ABSTRACT
The effects of hot water bath and steam pretreatments were investigated, considering color, anthocyanin profile and antioxidant activity of blueberry (Vaccinium ashei) juice. The juice maximum total phenolic content, anthocyanin, and antioxidant activity were observed using the steam pretreatment processing method. Eleven anthocyanins were isolated from the blueberry juice of steam treatment processing, while only 10 from the hot water bath and 8 from the control sample. After storage at 40°C for 10 days, the anthocyanin retention rate of the juice from steam treatment processing was higher than in that from hot water bath and control sample (30.71%, 23.77%, and 19.91%, respectively). The antioxidant capacity was also significantly higher and the hue angle (H°) was lower in the juice from steam pretreatment processing. Steam pretreatment process could prevent color deterioration, increase variety and content of anthocyanins, and improve antioxidant activity in the blueberry juice.
Introduction
Blueberry (genus Vaccinium, family Ericaceae) is a fruit native to North America, where it is widely cultivated and commercialized. Blueberry fruit with a dark-blue color is rich in health promoting compounds, including phenolic compounds such as anthocyanins, flavonoids, and tannins.[Citation1,Citation2]Anthocyanins and other phenolic compounds in blueberries can protect organisms against oxidative stress induced by free radicals and exhibit a wide range of health-promoting effects.[Citation3–Citation5] To extend the fruit’s commercial life, blueberries can be further processed into products such as jams and juices. Blueberry juice is increasingly promoted and consumed for its nutritional and health benefits.[Citation6,Citation7]
An attractive color is not only a main sensory characteristic of fruit products but also correlates with nutritional quality. Anthocyanin content is also an important indicator of nutritional value. However, anthocyanins are unstable and easily degraded.[Citation8,Citation9] Generally, the processing of berry juices alters the juice color and the content of bioactive compounds.[Citation10] Storage may further degrade anthocyanins and may affect antioxidant capacities in juice.[Citation11–Citation13]
Previous studies showed that a blanching pretreatment process could remarkably change the anthocyanins and phenolic contents of fruits and vegetables.[Citation14,Citation15] Blanching of fruits before making juice was effective in improving stability and recovery of bioactive phenolic compounds.[Citation15,Citation16] Blanched fruits and vegetables also held their attractive color by inactivating oxidative enzymes, more so than non-blanched ones.[Citation15,Citation17] In a previous study on blueberry juice processing, blanching pretreatment along the processing chain could improve extraction and stability of pigments.[Citation18,Citation19] However, to date, there is no report on whether the varieties of monomeric anthocyanins and its storage stability could be increased by hot water bath and steam pretreatments processing.
In this work, blueberry fruits (Vaccinium ashei) were subjected to steam pretreatment processing, hot water bath pretreatment processing, or without thermal pretreatments before pressing into juice. The anthocyanin content and varieties, color, total phenolic content, and antioxidant capacities of the blueberry juices were analyzed; furthermore, storage stability of the juices was investigated. These results will elucidate the relationship between pretreatment method and bioactive compound content in the processing of the blueberry juice process.
Materials and methods
Plant material
Rabbiteye blueberry (Vaccinium ashei) cultivar ‘Baldwin’ was handpicked on July 5, 2015, from a commercial plantation located in Hefei (31°52ʹN, 117°17ʹE) in central eastern China. Fruits were of uniform size and at physiological maturity. Blueberries were collected and quickly frozen (T = −40°C; air speed = 4.5 m/s; bed height = 3.6 cm). All fruits were stored at −20°C.
Standards and chemicals
The cyanidin-3-glucoside (C3G) standard, 2, 2-diphenyl-1-picrylhydrazyl (DPPH), and 2, 4, 6-Tris (2-pyridyl)-1, 3, 5-triazine (TPTZ) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The total antioxidant capacity (T-AOC) assay kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Other general chemicals with analytical grade were obtained from local suppliers.
Juice processing and storage
Frozen blueberry fruits were thawed at room temperature. Fruit was subjected to different pretreatments process (steam, hot water bath, and control) for 0, 1, 2, 3, 4, 5, or 6 min and then cooled to room temperature. Steam pretreatment processing was performed with one layer in a food steamer at atmospheric pressure. Hot water bath pretreatment processing was performed in thermostat water bath cauldron at 95ºC. Berries were then crushed and treated with pectinase (Laffort, Sydney, Australia) at a concentration of 0.05 g/kg fruit for 2 h at room temperature. The mixture was pressed with 200 mesh silk cloth and centrifuged at 3100 × g for 15 min to obtain blueberry juice. A pasteurization step (85°C, 5 min) was provided at the end of the process. Accelerated storage experiments of juices from different pretreatments process were carried out at 40°C for 10 days.[Citation20] Yields of blueberry juices were calculated according to the following equation:
Total phenolic content (TPC)
The TPC was measured according to Folin–Ciocalteu method.[Citation21] The absorbance of the sample was determined at 765 nm. The results were presented as mg gallic acid equivalents (GAE) per one liter of juice (mg GAE/L).
