?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In the study, lard, noninteresterified mixture of lard and rapeseed oil, and interesterified mixture of these fats were used in the production of meat batters. The quality (apparent viscosity, thermal drip, texture–penetration force, L*, a*, and b* color components) of model meat batters was determined. The researches also included an oxidative stability study of fat extracted from meat batters and fats used in their production process. Meat batters produced with noninteresterified fat or interesterified mixture of these fats were characterized by lower apparent viscosity (424 × 10−1 and 315 × 10−1 Pa s) and values of penetration force (6.1 and 5.3 N) in comparison with meat batter produced with lard (503 × 10−1 Pa s, 7.6 N, respectively). However, there was no significant influence of these fats addition on the thermal drip. Interesterification of lard and rapeseed oil mixtures can result in the production of a new fat with valuable functional properties. Interesterification caused a significant decrease in induction time (from 70.3 to 21.6 min) and onset oxidation temperatures (from 162–193.7 to 136–154°C) in obtained fat in each heating rate. Fats extracted from meat batters were characterized by lower onset oxidation temperatures (135.1–160°C for meat batters with noninteresterified fats and 132–152°C for batters with interesterified fats) and lower induction time (11.9 and 10.9 min) than fats used in meat batters. Oxidation parameters can help in the assessment of resistance of modified fat and meat batters to oxidative deterioration.
Introduction
Fat is added to meat products to provide good sensory attributes, especially those related with taste and texture.[Citation1] It also plays an important role in binding, rheological and structural properties.[Citation2] The technological application of fat used in meat products depends on its physical and chemical properties, which together with nutritional properties are limited by the composition of fatty acid. Pork fat is the most commonly used fat in meat production because of its superior technological characteristics. However, the fat is rich in saturated fatty acids (SFAs), which can be associated with increased risk of obesity and coronary heart disease.[Citation1] In order to reduce the SFA content in meat products, animal fat is altered by plant oil sources which are free of cholesterol and rich in unsaturated fatty acids.[Citation2] However, the incorporation of plant oils which have a high content of unsaturated fatty acid and are in a liquid state in room temperature seems to be technologically not suitable in meat products.[Citation1,Citation2] In order to improve technological attributes of plant oils, many methods of fat modification have been used, e.g., hydrogenation, blending, and interesterification.[Citation3,Citation4] The partial hydrogenation may lead to the production of trans fatty acids, which are known to have detrimental health effects. The adverse effect of hydrogenation makes the use of interesterification of fats and oils an attractable/attractive alternative for hydrogenation.[Citation2]
During interesterification, fatty acids are exchanged within and among triacylglycerols (TAGs) until a thermodynamic equilibrium is reached. The resulting products maintain the fatty acid profile and saturation degree of the starting blends, but a different TAG stereochemistry can be observed, which results in new physicochemical characteristics and nutritional properties.[Citation3] The interesterification of lipids catalyzed by lipases is an alternative to the chemical interesterification. In contrast to chemical methods, enzymatic synthesis as a tool for fat modification has many advantages, such as mild processing conditions, possibility of regiospecificity, and fatty acids specificity.[Citation5,Citation6] However, for the successful synthesis of the lipids for meat products, it is important not only to optimize the nutritional and physical properties but also to ensure that the oxidative stability of the final product is acceptable. Numerous methods have been developed for monitoring fat and oil autooxidation. Nowadays, instrumental methods are commonly used as they are faster, more precise, have wider scope of detection, and are more objective. Differential scanning calorimetry (DSC) is a nonchemical method to determine fat quality parameters.[Citation5,Citation7]
DSC can be used to record the heat released from a particular reaction in either isothermal or non-isothermal mode. The non-isothermal method is based on the linear correlation between the temperature that corresponds to a specific thermal event and a different heating rate. In general, non-isothermal methods are widely used in lipid oxidation because they can provide valuable analytical and kinetic information. The aim of the study was to determine the effects of fat: lard, noninteresterified mixture of lard and rapeseed oil, and interesterified mixture of lard and rapeseed oil-fats used in the process of model meat batters from chicken breast meat production. Such raw material is characterized by low fat content. The kinetic parameters of the oxidation process and oxidative stability were evaluated in fats used in the production of meat batters and fats extracted from meat batters. Moreover, the quality of meat batters was evaluated.
Materials and methods
Materials
Lard, rapeseed oil, and chicken breast muscles were provided by an industrial plant. Immobilized Lipozyme RM IM, used in this investigation as a catalyst of interesterification, porcine pancreatic lipase (Type II), and standard of fatty acid methyl ester, was procured from Sigma-Aldrich. All other solvents and reagents were purchased from Avantor Performance Materials Poland S.A. (Gliwice, Poland).
