?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
High-pressure processing (HPP) is a novel non-thermal processing technology, ensuring the safety of food products, as well as preserving their nutritional and functional characteristics, and avoiding the harmful effects of traditional thermal technologies. This study examined the inactivation of microorganisms and the HPP kinetic model in fermented pomegranate juice (FPJ) at different pressures (400 MPa, 500 MPa, and 600 MPa), and different treatment times (3 min, 5 min, 7 min, and 10 min). Moreover, HPP and thermal processing (TP) (65 °C/20 min) treatment responsible for a similar microbial inactivation activity, were compared by examining their impact on bioactive compounds, volatile compounds, and various quality attributes to provide a theoretical basis for FPJ processing. The inactivation curves of the microorganisms as a result of HPP were fitted using the Weibull model. Furthermore, HPP at or over 600 MPa/3 min inactivated the microorganisms and sufficiently retained the microbial populations investigated in this study below the detection limit. Following HPP and TP treatment, the total color difference (ΔE) values, total soluble solids (TSS), pH and titratable acidity (TA) had not significantly (p>0.05) changed. For total flavonoids, total phenols, anthocyanins, and antioxidant activity, the maximal retention of 91.18%, 97.52%, 95.69%, and 95.89% was achieved at 600 MPa/3 min, which was 4.62%, 24.61%, 26.83%, and 7.08% higher than in the TP-treated sample. The main volatile compounds in FPJ were esters and alcohols, and the FPJ treated at 600 MPa/3 min exhibited the highest alcohol content, while the esters were 2.27% higher than in the control. Considering the pasteurization effect and quality maintenance abilities of HPP and TP treatment, 600 MPa/3 min could be considered as an optimal condition in facilitating FPJ processing.
Introduction
Pomegranate (Punica granatum L) belongs to the Punicaceae family, which are widely distributed throughout the temperate and subtropical regions of Asia, Africa, America, and southern Europe.[Citation1] The juice of the pomegranate is a particularly popular fruit beverage due to its attractive color, and pleasant aroma, as well as its nutritional and bioactive characteristics. One such bioactive attribute of this juice is its antioxidative compounds, in particular, its phenolic compounds, with an abundance of anthocyanins.[Citation2,Citation3] Moreover, pomegranates reportedly play a role in the prevention of cancer, cardiovascular, and other chronic diseases,[Citation1,Citation4] due to their significant antioxidative activity in vivo against reactive oxygen species. TP is the most common method of extending the shelf life of juices by deactivating microorganisms and enzymes. However, this method significantly depreciates the quality of the juice by causing the loss of nutritional components, and inducing changes in color, flavor, and texture due to their heat sensitivity.[Citation5–Citation7] Subjecting pomegranate juice to TP might cause the degradation of anthocyanins, leading to a brownish color during processing and storage.[Citation8] The color changes substantially affect consumer behavior and result in a loss of the marketability of processed pomegranate products.[Citation8] These concerns sparked a growing interest in investigating the microbial inhibition and enzymatic inactivation using novel technologies that can circumnavigate or reduce heat during this process.
Since consumers demand food products that are fresh, or minimally processed the application of non-thermal technologies is gaining popularity.[Citation9] HPP represents a highly promising processing method that uses water as a pressure-transmitting medium, to expose foods to 100 MPa – 1000 MPa at room temperature, facilitating instantaneous transmittance of isostatic pressure to the products.[Citation5] Pathogens and vegetative spoilage microorganisms are inactivated by HPP treatment with minimal impact on the sensory and nutritional qualities of raw materials. This is due to its limited effect on the covalent bonds of low molecular-mass compounds, ensuring the safety of food products, as well as the retention of maximum freshness.[Citation10,Citation11] Previous studies have reported on the application of HPP in various fruit and vegetable products such as orange juice,[Citation12] green asparagus juice,[Citation6] mulberry juice,[Citation13] pomegranate juice,[Citation5,Citation11], and blueberry juice.[Citation14] Researchers focused on the effects of HPP on the anthocyanins, color, antioxidant capacity, and total phenols, as well as the inactivation of microorganisms or enzymes in those juices. Results indicated that HPP could improve the quality of juices, while preserving the natural anthocyanin content.[Citation5,Citation13] Therefore, HPP provides a unique opportunity for food processors to develop a new generation of value-added food products with superior quality to those processed conventionally.