Total anthocyanin content (TAC) and the retention rate of TAC
The pH differential method was used to estimate the TAC of the juices.[Citation22] Aliquots of each sample were diluted with pH 1.0 or pH 4.5 buffers to the same dilution. The absorbance was measured at 510 nm and 700 nm. The TAC and retention rate of TAC (%) were respectively calculated using the following formulas:
Where A is the difference in absorbance between pH 1.0 and 4.5, MW is the molecular weight of cyanidin-3-glucoside (449 g/mol); DF is the dilution factor; Ve is the extract volume, is the molar extinction coefficient of cyanidin-3-glucoside (29, 600), and M is the mass of the blueberry extracted. Total anthocyanin content (TAC) was expressed as mg cyanidin-3-glucoside (C3G) equivalents per one liter of juice (mg C3G/L). Further, TACt is the total anthocyanin content of storage for t day; TAC0 is the total anthocyanin content of storage for 0 day. Retention rate of TAC (%) expresses the retention rate of total anthocyanin content.
Analysis of individual anthocyanins by UPLC
Anthocyanin profiles in blueberry juice were analyzed using an Acquity UPLC system (Waters, Milford, US). The chromatographic system consisted of a pump, a Phenomenex C18 column (2.1 × 150 mm, 2.6 μm; FLM Scientific Instrument Co., Ltd., Guangzhou, China), and a Waters Tunable UV detector. The sample was filtered through 0.45 μm nylon membranes prior to UPLC analysis. The mobile phase consisted of an aqueous 6% formic acid (A) and acetonitrile containing 6% formic acid (B). The gradient elution program was as follows: 5–50% B (0–20 min), 50–5% B (20–22 min), and 5% B (22–25 min). The injection volume was 5 μL. The flow rate and column temperature were 0.3 mL/min and 30°C, respectively. Anthocyanins were analyzed by absorbance detection at 520 nm. All of the monomeric anthocyanins were quantified as C3G equivalents.
Anthocyanins identification by UPLC-DAD-ESI/MS
UPLC-DAD-ESI/MS was used to identify compounds extracted from control (without thermal pretreatment processing), hot water bath pretreatment processing and steam pretreatment processing juices. The chromatographic system consisted of an Acquity UPLC (Waters, Milford, US) equipped with a Diode Array Detector (DAD) and a Triple Quadrupole Mass Spectrometer. Samples were separated using a Phenomenex C18 column (2.1 × 150 mm, 2.6 μm; FLM Scientific Instrument Co., Ltd., Guangzhou, China). The column temperature was maintained at 30°C. The sample was filtered through 0.45 μm nylon membranes prior to UPLC analysis. The injection volume was 5 μL. The flow rate was 0.3 mL/min. The mobile phase consisted of an aqueous 0.2% formic acid (A) and acetonitrile containing 0.2% formic acid (B). The gradient program was as follows: 5–50% B (0–10 min), 50–5% B (10–11 min), and 5% B (11–14 min). Equilibrium time between runs was 10 min. Anthocyanins were detected at 520 nm. The conditions of MS analysis were as follows: electrospray ionization (ESI) interface, nitrogen drying, nebulizing gas and nebulizer pressure at 35 psi, dry gas flowing at 10 mL/min, 350ºC dry gas temperature, capillary voltage at 2500 V, and spectra recording in the positive ion mode with scans from m/z 10 to 1000.
Antioxidant activity
Antioxidant capacity was measured in terms of free radical scavenging capacity (DPPH), ferric reducing antioxidant power (FRAP), total antioxidant activity (T-AOC) and oxygen radical absorbance capacity (ORAC). A modified DPPH assay and a FRAP assay (Zhang, Li, Gao, 2016) were conducted, and the former results expressed as DPPH (%) were assessed by using the method described previously the latter was expressed as mmol ferrous ions per liter of juice (mmol Fe2+/L). T-AOC was determined by kit and expressed as T-AOC units per milliliter juice (U/mL).[Citation23]
The ORAC assay was performed according to the previous methods, with a slight modification. Briefly, juices from different pretreatments were diluted to 1: 50, 1: 100, 1: 200 and 1: 400 dilutions, respectively, with 75 mM pH7.4 phosphate buffer solution (PBS).[Citation24,Citation25] The 25 μL antioxidant (Trolox solution or diluted sample) was transferred to 96-well black polystyrene plate (Corning, America) containing 150μL 8.16 × 10−5 mM fluorescein solution. The plate was shaken for 2 min and after that incubated for 10 min at 37°C. Subsequently, 25 μL 153 mM AAPH solution was added, and the fluorescence reduction reaction was instantly started. Fluorescence intensity was measured every 2 min by a microplate reader (Molecular Devices SpectraMax M2e, California, USA) with 485 nm excitation wavelength and 530 nm emission wavelength. The plate was vibrated for 10 min before each determination. The measurement time was within 140 min. Instead of antioxidant, PBS was added in AAPH+ group, and instead of antioxidant and APPH, PBS was carried out in APPH-group. Final ORAC values were expressed as μmol Trolox equivalents per milliliter of juice (μmol TE/mL).
