ABSTRACT
The purpose of the study was to determine if pomegranate (Punica granatum L.) peel contains polyphenolic compounds with antibacterial activity against Cronobacter sakazakii. Minimum inhibitory and bactericidal concentrations of a methanolic extract were found to increase significantly (P < .05) with decreasing temperature (37, 21, 10 °C) and pH (6.0, 5.0, 4.0). Chemical analysis by high pressure liquid chromatography revealed that the elligitannins α- and β-punicalagin were most abundant hydrolysable polyphenolic compounds in the extract, followed by ellagic acid, ellagic acid derivatives and punicalin. Pomegranate peel is a potential source of natural preservatives for the control of Cronobacter sakazakii in food.
Introduction
Pomegranate (Punica granatum L.) is grown in many Middle Eastern, South Asian, Mediterranean countries and the USA, although Iran, China, India and Turkey are leading producers of fresh fruit and processed products including juices, jams, jellies or pastes.[Citation1] Consumer demand for pomegranate products is increasing in response to the nutritional quality and potential health benefits of the fruit attributed to the abundance of bioactive phytochemicals in the edible aril and seed.[Citation2] However, processing generates substantial waste in the form of inedible pomegranate peel (PoP), which represents approximately 50% of raw fruit weight.[Citation3] Considerable research has been directed at the development of means to dispose of PoP or to utilize it for the production of value added by-products. PoP contains several potentially valuable components including pectin, dietary fiber, micronutrients and an array of bioactive phenolic compounds ranging from simple phenolic acids (e.g. hydroxybenzoate) to complex, high molecular weight polyphenols (e.g. tannins) and a large pool of hydrolysable, water soluble polyphenolic compounds (e.g. ellagitannins).[Citation4–Citation6]
Extracts prepared from pomegranate plant parts have long been used in traditional medicines for the treatment of infectious diseases.[Citation7] Accordingly, the antimicrobial activity of extracts prepared from pomegranate tissues have been examined in some detail.[Citation8–Citation10] Aqueous, ethanolic or methanolic PoP extracts have been shown to inhibit Gram-negative and Gram-positive bacterial foodborne pathogens.[Citation9,Citation11] Chemical analysis indicates that antibacterial activity is strongly correlated with total phenolic content, the presence ellagic acid and of hydrolysable polyphenolic derivatives thereof.[Citation8,Citation12] Hence, PoP is a prospective source of antibacterial agents of potential utility as natural food preservatives.[Citation10,Citation13]
The opportunistic human pathogen Cronobacter sakazakii causes severe and occasionally fatal infections in neonates that are epidemiologically linked to the consumption of powdered infant formula.[Citation14] C. sakazakii has also been isolated from dehydrated foods fed to older infants, including follow-up formulas and cereals.[Citation15] The unusual osmotolerance, resistance to desiccation and mild thermotolerance of some strains of the species are conducive to survival in the manufacturing environment and in dehydrated foods.[Citation16–Citation18] Moreover, cells resuscitated during the reconstitution of infant formulas and cereals in water, milk or fruit juices can grow at temperatures ranging between 5.5 and 47°C.[Citation19,Citation20] Consequently, means to inactivate C. sakazakii during the manufacture of dehydrated foods, in the course of their preparation or during subsequent storage by the end-user are needed to control attendant risks.[Citation21] Conventional food preservatives could undoubtedly be used for this purpose. However, consumer concerns about the impact of synthetic chemicals on human health is leading food manufacturers to consider alternative antimicrobials derived from natural sources, notably phytochemicals.