Several published studies exist regarding the effects of HPP on pomegranate products.[Citation5,Citation11] Results indicate that the inactivation of microorganisms by HPP can be fitted using the Weibull model, while the HPP-treated samples display higher retention in color, anthocyanins, antioxidant activity, and an elevated total of phenols.[Citation5] HPP at room temperature has been reported to improve the quality of pomegranate juice, intensifying the red color of the fresh juice, and preserving the natural anthocyanin content.[Citation11] Furthermore, using probiotic bacteria to ferment pomegranate juice enhances its health benefits by increasing the antioxidant activity, producing acidic metabolites,[Citation15] increasing the microbial conversion of polyphenols,[Citation16] and promoting the flavor profile.[Citation17] However, no reports exist regarding the effects of HPP and TP on bioactive compounds, antioxidant activity, and volatile compounds in fermented pomegranate products.
Therefore, this study investigates the effect of different HPP conditions on the microbial population, color, pH, TSS, TA, total phenols, total flavonoids, anthocyanins, antioxidant capacity and volatile compounds of FPJ. These factors are compared to raw FPJ, as well as FPJ subjected to conventional TP, to evaluate the potential utilization of HPP for the production of high-quality FPJ. This study will provide technical support for the commercial application of HPP during FPJ production.
Materials and methods
Materials and bacterial strains
The concentrated pomegranate juice (pH 3.45, TSS 64.8 °Brix), concentrated mulberry juice (pH 3.74, TSS 62.3 °Brix), and concentrated acid pomegranate juice (pH 2.40, TSS 62.7 °Brix) were obtained from the Sichuan Xichang Guoguoguoye Co. Ltd and stored at −18 °C until use. The Lactobacillus plantarum CCTCC M207202 and Lactobacillus acidophilus CCTCC AB 2010208 were purchased from the Sichuan Gaofuji Biotech Co., Ltd. (Chengdu, China). These microbes were chosen as starters according to our previous study. Besides, the Saccharomyces cerevisiae CCTCC AY 92042 was purchased from the Angel Yeast Co., Ltd. (Hubei, China). These microbes were stored at −20 °C until use.
Chemicals
HPLC standards (Gallic acid, Cyanidin-3-glucoside (Cy-3-glu), and (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) and Rutin) were obtained from the Yuanye Biotechnology Co., Ltd. (Shanghai, China), while 2,2-diphenyl-1-picrylhydrazyl (DPPH) (HPLC grade) was obtained from the Aladdin Industrial Co., Ltd. (Shanghai, China). The Nutrient Agar (NA) and Rose Bengal Agar (RBA) (biological reagent) were purchased from the Beijing Aoboxing Biological Technology Co. Ltd. (Beijing, China), while all other chemicals (analytical grade) were purchased from the Chengdu Kelong Chemical Reagent Factory (Chengdu, China).
Preparation of FPJ
Based on the preliminary experimental results from the laboratory, the production process of FPJ commenced as follows: The concentrated pomegranate juice was diluted with distilled water to 15 °Brix (pH 3.89), and then pasteurized at 65 °C for 20 min to inactivate the initial microorganisms. First, 2 L diluted pomegranate juice was placed into sterilized borosilicate glass bottles (3 L) with polypropylene screw caps. Next, 240 g sugar liquid 50% (w/w) was added to a glass fermentation cylinder. The pomegranate juice (pH 3.93, TSS 23.98 °Brix) was fermented by adding L. plantarum (1.5%(w/w)), L. acidophilus (1.5%(w/w)), and S. cerevisiae (0.02% (w/w)) simultaneously in a sterile environment, while the amount of headspace in the glass bottles (7 cm) was maintained. The cell concentrations (1.0 × 106 CFU/mL, 1.0 × 106 CFU/mL, and 2.0 × 104 CFU/mL, respectively) in the prepared cultures were determined using a hemocytometer version XB-K-250 (Jianling Medical Device Co., Danyang, China). The subsequent mixture was incubated at 25 °C for 48 h to obtain FPJ. Finally, 1.11 mL of concentrated mulberry juice and 1.11 mL of concentrated acid pomegranate juice were added to 1 L of FPJ for coloring and seasoning, respectively.