Color measured
Color was measured with ColorQuest XE (Hunter Associates Laboratory Inc., Reston, VA, USA) (the 10° Standard Observer and Standard Illuminant D65). The parameters were lightness (L*), redness (a*) and yellowness (b*). The hue angle is expressed as:
Statistical analysis
Values were given as the means ± the standard deviation (SD) of triplicate experiments. Statistical analysis was performed with Prism™ v6.0 software. Means were further compared using Duncan’s range, and the differences were regarded as significant when P < .05.
Results and discussion
Juice yield, TPC and TAC of blueberry juices
The effect of thermal pretreatments processing on juice yield, TPC and TAC of blueberry juice was shown in . The results showed that juice yields improved with an increase of the pretreatments time (). The juice yield of control juice (0 min) was 68%. It was observed that hot water bath treatment and steam treatment process could increase juice yields, and steam pretreatment processing juice had higher yields than hot water bath juice. The juice yield of steam treatment juice increased to 78% at 3 min, while hot water bath treatment juice increased to a maximum of 71% at 5 min. Juice yield is one of the most important aspects determining the profitability of juice productions.[Citation26] It was reported that yields of five high-bush blueberry juices using steam blanching ranged from 68% to 72%.[Citation18]
Figure 1. Juice yield, total phenolic content (TPC), and anthocyanin content (TAC) of juices pressed from blueberries that were blanched by steaming or hot water bath for 0, 1, 2, 3, 4, 5, or 6 min. Different upper cases in the same series represent significant differences within one pretreatment group over time (P < .05). Error bar indicates mean value ± SD (n = 3)
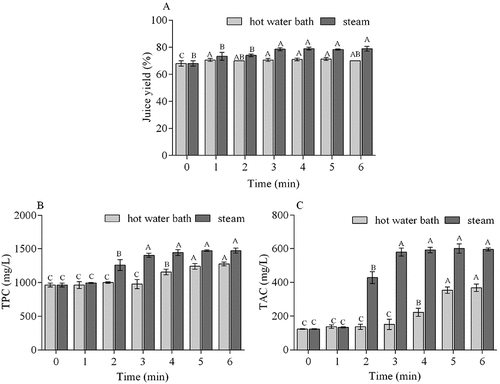
The TPC and TAC from steam and hot water bath pretreatment processing juices were observed ( and C). Significant (P < .05) increases in TPC and TAC was observed when steam treatment time increased from 0 to 3 min but did not significantly change after 3 min. Steam treatment for 3 min resulted in TPC and TAC values of 1471.26 mg GAE/L and 588.87 mg C3G/L, respectively. Meanwhile, significant (P < .05) increases of TPC and TAC were observed when hot water bath time increased from 0 to 5 min, but they did not significantly increase at 5 or 6 min. Hot water bath for 5 min resulted in average TPC and TAC values of 1240.20 mg GAE/L and 317.07 mg C3G/L, respectively. Compared with hot water bath treatment, steam pretreatment process could improve anthocyanin and phenolic content in blueberry juice. Several reporters have shown that heat pretreatment processing increased the extraction of anthocyanins and other phenolic compounds and color density of fruits.[Citation19,Citation27] And a positive correlation between blanching time and bioactive compound content has been confirmed.[Citation28]
Anthocyanins and phenols around blueberry are mainly distributed in the peel of fruits. The steam or hot water bath pretreatments processing could increase permeability of membranes in the peel tissue, which will facilitate the extraction of active components from the peel of blueberry; and subjecting to steam or hot water bath pretreatments process, the polyphenol oxidase (PPO) derived from blueberry fruits could be inactivated, which would bring helpful result of the reduction of enzyme-mediated anthocyanin degradation.[Citation19,Citation29,Citation30] Researches have suggested that a reasonable blanching process can cause the inactivation of oxidative enzymes, and thus leading to high retention of total vitamin C of vegetable or frozen fruit.[Citation31,Citation32]
Monomeric anthocyanins profile and content in blueberry juices
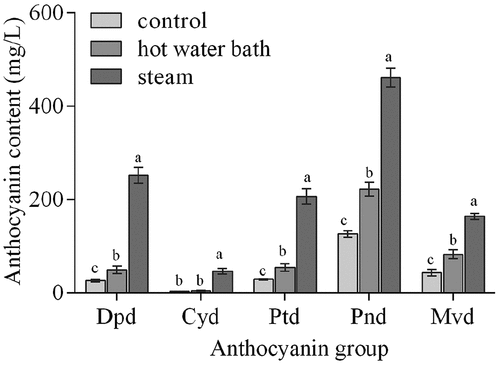
The different monomeric anthocyanins profile of blueberry juices were given in Figure. S1 and . Eleven and 10 monomeric anthocyanins were identified in steam pretreatment processing juice and hot water bath pretreatment processing juice, respectively, against 8 in control juice. Delphinidin-3-galactoside and peonidin-3-galactoside were found in hot water bath treatment juice, delphinidin-3-galactoside, cyanidin-3-galactoside, and peonidin-3-galactoside were discovered in steam treatment juice, while those monomeric anthocyanins were not found in control juice. There are five major classes of monomeric anthocyanins in blueberries, cyanidin glycosides (Cyd), delphinidin glycosides (Dpd), peonidin glycosides (Pnd), malvidin glycosides (Mvd) and petunidin glycosides (Ptd). Similar results have been reported in Prior et al. and You et al.[Citation33,Citation34]
Table 1. LC–MS data of anthocyanins in juice pressed from control, hot water bath (5 min) and steam (3 min) blanching blueberries