Past research indicates that crude phenolic-rich plant extracts and purified phenolic compounds have antibacterial activity against C. sakazakii. Simple phenolic compounds including vanillic acid, ethyl vanillin and vanillin can lessen the heat resistance of C. sakazakii and inhibit growth in reconstituted powdered infant formula.[Citation22] Comparatively little data is available on the antibacterial activity of higher molecular weight polyphenols against the species. Pina-Pérez et al.[Citation23] reported that a polyphenol-rich cocoa powder inhibited growth of C. sakazakii in reconstituted infant milk formula stored at 25°C. Kim et al.[Citation24] ascribed the bactericidal effects of water-soluble Muscatine (Vitis rotundifolia) seed extracts against the species to organic acids (primarily malic, tartaric) and tannic acid. Interestingly, both Muscatine seed and cocoa contain polyphenols that are also found in PoP extracts, such as catechin, epicatechin and ellagic acid.[Citation5] Consequently, we examined the chemical composition and antibacterial activity of a polyphenolic-rich extract to determine the potential value of PoP as a source of natural preservatives for the control of C. sakazakii in food.
Materials and methods
Pomegranate peels
Pomegranate fruit (Punica granatum L., cv. ‘Hicaznar’) from a local market was transported to the Food Research Laboratory at Sakarya University, Sakarya, Turkey. The fruit were washed in water and peeled manually. Peels were frozen at −45°C, lyophilized for 5 days (Labconco, FreeZone 6 Liter, Kansas City, USA) and stored at −20°C until used.
Chemicals and reagents
Punicalagin (97%) and punicalin (98%) standards were obtained from Biopurify Phytochemicals Ltd. (Sichuan, China), ellagic acid, gallic acid and Folin–Ciocalteu reagent from Sigma-Aldrich Canada Ltd. (Oakville, Canada). Methanol (MeOH) and acetonitrile used for extractions and liquid chromatography were HPLC grade (Fisher Scientific, Ottawa, Canada).
Preparation of a purified PoP extract
A 5 g sample of ground lyophilized PoP was stirred with 100 mL of an 80% MeOH + 0.01% HCl solution for 1 h at room temperature. The slurry was then spun at 10,000 rpm for 10 min in a centrifuge and the resulting supernatant was filtered through Whatman filter paper No. 1 into a rotary evaporator flask. The pellet was re-extracted with the same solvent under the same conditions and the filtrates were combined. Methanol was removed under vacuum (50 mm Hg) in a rotary evaporator operated at 40°C and the volume was restored to 100 mL with water + 0.01% HCl. Organic acids and sugars were removed by solid phase extraction (SPE) on reversed-phase C18 cartridges (Supelco ENVI-18, 50 μm particle size, 60 Å porosity, 5 g sorbent mass/20 mL reservoir volume, Sigma-Aldrich, Oakville, Canada) installed on a manifold system (Supelco, Visiprep DL). Each cartridge was conditioned with 25 mL of MeOH + 0.01% HCl followed by 25 mL water + 0.01% HCl prior to loading with 20 mL of the aqueous extract. The loaded cartridges were then washed with 25 mL water + 0.01% HCl and dried under vacuum for 3 min. Retained phenolic compounds were eluted with 25 mL MeOH + 0.01% HCl and the resulting purified PoP extract was dried under N2 at 35°C.
Total phenolic content
The total phenolic content (TPC) of the purified PoP extract was measured using the Folin–Ciocalteu microscale colorimetric method as described by Waterhouse.[Citation25] A standard curve prepared with gallic acid in the 50‒500 mg/L concentration range was used to determine TPC expressed as mg of gallic acid equivalents per dry weight (mg GAE/g DW).