HPP and TP treatments
The FPJ was processed using the method described by Wang et al.[Citation7] with slight modifications. For the HPP treatments, 40 mL of FPJ was poured into polyethylene/nylon vacuum bags (Huadun Plastics Co., Chengdu, China). The bags were then sealed using a heat sealer (SF-150, Afanlao Machinery Equipment Co., Ltd. Shanghai, China), taking care to expel as much air as possible. The bags were pressurized at 400 MPa, 500 MPa, and 600 MPa for 3 min, 5 min, 7 min, and 10 min, respectively at 20 °C (UHP-600, Baotou Kefa Co., Inner Mongolia, China). Water was employed as the pressure-transmitting medium, and the initial HPP treatment temperature was 20 °C. The temperature of the samples after HPP treatment ranged from 20 °C to 28 °C, and the necessary pressure was achieved at a rate of 400 MPa/min. Automatic depressurization occurred after 0–10 min of HPP treatment. Additionally, the depressurization time was less than 4 s. The treatment time reported in this study did not include the pressure increase and release time. For the TP treatments, 40 mL of FPJ was added to the pouches (120 × 170 × 0.16 mm). The pouches were placed in an autoclave (G154DWS, Zhiwei Equipment Co., Ltd. Xiamen, China), and maintained at 65 °C for 20 min. The heating- and cooling- rate were 4 °C/min and 0.5 °C/min, respectively. The temperature during the heating process was monitored by assessing a sample from the batch. Immediately after HPP and TP treatments, the samples were removed and cooled in an ice bath. All samples were stored at 4 ± 2 °C before analysis.
Microbiological assay
To detect the viable cells of natural microorganisms in FPJ, the total plate count method described by Chen et al.[Citation5] was used, with slight modifications. Untreated and treated samples were serially diluted with a sterile 0.85% NaCl solution, and 1.0 mL of each dilution was plated onto duplicate plates of the appropriate agar (NA, and RBA). NA was used for detecting the viable cells of the aerobic plate count (APC), and the plates were incubated at 37 °C for 48 ± 2 h. The RBA was used for counting the viable cells of yeasts and molds (Y&M) after incubation at 27 °C for 72 ± 2 h. After this period, the colonies were counted. Log N/N0 was calculated to determine the inactivation effect, where N0 was the number of initial microorganisms in the untreated sample, and N was the corresponding viable number of microorganisms after HPP and TP treatment. N0 was 1.45 × 104 CFU/mL, and 2.34 × 102 CFU/mL for APC and Y&M, respectively.
Kinetic study of microbial inactivation by HPP
The data that reflected the effect of microbial inactivation was fitted by the modified Weibull equation.[Citation5,Citation18]
Where: b, n, and t were the scale factors, shape factors, and treatment time (min), respectively.[Citation5,Citation18,Citation19] The b values represented the mean of the distribution describing the death times of the microbial population and have a probabilistic interpretation.[Citation18] The Weibull distribution corresponded to a concave upward survival curve, concave downward curve, and was linear for n < 1, n > 1, and n = 1, respectively.[Citation5]
Color
The color measurement of FPJ was conducted at 25 ± 2 °C using a Color Difference Meter (WF-32, Wave Optoelectronics Technology Co., Ltd. Shenzhen, China). For the assays, 20 mL of FPJ was poured into a 50 mL glass cup, taking care to remove air bubbles, and the color was analyzed using a colorimeter in the CIE L a b scale in reflectance mode in a dark room. The lightness values (L*), redness values (a*), and yellowness values (b*) of the FPJ were recorded. Three measurements were performed, and the results were averaged. In addition, ΔE was calculated using the following equations,[Citation2] where L0*, a0*, and b0* were the values of the control, and L*, a*, and b* were the values of the treated sample:
pH, TSS, and TA
The pH value was measured at 25 °C with a pH-meter, PHS 320 (Fangzhou Technology, Chengdu, China). The meter was calibrated with commercial buffer solutions at pH 6.8 and pH 4.0, respectively. Then, 20 mL of FPJ was inserted with a pH electrode, and the pH was recorded after stabilization. The TSS was determined as °Brix at ambient temperature (25 ± 1 °C) using an automatic refraction meter (A610, Hanon Equipment Co., Jinan, China). The TA was determined according to the method described by Chen et al.[Citation20] with slight modifications. A 10 mL volume of FPJ was titrated using standardized 0.1 mol/L NaOH to the phenolphthalein end point (pH = 8.2 ± 0.1). The TA was expressed as a percentage of the citric acid:
Where concentration of NaOH (C) = 0.1 mol/L, V = volume of NaOH (mL), the conversion factor of citric acid (K) = 0.064, the dilution factor (F) = 2, and m = weight of the samples (g).