Enzymatic interesterification
A mixture of lard and rapeseed oil at weight ratio 7:3 was interesterified in the presence of Lipozyme RM IM containing immobilized 1,3-specific lipase from Rhizomucor miehei. The interesterification was carried out at 50°C for 2 h. Interesterified fats were purified by neutralization of free fatty acids with 0.5 M KOH hydroethanolic solution (30% ethanol).[Citation8]
Preparation of meat batters
Three variants of meat batters were produced in duplicate and in three series. The recipes are given in . Chicken breast muscles were ground in a laboratory meat grinder (Diana 886.8, Zelmer, Rzeszów, Poland) equipped with ø3 mm plate and then cured (99.6% NaCl and 0.4% NaNO3) for 24 h under refrigeration (4–6°C). After this time, meat was homogenized with a fat and a half amount of crushed ice for 1 min using laboratory blender. Then, phosphates, soy protein isolate, sodium ascorbinate, and rest of crushed ice were added. Each batter was homogenized again for 5 min. In such prepared unheated meat batters, apparent viscosity was determined. After heat treatment, thermal drip was evaluated and after 24 h of cooling under refrigerated temperature (4–6°C), texture (penetration force) and color L*, a*, b* components were measured.
Table 1. Composition and technological properties of meat batters
Methods
Apparent viscosity
Meat batter apparent viscosity was measured in triplicate with a rotational viscometer (Rheotest-2, Germany). The measurement was made using a measuring set type H/H for high viscosity, consisting of a measuring cylinder and a rotating cylinder. The temperature of each sample (17 g) at the time of testing was 18 ± 1°C. The readings of α values in unit scale (µs) were made after 30 s of viscometer operation. Viscosity (η) was calculated from the following formula:
[10−1 Pa × s]
where τr is the shear stress , Dr is the shear rate = 16.20 s−1, and Z is the constant value for the used cylinder = 294.3
.
Thermal drip
Thermal drip was determined according to procedure described by Chmiel and Słowiński.[Citation9]
Instrumental texture of meat batters-penetration force
The penetration force of the examined model meat batters was determined after heat treatment and cooling (24 h). Prior to measurement, the samples were conditioned for 1.5 h at room temperature (approx. 20°C), and then 25 mm thick slices were prepared. The penetration force measurement was conducted using a universal testing machine ZWICKI type 1120. To measure the maximum force required for immersion inside the sample of metal, a cylindrical flat-felled mandrel of a diameter of 5 mm to a depth of 20 mm with the speed of movement of the measuring head of 50 mm/min was used from the obtaining of the pretension value of 0.5 N. The measurement was conducted every time in five different places of sample, and the calculated average value was assumed as the result.
L*, a*, b* color components
The L*, a*, and b* color components were determined with the use of CIEL*a*b* color scale using a Minolta CR200 colorimeter (Minolta, Osaka, Japan; light source D65, observer 2°, a measuring head hole 8 mm). The color parameters, such as L* (lightness), a* (redness), and b* (yellowness), were measured on the cross-section areas of batters after heat treatment. The instrument was calibrated using the reference values for white color (L* = 97.83, a* = −0.45, and b* = 1.88). All measurements were analyzed in five replicates.
Fat extraction from meat batters
The procedure described by Boselli et al.[Citation10] was used for fat extraction from meat batters. The collected fat sample was stored at −18°C until it was analyzed.
Determination of acid value
Acid values (AVs) were determined by titration of fat samples dissolved in the mixture of ethanol:diethyl ether (1:1, v/v) with 0.1 M ethanolic potassium hydroxide solution. Determination was done according to the Polish Standard.[Citation11]
Determination of fatty acid composition
The determination of fatty acid composition was carried out by gas chromatographic (GC) analysis of fatty acid methyl esters. Fatty acid methyl esters were prepared according to the Polish Standard.[Citation12] An YL6100 GC chromatograph equipped with a flame ionization detector and a BPX-70 capillary column of 0.20 mm (internal diameter) × 60 m length and 0.25 μm film thickness was used. The oven temperature was programmed as follows: 60°C for 5 min, then it was increased by 10°C/min to 180°C from 180 to 230°C by 3°C/min, and then kept at 230°C for another 15 min. The temperature of the split injector was 225°C, with a split ratio of 1:100; the detector temperature was 250°C. Nitrogen flowing at the rate of 1 mL/min was used as the carrier gas. Measurements were done in triplicate. The identification of fatty acids was carried out using the standards.
