, 80, 668–673. DOI: 10.1093/ajcn/80.3.668.
- Dos Santos, M. A.; Alicieo, T. V.; Pereira, C. M.; Ramis-Ramos, G.; Mendonça, C. R. Profile of Bioactive Compounds in Avocado Pulp Oil: Influence of the Drying Processes and Extraction Methods. J. Am. Oil Chem. Soc. 2014, 91, 19–27. DOI: 10.1007/s11746-013-2289-x.
- Ortiz, M. A.; Dorantes, A. L.; Gallndez, M. J.; Cárdenas, S. E. Effect of a Novel Oil Extraction Method on Avocado (Persea Americana Mill) Pulp Microstructure. Plant Foods Human Nutr. 2004, 59, 11–14.
- Bizimana, V.; Breene, W.; Csallany, A. Avocado Oil Extraction with Appropriate Technology for Developing Countries. J. Am. Oil Chem. Soc. 1993, 70, 821–822. DOI: 10.1007/BF02542610.
- Werman, M.; Neeman, I. Avocado Oil Production and Chemical Characteristics. J. Am. Oil Chem. Soc. 1987, 64, 229–232. DOI: 10.1007/BF02542007.
- Wang, W.; Bostic, T. R.; Gu, L. Antioxidant Capacities, Procyanidins and Pigments in Avocados of Different Strains and Cultivars. Food Chem. 2010, 122, 1193–1198. DOI: 10.1016/j.foodchem.2010.03.114.
- Ashton, O. B.; Wong, M.; McGhie, T. K.; Vather, R.; Wang, Y.; Requejo-Jackman, C.; Ramankutty, P.; Woolf, A. B. Pigments in Avocado Tissue and Oil. J. Agric. Food Chem. 2006, 54, 10151–10158. DOI: 10.1021/jf061809j.
- Soong, -Y.-Y.; Barlow, P. J. Antioxidant Activity and Phenolic Content of Selected Fruit Seeds. Food Chem. 2004, 88, 411–417. DOI: 10.1016/j.foodchem.2004.02.003.
- Gómez-López, V. M.;. Some Biochemical Properties of Polyphenol Oxidase from Two Varieties of Avocado. Food Chem. 2002, 77, 163–169. DOI: 10.1016/S0308-8146(01)00331-4.
- Lozano, Y.; Mayer, C. D.; Bannon, C.; Gaydou, E. M. Unsaponifiable Matter, Total Sterol and Tocopherol Contents of Avocado Oil Varieties. J. Am. Oil Chem. Soc. 1993, 70, 561–565. DOI: 10.1007/BF02545319.
- Jorge, T. D. S.; Polachini, T. C.; Dias, L. S.; Jorge, N.; Telis-Romero, J. Physicochemical and Rheological Characterization of Avocado Oils. Ciênc. Agrotec. 2015, 39, 390–400. DOI: 10.1590/S1413-70542015000400010.
- Yanty, N.; Marikkar, J.; Long, K. Effect of Varietal Differences on Composition and Thermal Characteristics of Avocado Oil. J. Am. Oil Chem. Soc. 2011, 88, 1997–2003. DOI: 10.1007/s11746-011-1877-x.
- Takenaga, F.; Matsuyama, K.; Abe, S.; Torii, Y.; Itoh, S. Lipid and Fatty Acid Composition of Mesocarp and Seed of Avocado Fruits Harvested at Northern Range in Japan. J. Oleo Sci. 2008, 57, 591–597.
- Azizi, S.; Najafzadeh, S. Fatty Acids and Volatile Compounds in Avocado Cultivated in North of Iran. World Appl. Sci. J. 2008, 5, 1–4.
- Ikhuoria, E.; Maliki, M. Characterization of Avocado Pear (Persea Americana) and African Pear (Dacryodes Edulis) Extracts. Afr. J. Biotechnol. 2007, 6, 950–952.
- Ozdemir, F.; Topuz, A. Changes in Dry Matter, Oil Content and Fatty Acids Composition of Avocado during Harvesting Time and Post-Harvesting Ripening Period. Food Chem. 2004, 86, 79–83. DOI: 10.1016/j.foodchem.2003.08.012.
- Vekiari, S. A.; Papadopoulou, P. P.; Lionakis, S.; Krystallis, A. Variation in the Composition of Cretan Avocado Cultivars during Ripening. J. Sci. Food Agric. 2004, 84, 485–492. DOI: 10.1002/(ISSN)1097-0010.
- Indriyani, L.; Rohman, A.; Riyanto, S. Physico-Chemical Characterization of Avocado (Persea Americana Mill.) Oil from Three Indonesian Avocado Cultivars. Res. J. Med. Plant. 2016, 10, 67–78. DOI: 10.3923/rjmp.2016.67.78.
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Washington, USA, 2007.
- Pearson, D.;. General Methods. In The Chemical Analysis of Foods; Pearson, D., Ed.; Longman Group Ltd.: Harlow, UK, 1976; pp 6–26.
- AOCS. Official and Tentative Methods of the American Oil Chemists’ Society, 5th ed.; the American Oil Chemists’ Society: Champaign, USA, 1999.
- Nina Naquiah, A. N.; Marikkar, J. M. N.; Mirghani, M. E. S.; F., N. A.; Yanty, N. A. M. Differentiation of Fractionated Components of Lard from Other Animal Fats Using Different Analytical Techniques. Sains Malays. 2017, 46, 209–216. DOI: 10.17576/jsm-2017-4602-04.
