?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of this study was to evaluate pathogenic and spoilage bacteria counts, physicochemical properties of fresh chilled pork treated with chitosan, nisin, and tea polyphenols along with their combination during storage at 4°C for 11 days. The result showed that treatments with chitosan, nisin, and tea polyphenols alone significantly (P < .05) reduced bacteria population (total viable counts (TVC), lactic acid bacteria, pseudomonas spp., staphylococcus spp., brochothrix thermosphacta, and enterobacteriaceae) on fresh chilled pork. The TVC of sample treated with a combination of nisin, tea polyphenols, and chitosan (N-TP-C) decreased from 7.2 to 5.95 log10 CFU/g at 11th day. It was also found from TVB-N, TBARS, pH, color, water-holding capacity, and texture measurement that the N-TP-C combination treatment significantly maintained good quality and extended the shelf life of fresh chilled pork up to 11 days. In conclusion, it could be suggested that the combination with nisin, tea polyphenols, and chitosan has great potential to preserve fresh chilled pork.
Introduction
At present pork has occupied approximately 64% of the meat consumption in China[Citation1] and the consumption of chilled pork has increased rapidly because of its better flavor, the improvement of meat quality, particularly tenderness, water-holding capacity, and nutrition.[Citation2,Citation3]With the increasing desire for fresh pork with good quality, the chilled pork will represent a wide market prospect. However, meat and meat products, especially chilled pork, are vulnerable to spoilage due to pathogenic and spoilage microorganism contamination (i.e., lactic acid bacteria, pseudomonas spp., and enterobacteriaceae) during sales and preservation, which causes economic loss and shortening of shelf life.[Citation2,Citation4,Citation5] Therefore, the contamination of microorganisms prevented and controlled in chilled pork to extend shelf life are the crucial concern.
It is well-known that many kinds of chemicals will kill or significantly inhibit the growth of spoilage and pathogenic bacteria in food.[Citation6] Although many chemicals are permitted to use as an antimicrobial agent in meat and meat products preservation, the long-term ingestion of excessive dose will cause hepatotoxicity and a minority of chemicals will even cause carcinogenicity or teratogenicity. Therefore, natural products were used as alternative preservatives in meat preservation become the increasingly demand by consumers. It is essential to develop new technological approaches for meat preservation. Natural compounds, such as nisin, chitosan, polyphenols and essential oils, have been investigated to replace chemical preservatives to control microbial contamination and extend shelf-life of food.[Citation7–Citation10] Nisin, a bacteriocin produced by Lactococcus lactis subsp. Lactis, is the first commercial bacteriocin and has been used in fresh meat preservation with a “generally recognized as safe” label.[Citation10,Citation11] However, nisin only shows excellent inhibitory activity to a broad range of Gram-positive bacteria and rarely activity to Gram-negative bacteria, which limits application area of nisin.[Citation12] Similarly, tea polyphenols, a natural preservative with antimicrobial capacity extracted from tea leaves, are also used for meat preservation.[Citation9,Citation13] In addition, chitosan, which is extracted from crustacean shells, exhibits a distinctly inhibitory effect to a number of food spoilage and pathogenic microorganisms.[Citation14] Chitosan has been widely used as an antimicrobial agent to prolong the shelf life of meat products.[Citation8,Citation15] Some previous researches have revealed that combination of natural antimicrobial agents shows more efficient capacity in inhibiting bacteria of meat products.[Citation8,Citation16,Citation17] Recent studies have shown that nisin combined with other antimicrobials can act synergistically antibacterial effects as they have different modes of action in the target cells.[Citation18,Citation19]
The objective of this study is to investigate the preservative effects of nisin, tea polyphenols, and chitosan on chilled pork under refrigerated storage, and evaluate the possible effects of nisin, tea polyphenols and chitosan alone and their combination on bacterial counts, lipid peroxidation, total volatile basic nitrogen (TVB-N), surface color, water-holding capacity and texture properties of fresh chilled pork.
Materials and methods
Preparation of nisin, tea polyphenols, and chitosan antimicrobial solution
A 20 g/L nisin solution (activity of 1 × 106 IU/g; Zhejiang Silver Elephant Bioengineering Ltd., Zhejiang, China) and a 20 g/L tea polyphenols solution (shanghai yuanye) were prepared. Then, a 10 g/L Chitosan solution (deacetylation degree > 92%; shanghai Aladdin) was prepared by dissolving 1.0 g of Chitosan into 100 mL sterile-distilled water with 1% (v/v) glacial acetic acid and stirring for 30 min. composite solution was prepared as followed by Cao et al.,[Citation8] with some modification: nisin and tea polyphenols were dissolved with sterile-distilled water with a concentration of 1% (w/v), separately, and this solution was defined as solution A. Chitosan was dissolved with 1% (v/v) glacial acetic acid and was defined as solution B. Ultimately, composite solution by mixing equivoluminal solution A and solution B. All solutions were sterilized by filtration through 0.22 um filters and. stored at 4°C.
Preparation of fresh chilled pork
Fresh pork tenderloin material was purchased from local superstore (Xinxiang, Henan province, China) and iced transportation to the laboratory. After removing the intramuscular fat and visible connective tissues, the chilled pork was sliced into pieces with uniform size (6 cm × 6 cm × 2 cm) of approximately 30 g. All samples were dipped into different antimicrobial solutions including 0.5% nisin, 0.5% TPs (tea polyphenols), 0.5% chitosan and N-TP-Combination (0.2% nisin, 0.2% tea polyphenols, 0.1% chitosan) for 10 min, respectively. Each treatment was divided into three groups (replicates), 30 slices in each replicate. Six samples were dipped into sterile-distilled water for 10 min and used as control. Finally, all samples were drained off the water by suspended on the plastic strips for 10 min after antimicrobial solution treatments. All treated samples were placed in a commercialized transparent crisper and stored at 4°C for a period of 11 days. All treated samples were prepared for experiments and 5 slices per group were taken at 0, 1, 3, 5, 7, 9, 11 days for evaluating pathogenic and spoilage bacteria counts, pH, TVB-N, lipid peroxidation, color, water-holding capacity, texture values, and sensory evaluation.