The monomeric anthocyanin content of juices from different pretreatments was significantly different (). The results demonstrated that Pnd were the most abundant compounds in all of the samples, followed by Mvd and Dpd. The content of five major anthocyanin glycosides in steam pretreatment juice was higher than juice from hot water bath treatment and control sample. Anthocyanins are rich in the skin of blueberries.[Citation35] The type and content of anthocyanins in fruit juices are influenced by fruit varieties and processing techniques.[Citation18,Citation34,Citation36] Pan et al. concluded that delphinidin-3-glucoside, petunidin-3-glucoside, and malvidin-3-glucoside were major anthocyanins in blueberry juice.[Citation37] Heat treatment increased permeability and the ability of anthocyanins diffusing into the liquid portion.[Citation19]
Figure 2. Contents of individual anthocyanins in juices from control, hot water bath (5 min) and steam blanching (3 min) on the day of pressing (0 day). Different lowercase letters represent significant differences between three pretreatment groups (P < .05). Error bar indicates mean value ± SD (n = 3). Dpd: Delphinidin glycosides; Cyd: Cyanidin glycosides; Ptd: Petunidin glycosides; Pnd: Peonidin glycosides; Mvd: Malvidin glycosides
TAC and TAC retention rate of blueberry juices during storage
TAC and TAC retention rate of juices from different fruit pretreatments during storage was shown in . The TAC of three samples decreased rapidly (). Declining rate was the fastest for control juice, followed by juice from hot water bath treatment and steam treatment juice. At 10 d of storage, the TAC was 24.96 mg C3G/L and 73.74 mg C3G/L for control juice and hot water bath treatment juice, respectively, against 181.09 mg C3G/L for steam treatment juice. The results showed that steam treatment processing juice maintained high TAC content during storage. Anthocyanins have low stability and easily degraded. The loss of TAC during storage might be attributed to comprehensive effects of pH, light, temperature, concentration, enzymes, and oxygen.[Citation37,Citation38] Hellstrom et al. reported that the stability of anthocyanins was affected by type of anhocyanins, origin of the juice, especially storage temperture.[Citation12]
Figure 3. The total anthocyanin content (TAC) and retention rate of TAC (%) in juices from control, hot water bath (5 min) and steam (3 min) blanching blueberries during storage. Different uppercase letters represent significant differences within one pretreatment group over storage time, and different lowercase letters represent significant differences between three pretreatment groups on the same day (P < .05). Error bar indicates mean value ± SD (n = 3)
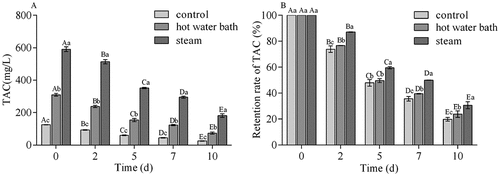
Effect of thermal pretreatments processing on TAC retention rate was predominant during storage (). The TAC retention rate of three juices decreased during storage. At 10 d, TAC retention rate was 23.77% for hot water bath treatment juice and 30.71% for steam treatment juice, as compared to 19.91% for control juice. Higher anthocyanin retention rate was observed in steam treatment juice than in control juice and hot water bath treatment juice. Skrede et al. revealed that the large loss of anthocyanins during blueberry juice processing was related to the action of native PPO.[Citation39] It has been reported earlier that natural enzymes activity was inhibited after blanching, and the degradation of anthocyanins became slow.[Citation8,Citation29,Citation40]
TPC and antioxidant capacity of blueberry juices during storage
The TPC of all juices was significantly reduced during storage (). After storage for 10 d, the highest TAC was found in the juice from steam pretreatment processing berries (181.09 mg C3G/L), while TAC of juices were low in the hot water bath treatment and control samples (73.74 mg C3G/L and 24.96 mg C3G/L, respectively). Mendes Lopes et al. evaluated the stability of steam-extracted grape juice during storage and observed that steam extraction resulted in higher soluble phenol.[Citation41]
Figure 4. The total phenolic content (TPC) and antioxidant capacity in juices from control, hot water bath (5 min) and steam (3 min) blanching blueberries during storage. Different uppercase letters represent significant differences within one pretreatment group over storage time, and different lowercase letters represent significant differences between three pretreatment groups on the same day (P < .05). Error bar indicates mean value ± SD (n = 3). DPPH: free radical-scavenging capacity; FRAP: ferric reducing antioxidant power; T-AOC: total antioxidant capacity; ORAC: oxygen radical absorbance capacity
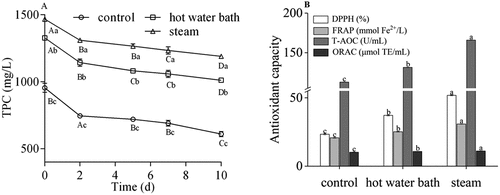