Total anthocyanin concentration
The total anthocyanin concentration was determined using the pH differential method described by Chen et al.[Citation5] and Kwaw et al.[Citation21] with slight modifications. In sum, two buffer solutions: KCl (0.025 mol/l) at pH 1.0 and CH3COONa (0.4 mol/l) at pH 4.5, were prepared. Then, 0.5 mL of FPJ was dispensed into two sets of tubes. A 4.5 mL volume of the KCl buffer was dispensed into one set of the tubes while 4.5 mL of CH3COONa was added to the other. The mixture was then set in the dark for 15 min. The absorbance of the solutions was read at 510 nm and 700 nm, respectively, against a distilled water blank, using a spectrophotometer (UV726, T6, PG General, Beijing, China). The total anthocyanin content was reported as mg of anthocyanin equivalents per liter of the sample (mg/L). The anthocyanin content was calculated using the following equation:
where A = (A510 − A700) pH 1.0 − (A510− A700) pH 4.5, the Cy-3-glu molecular weight (MW) = 449.2 g/mol, the dilution factor (DF) = 10, L (path length in cm) = 1, and ε (molar extinction coefficient) = 26,900 L.mol−1.cm−1.
Total phenol concentrations
In determining the concentration of total phenols, the Folin-Ciocalteu method was adopted as described by Kwaw et al.[Citation21], with slight modifications. For the assays, 1 mL of sample (previously diluted 100-fold with distilled water) was mixed with 0.5 mL of Folin–Ciocalteu reagent and 6.5 mL of 10% (w/v) sodium carbonate was added. The mixture was set for 1 h in the dark at room temperature. The absorbance was measured at 760 nm using a spectrophotometer (UV726, T6, PG General, Beijing, China). Quantification was based on the calibration curve of gallic acid and results were expressed as mg of gallic acid equivalents (GAE) per L of FPJ, according to the calibration curve (Y = 57.928X +0.0207, R2 = 0.999, in which Y was the absorbance at 760 nm and X was the concentration of gallic acid (mg/mL).
Total flavonoid content
The total flavonoid content was determined using the modified aluminum chloride colorimetric method described by Wang et al.[Citation7] A 0.5 mL volume of FPJ (previously diluted 10-fold with distilled water) was mixed with 0.15 mL of 5% NaNO2. Following incubation for 6 min, 0.15 mL of 10% AlCl3 was added to the mixture. After 5 min, 1 mL of 1 mol/L NaOH was added. The solution was left to sit in the dark for 15 min, after which the absorbance was measured at 510 nm. The total flavonoid content was calculated and expressed as mg of Rutin equivalent (RE)/L of FPJ, according to the calibration curve (Y = 2.2037X+0.0005, R2 = 0.999, in which Y was the absorbance at 510 nm and X was the concentration of Rutin (mg/mL).
Antioxidant capacity
The antioxidant capacity of FPJ was evaluated based on the DPPH radical scavenging activity, according to the method proposed by Kwaw et al.[Citation21] with some modifications. A 0.5 mL volume of FPJ was mixed with 4.5 mL of a DPPH methanolic solution (0.14 mmol/L). The mixture was set in the dark for 1 h at room temperature, after which the absorbance was measured at 517 nm with a spectrophotometer (UV-726, T6, PG General, Beijing, China). Trolox solutions within a range of 20 μM – 160 μM were used for calibration, and new Trolox calibration curve was created for each assay. The analyses were run in triplicate, and the results were expressed as μM Trolox/L of FPJ.
Identification and quantification of volatile compounds
The volatile compounds in the FPJ were analyzed using a headspace solid-phase microextraction gas chromatography-mass spectrometry (SPME-GC-MS) system as described by Wang et al.[Citation7] with some modifications. The GC–MS QP2010plus, (Shimadzu USA MANUFACTURING, Inc., Kyoto, Japan) was used. Initially, 6 mL of FPJ and 2 g of NaCl were transferred to a 15 mL SPME extraction bottle. The sample was preheated in a water bath at 40 °C for 10 min before extraction. Subsequently, a 65-μm divinylbenzene/polydimethylsiloxane (DVB/PDMS) fiber (Supelco, Bellefonte, PA, USA) was exposed to the sorption surface above the liquid surface for 60 min at 40 °C, to collect the analyses. The fiber was introduced into the GC injector where desorption was conducted at 250 °C for 3 min, followed by a start-up and collection of data.