Sn-2 positional fatty acid analysis
The positional distributions of fatty acids in the sn-2 and sn-1,3 positions of TAGs were determined according to the method described by Yüksel and Şahin Yeşilçubuk[Citation13] and Bryś et al.[Citation14] This method is based on the ability of the pancreatic lipase to selectively hydrolyze ester bonds in the sn-1,3 positions.
DSC measurements
The oxidative stability of tested fats was determined using isothermal mode of DSC (Q20, TA Instruments) coupled with a high-pressure cell. The isothermal temperature for each sample was 120°C. The non-isothermal (dynamic) mode of DSC was used to determine onset oxidation temperature (ton, °C). The kinetic parameters of the oxidation process (activation energy, pre-exponential factor) were also calculated. The procedure of determination of parameters listed above is described by Wirkowska–Wojdyła et al.[Citation15] Q200 DSC (TA Instruments, Newcastle, USA) was used to analyze melting characteristics of fats according to the procedure described by Aguedo et al.[Citation16]
Statistical analysis
The data were reported as the means ± standard deviation. One-way ANOVA was performed using the Statgraphics Plus, version 5.1 (Statistical Graphics Corporation, Warrenton, VA, USA). Differences were considered to be significant at a p-value <0.05, according to Tukey’s multiple range test.
Results and discussions
Technological quality of model meat batters
The quality characteristics of model chicken meat batters are given in . Modifications of fats significantly affected the apparent viscosity of produced meat batters (). Model meat batters MB_L:RSO and MB_iL:RSO were characterized by a significantly lower apparent viscosity in comparison with the viscosity of meat batter produced with lard (L). The significantly lowest viscosity was observed in meat batter in which an interesterified mixture of lard and rapeseed oil (MB_iL:RSO) was used. Modifications of fats significantly affected the viscosity of produced meat batters. The viscoelastic parameters of meat batters depend on, inter alia, the content of used fat and its structure. With the increase of fat content, the stickiness of stuffing is reduced. Higher harder fat content results in increase of rheological parameters, including viscosity. The above relationship is stronger when the temperature during, for example, production of frankfurters stuffing is lower. At the melting point of the fat, this relationship is reversed. There is not one optimal viscosity of meat batters, but it is assumed that the less viscous meat batter is, the better filling process into the casings will be. According to Yapar et al.,[Citation17] increasing emulsion viscosity is desired in high fat emulsion type meat products, because higher emulsion viscosity gives increased elasticity. However, in this case, more empty spaces in the casing may be formed. Other researchers have shown correlations between emulsion viscosity and emulsion stability, as high viscosity emulsions are not easily broken.[Citation18,Citation19]
There were no significant differences in thermal drip of produced model meat batters (). The replacing animal fat (lard) with noninteresterified mixture of lard and rapeseed oil or interesterified mixture of lard and rapeseed oil did not influence the amount of thermal drip; however, it significantly affected the texture of produced batters (). Batters with noninteresterified mixture of lard and rapeseed oil or interesterified mixture of lard and rapeseed oil were characterized by significantly lower values of penetration force in comparison with batter with lard (L). Since animal fats are hard and solid fats, animal fat in meat products plays an important role in textural characteristics of meat products.[Citation20,Citation21] Therefore, replacing animal fat with oils or interesterified vegetable oils in meat products may cause quality defects in which the most important problem is softening.[Citation22]
Replacing animal fat with vegetable oil can cause change in color. This change depends on the type of oil used and on the amount of fat.[Citation18] In our studies, the replacement of animal fat (lard) with noninteresterified mixture of lard and rapeseed oil or interesterified mixture of lard and rapeseed oil did not significantly affect the color components (L*, a*, and b*) of the model batters (). Some researchers reported similar results and indicated that the use of vegetable oil and interesterified vegetable oil in, for example, the fermented sausages or animal fat replacement with these kinds of fats did not create important changes in color components of meat products.[Citation20,Citation23] In contrast to our results, Park et al.[Citation24] found that as vegetable oil replaced animal fat in meat products, the yellowness values significantly increased. Also, as reported by Yıldız-Turp and Serdaroğlu,[Citation22] the use of vegetable oil and interesterified vegetable oil in the fermented meat products increased their yellowness and redness.