Microbial analysis
Test samples were prepared according to the method described by Helga et al.[Citation20] The determination of microbiological counts was performed according to Zhang et al.[Citation21] Meat samples (25 g) were homogenized 5 min using a homogenizer (Ultra-turax T25 basic, IKA-WERKE, Germany) and then diluted completely with 250 ml sterile saline solution (0.85% NaCl). One milliliter of each sample solution was spread on the surface of different selective agars. Plate count agar was used for total viable counts (TVC). MRS agar was used for lactic acid bacteria counts. Pseudomonas CFC selective agar was used for total pseudomonas counts. Mannitol salt agar (MSA) was used for total staphylococcus counts. Streptomycin thallous acetate actidione agar (STAA) was used for total brochothrix thermosphacta counts. Violate red bile dextrose agar (VRBDA) was used for total enterobacteriaceae counts. TVC, staphylococcus spp. counts and enterobacteriaceae counts were evaluated after incubating at 37°C for 48 h. Lactic acid bacteria counts and brochothrix thermosphacta counts were counted after at 30°C for 72 h. Pseudomonas.spp counts was counted after incubating at 25°C for 48 h. The results were reported as log 10 CFU/g and all the treatments were repeated twice and each test was performed in triplicate.
Total volatile basic nitrogen (TVB-N) measurements
The determination of TVB-N content in chilled pork was performed by a semimicro-quantitative nitrogen method according to Chinese standard GB/T 5009.228 (2016). In brief, all samples were made into ground pork by a meat grinder. Twenty grams of the each ground pork samples were dissolved into 100 ml trichloroacetic acid and vibrated 30 min for fully impregnation. Then, after filtration, 5 ml impregnation liquids, 5 ml MgO solution (10 g/L) and two drops of dimethicone were added into a Kjeldahl distillation unit. The mixture was distilled for 5 min and the distillate was absorbed with 10 ml boric (20 g/L) and then titrated with HCI (0.01 mol/L). The TVB-N content was calculated using the following equation. The result stated for each samples were the mean value of three measurements.
Where V1 is the titration volume for the tested sample (mL), V2 is the titration volume of blank (mL), and c is the actual concentration of HCl (mol/L), 14 is the molecular weight of nitrogen, and m is the weight of meat sample (g).
Ph and color analysis
Meat samples (5 g) were dissolved into 45 ml distilled water and then filtered. After 30 min standing, the pH values of filtrates of the meat samples were evaluated by a digital pH meter (Mettler Seven Compact™). Color change during the storage at 4°C was measured by Minolta chroma meter CR-400 (Konica Minolta sensing, Japan) according to the method of Cheng et al.[Citation22] The instrument was standardized with white tiles provided by the manufacture and a*,b*, L* value were described as redness, yellowness, and lightness, respectively. Each sliced was performed six measurements in different areas. All the tests were performed in triplicate.
Water-holding capacity (WHC) measurement
WHC analysis was conducted by centrifugation, cooking, and drip loss measurement.
Measurement of centrifugation loss of chilled pork was performed as follows the method described by Zheng et al.[Citation23] The meat samples (10 g) were packaged by absorbing papers and balanced with cottons, then, after centrifuging at 5000 g for 10 min, each meat samples were weighed again. The centrifugation loss (%) of meat samples was calculated as follows:
Where M1 is the initial weight of the meat and M2 is the final weight of the meat. Measurement of cooking loss of chilled pork was performed as follows method.[Citation24] Briefly, the meat samples were ground individually using a meat grinder. Then, ground meats (10 g) were put into a retort pouch and treated with 85°C water bath for 15 min and the core temperature of cooked samples reached to 72°C. After cooling down to room temperature, each meat samples was weighed again. The cooking loss (%) of meat samples was calculated as follows:
Where M1 is the initial weight of the meat and M2 is the final weight of the meat. Measurement of drip loss of chilled pork was performed as previous reported.[Citation25] The meat samples (10 g) were hoisted by silk sutures and packaged with one food preservation kits. Then, the bag mouths were enclosed tightly and hanged in 4°C for 24 h. After removing surface moisture of meat samples by absorbing papers, each meat samples was weighed again. The drip loss (%) of meat samples was calculated as follows:
Where M1 is the initial weight of the meat and M2 is the final weight of the meat.
Thiobarbituric acid value measure
Thiobarbituric acid (TBA) was determined as described by Jonberg[Citation26] with a few modifications. The meat samples (10 g) were ground individually using meat grinder and placed into 100 ml with 40 ml (8% w/v) trichloroacetic acid for a homogenization at 7500 rpm for 15 s. After standing for 1 h, the obtained supernatants were centrifuged at 3000 rpm for 10 min and then filtered through filter paper and diluted with distilled water to 50 ml. The filtrate (6 ml) was mixed with 6 ml TBA (0.02 mol/L) solution and heated with a 95°C water bath for 30 min to develop the rose-pink color, then cooled with cold flowing water. After centrifugation at 5000 rpm for 10 min at room temperature (25°C), the absorbance of the supernatant was measured at 532 nm by a UV-Vis spectrometer (Purkinje General Instrument, Beijing), 6 ml (8% w/v) trichloroacetic acid mixed with 6 ml TBA solution was used as the blank control. TBA values were calculated from a standard curve of malondialdehyde (MDA) with 1,1,1,3-teraetoxipropane and expressed as milligrams of MDA per kilogram of chilled pork samples.
Shearing force measurement
Shearing forces of different samples were determined by the methods of Chiavaro.[Citation27] Meat samples were sliced into cuboids with uniform size along myofiber parallel direction (2 cm × 1 cm× 1 cm) for shearing force measurement by TA.XT.plus texture analyzer with WBS probe. Myofiber direction was vertical to a cutter bit and testing parameters were as follows: pre-test speed 1 mm/s, test speed 1 mm/s, post-test speed 1 mm/s and shearing distance 25 mm.
Instrumental texture profile analysis (TPA)
Hardness, springiness, chewiness, and resilience of different samples were assessed by TPA and performed according to Millette with a few modification.[Citation28] Meat samples were sliced into a cube with uniform size along myofiber parallel direction (1 cm × 1 cm× 1 cm) to perform texture profile analysis by TA.XT.plus texture analyzer with the p36R probe. Myofiber direction was parallel to cutter bit and testing parameters were as follows: pre-test speed 2 mm/s, test speed 5 mm/s, post-test speed 2 mm/s, compression ratio 50% and dwell time 5 s.
