?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Malnutrition caused by iron deficiency can be overcome by fortification of food with iron. For the first time, metal (iron) bound/uptake food-grade microorganisms were used as fortificants in order to prevent deteriorative changes and to preserve the organoleptic acceptability of the fortified food. In the present study, biosorption capacity of Bacillus subtilis and uptake capacity of Saccharomyces cerevisiae for iron ions was determined by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) and found to be 7.1 ± 0.2 and 3.2 ± 0.2 mg per gram of the biosorbent respectively. Chocolate was fortified with various concentrations of biosorbed/uptaken iron, inorganic (free) iron and free biomass. Appearance, chemical and microbiological changes associated with fortification was monitored for 28 and 30 days of storage at 30°C respectively. Chocolate fortified with free (inorganic) iron showed significant increase in thiobarbituric acid (TBA) values when compared with the biosorbed/uptaken iron-fortified or free biomass-fortified chocolate samples. Black discoloration was observed in free iron-fortified samples when compared with samples fortified with metal enriched biomass or free biomass.
Introduction
Trace elements such as iron, iodine, fluoride, copper, zinc, chromium, selenium, manganese and molybdenum are vital for health.[Citation1] Under nutrition of these trace elements in food is termed as “Hidden hunger”, where it affects one in three people, globally. More than 2 billion people worldwide are suffering from this form of malnutrition.[Citation2]
Among the trace elements, iron is one of the essential elements required by all body cells, as it is a part of numerous highly complex processes occurs continuously in the body. Deficiency of iron causes decreased immune functions and leads to severe fatigue, improper maintaining of the body and can delay normal infant mental functions.[Citation3] Iron deficiency is a major health problem in the developing world, and recently WHO ranked it as 7 out of 10 major global preventable risks for disease, disability and death. About half of the people with iron deficiency are children because they require a high amount of iron during their growth from infancy to adolescence. The probability of iron deficiency is more in women than men because women lose iron during menstruation, and the need for iron is greater during periods of growth. Fortification of food with iron would be an effective approach and sustainable strategy in the present scenario for providing additional iron required to the body.[Citation4]
The efficacy of iron fortification depends on many factors such as iron status of the target population, suitable vehicle and the type of the iron compound.[Citation5] The inorganic form of iron is mostly used for fortification but may result in relatively high uptake which may cause detrimental effects and low bioavailability.[Citation6] The organic form of iron (complexed with protein, polysaccharide, ligand and or microorganism) can be a valuable source for fortification with better bioavailability. This observation was supported by many other studies where iron complexed with protein or polysaccharide showed better availability with increased iron status.[Citation7,Citation8] The advantages of these complexed proteins or polysaccharides is that they are composed of nutrients, which are absorbed or utilized by the body and allows the iron to be bioavailable.[Citation9]
Fortification of foods with iron is technically challenging because of off-color and off-flavor development, catalytic degradation of vitamins and oxidation of lipids occurring due to the reaction of inorganic form of iron with the food constituents[Citation10] which results in low consumer acceptance of the fortified food. However, in the organic form, the metal is complexed with amino acids, proteins, lipids,and polysaccharides,[Citation9,Citation11] and is unavailable for reaction with the food constituents. This eliminates or reduces the deteriorative changes associated with metal incorporation into foods. The organic form of the metal is thus not only more bioavailable than the inorganic form,[Citation11] it is also unreactive making the fortified food organoleptically acceptable.
Microbial biomass can act as an organic matrix, and enriched with the trace elements, can be employed as a medium for food fortification.[Citation12,Citation13] Many studies reported the use of microorganisms as natural biosorbent for metal ions.[Citation12] The increased bioavailability of the trace elements using yeast or bacteria as a vehicle compared with the inorganic forms of the trace element have been reported in many studies.[Citation11,Citation14–Citation16] The use of microorganisms for delivering of these nutrients is of relevance for the production of functional foods with these micronutrients.
In the present study, iron was biosorbed onto Bacillus subtilis by biosorption while it was incorporated into yeast by uptake. Bacillus subtilis and Saccharomyces cerevisiae (yeast) are non-pathogenic and non-toxic and has Generally Regarded as Safe (GRAS) status, and hence used in the fortification of foods. These microorganisms enriched with iron could be a valuable organic source of this element in food in bioavailable form that will also prevent deteriorative changes associated with metal incorporation. Supplementation of iron deficient groups (foods) with iron-enriched Bacillus/Yeast will be a new promising application.