Quality assessment of studied fats
Melting characteristic
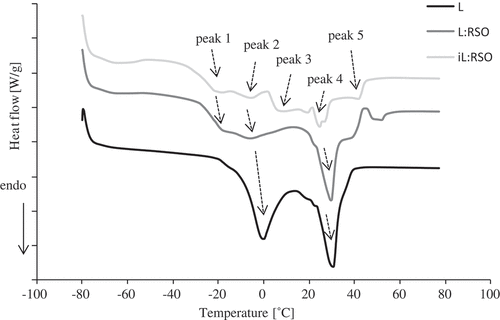
The thermal behavior of fats is usually described by melting and crystallization curves. The melting diagrams of the initial fats are presented in . The melting curves of the lard and noninteresterified fats show two and three distinct peaks, respectively. In L:RSO, peaks 1: −18.16 and 2: −6.20°C correspond to the lower melting TAG from RSO, peak 4: 29.50°C is associated with high-melting TAG from lard. Curves of interesterified mixture of lard and rapeseed oil exhibited a wide melting range, with five distinct peaks. This diversity in peaks in the melting behaviors suggests the presence of TAGs of different structure in the blend.[Citation25] The main consequences of interesterification were the decrease of peak 4 and the appearance of two new peaks: 3: 6.89 and 5: 41.77°C, which is related to the formation of new TAGs during interesterification. That can be attributed to the increase in amount of trisaturated and disaturated TAGs after interesterification, which also contributed to an increase of melting point for this blend.[Citation25]
Fatty acid composition and their positional distribution
The proportion of SFA, monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA) of the L, L:RSO, iL:RSO, and fat extracted from meat batters are given in and . In the mixture of lard and rapeseed oil, the content of SFAs decreased, whereas the amount of MUFAs and PUFAs increased. This is due to the fact that rapeseed oil is rich in oleic, linoleic, and α-linolenic fatty acid and is characterized by good balance between the omega-6 and omega-3 fatty acids.[Citation26] As a result of interesterification, the unsaturated fatty acids from rapeseed oil were incorporated into TAG structures of lard. Interesterification did not alter the amount of SFA, MUFA, PUFA related to the noninteresterified mixture. This is confirmed by Bryś et al. (2013, 2014). TAG of interesterified mixture contained 30.4% SFA, 50.4% MUFA, and 18.5% PUFA. Rapeseed oil has been used quite widely in enzymatic interesterification to increase the degree of unsaturation in fat rich in SFA.[Citation5,Citation27]
Table 2. Composition of the selected fatty acids and their distribution in triacylglycerols in fats used in model meat batters
The amount of SFA, MUFA, and PUFA in the MB_L, MB_L:RSO, MB_iL:RSO was at a similar level as in the fat used in meat batters. Cheong et al.[Citation26] and Javidipour and Vural[Citation2] observed that the use of interesterified fats containing vegetable oil in meat products resulted in a better ratio of unsaturated fatty acids to SFAs.
Stereochemistry plays a fundamental role in the rate and extent to which fatty acids are absorbed and become available for any metabolic and structural functions.[Citation3] Considering the result obtained, it can be concluded that interesterification affects the distribution of fatty acids in TAG mainly in external position due to the positional sn-1,3 specifity of the lipase. As the enzyme operated on the external positions of TAG, the percentages of given fatty acids in sn-2 position of interesterified TAG in comparison with initial blends remain nearly unchanged. Palmitic acid was the most abundant fatty acid located in the sn-2 position of TAG in the nonesterified and esterified mixture (). Taking into account the percentage of unsaturated fatty acids in the sn-2 position of TAG, it can be stated that after interesterification, PUFAs (mainly α-linolenic acid) were incorporated in external positions of TAG. The fatty acid distribution in model meat batters was determined by the type of fatty material used for their production (). In MB_L:RSO and MB_iL:RSO, the percentage of palmitic acid at the sn-2 position ranged from 76.7% to 76.8%, which confirms that it is located mainly in internal positions of TAG. Polyunsaturated α-linolenic acid in MB_L:RSO was located in sn-2 positions of TAG, whereas percentage of this acid in MB_iL:RSO does not exceeded 33%, which means that it is located mainly in external positions of TAG. The SFAs in the sn-2 position are absorbed more efficiently compared to the SFAs in the sn-1,3 positions. The more likely reason in the absorption difference may be the formation of insoluble calcium soaps since long-chain SFAs released from the sn-1,3 positions in the intestinal lumen tend to bind with Ca and Mg ions to form insoluble salts.[Citation3,Citation28] It confirms that enzymatic interesterification has been proved to be the method to achieve this modification in fats, thus, as an effective tool to introduce essential fatty acids that could be absorbed in the same way.