Sensory evaluation
Sensory evaluation of chilled pork treated were carried out during 4°C storage. A 10-member panel was recruit from graduate students who had prior experience on meat evaluation in the college of food science at Henan Institute of Science and Technology of china. The meat sample were cooked to the internal temperature of 70°C and presented to the panelists immediately. All samples were evaluated according to the Chinese standard GB/T22210-2008. The samples were randomly served to panelists in separate booths. Water was provided for cleaning the mouth between samples. The sensory attributes including color, odor, and texture were evaluated using a 9-point hedonic scale: 9, like extremely; 8, like very much; 7, like moderately; 6, like slightly; 5, acceptable; 4, dislike slightly; 3, dislike moderately; 2, like very much; 1, dislike extremely.
Statistical analysis
Each experiments were performed in triplicate and the mean ± standard deviations were used to compare differences between treatments. All data were analyzed using the program system of SPSS18.1 and the significant differences among means (p < .05) were based on Duncan’s multiple-range test. All figures were drawn by Origin 9.0.
Results and discussion
Bacterial count changes of fresh chilled fresh pork with different treatments during storage
Lactic acid bacteria, pseudomonas spp, staphylococcus spp. brochothrix thermosphacta and enterobacteriaceae were found in all fresh meat samples. Changes in microbiological count of fresh chilled pork during storage at 4°C for 11 days were shown in . The initial TVC of the control was approximately 4.76 log10 CFU/g, the result was higher than 3.82 log10 CFU/g and 4 log10 CFU/g which in the reports of Pogorzelska[Citation29] and Lorenzo[Citation30] respectively, indicating that the chilled fresh pork studied was slightly contaminated. The fresh chilled fresh pork might be contaminated by storage and sales process. On 5th day, the TVC in the control increased to 6.53 log10 CFU/g which was already higher than the required limits (less than or equal to 6.0 log10 CFU/g) of Chinese standard GB/T9959.2 (2008). It was shown that, according to the microbiological data, the shelf life of untreated samples was 3 days. The TVC of samples treated with nisin, TPs and chitosan reached the unacceptable limit on the 9th day(6.09 log10 CFU/g), 7th day(6.11 log10 CFU/g)and 9th day(6.16 log10 CFU/g), respectively. Nisin and chitosan treatments showed a significantly postponed microbial spoilage than TPs treatment, the shelf-life of nisin, TPs and chitosan treatment samples would be 9 days, 7days and 9days, respectively, while N-TP-C treatment samples could prolong this life to 11day when the TVC was 5.95 log10 CFU/g.
Table 1. Effect of different treatments on bacterial counts changes of fresh pork at 4°C for 11 days
The antimicrobial effect of nisin, TPs and chitosan treatment were investigated on lactic acid bacteria (LAB). The additions of nisin, TPS and chitosan led to a significantly (p < .05) decrease in the growth rate of LAB counts. The reductions of LAB number in meat samples caused by nisin, TPs, chitosan and N-TP-C combination treatments relative to control samples during refrigerated storage ranged from 5.12 to 4.57, 4.46, 4.22 and 4.07 log10 CFU/g, respectively. The N-TP-C treatments had the lowest LAB counts during 11 days storage, suggested that N-TP-C combination treatments were the best antibacterial treatment for inhibiting the LAB counts on fresh chilled meat.
A great many gram-positive and gram-negative bacteria grow very well in meat and meat products, and some can grow slowly in a refrigerated condition such as pseudomonas spp.[Citation31] Pseudomonas spp,as a common foodborn spoilage bacteria, was studied the growth inhibition by each different treatments. From , pseudomonas spp. counts showed significant increase from 2.75 to 5.71 log10 CFU/g than other treatments during storage for 11 days (p < .05). While N-TP-C treatments exhibited the best inhibitory effects on Pseudomonas spp. than other treatments (p < .05) in the 11th day. The pseudomonas spp. counts of nisin, TPs, chitosan and N-TP-C combination treatments were 4.59, 4.86, 4.37 and 4.13 log10 CFU/g, respectively. The results indicated that N-TP-C combination treatments showed excellent inhibitory effect on the growth of pseudomonas spp. in chilled pork during refrigerated storage.
The antimicrobial effect of nisin, TPs and chitosan treatment were also investigated on staphylococcus spp.as shown in . The staphylococcus spp.cuonts of control samples were increased gradually from 3.14 to 4.32 log10 CFU/g during storage for 11 days. Significant reductions to 3.63, 3.45, 3.21 and 3.09 log10 CFU/g of staphylococcus spp. at the end of storage (11th day), respectively, were obtained by nisin, TPs, chitosan and N-TP-C combination treatment. Therefore, the results showed that chitosan and N-TP-C combination treatment both exhibited better inhibitory capacity than nisin and TPs (p < .05).
Brochothrix thermosphacta counts of different treatments were increased slowly with the extension of storage time. The additions of nisin, TPS and chitosan led to a significantly (p < .05) decrease in the growth rate of Brochothrix thermosphacta counts. The Brochothrix thermosphacta counts of the control samples were approximately 2.32 log10 CFU/g on the initial day, and increased to 3.48 log10 CFU/g after 11days storage. In the 11th day, the Brochothrix thermosphacta counts of nisin, TPS, chitosan, and N-TP-C combination treatments were 2.73, 2.95, 2.82 and 2.53 log10 CFU/g respectively. So, the addition of different antimicrobial agents significantly affected the Brochothrix thermosphacta growth counts (p < .05).
The enterobacteriaceae growth counts were also inhibited by the addition of nisin, TPs and chitosan, as shown in . During the whole storage, the enterobacteriaceae counts treated with N-TP-C combination showed a lower values than other treatments (p < .05), especially at the beginning of storage, lower enterobacteriaceae counts (below 2 log10 CFU/g) were observed by N-TP-C combination treatment. Thus, the addition of N-TP-C reduced enterobacteriaceae counts growth in meat effectively.