Chocolate enriched with iron could be an attractive solution for many children since they constitute 50% of people with iron deficiency.[Citation17] Children prefer food products flavored with chocolate.[Citation18] Hence, in this context, the aim of the present study is to enrich the Bacillus and yeast with iron, its fortification into chocolate, and evaluate the chemical and microbiological changes in the fortified chocolate samples.
Materials and methods
Microorganism and growth conditions
The bacterium, Bacillus subtilis was maintained on nutrient agar plates, and the yeast Saccharomyces cerevisiae was maintained on yeast media agar plates (20 g/L dextrose; 10 g/L peptone; 5 g/L yeast extract and 20 g/L agar) at 4°C and subcultured every month.
Preparation of metal-enriched biomass by biosorption/uptake
Bacillus subtilis biomass was prepared by inoculating the strain into nutrient broth[Citation19] and incubating at 37°C for 24 hr at 150 rpm. The bacterial cells were harvested by centrifugation at 8000 rpm for 10 min, washed twice with ultrapure water before being used in the iron biosorption experiments.
Biosorption experiments were carried out in 250 ml Erlenmeyer flasks containing 100 mg/L of Fe and a biomass concentration of 1g/L at pH 4.5. The flasks were then agitated at 150 rpm on an orbital shaker at 30°C for 24 hr. After incubation, the solutions were centrifuged at 8000 rpm for 8 min, and the obtained supernatant was analyzed for the nonbiosorbed iron ions. To determine the amount of iron biosorbed onto the B. subtilis biomass, the iron biosorbed B. subtilis pellets (10*100 mg) was digested with 3% HNO3 at 98°C. After complete digestion, the vessel contents were dissolved in 50 ml of distilled water and analyzed.
A suspension (5 ml) of yeast culture grown from the yeast media agar plates at 30°C, 24 hr was used as the pre-inoculum. Yeast media were employed for biomass cultivation with and without iron supplementation. The inoculum was grown in 500 ml Erlenmeyer flasks containing 250 ml of yeast medium. The cultivation was started by adding the suspension of pre inoculum (5 ml) to the culture media, and the medium supplemented with iron was added with 25 mg ferrous sulfate heptahydrate (corresponding to 5 mg Fe/250ml). The flasks were then incubated in rotary shaker at 150 rpm, 30°C, 18 h. After incubation, the cells were harvested by centrifugation at 8000 rpm for 5 min. The cells were then washed with double-distilled water for two times to remove the contents of the medium. For iron quantification, the yeast pellets were digested using 3% HNO3 at 98°C to release the intracellular iron. The contents of the flask were mixed with 50 ml distilled water. The intracellular iron and residual iron in the cultivation media were then analyzed to determine the amount of iron uptake by the yeast.
Quantification of metal binding or incorporation into biomass
The concentration of the Fe (II) ions in the supernatant of B. subtilis after biosorption, culture media of yeast after incubation and the digested samples of the iron uptake yeast and iron biosorbed B. subtilis was measured by Inductive Coupled Plasma Optical Emission Spectroscopy (ICP-OES). The equilibrium biosorption capacity of B. subtilis and the uptake capacity of yeast at the corresponding equilibrium conditions was calculated using a mass balance equation[Citation20]:
where qe is the amount of the metal biosorbed/uptake by the biosorbent (mg g−1); Ci is initial metal ion concentration in solution (mg l−1); Ce is the equilibrium metal ion concentration in solution (mg l−1) V is volume of the medium(l); and m is the amount of the biosorbent used in the reaction mixture (g).
Chocolate making procedure and experimental design
Chocolate was prepared in the laboratory with common ingredients that people use in households. Sugar (30 g) was dissolved in 100 ml distilled water and boiled until string consistency was attained. To this, skim milk powder (3 g) and cocoa powder (3 g) were added while hot and thoroughly mixed. The obtained chocolate syrup (5 g) was mixed with butter (2 g) and distributed into individual plates. To each chocolate plate, one of the fortificant forms (free iron, biomass bound/uptake iron, or free biomass) were added according to the treatments. The components were mixed and allowed to cool by incubating at −80°C for 16 hr for solidification of the chocolate. Finally, the chocolate was stored at 30°C for 28 (Bacillus subtilis) and 30 (Saccharomyces cerevisiae) days and analyzed for color, appearance, chemical and microbiological changes at every seven (Bacillus subtilis) and 15 days (Saccharomyces cerevisiae). Chocolate was fortified with Bacillus subtilis and Saccharomyces cerevisiae with the treatments as described in and .