shows the antioxidant capacity of three juices over 10 d of storage. The DPPH was 37.16% and 51.81% for hot water bath treatment juice and steam treatment juice, while 23.38% for control juice. The FRAP of control and hot water bath treatment juice were 20.73 and 25.21 mmol Fe2+/L, against 30.81 mmol Fe2+/L for steam treatment juice. The T-AOC was 112.85, 131.35 and 165.88 U/mL for control juice, hot water bath treatment juice, and steam treatment juice, respectively. The ORAC was 10.74 μmol TE/mL for hot water bath treatment juice and 11.07 μmol TE/mL for steam treatment juice, as compared to 10.18 μmol TE/mL for control juice. Steam pretreatment processing juice showed higher antioxidant capacity than other two juices. The DPPH, FRAP, T-AOC, and ORAC of blueberry juice were significantly increased via steam treatment juice. Improvement of antioxidant capacity might be attributed to additional quenching of active oxygen species.[Citation42] Steam blanching has been reported as an extraction method for improving antioxidant capacity in raw garlic and olive leaves.[Citation43,Citation44] The antioxidant capacity of blueberry was related to its anthocyanin and total phenolic content.[Citation29,Citation45]
Color of blueberry juices during storage
Color parameters of juices, i.e., Lightness (L*) and Hue (H°) during storage were reported in . The L* and H° value are the indicators of darkening and browning, respectively.[Citation46] The values of L* and H° were observed to decrease in juices after hot water bath treatment juice and steam treatment juice. The L* and H° values were low in steam pretreatment juice, reflecting the dark and red color after steam treatment processing.[Citation37] The blanching of the stem before extrusion effectively prevented browning of fresh sugar cane juice at storage period.[Citation47]
Table 2. Color parameters of lightness (L*) and hue angle (H°) of stored juice pressed from control, hot water bath (5 min) and steam (3 min) blanching blueberries
Color appearance of juices varied significantly before and after storage (Figure S3). The color of three blueberry juices showed different fading extents during storage. The visual color appearance of steam treatment processing juice changed slowly, which indicated that steam treatment juice had good color stability. In this work, the color of blueberry juices kept deteriorating during storage, which was a link with anthocyanin retention. Anthocyanins have low stability and easily be degraded by heat, oxygen, pH, light, non-covalent interactions, and covalent reactions with other compounds; between the monomeric anthocyanins or the anthocyanin, and the other flavonoids in the juice can polymerize to turn into polymeric anthocyanins and led to the color change of products.[Citation37,Citation38,Citation48–Citation50]
Color deterioration was an important factor reflecting a quality defect in fruit products during the storage period.[Citation51] The color of packaged juice determined the consumer’s first impression directly, affecting the desire to purchase or consume the product.[Citation52] Fang et al. reported that the blanching process was beneficial to color stability of bayberry juice and other anthocyanin-containing products.[Citation53]
Conclusion
Blueberry juice via steam pretreatment processing contained 11 anthocyanins, more than juice from hot water bath pretreatment processing (10 anthocyanins) or without thermal pretreatment processing (8 anthocyanins) blueberries. Blueberry juice from steam pretreatment process significantly increased the content of total anthocyanins and total phenols. During storage, better color quality, more anthocyanins, and stronger antioxidant activity were found in juices from steam treatment blueberries compare with hot water bath and control sample. Therefore, steam pretreatment process could significantly delay color deterioration, improved the quality characteristics and increased antioxidant activity of blueberry juices.
Supplemental Material
Download MS Word (1.7 MB)Supplemental material
Supplementary data for this article can be accessed here.
Additional information
Funding
References
- Prior, R. L.; Cao, G. H.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant Capacity as Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium species.J. Agr. Food Chem. 1998, 46, 2686–2693. DOI: 10.1021/jf980145d.
- Giovanelli, G.; Brambilla, A.; Sinelli, N. Effects of Osmo-Air Dehydration Treatments on Chemical, Antioxidant and Morphological Characteristics of Blueberries. LWT–Food Sci. Tech. 2013, 54, 577–584. DOI: 10.1016/j.lwt.2013.06.008.
- Cho, M. J.; Howard, L. R.; Prior, R. L.; Clark, J. R. Flavonoid Glycosides and Antioxidant Capacity of Various Blackberry, Blueberry and Red Grape Genotypes Determined by High-Performance Liquid Chromatography/Mass Spectrometry. J. Sci. Food Agr. 2004, 84, 1771–1782. DOI: 10.1002/jsfa.1885.
- Siddiq, M.; Dolan, K. D.; Perkins-Veazie, P.; Collins, J. K. Effect of Pectinolytic and Cellulytic Enzymes on the Physical, Chemical, and Antioxidant Properties of Blueberry (Vaccinium Corymbosum,L.) Juice. LWT–Food Sci. Tech. 2018, 127–132. DOI: 10.1016/j.lwt.2018.02.008.
- Niu, S. Y.; Hao, F. G.; Mo, H. Z.; Jiang, J. F.; Wang, H.; Liu, C. H.; Fan, X. C.; Zhang, Y. Phenol Profiles and Antioxidant Properties of White Skinned Grapes and Their Coloured Genotypes during Growth. Biotechnol. Biotec. Eq. 2017, 31, 1–10. DOI: 10.1080/13102818.2016.1258329.