Owing to the rich nutrients of meat, the rapid growth of spoilage and pathogenic bacteria often accelerates the meat spoilage. Generally, the microbial counts for all treatments became slowly higher with storage time extension. But N-TP-C combination treatment exhibited a stronger inhibitory effect on the microbial growth and it implied N-TP-C combination treatment to be a very effective way for decreasing bacteria counts on fresh chilled pork and extending shelf life. In this study, a microbiological shelf life extension of 6 days was obtained for N-TP-C combination treated samples compared with the control. Previous studies have reported that nisin, TPs and chitosan exhibited the effective antimicrobial activities to most of spoilage and pathogenic microorganisms. Nisin has been reported to be antibacterial against to Gram-positive bacteria, but not Gram-negative bacteria. Because nisin destroy the cell membrane to lead the leakage of cellular content by absorbing to phospholipid on the cell membrane of Gram-positive bacteria, while Gram-negative bacteria are covered by outer membranes, which are far less permeable than those of Gram-positive bacteria. Tea polyphenols have shown inhibitory effects on Gram-positive and Gram-negative bacteria, the antibacterial mechanism of tea polyphenols includes cell normal physiological and morphological changes, the obstacles to protein synthesis and expression, and the damage to genetic material. The mode of the anti-microbial capacity of chitosan might be due to its destructive action of cell wall and membranes, resulting the formation of polymer membrane on cell surface. Then, the channel of nutrient acquisition for bacteria is affected which leading to the cell death.[Citation32–Citation34] Nisin, tea polyphenols, and chitosan combined treatment could displayed a synergistic inhibition on the growth of spoilage and pathogenic bacteria of fresh chilled pork .N-TP-C combination has been reported to be used as preservatives to efficiently inhibit the growth of spoilage microorganisms and pathogens of chilled mutton and improve the safety and shelf life of chilled mutton.[Citation17] In this study, the N-TP-C combination was investigated the preservation effect and mechanism on chilled pork by measuring microbiological, physiological and biochemical indexes.
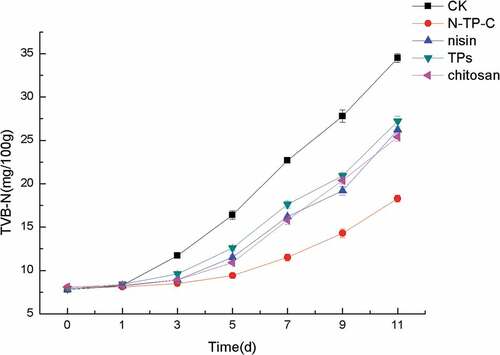
TVB-N changes of fresh chilled pork with different treatments during storage
TVB-N, which is mainly composed of trimethylamine (TMA), dimethylamine (DMA), ammonia and other compound, is mainly due to the activity of microbial enzymes for the degradation of proteins and nonprotein nitrogenous compounds.[Citation32,Citation35] TVB-N values of all different treatments during the storage were shown in . TVB-N values of all the pork samples during refrigerated storage gradually increased from initiation to 11 days. TVB-N in control, nisin, TPs, chitosan and N-TP-C combination reached to 16.4, 16.2, 17.6, 15.7, 18.3 mg/100 g at 5th,7th, 7th, 7th, 11th day, respectively, which had exceeded the limitation (15 mg/100 g of meat) of the Chinese hygienic standard (GB2707-2016) for fresh meat of livestock. Controls maintained a significantly higher TVB-N value than those of the others treated samples (p< .05). N-TP-C combination treatment exhibited excellent inhibitory effect for the TVB-N values rising of fresh chilled pork during refrigerated storage, which was in agreement with the TVC results (). Higher TVC values for the controls could account for its higher TVB-N values as compared with treated samples after 5 days storage. the results further illustrated that the decrease in TVB-N could be due to the inactivation of spoilage bacteria which produced specific enzymes involved in the autolytic degradation of proteins and amino acids. TVB-N is widely used as one of the important reference indices for assessing the spoilage and shelf life of meat products.[Citation31] The results of this study were in agreement with some previous studies. He et al.[Citation17] found that chilled mutton treated with chitosan, nisin and tea polyphenols combination decreased the TVB-N content of during refrigerated storage effectively. Yang et al.[Citation9] found a significant reduction in the formation of TVB-N content of tortoise meat with tea polyphenols and nisin combination treatment during chilled storage.
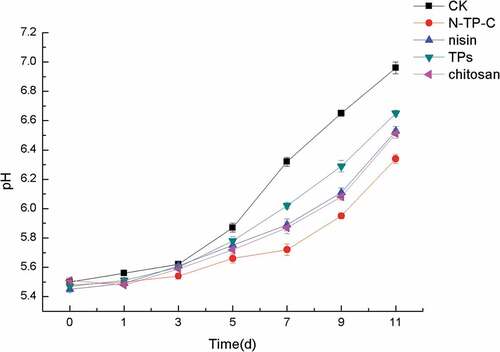
pH changes of fresh chilled fresh pork with different treatments during storage
Changes in the pH of fresh chilled fresh pork during storage at 4°C for 11 days were shown in . The pH of fresh chilled fresh pork with different treatments increased gradually with the extension of storage time. The initial pH values in control and different treatments were approximately 5.5 and the final pH values of different treatments were lower during the storage period compared with the control. On day 11 of storage, the pH values of the control, nisin, TPs, chitosan and N-TP-C treatments were 6.96, 6.53, 6.65, 6.51 and 6.34, respectively. TPs exhibited worst inhibitory effect on the final pH value increases. There was no significant difference between nisin and chitosan treatments (p > .05) which made the final pH value about 6.5 and N-TP-C combination treatments showed the best inhibitory efficacy for increasing of pH in chilled pork. These results were also in accord with the study by Wang et al.[Citation10] and Rahman.et al[Citation36] The rise of pH of fresh chilled pork during storage was due to the microorganisms growth on the surface of the meat, inducing the decomposition of different nutrients such as fat and amino acid.[Citation37] The lower pH in chilled pork with N-TP-C combination treatments might be due to inhibitory effect of combination on the growth of spoilage microorganisms.
