ABSTRACT
This work investigated the effect of controlled malolactic fermentation starters (Lactobacillus plantarum NoVA and Oenococcus oeni Oenos) and an un-inoculated culture on cherry wine’s physical, biochemical and sensory properties. NoVA promoted MLF most efficiently (15d), resulted in a large increase in the concentration of many esters and aromatic alcohols, and reinforced the cherry wine’s ‘fruity’ note and overall aroma. MLF carried out by Oenos lasted for 18 days, accompanied by the increase of many volatile compounds (with 2-phenylethyl acetate and benzaldehyde as major representatives) and several biogenic amines. With respect to spontaneous MLF, Tatumella, Pantoea, Lactococcus, and Lactobacillus were identified as the dominant genera, with a fermentation period of 25 d and the total biogenic amine amount 1.6-fold than that at the end of alcoholic fermentation. This study has proved that the use of Viniflora NoVA to conduct MLF is a worthwhile alternative to the traditional cherry wine-making.
Introduction
Malolactic fermentation (MLF) is a secondary fermentation process that usually takes place during or after the completion of alcoholic fermentation.[Citation1] This stage is characterized by the transformation of L-malic acid to L-lactic acid by lactic acid bacteria (LAB), resulting in deacidification, microbial stability and sensory improvement of the wines.[Citation2] MLF can be started by controlled LAB culture or leave it going spontaneously. Spontaneous MLF is often unpredictable. It may take place several months after the completion of alcoholic fermentation, or fail due to harsh wine environmental conditions (low pH, high ethanol and/or SO2 concentrations and low temperature).[Citation2,Citation3] Also, spontaneous MLF may lead to a higher production of off-flavors, a reduction in color and a higher synthesis of biogenic amines.[Citation4,Citation5] Therefore, nowadays, the use of LAB strains as malolactic starters is a common winemaking practice. Fortunately, a wide range of LAB strains are commercially available in the market, which offers us the opportunity to explore one or more suitable MLF starter to assure a rapid and reliable fermentation process, and give wines a consistent and predictable quality.
Currently, Oenococcus oeni is the main species used in MLF as a LAB starter, as it is well adapted to the low pH and high ethanol concentration of wine. But recent research has found that some Lactobacillus genera, such as L. plantarum, can also survive and be well adapted to the winemaking conditions, such as the ability to survive at low pH conditions, a similar SO2 tolerance to O. oeni and tolerance of ethanol up to 14%.[Citation6,Citation7] Additionally, they possess many favorable biological properties in comparison with O. oeni, including higher growth speed, formation of a more complex sensory profile and prevention of several undesired compounds.[Citation6,Citation7] All these advantages make L. plantarum as the up-to-date generation wine MLF starter culture. However, the metabolic activity as well as the kinetics of such genus over MLF and its influence on the quality of wines still need extensive investigation, and more evidence from fruit wine-making practice, other than grape wine, should be supplied, in order to explore the great potential of such genus in the wine industry.
Cherry wine is one of the most popular fruit wines consumed worldwide. This wine is attractive to consumers because of its color, taste, and flavor, as well as enrichment of antioxidant components.[Citation8,Citation9] To date, no studies have been reported in the literature concerning the comparison of commercial LAB strains including L. plantarum and O. oeni on the production of cherry wines. Therefore, in this study, the performance of the selected commercial LAB strains during MLF in cherry wines was assayed. Their influence on the modulation of cherry wine compositions (basic parameters and aromatic profile), sensory property, and biogenic amine concentrations was also evaluated. Besides, in order to exquisitely elucidate and compare the effect of commercial LAB on the MLF process, a spontaneous malolactic fermentation was conducted in a parallel experiment.
Materials and methods
Cherries and commercial microorganisms
Cherry fruits of “Lapins”, picked at commercial maturity from the local cherry orchard (Yantai, China) during the 2017 harvest season were used in this work. The analytical data of the initial musts were: total sugars 166 g/L, titratable acidity 6.7 g/L, pH 3.86. A commercial S. cerevisiae strain of Lalvin RC212 (Lallemand Inc., Montreal, Canada), a commercial O. oeni strain of Viniflora Oenos (Chr.-Han, Horsholm, Denmark) and a commercial L. plantarum of Viniflora NoVA (Chr.-Han, Horsholm, Denmark) were used for the fermentations.
Cherry wine production
A total of 36 kg cherries was destemmed, crushed, poured into 50 L stainless steel-jacketed fermenters, and treated with potassium metabisulphite (50 mg/L) and pectinolytic preparation of Lallzyme EX-V pectinase (20 mg/L). Sucrose was supplemented to a concentration of 210 g/L. The alcoholic fermentation was started by the inoculation of S. cerevisiae strain RC212 (250 mg/L) which was rehydrated according to the manufactures recommendations, and carried out under static conditions at 25°C until the sugar content was reduced below 4 g/L. At the end of alcoholic fermentation, yeast cells were removed by centrifugation at 7000 rpm for 10 min, and the resultant wines were placed homogeneously into nine 50-L stainless steel vessels.