- Yanty, N.; Marikkar, J.; Man, Y. C. Effect of Fractional Crystallization on Composition and Thermal Characteristics of Avocado (Persea Americana) Butter. J. Therm. Anal. Calorim. 2013, 111, 2203–2209. DOI: 10.1007/s10973-011-2055-y.
- Yanty, N.; Marikkar, J.; Miskandar, M. Comparing the Thermo-Physical Characteristics of Lard and Selected Plant Fats. Grasas y Aceites. 2012, 63, 328–334. DOI: 10.3989/gya.2012.v63.i3.
- ChiewWei, P.; YuenMay, C.; AhNgan, M.; ChengHock, C. The Effect of Physical Refining on Palm Vitamin E (Tocopherol, Tocotrienol and Tocomonoenol). Am. J. Appl. Sci. 2007, 4, 374–377. DOI: 10.3844/ajassp.2007.374.377.
- PORIM. PORIM Test Methods; Palm Oil Research Institute of Malaysia: Selangor, Malaysia, 1995.
- Nusantoro, B.; Xanthina, M.; Kadivar, S.; Yanty, N.; Dewettinck, K. Enzymatic Interesterification of Lauric Fat Blends Formulated by Grouping Triacylglycerol Melting Points. J. Am. Oil Chem. Soc. 2016, 93, 1051–1062. DOI: 10.1007/s11746-016-2851-4.
- Bora, P. S.; Narain, N.; Rocha, R. V.; Paulo, M. Q. Characterization of the Oils from the Pulp and Seeds of Avocado (Cultivar: Fuerte) Fruits. Grasas y Aceites. 2001, 52, 171–174.
- Quiñones-Islas, N.; Meza-Márquez, O. G.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Detection of Adulterants in Avocado Oil by Mid-FTIR Spectroscopy and Multivariate Analysis. Food Res. Int. 2013, 51, 148–154. DOI: 10.1016/j.foodres.2012.11.037.
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists, 5th ed.; the American Oil Chemists’ Society: Champaign, USA, 1998.
- Shahidi, F.; Wanasundara, U. N. Methods for Measuring Oxidative Rancidity in Fats and Oils. In Food Lipids: Chemistry, Nutrition and Biotechnology, 3rd ed.; Cc, A., Db, M., Eds.; CRC Press: Boca Raton, USA, 2008; pp 387–403.
- Moigradean, D.; Poiana, M.-A.; Gogoasa, I. Quality Characteristics and Oxidative Stability of Coconut Oil during Storage. J. Agroaliment. Processes Technol. 2012, 18, 272–276.
- Codex. Recommended Internal Standards Edible Fats and Oils; FAO corporate document repository: Rome, Italy, 1982.
- Cox, K. A.; McGhie, T. K.; White, A.; Woolf, A. B. Skin Colour and Pigment Changes during Ripening of ‘Hass’ Avocado Fruit. Postharvest. Biol. Technol. 2004, 31, 287–294. DOI: 10.1016/j.postharvbio.2003.09.008.
- Jiménez-Sotelo, P.; Hernández-Martínez, M.; Osorio-Revilla, G.; Meza-Márquez, O. G.; García-Ochoa, F.; Gallardo-Velázquez, T. Use of ATR-FTIR Spectroscopy Coupled with Chemometrics for the Authentication of Avocado Oil in Ternary Mixtures with Sunflower and Soybean Oils. Food Addit. Contam Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1105–1115. DOI: 10.1080/19440049.2016.1203073.
- Guillen, M.; Cabo, N. Characterization of Edible Oils and Lard by Fourier Transform Infrared Spectroscopy. Relationships between Composition and Frequency of Concrete Bands in the Fingerprint Region. J. Am. Oil Chem. Soc. 1997, 74, 1281–1286. DOI: 10.1007/s11746-997-0058-4.
- Tango, J. S.; Carvalho, C. R. L.; Soares, N. B. Physical and Chemical Characterization of Avocado Fruits Aiming Its Potencial for Oil Extraction. Rev. Bras. Frutic. 2004, 26, 17–23. DOI: 10.1590/S0100-29452004000100007.
- Tan, C. P.; Che Man, Y. Differential Scanning Calorimetric Analysis of Edible Oils: Comparison of Thermal Properties and Chemical Composition. J. Am. Oil Chem. Soc. 2000, 77, 143–155. DOI: 10.1007/s11746-000-0024-6.
- Jakab, A.; Heberger, K.; Forgacs, E. Comparative Analysis of Different Plant Oils by High-Performance Liquid Chromatography–Atmospheric Pressure Chemical Ionization Mass Spectrometry. J. Chromatogr. A. 2002, 976, 255–263.
- Lee, J. H.; Min, D. B. Nutraceuticals, Aging, and Food Oxidation. In Handbook of Functional Lipids; Akoh, C. C., Ed.; CRC Press: Boca Raton, USA, 2006; pp 325–350.
- Lu, Q.-Y.; Zhang, Y.; Wang, Y.; Wang, D.; Lee, R.-P.; Gao, K.; Byrns, R.; Heber, D. California Hass Avocado: Profiling of Carotenoids, Tocopherol, Fatty Acid, and Fat Content during Maturation and from Different Growing Areas. J. Agric. Food Chem. 2009, 57, 10408–10413. DOI: 10.1021/jf901839h.
- Wassell, P.; Young, N. W. G. Food Applications of Trans Fatty Acid Substitutes. Int. J. Food Sci. Technol. 2007, 42, 503–517. DOI: 10.1111/j.1365-2621.2007.01571.x.