Color changes of fresh chilled pork during storage with different treatments
Surface color is an important influencing factor for the purchasing chilled pork by many consumers and indicated the freshness and commercial acceptability of chilled pork during refrigerated storage. Changes in color values of chilled pork with different treatments during storage were depicted in . The L* and a* values in both controls and treatments decreased slowly during refrigerated storage. In control, samples exhibited an obvious lower L* and a* values than the other treatments. The L* and a* values of samples with N-TP-C combination treatments were highest until 11 days. Furthermore, the b* values of all untreated and treated samples slowly increased and N-TP-C combination treatments slightly increased b* values compared with other treatments during refrigerated storage for 11 days. These findings were in agreement with the previous report by Wang et al.,[Citation10] phage, nisin, and potassium sorbate combination treatments were able to significantly reduce the color change of fresh chilled pork. Hwang et al.[Citation38] reported that mugwort and rosemary combined with ascorbic acid treatment could inhibit the decrease of a* values of pork patties to improve shelf stability. In brief, all treated samples, especially N-TP-C combination treatments, showed higher L* and a* values and lower b* values than the controls, which demonstrated that natural preservatives had an advantage in slowing down color change of chilled pork during refrigerated storage.
Table 2. Effect of different treatments on Lightness (L),Redness (a) and Yellowness (b) changes of fresh pork at 4°C for 11 days
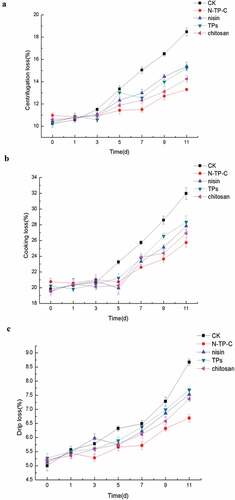
Water-holding capacity (WHC) of fresh chilled pork during storage with different treatments
Water loss often lead to texture loss of meat products which is attributed to meat spoilage. Centrifugation loss, cooking loss, and drip loss represented the content of water-holding capacity. The initial centrifugation, cooking and drip loss of fresh chilled pork was 10.5%, 19.8% and 5.1%, respectively. The centrifugation loss, cooking loss, and drip loss increased in both controls and different treatments during refrigerated storage (–). There was no significant difference in centrifugation loss, cooking loss and drip loss of all samples during the first 3 days of refrigerated storage, which could be due to the well freshness of chilled pork. In addition, centrifugation loss, cooking loss and drip loss of different treatments showed slightly decrease as compared to controls which remarkably decrease until the 11th day. Samples treated with N-TP-C combination showed the best water loss prevention (centrifugation loss 13.3%, cooking loss 25.74% and drip loss 6.69%) compared with controls (centrifugation loss 18.46%, cooking loss 31.97% and drip loss 8.67%). The WHC of nisin, TPs and chitosan exhibited inconspicuous difference and these values are distinctly higher than that of the controls. The results indicated that the fresh chilled pork treated with N-TP-C combination would be conducive to keeping better WHC. This beneficial result could be owing to the fact that the activities of proteolytic enzymes derived from pork and microorganisms, which contributed to the spoilage of muscle tissue and loss of water, were inhibited by the combination of nisin, TPs and chitosan.
Figure 3. Changes in centrifugation loss (a), cooking loss (b) and drip loss (c) of fresh pork samples at 4°C for 11 days. CK: control with no treatment; Nisin = nisin treatment; Tps = tea polyphenols treatment; Chitosan = chitosan treatment; N-TP-C = treatment with nisin, tea polyphenols and chitosan
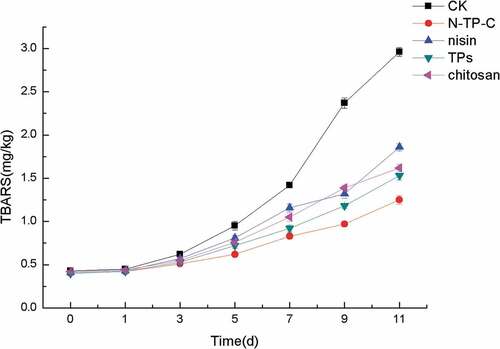
TBARS changes of fresh chilled pork during storage with different treatments
Lipid oxidation is a major responsibility for limiting the shelf-life of meat products. TBARS values represented the content of lipid oxidation, is mainly used as an important indicator to assess the quality of meat products account of its relatively simple measurement. As shown in , TBARS values in all the samples increased continuously and the different treatments exerted better effect to inhibit the rising of TBARS values of fresh chilled pork during refrigerated storage as compared to controls. The initial value of controls was 0.43 mg/kg and the final value reached to 2.96 mg/kg at the 11th day. The TBARS values of treatments with nisin, TPs and chitosan exceeded 1 mg/kg at the 7th day, 9th day and 7th day, respectively. However, these values were bigger than those of samples treated with N-TP-C combination, which showed the significant inhibition of the rising of TBARS value from 0.41 mg/kg to 1.25 mg/kg during refrigerated storage. Good inhibition effect of N-TP-C to TBARS value change could be attributed to the antioxidant activity of TPS. All above-mentioned results were in line with the previous reports. According to the finding of Song et al.,[Citation39] sodium alginate-based edible coating could inhibit the rising of TBARS value of refrigerated bream. Kim et al.[Citation40] also reported that fresh pork treated with garlic or onion juice reduced the lipid oxidation and extend the shelf-life to 7 days. Wang et al.[Citation10] found that nisin, potassium, and phage resulted in a decrease in TBARS values of fresh chilled pork and extended the shelf-life to 14 days. A multitude of natural preservatives has been used to control lipid oxidation in meat products.[Citation8,Citation9,Citation41] Our studies showed that N-TP-C combination could effectively inhibited the lipid oxidation of fresh chilled pork during refrigerated storage. Moreover, the content of lipid oxidation products is in connection with the color value of meat products. Xia et al.[Citation42] reported that the increased lipid oxidation could lead to a decrease in a* value (red color) and increase in b*(yellow color) value. Yellow color formation could be attributed to a color reaction between lipid oxidation products and amine in pork. Moreover, the red color increased as a result of the formation of metmyoglobin. In the current research, N-TP-C combination relieved the changes in lipid oxidation and surface color of chilled pork during refrigerated storage.