Quantification and identification of phenolic compounds by HPLC
Purified PoP extract was dissolved in 10 mL water, diluted 50-fold with a 95:5 (v:v) solution of 50 mM phosphoric acid:acetonitrile and passed through a 0.45 μm PVDF syringe filter (Chromatographic Specialties, Inc., Brockville, Canada). Hydrolysable phenolic compounds were analyzed with an Agilent 1100 series HPLC system equipped with a diode array detector and ChemStation™ software (Agilent Technologies Inc., Palo Alto, USA). Chromatographic separations were performed on a Kinetex 2.6 μm PFP-100Å 150 × 4.6 mm LC column (Phenomenex Inc., Torrance, USA) using 50 m mol−1 phosphoric acid (solvent A, pH 1.17) and acetonitrile (solvent B) as the mobile phase. Column temperature was set at 35°C and flow rate at 0.6 mL/min. The gradient elution program was as follows: from 5% to 22.3% B in 35 min, from 22.3% to 52.3% B in 10 min, from 52.3% to 100% B in 5 min, 100% B isocratic in 35 min, from 100% to 5% B in 3 min, and 100% B isocratic in 10 min.Identification and quantification was carried out at 258 nm for punicalin/punicalagins, and at 254 nm for ellagic acid/ellagic acid derivatives. Retention times and absorption spectra of external reference standards were used to identify punicalin, punicalagin, ellagic acid. Peaks that showed same absorption spectra as ellagic acid were assumed to be ellagic acid derivatives. Calibration curves generated from each external standard were used for the quantification of peaks. Concentrations of ellagic acid derivatives were expressed as ellagic acid equivalents.
Bacterial strains and cultural conditions
C. sakazakii strains HPB 2855, HPB 2871, HPB 3290 were obtained from the Bureau of Microbial Hazards culture collection (Health Canada, Ottawa, Canada). Stock cultures were maintained at −80°C in tryptic soy broth (TSB, BBL, Cockeysville, USA) containing 20% glycerol. Working cultures were grown in TSB supplemented with 5 g/L yeast extract (TSBYE) for 24 h at 37°C under constant agitation at 60 rpm.
Measurement of minimum inhibitory and minimum bactericidal concentrations of the purified PoP extract at different pH values and temperatures
Minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) were determined by a broth dilution method in ¼ strength TSBYE amended with 0.15% agar essentially as described by Delaquis et al.[Citation26] Cultures grown for 24 h at 37°C were diluted in ¼ strength TSBYE adjusted to pH 4.0 or 5.0 or 6.0 with either 5N HCl or 10N NaOH. Stock solutions of PoP extract dissolved in water adjusted to pH to 4.0, 5.0 or 6.0 were sterilized by passage through 0.22 µm cellulose acetate membrane filters (Fisher Scientific, Ottawa, Canada). The stock solutions were then diluted in a 1:1 ratio with ½ strength TSBYE + 0.30% agar adjusted to pH 4.0, 5.0 or 6.0. Appropriate amounts were dispensed into the wells of microtiter plates along with sterile ¼ strength TSBYE+0.15% agar adjusted to experimental pH to achieve concentrations ranging between 0.25 to 60 mg GAE total phenolics/mL. Each well was then inoculated with 10 µL of C. sakazakii culture (final concentration ~ 5 log CFU/mL). Minimum inhibitory concentrations (MIC) were defined as the lowest concentration that prevented visible growth. A loopful of medium from wells without evidence of growth was applied to tryptic soy agar supplemented with 5 g/L yeast extract (TSAYE) which was incubated for 24 h at 37°C to determine minimum bactericidal concentrations (MBC). Experiments were performed at 37, 21 and 10°C in media adjusted to pH 4.0, 5.0 and 6.0. Wells were examined for evidence of growth after 48 h incubation at 37°C, 48 h at 21°C and 8 days at 10°C.
Survival curves for C. sakazakii at different pH values and temperatures in TSBYE containing purified PoP extract
C. sakazakii was exposed to MBC concentrations (average values, ) of PoP in TSBYE adjusted to pH 4.0 or 5.0 or 6.0 at temperatures of 37, 21 and at 10°C. Stock solutions of aqueous phenolic extract were prepared using the methods described above. Inoculum consisted of a cocktail prepared by combining cultures of each C. sakazakii strain in ¼ strength TSBYE to achieve a cell density of approximately 5 log CFU/mL. Surviving cell populations were estimated by the spread plate method on TSAYE incubated at 37°C for 24 h. Recovery of cells below the detection limit was ensured by enrichment of a 1 mL sample in 10 mL TSBYE for 24 h at 37°C, followed by transfer to TSAYE incubated at 37°C for 24 h. Inoculated samples without any added aqueous phenolic extract served as controls.