GC-MS conditions
A DB-17MS chromatographic column (60 μm, 0.25 mm inside diameter and 0.25 μm film thickness; Agilent, USA) was used to separate volatiles. The temperature of the injector, GC-MS interface, and ion source was 250 °C, 250 °C, and 200 °C, respectively. The analyses were performed using helium as a carrier gas at a column flow of 0.7 mL/min. The electron impact ionization was 70 eV, in electronic ionization mode, and the filament current was 0.25 mA. The column oven temperature program was set for 3 min at 40 °C, raised to 130 °C at a rate of 10 °C/min, held for 4.5 min, then increased to 160 °C at a rate of 6 °C/min. The temperature was then raised significantly to 210 °C at a rate of 10 °C/min, held for 4.5 min, and finally increased to 280 °C at a rate of 15 °C/min. The temperature was maintained at isothermal for 3 min. The data was collected in a scanning range of 33–450. The mass spectra correlations were made using the National Institute of Standards and Technology library. Results were reported when the matching degree was higher than 80%.
Sensory evaluation
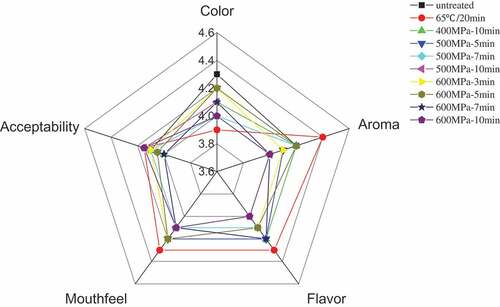
The sensory analysis of the FPJ was conducted according to the method described by Di Cagno et al.[Citation22] Ten trained sensory panelists ranging in age range from 20–30, were selected to form a sensory evaluation group (equal distribution between male and female). The panelists were placed in separate rooms to facilitate an unbiased evaluation of the sensory attributes. The FPJ samples were randomly coded, and served (20 mL) at room temperature, together with unsalted table biscuits and still water. Ten coded samples were randomly presented to the sensory panelists to prevent any flavor carryover effects. During their evaluation, the sensory panelists were asked to consider five aspects (color, aroma, flavor, mouthfeel, and acceptability) according to . The FPJs were scored on continuous 0–5 scales with 0 as the lowest and 5 as the highest score that could be ascribed to the sensory attributes. The scores were collected, and the average values were calculated.
Table 1. Sensory attributes and reference scores used for sensory evaluation of fermented pomegranate juice
Statistical analysis
All the data from three independent experiments were expressed as the mean ± standard deviation (SD), and assessed with one-way analysis of variance (ANOVA), employing the Pearson two-sided test using SPSS/PC version 23.0 (SPSS Inc., Chicago, USA). The ANOVA test was conducted for all experimental runs to determine the significance at the α = 0.05 level.
Results and discussion
The effects of TP and HPP treatments on microbial counts
shows the APC and Y&M of FPJ subjected to HPP and TP treatment. The APC and Y&M in HPP-treated samples decreased in conjunction with the pressure-treatment time and the increasing treatment pressure compared with the untreated samples. For APC, the reduction was rapid during the first 5 min of treatment but slowed between 5–10 min of treatment. Following HPP-treatment at 400 MPa for 10 min, 500 MPa for 5 min, 7 min, and 10 min, and 600 MPa for 3 min, 5 min, 7 min, and 10 min, no Y&M counts were detected, while the APC counts were below the detection limit (<100 CFU/mL), therefore, ensuring the safety of the FPJ. After TP treatment, both the APC counts and the Y&M counts failed to be detected in the FPJ. Similar results were obtained by others.[Citation5,Citation7] Wang et al.[Citation7] found that Y&M were not detected in mulberry juice treated at 300 MPa/10 min, and the minimum holding times sufficient to produce mulberry juice with no detectable TAB was located at 500 MPa/10 min. According to Chen et al.[Citation5], the Y&M were not detected in cloudy pomegranate juice, treated at 400 MPa/5 min, while the TAB were detected at 1.52 log10 CFU/mL. The inactivation of microorganisms by HHP treatment mainly resulted from changes in the membrane structures subjected to high pressure. Serment-Moreno et al.[Citation18] reported that microorganisms are impacted by several lethal effects occurring simultaneously, with cellular membrane damage frequently a dominant factor. At a sufficiently high level, the pressure could induce enzyme inactivation, membrane protein denaturation, as well as cell membrane rupture caused by a phase transition of the membrane and a change in its fluidity.