- Michalska, A.; Łysiak, G. Bioactive Compounds of Blueberries: Post-Harvest Factors Influencing the Nutritional Value of Products. Int. J. Mol. Sci. 2015, 16, 18642–18663. DOI: 10.3390/ijms160818642.
- Diaconeasa, Z.; Leopold, L.; Rugina, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and Antioxidant Properties of Anthocyanin Rich Extracts from Blueberry and Blackcurrant Juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. DOI: 10.3390/ijms16022352.
- White, B. L.; Howard, L. R.; Prior, R. L. Impact of Different Stages of Juice Processing on the Anthocyanin, Flavonol, and Procyanidin Contents of Cranberries. J. Agr. Food Chem. 2011, 59, 4692–4698. DOI: 10.1021/jf200149a.
- Laokuldilok, T.; Kanha, N. Microencapsulation of Black Glutinous Rice Anthocyanins Using Maltodextrins Produced from Broken Rice Fraction as Wall Material by Spray Drying and Freeze Drying. J. Food Process. Pres. 2017, 41. DOI: 10.1111/jfpp.12877.
- Hager, T. J.; Howard, L. R.; Prior, R. L. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Blackberry Products. J. Agr. Food Chem. 2008, 56, 689–695. DOI: 10.1021/jf071994g.
- Brownmiller, C.; Howard, L. R.; Prior, R. L. Processing and Storage Effects on Procyanidin Composition and Concentration of Processed Blueberry Products. J. Agr. Food Chem. 2009, 57, 1896–1902. DOI: 10.1021/jf803015s.
- Hellstrom, J.; Mattila, P.; Karjalainen, R. Stability of Anthocyanins in Berry Juices Stored at Different Temperatures. J. Food Compos. Anal. 2013, 31, 12–19. DOI: 10.1016/j.jfca.2013.02.010.
- Qiu, G.; Wang, D.; Song, X.; Deng, Y.; Zhao, Y. Degradation Kinetics and Antioxidant Capacity of Anthocyanins in Air-Impingement Jet Dried Purple Potato Slices. Food Res. Int. 2018, 105, 121–128. DOI: 10.1016/j.foodres.2017.10.050.
- Giuseppe, G.; Roberta, T.; Pietro, T. Berry Ripening, Pre‐Processing and Thermal Treatments Affect the Phenolic Composition and Antioxidant Capacity of Grape (Vitis Vinifera L.) Juice. J. Sci. Food Agr. 2016, 96, 664–671. DOI: 10.1002/jsfa.7138.
- Xin, Y.; Zhang, M.; Xu, B.; Adhikari, B.; Sun, J. Research Trends in Selected Blanching Pretreatments and Quick Freezing Technologies as Applied in Fruits and Vegetables: A Review. Int. J. Refrig. 2015, 57, 11–25. DOI: 10.1016/j.ijrefrig.2015.04.015.
- Saldivar, X.; Wang, Y. J.; Chen, P.; Mauromoustakos, A. Effects of Blanching and Storage Conditions on Soluble Sugar Contents in Vegetable Soybean. LWT-Food Sci. Tech. 2010, 43, 1368–1372. DOI: 10.1016/j.lwt.2010.04.017.
- Aguero, M. V.; Ansorena, M. R.; Roura, S. I.; Del Valle, C. E. Thermal Inactivation of Peroxidase during Blanching of Butternut Squash. LWT–Food Sci. Tech. 2008, 41, 401–407. DOI: 10.1016/j.lwt.2007.03.029.
- Brambilla, A.; Scalzo, R. L.; Bertolo, G.; Torreggiani, D. Steam-Blanched Highbush Blueberry (Vaccinium Corymbosum L.) Juice: Phenolic Profile and Antioxidant Capacity in Relation to Cultivar Selection. J. Agr. Food Chem. 2008, 56, 2643–2648. DOI: 10.1021/jf0731191.
- Rossi, M.; Giussani, E.; Morelli, R.; Scalzo, R. L.; Nani, R. C.; Torreggiani, D. Effect of Fruit Blanching on Phenolics and Radical Scavenging Activity of Highbush Blueberry Juice. Food Res. Int. 2003, 36, 999–1005. DOI: 10.1016/j.foodres.2003.07.002.
- Fan, L. H.; Martynenko, A.; Doucette, C.; Hughes, T.; Fillmore, S. Microbial Quality and Shelf Life of Blueberry Purée Developed Using Cavitation Technology. J. Food Sci. 2018, 83, 732–739. DOI: 10.1111/1750-3841.14073.
- Singleton, V. L.; Orthofer, R.; Lamuela-Raventós, R. M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178.
- Lee, S. G.; Vance, T. M.; Nam, T. G.; Kim, D. O.; Koo, S. I.; Chun, O. K. Evaluation of Ph Differential and HPLC Methods Expressed as Cyanidin-3-Glucoside Equivalent for Measuring the Total Anthocyanin Contents of Berries. J. Food Meas. Charact. 2016, 10, 562–568. DOI: 10.1007/s11694-016-9337-9.
- Zhang, L. L.; Li, N.; Gao, X. L. Phenolic Compounds and Antioxidant Activity of Wines Fermented Using Ten Blueberry Varieties. Am. J. Food Technol. 2016, 11, 291–297. DOI: 10.3923/ajft.2016.291.297.