MLF was carried out at 20°C in triplicate. Controlled MLF was started by inoculation of LAB species (NoVA or Oenos) at a concentration of 30 mg/L according to the manufactures instructions. Spontaneous MLF was conducted without inoculations, and samples were taken at the 4th, 12th, and 20th day in order to evaluate the bacterial diversity during this process. For simplicity, we define M4d, M12d, and M20d as the cherry wines sampled in those days, respectively. For all malolactic cultures, the progress was followed by measuring wine L-malic acid content (L-malic acid Enzymatic BioAnalysis, Boehringer-Mannheim/R-Biopharm, Darmstadt, Germany) until the L-malic acid concentration was reduced below 0.5 g/L. When MLF was finished, young cherry wines were sulfited and stored at 4°C until the analysis.
Bacterial count
Serial decimal dilutions were prepared in sterile saline solution (0.9% NaCl), and appropriate volumes were spread in duplicate onto fresh MRS agar plates (Scharlau Chemie S.A., Barcelona, Spain) with 200 μg/mL of nystatin (Acofarma, S. Coop., Terrassa. Spain) for bacterial counts. These plates were incubated at 30°C under anaerobic conditions for at least 5 days.[Citation8] Additionally, 32 colonies (15 out of NoVA, 17 out of Oenos) were randomly picked up from the agar plate and their taxonomic identities at species were confirmed by the amplification of rpoB gene using PCR-DGGE.[Citation10]
Next-generation sequencing
The evaluation of bacterial diversity during cherry wine spontaneous MLF was carried out using a next-generation sequencing method, according to Xu.[Citation11] DNA of samples was extracted using E.Z.N.ATM Mag-Bind Soil DNA Kit (OMEGA, USA), and sent to Sangon Biotech (Shanghai Co., Ltd., China) for amplification and sequence determination. V3-V4 region of the 16S rRNA gene was amplified using the primer pair 341F/805R with adapter sequences (lower case) for Illumina Miseq 2 × 300. Raw Illumina fastq files were then processed by Cutadapt, Pear, Prinseq, Usearch, and Uchime software. Then, the multiple sequences were grouped into Operational Taxonomic Units (OTUs) using the Usearch with a threshold of 97% pairwise identity. The most abundant sequence of each OUT was picked and submitted to Ribosomal Database Project (RDP) classifier for taxonomic identity using a 0.80 confidence threshold. The Shannon, Chao1, and Simpson indexes were calculated by Mothur to investigate the abundance and diversity of microbial communities. Venn Diagram in the R statistical environment was used in calculating the number of shared and unique OTUs in the different datasets.
Basic analysis
Conventional enological parameters, including total reducing sugars, titratable acidity (expressed as g/L of malic acid), pH, ethanol, and volatile acidity, were determined according to the OIV official methods.[Citation12]
Volatile compounds determination
Volatile compounds in cherry wines were extracted by HS-SPME followed by identification and quantification on GC-MS, according to Sun et al.[Citation13]
Biogenic amine analysis
The concentration of the biogenic amine in cherry wines was assayed according to the Chinese national standards GB5009.208–2016, as stated in previous publication.[Citation14] The concentration of the biogenic amine in cherry wines was assayed according to the Chinese national standards GB5009.208–2016. Before derivatization, fat in the samples was removed by hexane, and solid NaCl was added to fat-free samples to saturation and pH adjustment to 12.0 by 0.1mol/L NaOH. Extraction solvent (butanol:trichloromethane = 1:1, v/v) was added to the equal amount of fat-free sample, followed by vigorous stirring and centrifugation at 3100 × g for 10 min. Precipitate was discarded and supernatant was treated with 0.2 mL 1mol/L HCl. Excessive extraction solvent was removed by nitrogen flowing at 40°C, and the residues were dissolved in 1 mL of 0.1 mol/L HCl, ready for the derivatization.
An aliquot of 0.5 mL pretreated cherry wine, 1.5 mL saturated sodium bicarbonate and 1 mL Dns-Cl solution (10 g/L in acetone) were added to a 10 mL vial. After agitation, this mixture was subjected to incubation at 60°C for 30 min, followed by addition of 0.1 mL sodium glutamate (50 g/L in saturated sodium bicarbonate) and an extended incubation at 60°C for 15 min. After incubation, acetone was removed by nitrogen flowing and 3 mL ether was added, excessive of which was removed by stream of nitrogen. The residues were reconstituted by 1 mL methanol, filtered through 0.2 μm filters and injected into HPLC. All analyses were in triplicate.