Table 1. Different treatments of chocolate fortified with different concentrations of free iron, iron biosorb onto Bacillus subtilis biomass, and biomass without iron
Table 2. Different treatments of chocolate fortified with different concentrations of free iron, iron uptake yeast, and biomass without iron
Microbiological analysis of chocolate samples
Different treatments of chocolate fortified with B. subtilis and yeast were subjected to microbiological analysis. For this chocolate was homogenized with double-distilled water and spreaded on Potato Dextrose agar (PDA) plates for yeast and mold counts and on Eosin Methylene Blue (EMB) agar plates for coliforms counts. The plates were than observed after incubation at 37°C (PDB plates) and 25.5°C (EMB plates) for 24–48 h.
Chemical changes (TBA test) in chocolate during storage
The chocolate fortified with both Bacillus and yeast was analyzed chemically by thiobarbituric acid (TBA) to estimate the amount of product formed as a result of oxidation spectrophotometrically. For this, 2 g of chocolate was melted and allowed to dissolve in 2 ml of water. Then, freshly prepared 2 ml of 20 mM TBA reagent was added before incubating in boiling water bath for 30 min. Then, the samples were allowed to cool and, the obtained orange-red supernatant was separated, and its absorbance was measured at 531 nm.
Determination of iron from chocolate samples (B. subtilis)
To determine iron in fortified chocolate samples, 2 g of freshly prepared chocolate was fortified with highest concentration of inorganic iron, iron biosorbed B. subtilis biomass and free biomass as presented in and incubated at 30°C for 28 days. Then, the chocolate was powdered by using sterile glass motor and pestle. Weigh 1 g of powdered chocolate into clean dry digestion vessel and add HNO3 and H2SO4 in 10:5 ratios, respectively. The vessel was heated at 100°C after mixing thoroughly. To avoid carbonization during heating 1 ml of HNO3 can be added throughout the process if needed. After completion of digestion, the remained portion of the vessel contents (4 ml of acid) was made up to 50 ml with distilled water. Since ICP-OES was sensitive to highest concentration of acids, from the above sample 10 ml was taken and further made up to 50 ml with distilled water. Then, the diluted sample was analyzed for the determination of iron in all the fortified treatments including control. Acid digestion was performed by the method described in other studies.[Citation21]
Statistical analysis
All determinations were performed in replicate. The results were expressed as mean ± SD (standard deviation). Figures were represented using GraphPad Prism version 5.01.
Results and discussion
Application of Bacillus subtilis and Saccharomyces cerevisiae for iron fortification through chocolate
Based on the results obtained by ICP-OES, the biosorption potential of B. subtilis was found to be 7.05 ± 0.212 mg per gram of the biosorbent, and the uptake capacity of yeast was found to be 3.18 ± 0.2 mg per gram of the biomass. While in other studies maximum biosorption efficiency of 90–100% was achieved with other strains of Bacillus for Fe ions[Citation22], and the uptake capacity of yeast Saccharomyces cerevisiae was found to be 2.83 mg per gram of the biomass.[Citation13] From we can elucidate that the amount of the iron biosorbed was same with the amount of iron released as a result of acid digestion which confirms that there was no loss of iron during the biosorption process.
Table 3. Amount of Fe biosorbed after biosorption and release of iron from iron biosorbed B. subtilis after digesting with 3% HNO3.
The obtained iron enriched B. subtilis and yeast was used for the fortification of chocolate. The chocolate samples were analyzed chemically and microbiologically when fresh and after 28 and 30 days of storage with 7 and 15 days interval, respectively.
Sensory changes in chocolate
Besides bioavailability, other aspects have to be taken into consideration for fortification of food like form and source of the iron. Iron when present in inorganic form, reacts with the food constituents such as lipids and carbohydrates and lead to several chemical processes like lipid peroxidation, unacceptable taste and discoloration of the iron-fortified food resulting in poor consumer acceptance.