Shear force and texture changes of fresh chilled fresh pork with different treatments during storage
Tenderness, an important factor affecting the quality of pork, is commonly assessed by a method of shear force. Tenderness is anticorrelated with shear force, which means a decrease of shear force stood for an increase of tenderness.[Citation43] The differences between controls and different treatments are shown in . Shear force values in all treatments decreased gradually during whole-refrigerated storage as compared to controls. Shear force value of samples treated with N-TP-C combination showed the significant loss prevention compared with controls from the 5th day to the 11th day. There was no significant difference among the different treatments with nisin, TPs and chitosan to shear force values of fresh chilled pork during refrigerated storage (p > .05), but these values exhibited significant difference as compared to controls (p < .05). The above results indicated that the fresh chilled pork treated with N-TP-C combination would result in lower tenderness, which could be due to the fact that combination is possible to improve the water-holding capacity and reduce protein degradation of fresh chilled pork during refrigerated storage.
Table 3. Effect of different treatments on shear force and texture changes of fresh pork at 4°C for 11 days
Pork texture was assessed by texture profile analysis (TPA). Hardness, springiness, chewiness, and resilience were used to specifically depict the texture changes of different-treated samples during refrigerated storage. As shown in , there was a significant difference in hardness, springiness, chewiness, and resilience of controls samples from the 1st day to 11th day during refrigerated storage (P < 0.05), a decrease of hardness, springiness, chewiness, and resilience of controls were obtained. The hardness, springiness, chewiness and resilience changes of fresh chilled pork of all treatments were significantly slowed down during the storage period compared to control (p < .05). Especially, the N-TP-C combination treatments showed the best improvement of hardness, springiness, chewiness, and resilience of fresh chilled pork during refrigerated storage for 11days. This work showed that the N-TP-C combination treatments, which had been approved and widely used in the food industry, was beneficial to improve edible quality of fresh chilled pork during refrigerated storage. Hence, the combination of nisin, TPs and chitosan could be used as a promising natural antimicrobial biopreservative and extend the shelf time of fresh chilled pork to 11days.
Sensory evaluation of fresh chilled pork with N-TP-C treatments during storage
The sensory attribute is a very important index for evaluating meat quality. The sensory properties of chilled pork, including color, odor and texture, with N-TP-C treatments were shown in . All sensory properties in N-TP-C treatments decreased gradually during whole-refrigerated storage and showed the significant difference from the 5th day to 11th day (p < .05). Sensory panelists founded that the trend of the changes of sensory color and texture were in accordance with the instrumental color and texture of N-TP-C treatments. The slow rate of decline in the sensory scores of the color and texture might be due to nisin, TPs, and chitosan inhibiting enzyme activity and microbial growth in fresh chilled pork. The sensory odor characteristics might be related to pH changes and microbial growth in fresh chilled pork. After storage for 11 days, the sensory scores of the color, odor, and texture was 6.24, 5.91, and 6.13, respectively. Since the meat was to be considered acceptable when the sensory score is above 6.00, the fresh chilled pork with N-TP-C treatments, storing for 11 days, remained acceptable.
Table 4. Effect of N-TP-C treatments on sensory characteristics to fresh pork at 4°C for 11 days
Conclusion
In this study, it was found that combination treatment of nisin, Tps and chitosan exhibited superior antimicrobial activity and shelf life extension ability on fresh chilled pork compared to individual treatment with nisin, TPs and chitosan. Combination treatment of nisin, TPs and chitosan had a possible synergistic effect on microbial inhibition and could reduce the value of pH, TVB-N and TBARS in fresh chilled pork during 11 days of storage at 4°C. Moreover, the combined treatment also exhibited a protective effect against the decrease of texture properties and the degradation of color. Therefore, nisin and TPs in combination with chitosan can be utilized effectively to potentially improve shelf-life and sensory quality of fresh chilled pork.
Disclosure statement
The authors declare that they have no competing interests. The authors declare that they have no competing interests.
Additional information
Funding
References
- Verbeke, W.; Liu, R. The Impacts of Information about the Risks and Benefits of Pork Consumption on Chinese Consumers’ Perceptions Towards, and Intention to Eat, Pork[J]. Meat Sci. 2014, 98(4), 766–772. DOI:10.1016/j.meatsci.2014.07.023
- Wang, K.; Ye, K.; Zhu, Y.; Huang, Y.; Wang, G.; Wang, H.; Zhou, G. Prevalence, Antimicrobial Resistance and Genetic Diversity of Listeria Monocytogenes Isolated from Chilled Pork in Nanjing, China[J]. LWT - Food Sci. Technol. 2015, 64(2), 905–910. DOI: 10.1016/j.lwt.2015.06.015.
- Lavieri, N.; Williams, S. K. Effects of Packaging Systems and Fat Concentrations on Microbiology, Sensory and Physical Properties of Ground Beef Stored at 4±1°C for 25 days[J]. Meat Sci. 2014, 97(4), 534–541. DOI: 10.1016/j.meatsci.2014.02.014.
- Ayari, S.; Han, J.; Vu, K. D.; Lacroix, M. Effects of Gamma Radiation, Individually and in Combination with Bioactive Agents, on Microbiological and Physicochemical Properties of Ground beef[J]. Food Control 2016, 64, 173–180. DOI: 10.1016/j.foodcont.2015.12.034.
- Tang, X.; Sun, X.; Wu, V. C. H.; Xie, J.; Pan, Y.; Zhao, Y.; Malakar, P. K. Predicting Shelf-Life of Chilled Pork Sold in China[J]. Food Control 2013, 32(1), 334–340. DOI: 10.1016/j.foodcont.2012.12.010.
- Purnell, G.; James, C.; James, S. J.; Howell, M.; Corry, J. E. L. Comparison of Acidified Sodium Chlorite, Chlorine Dioxide, Peroxyacetic Acid and Tri-Sodium Phosphate Spray Washes for Decontamination of Chicken carcasses[J]. Food Bioprocess. Technol. 2014, 7(7), 2093–2101. DOI: 10.1007/s11947-013-1211-8.
- Smaoui, S.; Hsouna, A. B.; Lahmar, A.; Ennouri, K.; Mtibaa-Chakchouk, A.; Sellem, I.; Najah, S.; Bouaziz, M.; Mellouli, L. Bio-Preservative Effect of the Essential Oil of the Endemic Mentha Piperita Used Alone and in Combination with BacTN635 in Stored Minced Beef meat[J]. Meat Sci. 2016, 117, 196–204. DOI: 10.1016/j.meatsci.2016.03.006.