The quantification of biogenic amines was carried out on a Shimadzu Prominence LC-20A HPLC (Shimadzu Corporation, Kyoto, Japan) coupled with a variable wavelength UV-Vis spectrophotometer. Separation was performed on an Inertsil ODS-SP (4.6 × 250 mm, particle diameter 5 μm) column (GL Sciences, Tokyo, Japan). Methanol and ultra-pure water were used as solvents A and B, respectively. The gradient profile was: 0–7 min, 55–65% A; 7–14 min, 65–70% A; 14–27 min, 70–90% A; 27–30 min, 90–100% A; 30–36 min, 100–55% A; 36–45 min, 55% A). The dansylamides were detected by monitoring their UV absorbance at 254 nm.
Sensory analysis
Quantitative descriptive analysis (QDA) of the tested cherry wines was carried out by a sensory expert panel comprising nine tasters (six females and three males, ranging in ages from 27 to 42, with an average age of 34). The odors perceived were classified into six attributes (floral, fruity, vegetable, nutty, alcohol, and global aroma). Samples were served at 18°C in standard wine-testing glasses (ISO 3591, 1997) labeled with three numbers, and presented to the judges in a random order. And, the judges were asked to rate the intensity of each of the attributes on the basis of a 9-point scale where zero represented nonexistence and nine corresponded to the highest intensity. Each sample was judged in duplicate.
Statistical analysis
Statistical analyses were performed using the SPSS version 16.0 statistical package for windows (SPSS Inc., Chicago, Illinois). ANOVA and Duncan’s multiple range tests were applied to the data to determine significant differences, and the model was statistically significant with a value of P< .05
Results and discussion
Evolution of wine-related microorganisms during MLF
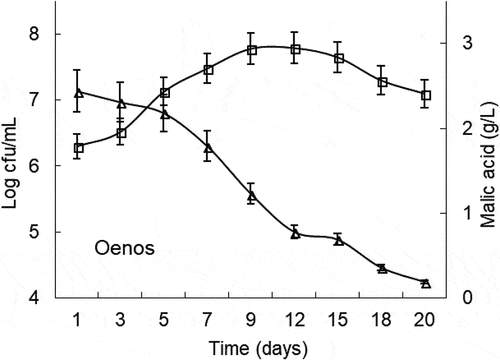
The alcoholic fermentation lasted for approximately 8 days, and the resulting cherry wines reached an alcoholic degree of 10.9% v/v and 0.18 g/L of volatile acidity (). With respect to MLF, the relationship between the L-malic acid degradation rate and the controlled LAB culture viability in the cherry wines is presented in and . A distinct difference in the behavior of the wines made with L. plantarum NoVA and O. oeni Oenos can be seen from the data. NoVA was quickly adapted to the fermenting musts, exhibited rapid growth and provided a complete degradation of L-malic acid. At full population, NoVA reached a maximal cell population of around 6.3 × 107 CFU/mL, and thereafter the bacterial viability declined quickly, which helps to prevent the formation of putative undesirable compounds at the end of winemaking. MLF conducted by L. plantarum NoVA was completed in less than 15 days. In the case of Oenos, it was slightly inhibited at the beginning of MLF and a lag of almost 48 h occurred in the initiation of L-malic acid metabolism. Then, Oenos began to develop prosperously and L-malic acid began to decline. At full MLF, Oenos acquired a maximal concentration of around 4.9 × 107 CFU/mL, and maintained a higher cell viability till the completion of MLF. MLF carried out by O. oeni Oenos lasted for 18 days, 3 days longer than that of NoVA.
Table 2. Basic composition of cherry wines
Figure 1. NoVA counts and time courses of malic acid concentrations during MLF in cherry wines. Values are mean of triplicates±SE. (■) denotes log CFU/mL and (▲) represents malic acid concentration
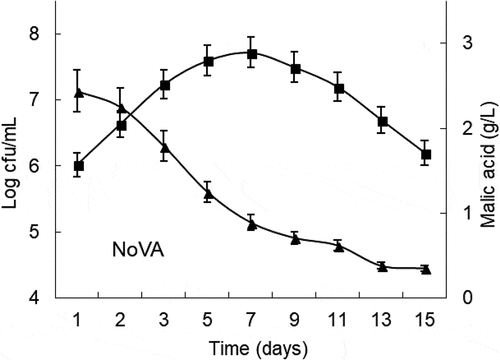
Figure 2. Oenos counts and time courses of malic acid concentrations during MLF in cherry wines. Values are mean of triplicates±SE. (□) denotes log CFU/mL and (Δ) represents malic acid concentration
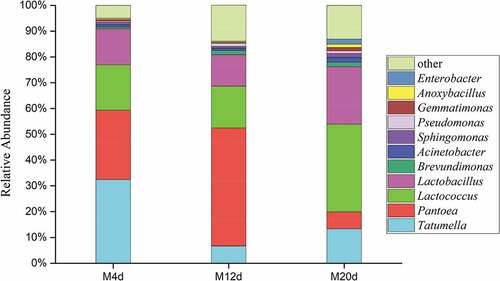
With regard to spontaneous MLF, a next-generation sequencing method was used to evaluate the autochthonous bacterial communities present in the cherry wines. Samples were taken at the 4th, 12th and 20th day of cherry wine spontaneous MLF. As seen in , a total of 805, 1193, and 1627 operational taxonomic units (OTUs; 97% nucleotide identity) were obtained from MLF 4d, 12d and 20d samples, respectively, and among which, 141 OTUs were found to be common to all. The bacterial diversity of cherry wines assessed in this study was characterized by 11 main different genera (). And, these genera were classified according to family and Gram staining (). This is not surprising as it is well reported that only a fraction of most environmental bacteria have been cultivated, at least on standard culture media.[Citation15] The predominant groups were Tatumella, Pantoea, Lactococcus, and Lactobacillus genera. Other isolates were members of the genera Brevundimonas, Acinetobacter, Sphingomonas, Pseudomonas, and Enterobacter.