Table 1. Concentrations of phenolic compounds in the purified methanolic extract of pomegranate peel
Table 2. Minimum inhibitory (MIC) and minimum bactericidal (MBC) concentrations of the aqueous phenolic extract from pomegranate peel against three Cronobacter sakazakii isolates in tryptic soy broth + 0.5 g/L yeast extract adjusted to pH 4.0, 5.0 and 6.0 and incubated at 10°C, 21°C, and 37°C.a.
Statistical analysis
Three independent replicates were performed for each experimental condition. Duncan’s multiple-range tests were used for statistical analysis using SPSS version 20.0 (IBM Corp, Sommers, NY, USA). Differences between means were considered significant at p < .05.
Results and discussion
Composition of the PoP extract
The total phenolic content (TPC) of purified extract prepared from lyophilized PoP was 129.7 ± 1.0 mg GAE/g DW. Variable TPCs have been reported in methanolic extracts prepared from lyophilized PoP, ranging from 80 mg GAE/g[Citation27], to 101.9 mg GAE/g[Citation28], 118 mg GAE/g[Citation29] and 262.5 mg GAE/g.[Citation9] Differences in TPC were not unexpected as both the content and relative proportion of specific phenolic compounds in pomegranate tissues are known to be affected by cultivar, agronomic practice, fruit maturity, drying method and extraction procedure.[Citation5] In addition, post extraction handling and analytical procedures can affect the outcome of the TPC measurement. The extract prepared in the present work was purified by solid phase extraction to avoid confounding antimicrobial effects caused by non-phenolic constituents, notably organic acids and sugars that may interfere with the Folin–Ciocalteu reaction but which are seldom removed prior to analysis.[Citation30] Since none of the extracts examined in the aforementioned studies were purified, the actual TPCs were possibly higher than reported.
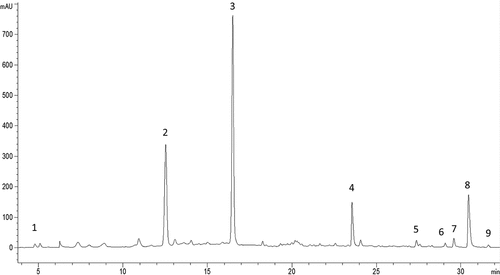
Chromatograms of the purified extract () revealed that nine compounds including ellagic acid and 5 co-eluted species (tentatively identified as derivatives of ellagic acid), and the ellagitannins punicalin, α-punicalagin and β-punicalagin were separated by HPLC. Concentrations provided in showed that the elligitannins α- and β-punicalagin (39.1 mg/g) were most abundant, followed by ellagic acid (4.0 mg/g) and punicalin (0.9 mg/g). Similar concentrations (38.6 mg/g, 2.8 mg/g and 0.8 mg/g, respectively) were measured in a methanolic extract of lyophilized PoP by John et al.[Citation27] The extract prepared in the present work also contained 3.7 mg/g of ellagic acid derivatives that could not be identified using analytical tools at our disposal. It should be noted that Fisher et al.[Citation28] detected five ellagic acid derivatives (ellagic acid -pentoside, -hexoside, – acid-dihexoside,- deoxyhexoside and – galloyl-HHDP-hexoside) in a methanolic PoP extract analyzed by mass spectrometry.