Oxidative stability
The content of free fatty acid (measured by AV) is presented in . Interesterification caused a statistically significant increase in AV (from 1.03 mg KOH/g of fat in L:RSO to 5.06 mg KOH/g of fat in iL:RSO). Bryś et al.[Citation5,Citation27] also observed that interesterified mixtures were characterized by a higher content of free fatty acid than noninteresterified fats. This is due to the fact that the natural function of lipases is to catalyze the hydrolysis of fats. The fats extracted from model meat batters were characterized by a statistically significant higher AV than fats used in production of meat batters.
Table 3. Parameters of the L, L:RSO, iL:RSO, and fat extracted from meat batters and regression analysis of the DSC data, activation energies (Ea), pre-exponential factors (Z) for L, L:RSO, iL:RSO, and fats extracted from meat batters
The production of interesterified fats can be impeded by their high susceptibility to oxidative deterioration.[Citation29] Modified fats containing unsaturated fatty acids can deteriorate during storage and produce off-flavors and odors characteristic for oxidation.[Citation30] The induction time and onset oxidation temperatures values obtained for L, L:RSO, iL:RSO, MB_L, MB_L:RSO, MB_iL:RSO were used as parameters for the assessment of the resistance of tested fats to their thermal oxidative decomposition. The results are given in (induction time) and in (ton). The results presented in , for fats used in meat batters, show that the studied fats have relatively high onset oxidation temperatures, which are associated with a high content of SFA and MUFA. Noninteresterified mixture of lard and rapeseed oil was characterized by a higher induction time and higher oxidation onset temperatures than lard. L:RSO is more unsaturated than lard and should oxidize more quickly, but on the other hand, rapeseed oil is rich in natural antioxidants.[Citation31] Interesterification resulted in the reduction of oxidative stability. Interesterified mixture of lard and rapeseed oil, with lower induction time and lower ton, is less stable than noninteresterified mixture. Most studies have reported a decrease in oxidative stability of interesterified fats compared to the initial mixture. Bryś et al.[Citation5] showed that the PDSC (pressure differential scanning calorimetry) tests performed for interesterified mixtures of lard, rapeseed oil, and concentrate of fish oil and mixtures of milkfat, rapeseed oil, and concentrate of fish oil resulted in reduced induction time in comparison to original fats. Kowalska et al.[Citation32] also showed lower oxidative stability of enzymatic interesterified mixtures of goose fat with rapeseed oil compared to the noninteresterified mixtures. The presence of antioxidants, prooxidants, composition, and structure (unsaturation) of fatty acids and chemical structure of TAG influences on the oxidative stabilities. During enzymatic interesterification and product purification, antioxidants and prooxidants can be removed or at least their concentrations are reduced.[Citation32] Martin et al.[Citation30] also suggested that the loss of endogenous antioxidant, when vegetable oil is used in the modification, is the main reason for explaining a worse oxidative stability of interesterified fats. Kowalska et al.[Citation32] found that the loss of α-tocopherol was in the range of 10.2–14.9% for interesterified mixtures and for the sum of (β and γ)-tocopherols and for δ-tocopherol, the losses after interesterification were 22.4–28.3% and 24.9–28.5%, respectively.
Table 4. DSC heating rates (β) and oxidation onset temperatures (ton) for L, L:RSO, iL:RSO, and fats extracted from meat batters
Fats extracted from meat batters were characterized by a lower induction time than fats used in the production of them. Induction time for fats extracted from MB_L, MB_L:RSO, MB_iL:RSO was at the level of 10.67, 11.95, 10.92 min, respectively, whereas for fats used in meat batters: L, L:RSO, iL:RSO, 56.90, 70.31, 21.56 min, respectively. For fat extracted from MB_L, MB_L:RSO, and MB_iL:RSO, lower onset oxidation temperatures in each heating rates, than for L, L:RSO, and iL:RSO, were also observed.
Lower resistance to oxidation of fats extracted from meat batters may be associated with a higher content of free fatty acids compared to the L, L:RSO, and iL:RSO. Martin et al.[Citation30] reported than increase in the content of free fatty acids and polar fraction in interesterified fat may reduce resistance of fat to oxidation. The higher the level of FFAs (free fatty acids), monoacylglycerols, and diacylglycerols in the obtained product after interesterification with respect to the level of TAG is, the higher reduction in oxidative stability is observed. Hamam and Shahidi[Citation33] suggested that the presence of FFAs in the reaction mixture may induce oxidation due to a catalytic effect of the carboxylic groups of the FFA on the formation of free radicals.