Figure 3. The VEEN diagrams of the three cherry wine samples resulting from MLF according to bacterial biodiversity
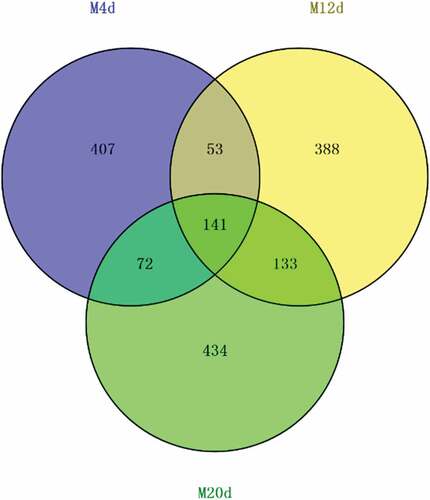
Table 1. Classification of dominant bacterial genera to family and Gram staining
Although the major genera among the three samples were uniform, their relative abundances were different. In M4d sample, the dominant genus was Tatumella (32.46%) and Pantoea (26.91%), followed by Lactococcus (17.54%) and Lactobacillus (14.12%). In M12d sample, the percentage of Pantoea was increased by 18.9% while Tatumella was decreased by 25.75%, and Lactococcus (16.19%) and Lactobacillus (12.26%) both had slight decreases. At near end of MLF (in M20d sample), Lactococcus (34.02%) and Lactobacillus (22.29%) were found to be the most prevalent genera; the Tatumella content was increased again and reached a level of about 13.27% whereas Pantoea concentration decreased sharply to 6.57%; genera like Brevundimonas, Acinetobacter, Sphingomonas, Pseudomonas, Gemmatimonas, Anoxybacillus, and Enterobacter were detected still at minor proportions (<2%). As for the reduction of malic acid, it was found a delay of almost 9 days occurred before the onset of MLF. Then, the concentration of L-malic acid began to slowly decline and the MLF period was extended to 25 days (data not shown).
From the taxonomic analysis results, it should be pointed out that the next-generation sequencing method used in the current study revealed a very heterogeneous microflora in spontaneously fermented cherry wines, and the microbes detected are widely distributed in the environment, including soil and air.[Citation16] Lactococcus and Lactobacillus, usually associated with MLF of wine, showed an overall increasing tendency along with the fermentation process, till they became the dominant genera at the end. Pantoea and Acinetobacter have been isolated from other fermenting materials, such as grapes, but were reported as innocuous contaminants in musts and wines.[Citation17,Citation18] Sphingomonas was also detected previously during grape wine fermentation, but its role and impact on wine quality and organoleptic properties remains unknown.[Citation15] Acetic bacteria, related to spoilage of the wine, was not detected in the current study.
Chemical composition of cherry wines
summarizes the mean values ± standard deviation of the classic enological parameters of the cherry wines before and after MLF. There were negligible or slight differences in the ethanol, L-malic acid and titratable acidity content among the tested samples following MLF, with an average value of 10.9% (v/v), 0.35 g/L, and 8.00 g/L, respectively. An increase in the range of 0.02–0.11 in pH was observed, in response to the decrease of L-malic acid and titratable acidity levels. The least pH increase was found in NoVA derived sample whereas the largest increase was in spontaneous fermentation. The former observation was in accordance with the manufacturers’ statement that NoVA will complete the MLF in the must without increasing the pH, which aids the winemaker in keeping the wine pH down. A measure of volatile acidity is used routinely as an indicator of wine spoilage. Such parameter showed a significant increase as a result of spontaneous fermentation, and only had small changes in the other two controlled MLF wines.