Antibacterial activity of the PoP extract
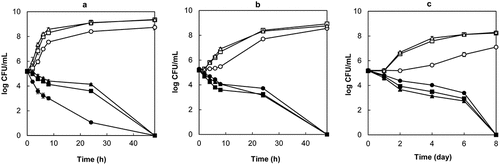
Antibacterial activity of the purified PoP extract against C. sakazakii was examined in tryptic soy broth supplemented with 5 g/L yeast extract (TSBYE). Results of MIC and MBC measurements presented in show that the extract exerted concentration-dependent bacteriostatic and bactericidal effects that increased significantly (p < .05) with decreasing temperature and pH. While MIC values obtained were comparable some strain-associated differences in MBC were apparent, notably at pH 5.0 and 6.0 where C. sakazakii HPB 3290 was more susceptible to the bactericidal effects of the extract than strains HPB 2855 and 3290. Inter-species variability in sensitivity to the antibacterial effects of polyphenols has been reported previously.[Citation31] The differences observed in the present study were small, however, and exposure to the extract was clearly lethal to all the experimental strains. C. sakazakii was also incubated in TSBYE supplemented with MBC concentrations of the extract to assess the rapidity and duration of antibacterial effects. Survival curves () showed that lethality was enhanced at 37 °C but not at 21 or 10 °C. The most rapid population decline was observed at pH 4.0 and a temperature of 37 °C, where the population was reduced by 4 log CFU/mL after 24 h and C. sakazakii was not recovered by enrichment after 48 h. In contrast, the population fell by 0.5 log CFU/mL after 24 h at pH 4.0/10 °C, and 8 days of incubation were required to achieve non-recovery by enrichment. Hence, the rate of C. sakazakii inactivation by PoP extract was likely affected by complex interactions between pH and temperature.
Figure 2. Survivor curves for C. sakazakii (cocktail of 3 strains) at 37°C (A), 21°C (B), 10°C (C) in tryptic soy broth +0.5 g/L yeast extract adjusted to pH 4.0 (●), 5.0 (■), and 6.0 (▲) and supplemented with MBC concentrations of PoP extract. Controls: tryptic soy broth +0.5 g/L yeast extract adjusted to pH 4.0 (○), 5.0 (□), and 6.0 (∆)
The present report provides the first evidence that the polyphenolic fraction of a PoP extract exerts antibacterial activity against the opportunistic foodborne pathogen C. sakazakii. Prior research has shown that other species from the family Enterobacteriaceae are inhibited or inactivated by exposure to polyphenol-rich methanolic extracts. For example, Al-Zoreky[Citation9] measured MIC values of 0.25 mg/mL for Yersinia enterocolitica, 1 mg/mL E. coli and 4 mg/mL for Salmonella enterica on Müller-Hinton agar (medium pH 7.3). These values are lower than the average MIC of 6.67 mg/mL at pH 6.0 reported here for three strains of C. sakazakii but the extract was not purified and had a TPC of 262.5 mg GAE/g, or approximately twice that used in the present study. The antibacterial activity of crude plant extracts generally increases with TPC. Moreover, non-phenolic constituents, such as organic acids, can contribute additional antibacterial effects.[Citation32] Because such constituents were removed from the extract prepared for the present work, activity against C. sakazakii could be ascribed to the polyphenolic fraction of PoP.
Some of the polyphenolic compounds identified in the PoP extract have been shown to inhibit Gram negative bacteria, including species of Enterobacteriaceae. For example, the ellagitannin punicalagin was reported to inhibit 10 strains of S. enterica at MICs ranging between 0.25 and 1.0 mg/mL[Citation33], six S. enterica strains at MICs between 0.6 and 0.8 mg/mL and six E. coli strains at MICs of 1.0 to 3.2 mg/mL.[Citation31] The antibacterial effects of ellagic acid, the second most abundant polyphenolic compound in the extract, have been studied in detail against the bacterium Helicobacter pylori.[Citation34] Evidence of activity against other Gram negative species is comparatively scarce, although Panichayupakaranant[Citation35] and Rosa-Burgos et al.[Citation3] found that ellagic acid rich PoP extracts inhibit S. enterica, Shigella sonnei and E. coli. Hence, the antibacterial effects of PoP extract against C. sakazakii may stem from the combined activity of ellagitannins, ellagic acid and derivatives thereof.
Conclusion
A purified PoP extract was shown to contain polyphenolic compounds with bacteriostatic and bactericidal activity against C. sakazakii. Hence, waste derived from the processing of this fruit could serve as a source of natural food preservatives for the control of C. sakazakii. Future work will address the level of supplementation required to inactivate C. sakazakii in foods, notably during rehydration or post-preparation handling of dehydrated products.