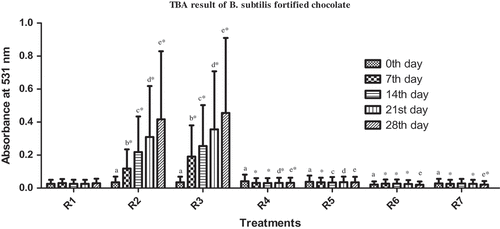
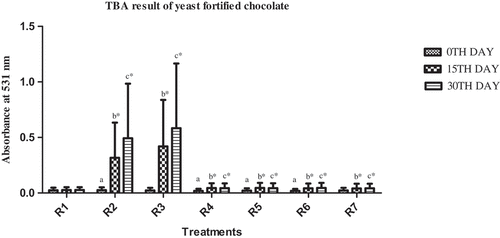
The fortified chocolate treatments were observed for sensory changes during storage ( and ). The chocolate fortified with inorganic (free) iron (R2 & R3) showed black discoloration upon storage. Greater degree of discoloration was noticed in chocolate with higher amount of free iron. No discoloration was observed in the chocolate containing iron biosorbed/uptake biomass (R4 & R5) or with only biomass (R6 & R7) or in control (R1). The intensity of the black color was increased with the increase in storage period and by the end of the 28th/30th day, large dark color spots were observed in the free iron-fortified chocolate samples in both experiments. The increased rate of oxidation upon storage was evaluated by TBA test. Darkening of food samples when fortified with inorganic iron was also observed in other studies. The tortillas fortified with iron were significantly darker than control tortillas.[Citation23] Darkening was observed in instant noodles when fortified with iron.[Citation24] Hence, iron biosorbed/uptaken biomass can prevent the deteriorative and unacceptable sensory changes observed during fortification of food with inorganic (free) metals.
Figure 1. Sensory changes of chocolate as a result of fortification with free iron, iron biosorb to the biomass and the biomass. R1- control; R2&R3- free iron-fortified chocolate; R4&R5- iron biosorbed B. subtilis biomass-fortified chocolate; R6&R7- free B. subtilis biomass fortified chocolate

Figure 2. Sensory changes of chocolate as a result of fortification with free iron, iron uptake by the biomass and the biomass. R1- control; R2&R3- free iron-fortified chocolate; R4&R5- iron uptake S. cerevisiae biomass-fortified chocolate; R6&R7- free S. cerevisiae biomass-fortified chocolate

The chocolate made with yeast showed holes/eyes. Although this may be considered a defect, alternatively the product can be marketed as a special kind of chocolate (as in case of Swiss cheese). Appropriate technological solutions, like overlaying with a normal coat of chocolate can be done. Such change was not noticed with chocolate containing B. subtilis. Yeast, molds and coliforms were absent in all the fortified treatments either fresh or during storage.
TBA analysis of iron-fortified chocolate
The chemical changes of chocolate as a result of fortification during 28 and 30 days of storage are shown in and for B. subtilis and yeast. TBA assay indicates the extent of oxidative changes.[Citation25] In both the experiments, upon comparing the TBA values, significantly higher values were observed in free iron-fortified chocolate when compared to control and biosorbed/uptake iron-fortified chocolate (lower TBA values). During storage, the TBA values increased significantly. From these results, we can infer that the oxidation process was higher in chocolate samples containing free iron than in that containing biosorbed/uptake iron. The reason for high TBA values is that iron, when present in free state, reacts with the food constituents and triggers lipid peroxidation reactions (and higher TBA values) making the food organoleptically unacceptable to consume. However, iron biosorbed/uptake to biomass is unavailable for reaction with food constituents resulting in lower TBA values, indicating lower oxidative changes. Similar results were observed with other studies where free iron-fortified cheese showed higher TBA values when compared with bound or encapsulated iron-fortified cheese samples (not in free state).[Citation26–Citation30] Hence, iron biosorbed/uptaken to biomass is unavailable for reaction with food constituents resulting in lower TBA values, indicating lower oxidative changes.
Determination of iron from chocolate samples
Based on the results obtained by ICP-OES () it was concluded that the amount of iron-fortified was the same with the amount of the iron released in the chocolate samples as a result of acid digestion. Hence, we can interpret that the iron will be in bioavailable form when fortified products were consumed. As a result, the malnutrition caused by trace elements can be overcome by taking metal-fortified food products without any harmful side effects routinely.
Table 4. Amount of Fe released as result of acid digestion of fortified chocolate samples
Conclusion
This study proves that the biomass of B. subtilis and yeast can biosorb/uptake iron. This is only the first study employing the microbial biomass enriched with metal as fortificant. In both the studies, chocolate fortified with free iron showed discoloration and higher TBA values during storage. However, chocolate fortified with biomass biosorb/uptake iron did not show any discoloration and had lower TBA values as control. Thus, iron-enriched yeast biomass and Bacillus biomass can serve as a vehicle for metals during fortification of foods without causing any organoleptic/deteriorative changes. They can be employed in the preparation and commercial manufacture of fortified food products to supplement iron or other trace elements. The savings due to avoiding product spoilage due to oxidative damage may compensate for cost of biomass biosorb/uptake iron production.