Aromatic profile of cherry wines
lists the concentrations of volatile compounds identified in the cherry wines before and after MLF, including alcohols, acids, esters, aldehydes, ketones, and phenols. Esters are the main volatile compounds, which evidenced changes due to wine fermentation with LAB strains. The inoculation of NoVA into cherry musts resulted in an increase in the concentration of many esters, such as ethyl butyrate, ethyl 3-methylbutanoate, 3-methylbutyl acetate, and ethyl hexanoate. These esters contribute desirable and fruity aromas to the cherry wine, including sweet, banana, pineapple, and pear notes, and many of which are characterized by very low olfactory detection thresholds; therefore, the enhancement of these compounds following MLF in NoVA cultured cherry wines could have important sensory implications. Similarly, the ester concentrations in the samples inoculated with Oenos were also increased. Particularly, the contents of diethyl succinate and 2-phenylethyl acetate showed marked increase of 33% and 100% in Oenos than that of initial cherry wine after alcoholic fermentation. The generalized increase in many esters occurred not only in the cherry wines with controlled MLF but also in the sample with spontaneous MLF, as evidenced that a notably higher production of ethyl acetate, ethyl decanoate, and ethyl benzoate was detected in such a wine.
Table 3. Concentrations of volatile compounds (mg/L) in cherry wines before and after MLF
A total of nine compounds were identified in the volatile alcohol group. They mainly are produced through yeast metabolism during wine fermentation[Citation19], while during MLF their concentrations may be increase or decrease. Both spontaneous and controlled MLF resulted in the decrease of most assayed alcohols, particularly in the case of 3-methyl-1-butanol and β-phenylethyl alcohol. Benzyl alcohol is the only compound that showed growth (4.8 ~ 38.1%) among all the wine samples. 1-Propanol is another alcohol that should be noted, since spontaneous MLF significantly increased its concentration whereas controlled MLF decreased its production.
Acetic, hexanoic, and octanoic acids are three volatile acids quantified in the cherry wines. Wine sample made from spontaneous fermentation showed the highest level of acetic acid, followed by Oenos and NoVA. As for the reason why Oenos produced more such compound than NoVA, it should be ascribed to the heterofermentative activity that O. oeni strain possesses, and acetic acid can be synthesized from hexoses as a result, whereas L. plantarum is homofermentative and is, therefore, unable to.[Citation6]
Three aldehydes were identified in this study: acetaldehyde, furfural, and benzaldehyde. Controlled MLF and spontaneous fermentation all resulted in a decrease of acetaldehyde in the cherry wines. This is beneficial from a sensory perspective as the decrease of this compound may cause a reduction of the herbaceous and green aroma of wines.[Citation20] Benzaldehyde is a characteristic aromatic compound in the cherry wine.[Citation21] Oenos increased its amount following MLF compared to the level at the exact end point of alcoholic fermentation, whereas NoVA resulted in a significantly lower concentration.
Two ketones were quantified in the cherry wines: 2,3-butanedione and 3-hydroxy-2-butanone. They are two typical volatile compounds associated with MLF. At a low amount (≤5–7 mg/L), they contribute to the bouquet of the wine and add complexity to the wine aroma. Whereas if the concentration rises above the limit, a detrimental effect could be exerted.[Citation22] Oenos synthesized more of these two compounds than NoVA in the same wine condition. This could be correlated with the citrate lyase complex that O. oeni strain possesses, and 2,3-butanedione can be produced from citric acid as a result, whereas some Lb. plantarum strains did not have such a complex and therefore led to a very low production of such compound.[Citation23]
Only one volatile phenol (4-ethyl phenol) was detected from the cherry wine samples, but its presence is very important because it may undermine the final quality of wine.[Citation24] Spontaneous MLF and NoVA led to its formation, but its concentration in this study was quite small, far below its sensory threshold. Such an observation is in agreement with previous reports that a few Lactobacillus species could produce very small amounts of volatile phenols over MLF[Citation25], due to the presence of a putative pad gene in these strains.
In addition, odor activity value (OAV) was used to evaluate the contribution of each compound to the aroma of cherry wines () in this study. This parameter is expressed as the ratio between the volatile concentration and its odour threshold, which is very useful in assessing the relative importance of individual chemical components present in food[25,.[Citation29][Citation24] Five ester components, i.e., ethyl butyrate, ethyl 3-methylbutanoate, 3-methylbutyl acetate, ethyl hexanoate, and ethyl octanoate were found with OAV > 1 in this study, contributing apple, strawberry, pineapple and other fruity notes to cherry wine. They showed higher values in the NoVA cultured samples, suggesting a more intense fruity aroma might be perceived in such a wine. Octanoic acid had an OAV > 1 in all the samples, and would probably exert a cheesy note to the wine samples. The inoculations of NoVa and Oenos evidently enhanced the synthesis of α-terpineol which was responsible for floral aroma, implying that a stronger floral nuance could be detected from these two cherry wines. Benzaldehyde, associated with an almond and burnt sugar note, had OAV > 3 in all the samples. Oenos cultured cherry wine showed highest OAVs of such compound[Citation25,Citation29,Citation30], indicating that the most intense almond nuance might be sensed in this wine.