Acknowledgments
The lead author is grateful to the Sakarya University Scientific Research Projects Unit for the scholarship which supported this study. The authors wish to thank Oktay Yemiş, Kevin Usher, Steve Orban, and John Drover for valuable technical assistance and suggestions throughout this study.
References
- Teixeira Da Silva, J. A.; Rana, T. S.; Narzary, D.; Verma, N.; Meshram, D. T.; Ranade, S. A. Pomegranate Biology and Biotechnology: A Review. Sci. Hort. 2013, 160, 85–107. DOI: 10.1016/j.scienta.2013.05.017.
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J. B.; Sreeharshan, S. Pomegranate Fruit as a Rich Source of Biologically Active Compounds. Biomed. Res. Int. 2014, 2014, 686921. DOI: 10.1155/2014/686921.
- Rosas-Burgos, E. C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kacániová, M.; Hernández- García, F.; Cárdenas-López, J. L.; Carbonell-Barrachina, Á. A. Antimicrobial Activity of Pomegranate Peel Extracts as Affected by Cultivar. J. Sci. Food Agric. 2017, 97, 802–810. DOI: 10.1002/jsfa.7799.
- Hasnaoui, N.; Wathelet, B.; Jimenez-Araujo, A. Valorization of Pomegranate Peel from 12 Cultivars: Dietary Fibre Composition, Antioxidant Capacity and Functional Properties. Food Chem. 2014, 160, 196–203. DOI: 10.1016/j.foodchem.2014.03.089.
- Akhtar, S.; Ismail, T.; Fraternalem, D.; Sestili, P. Pomegranate Peel and Peel Extracts: Chemistry and Food Features. Food Chem. 2015, 174, 417–425. DOI: 10.1016/j.foodchem.2014.11.035.
- Talekar, S.; Patti, A. F.; Vijayraghavan, R.; Arora, A. An Integrated Green Biorefinery Approach Towards Simultaneous Recovery of Pectin and Polyphenols Coupled with Bioethanol Production from Waste Pomegranate Peels. Bioresour. Technol. 2018, 266, 322–334. DOI: 10.1016/j.biortech.2018.06.072.
- Viuda-Martos, M.; Fernandez-Lopez, L.; Perez-Alvarez, J. A. Pomegranate and Its Many Functional Components as Related to Human Health: A Review. Comp. Rev. Food Sci. 2010, B, 635–654. DOI: 10.1111/j.1541-4337.2010.00131.x.
- Reddy, M. K.; Gupta, S. K.; Jacob, M. R.; Khan, S. I.; Ferreira, D. Antioxidant, Antimalarial and Antimicrobial Activities of Tannin-Rich Fractions, Ellagitannins and Phenolic Acids from Punica Granatum L. Planta Med. 2017, 73, 461–467. DOI: 10.1055/s-2007-967167.
- Al-Zoreky, N.;. Antimicrobial Activity of Pomegranate (Punica Granatum L.) Fruit Peels. Int. J. Food Microbiol. 2017, 134, 244–248. DOI: 10.1016/j.ijfoodmicro.2009.07.002.
- Howell, A. B.; D’Souza, D. H. The Pomegranate: Effects on Bacteria and Viruses that Influence Human Health. J. Evid. Based Complementary Altern. Med. 2013, 2013, 1–11. Article ID 606212 DOI: 10.1155/2013/606212.
- Hayrapetyan, H.; Hazeleger, W. C.; Beumer, R. R. Inhibition of Listeria Monocytogenes by Pomegranate (Punica Granatum) Peel Extract in Meat Paté at Different Temperatures. Food Control. 2012, 23, 66–72. DOI: 10.1016/j.foodcont.2011.06.012.
- Gullon, B.; Pintadoa, M.; Pérez-Álvarez, J.; Viuda-Martos, V. M. Assessment of Polyphenolic Profile and Antibacterial Activity of Pomegranate Peel (Punica Granatum) Flour Obtained from Co-Product of Juice Extraction. Food Control. 2016, 59, 94–98. DOI: 10.1016/j.foodcont.2015.05.025.