- Huang, D. J.; Ou, B. X.; Hampsch-Woodill, M.; Flanagan, J. A.; Prior, R. L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agr. Food Chem. 2002, 50, 4437–4444. DOI: 10.1021/jf0201529.
- Monagas, M.; Garrido, I.; Lebrónaguilar, R.; Bartolome, B.; Gómezcordovés, C. Almond ((Prunus Dulcis (Mill.) D.A. Webb) Skins as a Potential Source of Bioactive Polyphenols. J. Agr. Food Chem. 2007, 55, 8498–8507. DOI: 10.1021/jf071780z.
- Bobinaite, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of Pulsed Electric Field in the Production of Juice and Extraction of Bioactive Compounds from Blueberry Fruits and Their By-Products. J. Food Sci. Tech. 2015, 52, 5898–5905. DOI: 10.1007/s13197-014-1668-0.
- Liu, S.; Chang, X.; Liu, X.; Shen, Z. Effects of Pretreatments on Anthocyanin Composition, Phenolics Contents and Antioxidant Capacities during Fermentation of Hawthorn (Crataegus Pinnatifida) Drink. Food Chem. 2016, 212, 87–95. DOI: 10.1016/j.foodchem.2016.06.011.
- Lambri, M.; Torchio, F.; Colangelo, D.; Segade, S. R.; Giacosa, S.; De Faveri, D. M.; Gerbi, V.; Rolle, L. Influence of Different Berry Thermal Treatment Conditions, Grape Anthocyanin Profile, and Skin Hardness on the Extraction of Anthocyanin Compounds in the Colored Grape Juice Production. Food Res. Int. 2015, 77, 584–590. DOI: 10.1016/j.foodres.2015.08.027.
- Kalt, W.; Mcdonald, J. E.; Donner, H. Anthocyanins, Phenolics, and Antioxidant Capacity of Processed Lowbush Blueberry Products. J. Food Sci. 2000, 65, 390–393. DOI: 10.1111/j.1365-2621.2000.tb16013.x.
- Spanos, G. A.; Wrolstad, R. E., . Influence of Processing and Storage on the Phenolic Composition of Thompson Seedless Grape Juice. J. Agr. Food Chem.. 1990, 38, 1572–1589. DOI: 10.1021/jf00097a031.
- Epameinondas, X.; Eleni, G.; Petros, T.; Lilia, A. Effect of Microwave Assisted Blanching on the Ascorbic Acid Oxidase Inactivation and Vitamin C Degradation in Frozen Mangoes. Innov. Food Sci. Emerg. Technol. 2018, 48, 248–257. DOI: 10.1016/j.ifset.2018.06.012.
- Munyaka, A. W.; Oey, I.; Loey, A. V.; Hendrickx, M. Application of Thermal Inactivation of Enzymes during Vitamin C Analysis to Study the Influence of Acidification, Crushing and Blanching on Vitamin C Stability in Broccoli (Brassica Oleracea L Var. Italica). Food Chem. 2010, 120, 591–598. DOI: 10.1016/j.foodchem.2009.10.029.
- Prior, R. L.; Lazarus, S. A.; Cao, G.; Muccitelli, H.; Hammerstone, J. F. Identification of Procyanidins and Anthocyanins in Blueberries and Cranberries (Vaccinium Spp.) Using High-Performance Liquid Chromatography/Mass Spectrometry. J. Agr. Food Chem. 2001, 46, 2686–2693. DOI: 10.1021/jf980145d.
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P. G. Comparison of Anthocyanins and Phenolics in Organically and Conventionally Grown Blueberries in Selected Cultivars. Food Chem. 2011, 125, 201–208. DOI: 10.1016/j.foodchem.2010.08.063.
- Kim, H.; Bartley, G. E.; Rimando, A. M.; Yokoyama, W. Hepatic Gene Expression Related to Lower Plasma Cholesterol in Hamsters Fed High-Fat Diets Supplemented with Blueberry Peels and Peel Extract. J. Agr. Food Chem. 2010, 58, 3984–3991. DOI: 10.1021/jf903230s.
- Jiang, B.; Mantri, N.; Hu, Y.; Lu, J.; Jiang, W.; Lu, H. Evaluation of Bioactive Compounds of Black Mulberry Juice after Thermal, Microwave, Ultrasonic Processing, and Storage at Different Temperatures. Food Sci. Tech. Int. 2015, 21, 392–399. DOI: 10.1177/1082013214539153.
- Pan, Y. Z.; Guan, Y.; Wei, Z. F.; Peng, X.; Li, T. T.; Qi, X. L.; Zu, Y. G.; Fu, Y. J. Flavonoid C -Glycosides from Pigeon Pea Leaves as Color and Anthocyanin Stabilizing Agent in Blueberry Juice. Ind. Crop Prod. 2014, 58, 142–147. DOI: 10.1016/j.indcrop.2014.04.029.