Acknowledgments
The authors would like to thank NCCCM, Hyderabad for their support in ICP-OES analysis.
References
- Stein, A. J.; Meenakshi, J.; Qaim, M.; Nestel, P.; Sachdev, H.; Bhutta, Z. A. Potential Impacts of Iron Biofortification in India. Soc. Sci. Med. 2008, 66, 1797–1808. DOI: 10.1016/j.socscimed.2008.01.006.
- Fao, I.; Wfp, the State of Food Insecurity in the World. The Multiple Dimensions of Food Security, FAO; Rome, 2013.
- Grantham-McGregor, S.; Ani, C. A Review of Studies on the Effect of Iron Deficiency on Cognitive Development in Children. J. Nutr. 2001, 131, 649S–668S. DOI: 10.1093/jn/131.2.649S.
- Huma, N.; Salim-Ur-Rehman,; Anjum, F. M.; Murtaza, M. A.; Sheikh, M. A. Food Fortification Strategy—Preventing Iron Deficiency Anemia: A Review. Crit. Rev. Food Sci. Nutr. 2007, 47, 259–265. DOI: 10.1080/10408390600698262.
- Allen, L. H.;. Iron Supplements: Scientific Issues Concerning Efficacy and Implications for Research and Programs. J. Nutr. 2002, 132, 813S–819S. DOI: 10.1093/jn/132.4.813S.
- Liyanage, C.; Hettiarachchi, M. Food Fortification. Ceylon Med. J. 2011, 56, 124–127.
- Kwak, H.; Yang, K.; Ahn, J. Microencapsulated Iron for Milk Fortification. J. Agric. Food. Chem. 2003, 51, 7770–7774. DOI: 10.1021/jf030199+.
- Kim, S.; Ahn, J.; Seok, J.; Kwak, H. Microencapsulated Iron for Drink Yogurt Fortification. Asian. Australas. J. Anim. Sci. 2003, 16, 581–587. DOI: 10.5713/ajas.2003.581.
- Della Lucia, C. M.; Tostes, M. D. G. V.; Silveira, C. M. M.; Bordalo, L. A.; Rodrigues, F. C.; Pinheiro-Sant’Ana, H. M.; Martino, H. S.; Costa, N. M. B. Iron Bioavailability in Wistar Rats Fed with Fortified Rice by Ultra Rice? Technology with or without Addition of Yacon Flour (Smallanthus Sonchifolius). Arch. Latinoam. Nutr. 2013, 63, 64–73.
- Mellican, R. I.; Li, J.; Mehansho, H.; Nielsen, S. S. The Role of Iron and the Factors Affecting Off-Color Development of Polyphenols. J. Agric. Food Chem. 2003, 51, 2304–2316. DOI: 10.1021/jf020681c.
- Leonardi, A.; Zanoni, S.; De Lucia, M.; Amaretti, A.; Raimondi, S.; Rossi, M. Zinc Uptake by Lactic Acid Bacteria. ISRN. Biotechnol. 2013, 2013. DOI: 10.5402/2013/312917.
- Mrvčić, J.; Stanzer, D.; Šolić, E.; Stehlik-Tomas, V. Interaction of Lactic Acid Bacteria with Metal Ions: Opportunities for Improving Food Safety and Quality. World J. Microbiol Biotechnol. 2012, 28, 2771–2782. DOI: 10.1007/s11274-012-1094-2.
- Gaensly, F.; Picheth, G.; Brand, D.; Bonfim, T. The Uptake of Different Iron Salts by the Yeast Saccharomyces Cerevisiae. Braz. J. Microbiol. 2014, 45, 491–494. DOI: 10.1590/s1517-83822014000200016.
- Qin, S.; Gao, J.; Huang, K. Effects of Different Selenium Sources on Tissue Selenium Concentrations, Blood Gsh-Px Activities and Plasma Interleukin Levels in Finishing Lambs. Biol. Trace Elem. Res. 2007, 116, 91–102. DOI: 10.1007/BF02685922.
- Adamo, G. M.; Brocca, S.; Passolunghi, S.; Salvato, B.; Lotti, M. Laboratory Evolution of Copper Tolerant Yeast Strains. Microb. Cell. Fact. 2012, 11, 1–11. DOI: 10.1186/1475-2859-11-1.