- Cao, Y.; Gu, W.; Zhang, J.; Chu, Y.; Ye, X.; Hu, Y.; Chen, J. Effects of Chitosan, Aqueous Extract of Ginger, Onion and Garlic on Quality and Shelf Life of Stewed-Pork during Refrigerated storage[J]. Food Chem. 2013, 141(3), 1655–1660. DOI: 10.1016/j.foodchem.2013.04.084.
- Yang, A.; Cheng, F.; Tong, P.; Chen, H. Effect of Tea Polyphenol and Nisin on the Quality of Tortoise (Trachemys Scripta Elegans) Meat during Chilled storage[J]. J. Food Process. Preserv. 2017, 41(6), 1–8. DOI: 10.1111/jfpp.2017.41.issue-6.
- Wang, C.; Yang, J.; Zhu, X.; Lu, Y.; Xue, Y.; Lu, Z. Effects of Salmonella Bacteriophage, Nisin and Potassium Sorbate and Their Combination on Safety and Shelf Life of Fresh Chilled pork[J]. Food Control. 2017, 73, 869–877. DOI: 10.1016/j.foodcont.2016.09.034.
- Pawar DD, Malik SV, Bhilegaonkar KN, Barbuddhe SB. Effect of nisin and its combination with sodium chloride on the survival of Listeria monocytogenes added to raw buffalo meat mince[J]. Meat Science. 2017, 2000, 56(3): 215–21. DOI: 10.1016/S0309-1740(00)00043-7
- Gough, R.; O’Connor, P. M.; Rea, M. C.; Gómez-Sala, B.; Miao, S.; Hill, C.; Brodkorb, A. Simulated Gastrointestinal Digestion of Nisin and Interaction between Nisin and bile[J]. LWT - Food Sci. Technol. 2017, 86, 530–537. DOI: 10.1016/j.lwt.2017.08.031.
- Dembele, S.; Wang, D. F.; Sun, J. P.; Dong, S. Y. Comparison Study of the Effects of Different Crude Green Tea Polyphenols on the Quality of Dried Catfish during Ambient storage[J]. J. Food Process Eng. 2011, 34(3), 566–579. DOI: 10.1111/jfpe.2011.34.issue-3.
- Huang, W.; Xu, H.; Xue, Y.; Huang, R.; Deng, H.; Pan, S. Layer-By-Layer Immobilization of Lysozyme-Chitosan-Organic Rectorite Composites on Electrospun Nanofibrous Mats for Pork preservation[J]. Food Res. Int. 2012, 48(2), 784–791. DOI: 10.1016/j.foodres.2012.06.026.
- Fan, W.; Sun, J.; Chen, Y.; Qiu, J.; Zhang, Y.; Chi, Y. Effects of Chitosan Coating on Quality and Shelf Life of Silver Carp during Frozen storage[J]. Food Chem. 2009, 115(1), 66–70. DOI: 10.1016/j.foodchem.2008.11.060.
- Branen, J. K.; Davidson, P. M. Enhancement of Nisin, Lysozyme, and Monolaurin Antimicrobial Activities by Ethylenediaminetetraacetic Acid and lactoferrin[J]. Int. J. Food Microbiol. 2004, 90(1), 63–74.
- He L, Zou L, Yang Q, Xia J, Zhou K, Zhu Y, Han X, Hu B, Deng W, Liu S. Antimicrobial Activities of Nisin, Tea Polyphenols, and Chitosan and their Combinations in Chilled Mutton[J]. Journal of Food Science, 2016, 86(6):1466–1471.DOI:10.1111/1750-3841.13321.
- Field, D.; Daly, K.; O’Connor, P. M.; Cotter, P. D.; Hill, C.; Ross, R. P. Efficacies of Nisin A and Nisin V Semipurified Preparations Alone and in Combination with Plant Essential Oils for Controlling Listeria monocytogenes[J]. Appl. Environ. Microbiol. 2015, 81(8), 2762–2769. DOI: 10.1128/AEM.00070-15.
- Turgis, M.; Vu, K. D.; Dupont, C.; Lacroix, M. Combined Antimicrobial Effect of Essential Oils and Bacteriocins against Foodborne Pathogens and Food Spoilage bacteria[J]. Food Res. Int. 2012, 48(2), 696–702. DOI: 10.1016/j.foodres.2012.06.016.
- Medić, H.; Kušec, I. D.; Pleadin, J.; Kozačinski, L.; Njari, B.; Hengl, B.; Kušec, G. The Impact of Frozen Storage Duration on Physical, Chemical and Microbiological Properties of pork[J]. Meat Sci. 2018, 140, 119–127. DOI: 10.1016/j.meatsci.2018.03.006.
- Zhang, H.; He, P.; Kang, H.; Li, X. Antioxidant and Antimicrobial Effects of Edible Coating Based on Chitosan and Bamboo Vinegar in Ready to Cook Pork chops[J]. LWT - Food Sci. Technol. 2018, 93, 470–476. DOI: 10.1016/j.lwt.2018.04.005.
- Cheng, J. R.; Liu, X. M.; Zhang, Y. S.; Zhang, Y.-H.; Chen, Z.-Y.; Tang, D.-B.; Wang, J.-Y. Protective Effects of Momordica Grosvenori Extract against Lipid and Protein Oxidation-Induced Damage in Dried Minced Pork slices[J]. Meat Sci. 2017, 133, 26–35. DOI: 10.1016/j.meatsci.2017.04.238.
- Zheng, H. B.; Han, M. Y.; Yang, H. J.; Tang, C.-B.; Xu, X.-L.; Zhou, G.-H. Application of High Pressure to Chicken Meat Batters during Heating Modifies Physicochemical Properties, Enabling Salt Reduction for High-Quality products[J]. LWT - Food Sci. Technol. 2017, 84, 693–700. DOI: 10.1016/j.lwt.2017.06.006.
- Ha, M.; Dunshea, F. R.; Warner, R. D. A Meta-Analysis of the Effects of Shockwave and High Pressure Processing on Color and Cook Loss of Fresh meat[J]. Meat Sci. 2017, 132, 107–111. DOI: 10.1016/j.meatsci.2017.04.016.