Table 4. Mean values of OAV for volatile compounds which presented OAV > 1 in cherry wines
Biogenic amine profile of cherry wines
shows the means and standard deviation of the seven biogenic amines determined in the cherry wines, before and after MLF. Spermine and tyramine were two most abundant BAs detected in the samples when alcoholic fermentation was completed, while the other five all covered in medium or lesser levels. Spontaneous MLF resulted in a marked increase in the concentration of most amines, with the total amount 1.6-fold than that at the exact end of alcoholic fermentation. In particular, the content of tyramine, tryptamine, and histamine increased by 69.6%, 88.5% and 172.3% following MLF. It is assumed that the wine condition favored the growth of some autochthonous surviving strains with the relevant decarboxylase enzymes, and/or directly stimulated the enzyme activity and facilitated the conversion of the amino acids to biogenic amines. Together with the results obtained from taxonomic analysis in Section “Evolution of wine-related microorganisms during MLF,” it was found that many genera identified in this study have been reported associated with the synthesis of BA, particularly Lactobacillus, Lactococcus, Pseudomonas, and Enterobacter.[Citation31,Citation32] Further studies are still required for the intensive investigation on the relationship between these autochthonous microbes and the formation of various amine compounds.
Table 5. Concentrations of biogenic amines (mg/L) in cherry wines before and after MLF
The use of Oenos also led to an increase in the total biogenic amine amount, but the extent was significantly less than that of spontaneous MLF. Tryptamine and histamine presented the largest increase (>30%) following MLF, and spermine (11.9%) and tyramine (13.2%) showed medium increase. With respect to NoVA cultured cherry wines, MLF slightly affected these BAs analyzed, since five of these amine compounds including histamine, phenylethylamine, cadaverine, spermidine, and tyramine remained nearly unchanged following MLF.
Sensory evaluation
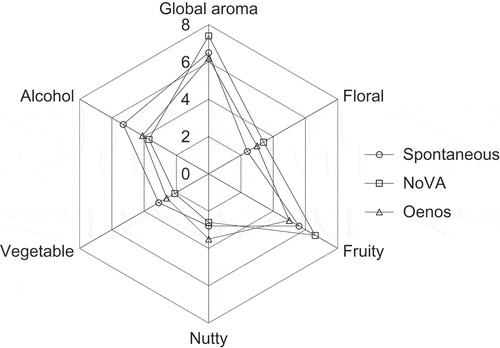
Cherry wines were analyzed for selected aroma attributes by a trained panel, and the results are presented in . As seen, cherry wines resulting from spontaneous fermentation were characterized by a moderate sense of ‘fruity’ and ‘alcohol’, and slight notes of ‘floral’, ‘vegetable’ and ‘nutty’. In comparison with that wine, samples inoculated with NoVA were graded with higher scores for ‘fruity’ and ‘floral’ and lower values for ‘alcohol’ and ‘vegetable’, showing an increase of the overall aroma. In the case of Oenos cultured cherry wine, it was found that this wine scored the lowest for the ‘fruity’ and ‘global aroma’, but was distinguished by its higher perception of ‘nutty’ descriptor. All these observations suggested that the contribution of LAB to the wine sensory quality was quite significant. Understanding the mechanism of LAB species in the cherry wine-making process, especially in the MLF process, will help to explain their influences on the sensory profile.
From the aroma analysis and sensory evaluation result, we assume that the sensory differences can be ascribed to the variable concentrations of some aromatic compounds produced by different LAB species. The ‘fruity’ character rating 18% and 32% higher in NoVA derived cherry wine, relative to that of spontaneous MLF and Oenos, could be linked to higher concentrations of several fruity ethyl esters synthesized by NoVA, such as ethyl butyrate, 3-methylbutyl acetate, and ethyl hexanoate. The higher sense of ‘alcohol’ perceived in the spontaneous fermentation wine may be associated with a higher concentration of 1-propanol in such a wine. Benzaldehyde, responsible for an almond and burnt sugar note, was determined in its highest concentration in Oenos cultured samples, which would probably agree with the highest perception intensity of ‘nutty’ in this wine.
Conclusion
In this study, we compared the effect of controlled MLF by selected LAB starter and uninoculated culture on cherry wine production. Results showed that all these MLF processes can induce a wide range of modulation to the wine’s basic composition, aromatic and sensory profiles as well as biogenic amine concentration. Finally, L. plantarum Viniflora NoVA was proved to be a better LAB starter due to the enhanced overall aroma of the resulting wines, a complete and rapid MLF, as well as the benefits of almost no formation of biogenic amines. Future research should investigate the performance of L. plantarum NoVA at an industrial scale, and the possibility of using this wine-making practice with other cherry varieties.
Acknowledgments
We are grateful to the National Youth Science Foundation of China (Nos. 31701570 and 31501577).
Additional information
Funding
References
- Bartowsky, E. J.; Costello, P. J.; Chambers, P. J. Emerging Trends in the Application of Malolactic Fermentation. Aus. J. Grap. Wine Res. 2015, 21, 663–669. DOI: 10.1111/ajgw.12185.