- Zhang, S.; Zhang, Y.; Peng, N.; Zhang, H.; Yao, J.; Li, Z.; Liu, L. Pharmacokinetics and Biodistribution of Zinc-Enriched Yeast in Rats. Sci. World. J. 2014, 2014, 4. DOI: https://doi.org/10.1155/2014/217142.
- Ghosh, A.; Ghartimagar, D.; Thapa, S.; Sathian, B.; De, A. Microcytic Hypochromic Anemia in Pediatric Age Group: A Hospital Based Study in Nepal. Am. J. Public. Health. Res. 2015, 3, 57–61.
- Douglas, F. W.; Rainey, N.; Wong, N.; Edmondson, L.; LaCroix, D. Color, Flavor, and Iron Bioavailability in Iron-Fortified Chocolate Milk. J. Dairy. Sci. 1981, 64, 1785–1793. DOI: 10.3168/jds.S0022-0302(81)82767-1.
- Şahin, Y.; Öztürk, A. Biosorption of Chromium (Vi) Ions from Aqueous Solution by the Bacterium Bacillus Thuringiensis. Process. Biochem. 2005, 40, 1895–1901. DOI: 10.1016/j.procbio.2004.07.002.
- Bueno, B.; Torem, M.; Molina, F.; De Mesquita, L. Biosorption of Lead (Ii), Chromium (Iii) and Copper (Ii) by R. Opacus: Equilibrium and Kinetic Studies. Miner. Eng. 2008, 21, 65–75. DOI: 10.1016/j.mineng.2007.08.013.
- Momen, A. A.; Zachariadis, G. A.; Anthemidis, A. N.; Stratis, J. A. Investigation of Four Digestion Procedures for Multi-Element Determination of Toxic and Nutrient Elements in Legumes by Inductively Coupled Plasma-Optical Emission Spectrometry. Anal. Chim. Acta. 2006, 565, 81–88. DOI: 10.1016/j.aca.2006.01.104.
- Maruthi, Y.; Dadhich, A. S.; Chaitnya, D. A.; Hosssain, K.; Avanthi, M. Biosorption of Iron (Ii & Iii) by Thiobacillus Ferrooxidans and Aspergillus Niger-Fungi Isolated from Soil in the Vicinity of Steel Plant. J. Ind. Poll. Cont. 2010, 26, 211–216.
- Richins, A.; Burton, K.; Pahulu, H. F.; Jefferies, L. K.; Dunn, M. L. Effect of Iron Source on Color and Appearance of Micronutrient-Fortified Corn Flour Tortillas. Cereal. Chem. 2008, 85, 561–565. DOI: 10.1094/CCHEM-85-4-0561.
- Kongkachuichai, R.; Kounhawej, A.; Chavasit, V.; Charoensiri, R. Effects of Various Iron Fortificants on Sensory Acceptability and Shelf-Life Stability of Instant Noodles. Food Nutr. Bull. 2007, 28, 165–172. DOI: 10.1177/156482650702800205.
- Guillen-Sans, R.; Guzman-Chozas, M. The Thiobarbituric Acid (Tba) Reaction in Foods: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 315–350. DOI: 10.1080/10408699891274228.
- Jalili, M.;. Chemical Composition and Sensory Characteristics of Feta Cheese Fortified with Iron and Ascorbic Acid. Dairy. Sci. Technol. 2016, 96, 579–589. DOI: 10.1007/s13594-016-0280-7.
- Majeed, H.; Qazi, H. J.; Safdar, W.; Fang, Z. Microencapsulation Can Be a Novel Tool in Wheat Flour with Micronutrients Fortification: Current Trends and Future Applications–A Review. Czech. J. Food Sci. 2013, 31, 527–540. DOI: 10.17221/110/2013-CJFS.
- Zhang, D.; Mahoney, A. W. Iron Fortification of Process Cheddar Cheese. J. Dairy Sci. 1991, 74, 353–358. DOI: 10.3168/jds.S0022-0302(91)78177-0.
- Azzam, M.;. Effect of Fortification with Iron-Whey Protein Complex on Quality of Yoghurt. Egypt. J. Dairy. Sci. 2009, 37, 55–63.
- El-Kholy, A. M.; Osman, M.; Gouda, A.; Ghareeb, W. A. Fortification of Yoghurt with Iron. World J. Dairy. Food. Sci. 2011, 6, 159–165.