- Tanveer, A.; Farooq, U.; Akram, K.; Hayat, Z.; Shafi, A.; Nazar, H.; Ahmad, Z. Pomegranate Extracts: A Natural Preventive Measure against Spoilage and Pathogenic Microorganisms. Food Rev. Int. 2014, 31, 29–51. DOI: 10.1080/87559129.2014.961074.
- Drudy, D.; Mullane, N. R.; Quinn, T.; Wall, P. G.; Fanning, S. Enterobacter Sakazakii: An Emerging Pathogen in Powdered Infant Formula. Clin. Infect. Dis. 2006, 42, 996–1002. DOI: 10.1086/501019.
- Chap, J.; Jackson, P.; Siqueira, R.; Gaspar, N.; Quintas, C.; Park, J.; Osaili, T.; Shaker, R.; Jaradat, Z.; Hartantyo, S. H.; et al. International Survey of Cronobacter Sakazakii and Other Cronobacter Spp. In Follow up Formulas and Infant Foods. Int. J. Food Microbiol. 2009, 136, 185–188. DOI: 10.1016/j.ijfoodmicro.2009.08.005.
- Osaili, T.; Forsythe, S. Desiccation Resistance and Persistence of Cronobacter Species in Infant Formula. Int. J. Food Microbiol. 2009, 136, 214–220. DOI: 10.1016/j.ijfoodmicro.2009.09.005.
- Walsh, D.; Molloy, C.; Iversen, C.; Carroll, J.; Cagney, C.; Fanning, S.; Duffy, G. Survival Characteristics of Environmental and Clinically Derived Strains of Cronobacter Sakazakii in Infant Milk Formula (IMF) and Ingredients. J. Appl. Microbiol. 2011, 110, 697–703. DOI: 10.1111/j.1365-2672.2010.04921.x.
- Yan, Q. Q.; Condell, O.; Power, K.; Butler, F.; Tall, B. D.; Fanning, S. Cronobacter Species (Formerly Known as Enterobacter Sakazakii) in Powdered Infant Formula: A Review of Our Current Understanding of the Biology of This Bacterium. J. Appl. Microbiol. 2012, 113, 1–15. DOI: 10.1111/j.1365-2672.2012.05281.x.
- Beuchat, L. R.; Kim, H.; Gurtler, J. B.; Lin, L. C.; Ryu, J. H.; Richards, G. M. Cronobacter Sakazakii in Foods and Factors Affecting Its Survival, Growth, and Inactivation. Int. J. Food Microbiol. 2009, 136, 204–213. DOI: 10.1016/j.ijfoodmicro.2009.02.029.
- Richards, G. M.; Gurtler, J. B.; Beuchat, L. R. Survival and Growth of Enterobacter Sakazakii in Infant Rice Cereal Reconstituted with Water, Milk, Liquid Infant Formula, or Apple Juice. J. Appl. Microbiol. 2005, 99, 844–850. DOI: 10.1111/jam.2005.99.issue-4.
- Pina-Pérez, M. C.; Rodrigo, D.; Martínez, A. Non-Thermal Inactivation of Cronobacter Sakazakii in Infant Formula Milk: A Review. Crit. Rev. Food Sci. Nutr. 2015, 56, 1620–1629. DOI: 10.1080/10408398.2013.781991.
- Yemiş, G. P.; Pagotto, F.; Bach, S.; Delaquis, P. Effect of Vanillin, Ethyl Vanillin, and Vanillic Acid on the Growth and Heat Resistance of Cronobacter Species. J. Food Prot. 2011, 74, 2062–2069. DOI: 10.4315/0362-028X.JFP-11-230.
- Pina-Pérez, M. C.; Rodrigo, D.; Martinez, A. Bacteriostatic Effect of Cocoa Powder Rich in Polyphenols to Control Cronobacter Sakazakii Proliferation in Infant Milk Formula. Science and Technology against Microbial Pathogens. Research, Development and Evaluation. Proceedings of the International Conference on Antimicrobial research (ICAR 2010). Valladolid, Spain November 2010. ISBN-13 978-981-42354-85-1. World Scientific Publishing Co. Pte. Ltd. 2011, pp. 85–88.