[Citation23] The acyl chains of the phospholipid bilayer might experience crystallization, leading to bud formation, membrane rupture, and intracellular material leakage.[Citation18] The occurrence of irreversible protein/enzyme denaturation, and intracellular content leakage caused the inactivation of a large variety of pathogenic and spoilage bacteria.[Citation18] These results indicated that the Y&M were more sensitive to HPP than APC, which was primarily due to their cell wall type and cellular morphology.[Citation6,Citation18,Citation23] In general, prokaryotic cells exhibited a higher resistance to pressure than eukaryotic cells, which might be due to the presence of a thicker peptidoglycan layer.[Citation23] Within prokaryotes, gram-positive microorganisms such as Bacillus, Listeria, Staphylococcus, and Clostridium have a thicker peptidoglycan layer and are, therefore, generally more pressure resistant than gram-negative microorganisms.[Citation23] Furthermore, some microbes present in the FPJ might display high resistance to HPP, and can adapt to the pressure, therefore, rendering the APC more sensitive to HPP than the Y&M.[Citation5]
Table 2. Microbial inactivation of fermented pomegranate juice by high pressure processing and thermal processing
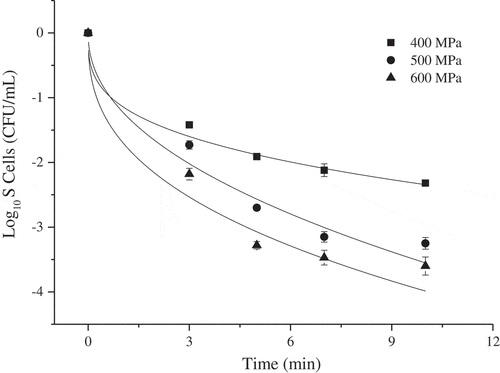
shows the kinetic curves of the APC inactivation fitted by the Weibull model. The values of the model parameters are presented in . R2 of the fitting curves exceeded 0.95, which indicated that the APC inactivation by HPP was well fitted. At 600 MPa, the b value was higher than at 400 MPa and 500 MPa, indicating that the effect of microbial inactivation at 600 MPa was better than at 400 MPa and 500 MPa at the same holding times. This value placement within the model was, indeed, consistent with the experimental results of this study. The n values under 400 MPa, 500 MPa, and 600 MPa were all lower than 1, demonstrating that the remaining cells could adapt to the applied stress, while the decreasing trend of the APC became slower with time. As noted previously, similar results were obtained by others.[Citation5,Citation19] Chen et al.[Citation5] observed that the effect of HPP on the APC and Y&M inactivation in cloudy pomegranate juice was fitted well by the Weibull model. According to Kaur et al.[Citation19] the inactivate curves of Escherichia coli, Listeria innocua, and Staphylococcus aureus in black tiger shrimp subjected to HPP (300 MPa to 600 MPa) and specific temperatures (30 °C to 50 °C), were also fitted well by the Weibull model. Moreover, S. aureus was found to be the most baro-resistant species among the three pathogens, and the minimum processing intensity required for the destruction of S. aureus was 500 MPa/9 min/50 °C.[Citation19] Survival curves for HPP treatments show pronounced tails (), which could be primarily attributed to the reflection of resistance heterogeneity within the population, either inherent to the bacterial cells or acquired during the treatment.[Citation18,Citation24] It was also possible that there was a small amount of pressure-resistant bacteria in the original iteration of the material during the process of HPP treatment germination.[Citation5,Citation19] Nevertheless, the curve fitting presented in this study will contribute in stimulating the processing parameters that could achieve microbial safety in HPP-treated FPJ.
Table 3. The Weibull model kinetic parameters of microbial inactivation of fermented pomegranate juice treated by high pressure processing
Figure 1. Microbial inactivation of fermented pomegranate juice treated by high pressure processing fitting by the Weibull model
In this study, no Y & M counts were detected in the FPJ. Furthermore, the APC counts were below the limit of detection (<100 CFU/mL) after HHP treatments at 400 MPa for 10 min, 500 MPa for 5 min, 7 min, and 10 min, 600 MPa for 3 min, 5 min, 7 min, and 10 min and TP treatment at 65 °C for 20 min. To examine the effect of HPP and TP on the quality of the FPJ, these particular conditions were selected as pasteurization parameters, and the quality attributes were determined.