The kinetic parameters for the fats tested are listed in . The activation energies calculated from the experimental data were 96.05 kJ/mol for L, 79.55 kJ/mol for L:RSO, and 128.74 kJ/mol for iL:RSO. Activation energy for fat extracted from meat batters remained at the same level as the activation energy in fat used to prepare them. Fat extracted from meat batters produced with interesterified mixtures of lard and rapeseed oil was characterized by a higher activation energy and pre-exponential factor, than interesterified mixtures iL:RSO. Kowalska et al.[Citation32] also observed that activation energy and pre-exponential factor for fats after enzymatic interesterification assisted with microwaves were on higher level than in initial mixtures. On the other hand, Wirkowska–Wojdyła et al.[Citation7] noted that interesterified fats were characterized by a lower value of activation energy and pre-exponential factor than initial mixtures. The fat oxidation is multifaceted reaction which depends on many factors including endogenous antioxidants, catalysts, and primary and secondary oxidation products especially in the case when the vegetable oils are used in the modification and technology. Adhvaryu et al.[Citation34] reported that a high PUFA content would lower, while high MUFA and SFA content would increase the Ea value for lipid oxidation. However, in the present study, no conclusively correlation between fatty acids composition and Ea values was observed.
Conclusion
Interesterification of lard and rapeseed oil mixtures can result in the production of a new fat with valuable functional properties. Such modified fats can be successfully used in the meat batters formulations, with no influence on the thermal drip or color components. However, usage of modified fats influenced the apparent viscosity and texture chicken meat batters. This should be investigated during sausage production. The kinetic information, induction time, and onset oxidation temperatures can help in the assessment of resistance of modified fat and meat batters to oxidation. The PDSC method has a great potential to be successfully applied and is competitive as routine quality control analysis of food products.
Nomenclature
| L | = | Lard |
| L:RSO | = | Noninteresterified mixture of lard and rapeseed oil |
| iL:RSO | = | Interesterified mixture of lard and rapeseed oil |
| MB_L | = | Meat batters produced with lard |
| MB_L:RSO | = | Meat batters produced with noninteresterified mixture of lard and rapeseed oil |
| MB_iL:RSO | = | Meat batters produced with interesterified mixture of lard and rapeseed oil |
References
- Ospina-E, J. C.; Cruz-S, A.; Pérez-Álvarez, J. A.; Fernández-López, J. Development of Combinations of Chemically Modified Vegetable Oils as Pork Backfat Substitutes in Sausages Formulation. Meat Sci. 2010, 84, 491–497. DOI: 10.1016/j.meatsci.2007.06.012.
- Javidipour, I.; Vural, H. Effects of Incorporation of Interesterified Plant Oils Quality and Fatty Acid Composition of Turkish-Type Salami. Nahrung/Food. 2002, 46, 404–407. DOI: 10.1002/1521-3803(20021101)46:6<404::AID-FOOD404>3.0.CO;2-8.
- Farfán, M.; Villalón, M. J.; Ortíz, M. E.; Nieto, S.; Bouchon, P. The Effects of Interesterification on the Bioavailability of Fatty Acids. Food Chem. 2013, 139, 571–577. DOI: 10.1016/j.foodchem.2013.01.024.
- Sharma, M.; Lokesh, B. R. Effect Enzymatic Trans- and Interesterification on the Thermal Properties of Groundnut and Linseed Oil and Their Blends. J. Am. Oil Chem. Soc. 2012, 89, 805–813. DOI: 10.1007/s11746-011-1985-7.
- Bryś, J.; Wirkowska, M.; Górska, A.; Ostrowska-Ligęza, E.; Bryś, A. Application of the Calorimetric and Spectroscopic Methods in Analytical Evaluation of the Human Milk Fat Substitutes. J. Therm. Anal. Calorim. 2014, 118, 841–848. DOI: 10.1007/s10973-014-3893-1.
- Danthine, A.; De Clercq, N.; Dewettinck, K.; Gibon, V. Monitoring Batch Lipase Catalyzed Interesterification of Palm Oil and Fractions by Differential Scanning Calorimetry. J. Therm. Anal. Calorim. 2014, 115, 2219–2229. DOI: 10.1007/s10973-014-3645-2.
- Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A.; Ostrowska-Ligęza, E. Oxidation Kinetics and Melting Profiles of the Structured Lipids Used in Infant Cookies. Eur. J. Lipid Sci. Technol. 2014, 116, 1546–1552. DOI: 10.1002/ejlt.201400081.