- Otto, G.; Roehe, R.; Looft, H.; Thoelking, L.; Henning, M.; Plastow, G. S.; Kalm, E. Drip Loss of Case-Ready Meat and of Premium Cuts and Their Associations with Earlier Measured Sample Drip Loss, Meat Quality and Carcass Traits in pigs[J]. China Meat Sci. 2006, 72(4), 680–687. DOI: 10.1016/j.meatsci.2005.10.001.
- Jongberg, S.; Skov, S. H.; Tørngren, M. A.; Skibsted, L. H.; Lund, M. N. Effect of White Grape Extract and Modified Atmosphere Packaging on Lipid and Protein Oxidation in Chill Stored Beef patties[J]. Food Chem. 2011, 128(2), 276–283. DOI: 10.1016/j.foodchem.2011.03.015.
- Emma C, Massimiliano R, Elena V; et al. Cooking of Pork Longissimus Dorsi at Different Temperature and Relative Humidity Values: Effects on Selected Physico-Chemical properties[J]. J. Food Eng. 2009, 93(2), 158–165.
- Millette, M.; Tien, L.; Smoragiewicz, W.; Lacroix, M. Inhibition of Staphylococcus Aureus on Beef by Nisin-Containing Modified Alginate Films and beads[J]. Food Control 2007, 18(7), 878–884. DOI: 10.1016/j.foodcont.2006.05.003.
- Pogorzelska, E.; Godziszewska, J.; Brodowska, M.; Wierzbicka, A. Antioxidant Potential of Haematococcus Pluvialis Extract Rich in Astaxanthin on Colour and Oxidative Stability of Raw Ground Pork Meat during Refrigerated storage[J]. Meat Sci. 2017, 135, 54–61. DOI: 10.1016/j.meatsci.2017.09.002.
- Lorenzo, J. M.; Jorge, S.; Amado, I. R.; Franco, D. Influence of Natural Extracts on the Shelf Life of Modified Atmosphere-Packaged Pork patties[J]. Meat Sci. 2014, 96(1), 526–534. DOI: 10.1016/j.meatsci.2013.08.007.
- Miao, J.; Peng, W.; Liu, G.; Chen, Y.; Chen, F.; Cao, Y. Biopreservative Effect of the Natural Antimicrobial Substance from Lactobacillus Paracasei Subsp. Tolerans FX-6 on Fresh Pork during Chilled storage[J]. Food Control 2015, 56, 53–56. DOI: 10.1016/j.foodcont.2015.03.013.
- Yi, S.; Li, J.; Zhu, J.; Lin, Y.; Fu, L.; Chen, W.; Li, X. Effect of Tea Polyphenols on Microbiological and Biochemical Quality of Collichthys Fish ball[J]. J. Sci. Food Agric. 2011, 91(9), 1591–1597. DOI: 10.1002/jsfa.v91.9.
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main uses[J]. Crit. Rev. Food Sci. Nutr. 2016, 56(8), 1262–1274. DOI: 10.1080/10408398.2013.763765.
- Sagoo, S.; Board, R.; Roller, S. Chitosan Inhibits Growth of Spoilage Micro-Organisms in Chilled Pork products[J]. Food Microbiol. 2002, 19(2–3), 175–182. DOI: 10.1006/fmic.2001.0474.
- Balamatsia, C. C.; Patsias, A.; Kontominas, M. G.; Savvaidis, I. Possible Role of Volatile Amines as Quality-Indicating Metabolites in Modified Atmosphere-Packaged Chicken Fillets: Correlation with Microbiological and Sensory attributes[J]. Food Chem. 2007, 104(4), 1622–1628. DOI: 10.1016/j.foodchem.2007.03.013.
- Rahman, S. M. E.; Wang, J.; Oh, D. H. Synergistic Effect of Low Concentration Electrolyzed Water and Calcium Lactate to Ensure Microbial Safety, Shelf Life and Sensory Quality of Fresh pork[J]. Food Control 2013, 30(1), 176–183. DOI: 10.1016/j.foodcont.2012.06.041.
- Masniyom, P.; Benjakul, S.; Visessanguan, W. Shelf-Life Extension of Refrigerated Seabass Slices under Modified Atmosphere packaging[J]. J. Sci. Food Agric. 2002, 82(8), 873–880. DOI: 10.1002/(ISSN)1097-0010.
- Hwang, K. E.; Kim, H. W.; Song, D. H.; Kim, Y.-J.; Ham, Y.-K.; Choi, Y.-S.; Lee, M.-A.; Kim, C.-J. Effect of Mugwort and Rosemary either Singly, or Combination with Ascorbic Acid on Shelf Stability of Pork patties[J]. J. Food Process. Preserv. 2017, 41(4), 1–9. DOI: 10.1111/jfpp.2017.41.issue-4.
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of Sodium Alginate-Based Edible Coating Containing Different Anti-Oxidants on Quality and Shelf Life of Refrigerated Bream (Megalobrama amblycephala)[J]. Food Control 2011, 22(3–4), 608–615. DOI: 10.1016/j.foodcont.2010.10.012.
- Kim, Y. J.; Jin, S. K.; Park, W. Y.; Kim, B. W.; Joo, S. T.; Yang, H. S. The Effect of Garlic or Onion Marinade on the Lipid Oxidation and Meat Quality of Pork during Cold storage[J]. J. Food Qual. 2010, 33(s1), 171–185. DOI: 10.1111/j.1745-4557.2010.00333.x.
- Xi, Y.; Sullivan, G. A.; Jackson, A. L.; Zhou, G. H.; Sebranek, J. G. Use of Natural Antimicrobials to Improve the Control of Listeria Monocytogenes in a Cured Cooked Meat Model system[J]. Meat Sci. 2011, 88(3), 503–511. DOI: 10.1016/j.meatsci.2011.01.036.
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical Change and Protein Oxidation in Porcine Longissimus Dorsi as Influenced by Different Freeze-Thaw cycles[J]. Meat Sci. 2009, 83(2), 239–245. DOI: 10.1016/j.meatsci.2009.05.003.
- Shorthose, W. R.; Harris, P. V. Effect of Animal Age on the Tenderness of Selected Beef muscles[J]. J. Food Sci. 1990, 55(1), 1–8. DOI: 10.1111/jfds.1990.55.issue-1.