The effect of TP and HPP treatments on the color, pH, TSS, and TA of the FPJ
The effect of HPP and TP on the color of the FPJ is shown in . A significant change (p<0.05) in the L* value, the a* value and the b* value were found in the HPP-treated samples compared with the untreated sample and the TP-treated sample. Although the ΔE increased in all processed samples, it was below 2, indicating that both HPP and TP had little impact on the color of the FPJ. The results were in agreement with previous studies.[Citation9,Citation20] Barba et al.[Citation9] reported that no color changes were visually noticeable in pressurized blueberry juice for any of the HPP treatments (200 MPa, 400 MPa, and 600 MPa for 5 min, 9 min and, 15 min, respectively). Chen et al.[Citation20] reported that the color of ginger juice was not influenced by HPP treatment (500 MPa/10 min). These results demonstrated that the color of the juices was stabilized during HPP treatment. Moreover, the color changes during TP might be attributed to the sugar degradation products (furfurals) from reactions like the Maillard reaction, which accelerated the rate of anthocyanin deterioration.[Citation25]
Table 4. Effects of thermal processing and high pressure processing on physicochemical parameters of fermented pomegranate juice
As shown in , no significant (p>0.05) difference was evident in the TA, pH, and TSS between HPP-treated, TP-treated, and untreated samples, indicating that the HPP and TP had little impact on the TA, pH, and TSS of the FPJ. These results corresponded with those of previous studies.[Citation2,Citation26] Varela-Santos et al.[Citation2] reported that no significant (p0.05) changes were observed in the TA, pH, and TSS of the pomegranate juice subjected to HPP at 350 MPa, 450 MPa, and 550 MPa for 30 s, 90 s, and 150 s, respectively. According to Xu et al.[Citation26], HPP (500 MPa/10 min) and TP (90 °C/2 min) did not change the TA, pH, and TSS in banana puree, indicating that these elements were stabilized during processing.
The effect of TP and HPP treatments on the total phenol content, total anthocyanins, total flavonoids, and antioxidant capacity
Both HPP and TP resulted in a significant (p<0.05) decrease in the total phenols in the FPJ (). When the treatment time or the pressure was increased, the total phenols decreased in all HPP-treated samples. A decrease of 13.31%, 18.58%, and 23.88% was observed in samples treated with HPP at 400 MPa, 500 MPa, and 600 MPa for 10 min, respectively. A distinct total phenol retention of 97.52% was achieved by HPP at 600 MPa/3 min, while the retention in the TP-treated sample was 72.91%, indicating that HPP could maintain the phenolic content more effectively than TP. The experimental results generated in this study have been confirmed by similar results from other publications.[Citation5,Citation7] According to Wang et al.[Citation7] a 32.11% and 7.97% reduction the total phenols of mulberry juice was achieved by TP (85 °C/15 min) and HPP (500 MPa/10 min), respectively. Chen et al.[Citation5] found that TP-treated (110 °C/8.6 s) cloudy pomegranate juice displayed a significant decrease in total phenols compared to the HPP-treated sample (400 MPa/5 min). The decrease of total phenols by HPP might be attributed to a balance between higher extractability and non-enzymatic oxidation degradation of phenolic compounds.[Citation27] Alternatively, the decrease of total phenols by TP might be caused by the thermal instability of these compounds. The high temperature accelerated the progress of chemical reactions, which effectively promoted the oxidative degradation of the total phenols.[Citation28]
Table 5. Effects of thermal processing and high pressure processing on total phenols, total flavonoids, anthocyanins and total antioxidant activity in fermented pomegranate juice
As shown in , a significant decrease (p<0.05) was apparent in the total flavonoids of all processed samples compared with the control, while no significant difference (p>0.05) was evident in the total flavonoids of the HPP and TP treatments. A reduction of 12.93%, 20.05%, and 20.21% was found in samples treated with HPP at 400 MPa, 500 MPa, and 600 MPa for 10 min, respectively. A pronounced total flavonoid retention of 91.18% was achieved by HPP at 600 MPa/3 min, while it was 86.57% in the TP-treated sample. The results recorded in these experiments were in agreement with previous studies.[Citation7,Citation29] As reported by Wang et al.[Citation7] the HPP (500 MPa/10 min) and TP (85 °C/15 min) treatments significantly (p<0.05) reduced the total flavonoids of mulberry juice. Nayak et al.[Citation29] reported that the HPP treatment (600 MPa/5 min) and TP treatment (80 °C/60 s) decreased the total flavonoids of elephant apple juice. The decrease of total flavonoids by TP might be attributed to the thermal instability of the phenolic compounds and flavonoid compounds.[Citation30] The decrease of the total flavonoids in the HPP samples might be associated with the non-enzymatic oxidation degradation of the phenolic compounds.[Citation27]