- Jiménez, M. J.; Esteban, L.; Robles, A.; Hita, E.; González, P. A.; Muñío, M. M.; Molin, E. Production of Triacylglycerols Rich in Palmitic Acid at Sn-2 Position by Lipase-Catalyzed Acidolysis. Biochem. Eng. J. 2010, 51(3), 95–198. DOI: 10.1016/j.bej.2010.06.015.
- Chmiel, M.; Słowiński, M. The Use of Computer Vision System to Detect Pork Defects. LWT - Food Sci. Technol. 2016, 73, 473–480. DOI: 10.1016/j.lwt.2016.06.054.
- Boselli, E.; Velazco, V.; Caboni, M. F.; Lercker, G. Pressurized Liquid Extraction of Lipids for the Determination of Oxysterols in Egg-Containing Food. J. Chromatogr. A. 2001, 917, 239–244. DOI: 10.1016/S0021-9673(01)00688-4.
- PN-EN ISO 660 (2010) Oleje i tłuszcze roślinne oraz zwierzęce. Oznaczanie liczby kwasowej.
- 14. PN-EN ISO 12966-2 (2011) Oleje i tłuszcze roślinne oraz zwierzęce – Chromatografia gazowa estrów metylowych kwasów tłuszczowych – Część 2: Przygotowanie estrów metylowych kwasów tłuszczowych.
- Yüksel, A.; Şahin Yeşilçubuk, N. Enzymatic Production of Human Milk Fat Analogues Containing Stearidonic Acid and Optimization by Response Surface Methodology. LWT - Food Sci. Technol. 2012, 46, 210–216. DOI: 10.1016/j.lwt.2011.10.004.
- Bryś, J.; Vaz Flores, I.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, A. Use of GC and PDSC Methods to Characterize Human Milk Fat Substitutes Obtained from Lard and Milk Thistle Oil Mixtures. J. Therm. Anal. Calorim. 2017, 130, 319–327. DOI: 10.1007/s10973-017-6452-8.
- Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A.; Ostrowska-Ligęza, E. Effect of Enzymatic Interesterification on Physiochemical and Thermal Properties of Fat Used in Cookies. LWT - Food Sci. Technol. 2016, 74, 99–105. DOI: 10.1016/j.lwt.2016.07.040.
- Aguedo, M.; Giet, J.-M.; Hanon, E.; Logany, G.; Wathelet, B.; Destain, J.; Brasseur, R.; Vandenbol, M.; Danthine, S.; Blecker, C.; et al. Calorimetric Study of Milk Fat/Rapeseed Oil Blends and Their Interesterification Products. Eur. J. Lipid Sci. Technol. 2009, 111, 376–385. DOI: 10.1002/ejlt.200800184.
- Yapar, A.; Atay, S.; Kayacier, A.; Yetim, H. Effects of Different Levels of Salt and Phosphate on Some Emulsion Attributes of the Common Carp (Cyprinus Carpio L., 1758). Food Hydrocolloids. 2006, 20(6), 825–830. DOI: 10.1016/j.foodhyd.2005.08.005.
- Choi, Y. S.; Choi, J. H.; Han, D. J.; Kim, H. Y.; Lee, M. A.; Kim, H. W. Characteristics of Low-Fat Meat Emulsion Systems with Pork Fat Replaced by Vegetable Oils and Rice Bran Fiber. Meat Sci. 2009, 82(2), 266–271. DOI: 10.1016/j.meatsci.2009.01.019.
- Lee, M. A.; Han, D. J.; Jeong, J. Y.; Choi, J. H.; Choi, Y. S.; Kim, H. Y.; Paik, H.-D.; Kim, C. J. Effect of Kimchi Powder Level and Drying Methods on Quality Characteristics of Breakfast Sausage. Meat Sci. 2008, 80(3), 708–714. DOI: 10.1016/j.meatsci.2008.03.010.
- Ospina-E, J. C.; Sierra-C, A.; Ochoa, O.; Perez-Alvarez, J. A.; Fernandez-Lopez, J. Substitution of Saturated Fat in Processed Meat Products: A Review. Crit. Rev. Food Sci. 2012, 52(1–3), 113–122. DOI: 10.1080/10408398.2010.493978.
- Kılıç, B.; Özer, C. O. Effects of Replacement of Beef Fat with Interesterified Palm Kernel Oil on the Quality Characteristics of Turkish Dry-Fermented Sausage. Meat Sci. 2017, 131, 18–24. DOI: 10.1016/j.meatsci.2017.04.020.