- Reque, P. M.; Steffens, R. S.; Jablonski, A.; Flores, S. H.; Rios, A. D. O.; De Jong, E. V. Cold Storage of Blueberry (Vaccinium Spp.) Fruits and Juice: Anthocyanin Stability and Antioxidant Activity. J. Food Compos. Anal. 2014, 33, 111–116. DOI: 10.1016/j.jfca.2013.11.007.
- Skrede, G.; Wrolstad, R. E.; Durst, R. W. Changes in Anthocyanins and Polyphenolics during Juice Processing of Highbush Blueberries (Vaccinium Corymbosum L.). J. Food Sci. 2000, 65, 357–364. DOI: 10.1111/j.1365-2621.2000.tb16007.x.
- Lee, J.; Durst, R. W.; Wrolstad, R. E. Impact of Juice Processing on Blueberry Anthocyanins and Polyphenolics: Comparison of Two Pretreatments. J. Food Sci. 2002, 67, 1660–1667. DOI: 10.1111/j.1365-2621.2002.tb08701.x.
- Mendes Lopes, M. L.; Lemos Miguel, M. A.; Fialho, E.; Valente‐Mesquita, V. L. Grape Juice Obtained Using Steam Extraction and Other Small‐Scale Extraction Methods: Phenolic Content, Antioxidant Capacity and Stability during Storage. Int. J. Food Sci. Tech. 2016, 51, 1696–1702. DOI: 10.1111/ijfs.13144.
- Sahin, S.; Demir, C. Determination of Antioxidant Properties of Fruit Juice by Partial Least Squares and Principal Component Regression. Int. J. Food Prop. 2016, 19, 1455–1464. DOI: 10.1080/10942912.2014.968791.
- Romero-Garcia, J. M.; Lama-Munoz, A.; Rodriguez-Gutierrez, G.; Moya, M.; Ruiz, E.; Fernandez-Bolanos, J.; Castor, E. Obtaining Sugars and Natural Antioxidants from Olive Leaves by Steam-Explosion. Food Chem. 2016, 210, 457–465. DOI: 10.1016/j.foodchem.2016.04.113.
- Noda, Y.; Asada, C.; Sasaki, C.; Hashimoto, S.; Nakamura, Y. Extraction Method for Increasing Antioxidant Activity of Raw Garlic Using Steam Explosion. Biochem. Eng. J. 2013, 73, 1–4. DOI: 10.1016/j.bej.2013.01.013.
- Castrejón, A. D. R.; Eichholz, I.; Rohn, S.; Kroh, L. W.; Huyskens-Keil, S. Phenolic Profile and Antioxidant Activity of Highbush Blueberry (Vaccinium Corymbosum L.) During Fruit Maturation and Ripening. Food Chem. 2008, 109, 564–572. DOI: 10.1016/j.foodchem.2008.01.007.
- Conner, P. J.; MacLean, D. Fruit Anthocyanin Profile and Berry Color of Muscadine Grape Cultivars and Muscadinia Germplasm. Hortscience. 2013, 48, 1235–1240. DOI: 10.21273/HORTSCI.48.10.1235.
- Mao, L. C.; Xu, Y. Q.; Que, F. Maintaining the Quality of Sugarcane Juice with Blanching and Ascorbic Acid. Food Chem. 2007, 104, 740–745. DOI: 10.1016/j.foodchem.2006.09.055.
- West, M. E.; Mauer, L. J. Color and Chemical Stability of a Variety of Anthocyanins and Ascorbic Acid in Solution and Powder Forms. J. Agr. Food Chem. 2013, 61, 4169–4179. DOI: 10.1021/jf400608b.
- Casati, C. B.; Baeza, R.; Sanchez, V. Comparison of the Kinetics of Monomeric Anthocyanins Loss and Colour Changesin Thermally Treated Blackcurrant, Maqui Berry and Blueberrypulps from Argentina. J. Berry Res. 2017, 7, 85–96. DOI: 10.3233/JBR-170151.
- Rivas-Gonzalo, J. C.; Bravo-Haro, S.; Santos-Buelga, C. Detection of Compounds Formed through the Reaction of Malvidin 3-Monoglucoside and Catechin in the Presence of Acetaldehyde. J. Agr. Food Chem. 1995, 43, 1444–1449. DOI: 10.1021/jf00054a006.
- Buvé, C.; Kebede, B. T.; Batselier, C. D.; Carrillo, C.; Pham, H. T. T.; Hendrickx, M.; Grauwet, T.; Loey, A. V. Kinetics of Colour Changes in Pasteurised Strawberry Juice during Storage. J. Food Eng. 2017, 216, 42–45. DOI: 10.1016/j.jfoodeng.2017.08.002.
- Nowicka, P.; Wojdyło, A. Bioactive Compounds and Sensory Attributes of Sour Cherry Puree Sweetened with Natural Sweeteners. Int. J. Food Sci. Tech. 2015, 50, 585–591. DOI: 10.1111/ijfs.12685.
- Fang, Z.; Zhang, M.; Sun, Y.; Sun, J. How to Improve Bayberry (Myrica Rubra Sieb. Et Zucc.) Juice Color Quality: Effect of Juice Processing on Bayberry Anthocyanins and Polyphenolics. J. Agr. Food Chem. 2006, 54, 99–106. DOI: 10.1021/jf051943o.