- Kim, T. J.; Silva, J. L.; Weng, W. L.; Chen, W. W.; Corbitt, M.; Jung, Y. S.; Chen, Y. S. Inactivation of Enterobacter Sakazakii By Water-Soluble Muscadine Seed Extracts. Int. J. Food Microbiol. 2009, 129, 3295–3299. DOI: 10.1016/j.ijfoodmicro.2008.12.014.
- Waterhouse, A. L. Unit I1.1: Polyphenolics: determination of total phenolics. In Current Protocols in Food Analytical Chemistry, ed. by Wrolstad, R.E., Wiley New York, 2002, pp. 1–4.
- Delaquis, P. J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial Activity of Individual and Mixed Fractions of Dill, Cilantro, Coriander and Eucalyptus Essential Oils. Int. J. Food Microbiol. 2002, 74, 101–109.
- John, K. M. M.; Bhagwat, A. A.; Luthria, D. Swarm Motility Inhibitory and Antioxidant Activities of Pomegranate Peel Processed under Three Drying Conditions. Food Chem. 2017, 235, 145–153. DOI: 10.1016/j.foodchem.2017.04.143.
- Fischer, U. A.; Carle, R.; Kammerer, D. R. Identification and Quantification of Phenolic Compounds from Pomegranate (Punica Granatum L.) Peel, Mesocarp, Aril and Differently Produced Juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. DOI: 10.1016/j.foodchem.2010.12.156.
- Calín-Sánchez, Á.; Figiel, A.; Hernández, F.; Melgarejo, P.; Lech, K.; Carbonell-Barrachina, Á. A. Chemical Composition, Antioxidant Capacity, and Sensory Quality of Pomegranate (Punica Granatum L.) Arils and Rind as Affected by Drying Method. Food. Bioproc. Tech. 2013, 6, 1644–1654. DOI: 10.1007/s11947-012-0790-0.
- Wrolstad, R. E.; Acree, T. E.; Decker, E. A.; Penner, M. H.; Reid, D. S.; Schwartz, S. J.;Shoemaker, C. F.; Smith, D. M.; Sporns, P. Unit I1: Polyphenolics. In Handbook of Food Analytical Chemistry Volume 2: Pigments, Colorants, Flavors, Texture, and Bioactive Food Components, Wiley Interscience: New Jersey, 2005, pp. 5–70.
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial Activity of 10 Different Plant Polyphenols against Bacteria Causing Food-Borne Disease. Biol. Pharm. Bull. 2004, 27, 1965–1969.
- Shan, B.; Cai, Y. Z.; Brooks, J.; Corke, H. The in Vitro Antibacterial Activity of Dietary Species and Medicinal Herb Extracts. Int. J. Food Microbiol. 2007, 117, 112–119. DOI: 10.1016/j.ijfoodmicro.2007.03.003.
- Li, G.; Yan, C.; Xu, Y.; Feng, Y.; Wu, Q.; Lu, X.; Yang, B.; Wang, X.; Xia, X. Punicalagin Inhibits Salmonella Virulence Factors and Has Anti-Quorum-Sensing Potential. Appl. Environ. Microbiol. 2014, 80, 6204–6211. DOI: 10.1128/AEM.01458-14.
- De, R.; Sarkar, A.; Ghosh, P.; Ganguly, M.; Karmakar, B. C.; Saha, D. R.; Halder, A.; Chowdhury, A.; Mukhopadhyay, A. K. Antimicrobial Activity of Ellagic Acid against Helicobacter Pylori Isolates from India and during Infections in Mice. J. Antimicrob. Chemother. 2018, 73, 1595–1603. DOI: 10.1093/jac/dky079.
- Panichayupakaranant, P.;. Antibacterial Activity of Ellagic Acid-Rich Pomegranate Rind Extracts. Planta Med. 2010, 76, P408. DOI: 10.1055/s-0030-1264706.