- Yıldız-Turp, G.; Serdaroğlu, M. Effect of Replacing Beef Fat with Hazelnut Oil on Quality Characteristics of Sucuk - a Turkish Fermented Sausage. Meat Sci. 2008, 78(4), 447–454. DOI: 10.1016/j.meatsci.2007.07.013.
- Soyer, A.; Ertaş, A. H.; Üzümcüogˇlu, Ü. Effect of Processing Conditions on the Quality of Naturally Fermented Turkish Sausages (Sucuks). Meat Sci. 2005, 69(1), 135–141. DOI: 10.1016/j.meatsci.2004.06.015.
- Park, J. C.; Jeong, J. Y.; Lee, E. S.; Choi, J. H.; Choi, Y. S.; Yu, L. H.; Paik, H. D.; Kim, C. J. Effects of Replaced Plant Oils on the Quality Properties in Low-Fat Hamburger Patties. Korean J. Food Sci. Tech. 2005, 37(3), 412–417. DOI: 10.5851/kosfa.2015.35.1.130.
- Oliveira, P. D.; Rodrigues, A. M. C.; Bezerra, C. V.; Silva, L. H. M. Chemical Interesterification of Blends with Palm Stearin and Patawa Oil. Food Chem. 2017, 215, 369–376. DOI: 10.1016/j.foodchem.2016.07.165.
- Cheong, L.-Z.; Zhang, H.; Nersting, L.; Jensen, K.; Haagensen, J. A. J.; Xu, X. Physical and Sensory Characteristic of Pork Sausages from Enzymatically Modified Blends of Lard and Rapeseed Oil during Storage. Meat Sci.. 2010, 85, 691–699. DOI: 10.1016/j.meatsci.2010.03.026.
- Bryś, J.; Wirkowska, M.; Górska, A.; Ostrowska-Ligęza, E.; Bryś, A.; Koczoń, P. The Use of DSC and FT-IR Spectroscopy for Evaluation of Oxidative Stability of Interesterified Fats. J. Therm. Anal. Calorim. 2013, 113, 481–487. DOI: 10.1007/s10973-012-2794-4.
- Wang, T.; Wang, X.; Wang, X. Effects of Lipid Structure Changed by Interesterification on Melting Property and Lipemia. Lipids. 2016, 51, 1115–1126. DOI: 10.1007/s11745-016-4184-3.
- Claro Da Silva, R.; Schaffer De Martini Soares, F. A.; Hazzan, M.; Capacla, I. R.; Almeida Gonçalves, M. I.; Gioielli, L. A. Continuous Enzymatic Interesterification of Lard and Soybean Oil Blend: Effects of Different Flow Rates on Physical Properties and Acyl Migration. J Mol. Catal. B. 2012, 76, 23–28. DOI: 10.1016/j.molcatb.2011.11.021.
- Martin, D.; Reglero, G.; Senorans, F. J. Oxidative Stability of Structured Lipids. Eur. Food Res. Tech. 2010, 23, 635–653. DOI: 10.1007/s00217-010-1324-5.
- Szydłowska-Czerniak, A.; Karlovits, G.; Hellner, G.; Dianoczki, C.; Szłyk, E. Effect of Enzymatic and Hydrothermal Treatments of Rapeseeds on Quality of the Pressed Rapeseed Oils. Part I: Antioxidant Capacity and Antioxidant Content. Process Biochem. 2010, 45, 7–17. DOI: 10.1016/j.procbio.2009.07.016.
- Kowalska, D.; Kostecka, M.; Tarnowska, K.; Kowalski, B. Oxidative Stabilities of Enzymatically Interesterified Goose Fat and Rapeseed Oil Blend by Rancimat and PDSC. J. Therm. Anal. Calorim. 2014, 115, 2063–2070. DOI: 10.1007/s10973-013-3125-0.
- Hamam, F.; Shahidi, F. Enzymatic Acidolysis of an Arachidonic Acid Single-Cell Oil with Capric Acid. J. Am. Oil Chem. Soch. 2004, 81, 887–892. DOI: 10.1007/s11746-004-0996-2.
- Adhvaryu, A.; Erhan, S. Z.; Liu, Z. S.; Perez, J. M. Oxidation Kinetic Studies of Oils Derived from Unmodified and Genetically Modified Vegetables Using Pressurized Differential Scanning Calorimetry and Nuclear Magnetic Resonance Spectroscopy. Thermochim. Acta. 2000, 364, 87–97. DOI: 10.1016/S0040-6031(00)00626-2.