- Lucio, O.; Pardo, I.; Heras, J. M.; Krieger-Weber, S.; Ferrer, S. Use of Starter Cultures of Lactobacillus to Induce Malolactic Fermentation in Wine. Aus. J. Grap Wine Res. 2017, 23, 15–21. DOI: 10.1111/ajgw.2017.23.issue-1.
- Berbegal, C.; Peña, N.; Russo, P.; Grieco, F.; Pardo, I.; Ferrer, S.; Spano, G.; Capozzi, V. Technological Properties of Lactobacillus Plantarum Strains Isolated from Grape Must Fermentation. Food Microbiol. 2016, 57, 187–194. DOI: 10.1016/j.fm.2016.03.002.
- Guo, -Y.-Y.; Yang, Y.-P.; Peng, Q.; Han, Y. Biogenic Amines in Wine: A Review. Int. J. Food Sci. Technol. 2015, 50, 1523–1532. DOI: 10.1111/ijfs.12833.
- Henríquez-Aedo, K.; Durán, D.; Garcia, A.; Hengst, M. B.; Aranda, M. Identification of Biogenic Amines-Producing Lactic Acid Bacteria Isolated from Spontaneous Malolactic Fermentation of Chilean Red Wines. LWT - Food Sci. Technol. 2016, 68, 183–189. DOI: 10.1016/j.lwt.2015.12.003.
- Brizuela, N. S.; Bravo-Ferrada, B. M.; La Hens, D. V.; Hollmann, A.; Delfederico, L.; Caballero, A.; Tymczyszyn, E. E.; Semorile, L. Comparative Vinification Assays with Selected Patagonian Strains of Oenococcus Oeni and Lactobacillus Plantarum. LWT - Food Sci. Technol. 2017, 77, 348–355. DOI: 10.1016/j.lwt.2016.11.023.
- Du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The Next Generation of Malolactic Fermentation Starter Cultures—An Overview. Food Bioprocess. Technol. 2011, 4, 876–906. DOI: 10.1007/s11947-010-0448-8.
- Sun, S. Y.; Che, C. Y.; Sun, T. F.; Lv, Z. Z.; He, S. X.; Gu, H. N.; Shen, W. J.; Chi, D. C.; Gao, Y. Evaluation of Sequential Inoculation of Saccharomyces Cerevisiae and Oenococcus Oeni Strains on the Chemical and Aromatic Profiles of Cherry Wines. Food Chem. 2013, 138, 2233–2241. DOI: 10.1016/j.foodchem.2012.12.032.
- Xiao, Z.-B.; Zhang, N.; Niu, Y.-W.; Feng, T.; Tian, H.-X.; Zhu, J.-C.; Yu, H.-Y. Multivariate Classification of Cherry Wines Based on Headspace Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry of Volatile Compounds. Int. J. Food Prop. 2015, 18, 1272–1287. DOI: 10.1080/10942912.2012.710286.
- Spano, G.; Lonvaud-Funel, A.; Claisse, O.; Massa, S. In Vivo PCR-DGGE Analysis of Lactobacillus Plantarum and Oenococcus Oeni Populations in Red Wine. Curr. Microbiol. 2007, 54, 9–13. DOI: 10.1007/s00284-006-0136-0.
- Xu, J.; He, J.; Wang, M.; Cultivation, L. L. Stable Operation of Aerobic Granular Sludge at Low Temperature by Sieving Out the Batt-like Sludge. Chemosphere. 2018, 211, 1219–1227. DOI: 10.1016/j.chemosphere.2018.08.018.
- OIV. Compendium of International Methods of Wine and Must Analysis; Organisation Internationale de la Vigne et du Vin: Paris, 2005.
- Sun, S. Y.; Jiang, W. G.; Zhao, Y. P. Evaluation of Different Saccharomyces Cerevisiae Strains on the Profile of Volatile Compounds and Polyphenols in Cherry Wines. Food Chem. 2011, 127, 547–555. DOI: 10.1016/j.foodchem.2011.01.039.
- Sun, S. Y.; Chen, Z. X.; Jin, C. W. Combined Influence of Lactic Acid Bacteria Starter and Final pH on the Induction of Malolactic Fermentation and Quality of Cherry Wines. LWT Food Sci. Technol. 2018, 89, 449–456. DOI: 10.1016/j.lwt.2017.11.023.
- Portillo, M. D. C.; Franquès, J.; Araque, I.; Reguant, C.; Bordons, A. Bacterial Diversity of Grenache and Carignan Grape Surface from Different Vineyards at Priorat Wine Region (catalonia, Spain). Int. J. Food Microbiol. 2016, 219, 56–63. DOI: 10.1016/j.ijfoodmicro.2015.12.002.
- Gilbert, J. A.; van der Lelie, D.; Zarraonaindia, I. Microbial Terroir for Wine Grapes. Proc. National Academy Sci. 2014, 111, 5–6. DOI: 10.1073/pnas.1320471110.
- Subden, R. E.; Husnik, J. I.; van Twest, R.; van der Merwe, G.; van Vuuren, H. J. J. Autochthonous Microbial Population in a Niagara Peninsula Icewine Must. Food Res. Int. 2003, 36, 747–751. DOI: 10.1016/S0963-9969(03)00034-6.
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The Microbial Ecology of Wine Grape Berries. Int. J. Food Microbiol. 2012, 153, 243–259. DOI: 10.1016/j.ijfoodmicro.2011.11.025.
- Duan, W.-P.; Zhu, B.-Q.; Song, -R.-R.; Zhang, B.; Lan, Y.-B.; Zhu, X.; Duan, C.-Q.; Han, S.-Y.; Composition, V. Aromatic Attributes of Wine Made with Vitisvinifera L.cv Cabernet Sauvignon Grapes in the Xinjiang Region of China: Effect of Different Commercial Yeasts. International. J. Food Prop. 2018, 21, 1423–1441. DOI: 10.1080/10942912.2018.1479860.
- Izquierdo Cañas, P. M.; García Romero, E.; Gómez Alonso, S.; Palop Herreros, M. L. L. Changes in the Aromatic Composition of Tempranillo Wines during Spontaneous Malolactic Fermentation. J. Food Compost. Anal. 2008, 21, 724–730. DOI: 10.1016/j.jfca.2007.12.005.
- Xiao, Z.; Zhou, X.; Niu, Y.; Yu, D.; Zhu, J.; Optimization, Z. G. Application of Headspace-Solid-Phase Micro-Extraction Coupled with Gas Chromatography–Mass Spectrometry for the Determination of Volatile Compounds in Cherry Wines. J. Chromatogr. B. 2015, 978, 122–130. DOI: 10.1016/j.jchromb.2014.12.006.
- Bartowsky, E. J.; Henschke, P. A. The ‘buttery’ Attribute of Wine-diacetyl-desirability, Spoilage and Beyond. Int. J. Food Microbiol. 2004, 96, 235–252. DOI: 10.1016/j.ijfoodmicro.2004.05.013.
- Mtshali, P. S.; Divol, B.; Du Toit, M. Identification and Characterization of Lactobacillus Florum Strains Isolated from South African Grape and Wine Samples. Int. J. Food Microbiol. 2012, 153, 106–113. DOI: 10.1016/j.ijfoodmicro.2011.10.023.
- He, Y.-N.; Ning, P.-F.; Yue, T.-X.; Zhang, Z.-W. Volatile Profiles of Cabernet Gernischet Wine under Rain-shelter Cultivation and Open-field Cultivation Using Solid-phase Micro-extraction–Gas chromatography/Mass Spectrometry. Int. J. Food Prop. 2017, 20, 2181–2196. DOI: 10.1080/10942912.2016.1174711.
- Couto, J. A.; Campos, F. M.; Figueiredo, A. R.; Hogg, T. A. Ability of Lactic Acid Bacteria to Produce Volatile Phenols. Am. J. Enol. Vitic. 2006, 57, 166–171.
- Capone, S.; Tufariello, M.; Siciliano, P. Analytical Characterisation of Negroamaro Red Wines by “aroma Wheels”. Food Chem. 2013, 141, 2906–2915. DOI: 10.1016/j.foodchem.2013.05.105.
- González Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Relationships between Godello White Wine Sensory Properties and Its Aromatic Fingerprinting Obtained by GC-MS. Food Chem. 2011, 129, 890–898. DOI: 10.1016/j.foodchem.2011.05.040.
- Etiévant P. X., “Wine,” In: Maarse H., Ed., Volatile Compounds in Foods and Beverages, Marcel Dekker, New York, 1991, pp. 483-546.
- Kim, B.-H.; Park, S. K.; Aroma, V. Sensory Analysis of Black Raspberry Wines Fermented by Different Yeast Strains. J. Inst. Brew. 2015, 121, 87–94. DOI: 10.1002/jib.199.
- Ferreira, V.; López, R.; Cacho, J. F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. DOI: 10.1002/1097-0010(20000901)80:11<1659::AID-JSFA693>3.0.CO;2-6.
- Özogul, F.; Kacar, Ç.; Hamed, I. Inhibition Effects of Carvacrol on Biogenic Amines Formation by Common Food-borne Pathogens in Histidine Decarboxylase Broth. LWT - Food Sci. Technol. 2015, 64, 50–55. DOI: 10.1016/j.lwt.2015.05.027.
- Guillén-Velasco, S.; Ponce-Alquicira, E.; Farrés-González Saravia, A.; Guerrero-Legarreta, I. Histamine Production by Two Enterobacteriaceae Strains Isolated from Tuna (thunnus Thynnus) and Jack Mackerel (trachurus Murphyii). Int. J. Food Prop. 2004, 7, 91–103. DOI: 10.1081/JFP-120022984.