The changes in the anthocyanin content by HPP and TP are shown in . A significant decrease (p<0.05) was observed in the anthocyanin content of all processed samples compared to the untreated sample. Moreover, increasing the treatment pressure and prolonging the treatment time, induced a significant decrease (p<0.05) in the anthocyanin content. A reduction of 6.88%, 7.68% and 13.61% were found in samples treated at 400 MPa, 500 MPa, and 600 MPa for 10 min, respectively. A significantly (p <0.05) higher anthocyanin retention of 97.31% was achieved by HPP at 500 MPa/5 min, while it was 68.86% in the TP-treated sample, demonstrating that the HPP treatment maintained higher anthocyanins than the TP treatment. It has been noted elsewhere that others obtained similar results.[Citation3,Citation13] Patras et al.[Citation3] found that all HPP treatments (400 MPa, 500 MPa, and 600 MPa/15 min/10–30 °C) maintained a significantly (p<0.05) higher anthocyanin content in strawberry and blackberry purées than TP treatment (70 °C/2 min). Zou et al.[Citation13] also reported that HPP treatment (500 MPa/5 min) maintained significantly (p<0.05) higher anthocyanin levels in mulberry juice than TP treatment (110 °C/8.6 s). The anthocyanins represented the main flavonoids (1.6 − 23.6%), found in pomegranate juice, and was responsible for its red color.[Citation1] The anthocyanins were found to be the most unstable during processing, being easily oxidized under various processing conditions (oxygen, light, and pH), and they are known to be sensitive to high temperatures.[Citation1] The decrease in the anthocyanin content during TP might be caused by the high temperature, effectively inducing the degradation of the anthocyanins.[Citation1] On the one hand, the degradation product of organic acids produced by heat could form condensation products with anthocyanins.[Citation31] On the other hand, due to the condensation reactions involving the covalent association of anthocyanins with other flavanols or organic acids present in juices leading to the formation of a new pyran ring by cycloaddition, which could be responsible for changes in the colour of product.[Citation32] In this study, the decrease in the anthocyanin content by HPP might be due to the condensation reactions of anthocyanins with other phenolic compounds that naturally occur in juices. However, the condensation products were unstable, and further degraded to colorless compounds.[Citation33]
The DPPH analysis indicated that the antioxidant activity of the FPJ decreased significantly (p<0.05) in all processed samples (). A significant (p<0.05) decrease of 6.63%, 6.93%, and 9.33% was found in samples treated with HPP at 400 MPa, 500 MPa, and 600 MPa for 10 min, respectively. The behavior of total antioxidant activity was similar to that observed for anthocyanins and total phenolic content. A significantly (p< 0.05) higher retention of 95.89% was achieved by HPP treatment at 600 MPa/3 min for antioxidant activity, while it was 88.81% in the TP-treated sample, indicating that HPP maintained higher antioxidant activity than TP. The results yielded here corresponded with those of previous studies.[Citation3,Citation5] According to Patras et al.[Citation3] the antioxidant activity of HPP-treated (400 MPa, 500 MPa, and 600 MPa/15 min/10–30 °C) strawberry and blackberry purées were significantly higher (p< 0.05) than in the TP-processed (70 °C/2 min) samples. Chen et al.[Citation5] reported that the reduction in the antioxidant content of an HPP-treated (400 MPa/5 in) cloudy pomegranate juice sample was significantly (p<0.05) lower than in the TP-treated (110 °C/8.6 s) sample. Statistical analysis showed that the antioxidant activity had a positive correlation with the content of total flavonoids, total phenols and anthocyanins () with r = 0.875, 0.799, 0.792, respectively. Therefore, the decrease in the antioxidant capacity might be attributed to the degradation of these compounds during HPP and TP processing.[Citation6] Furthermore, it was possible that the decrease in antioxidant activity during processing might be due to the highly oxidative conditions that resulted from the remaining activity of the enzymes, such as polyphenol oxidase.[Citation9] Another reason for the decrease in the antioxidant activity by HPP might be the decline in FPJ volume that occurred during pressurization. This might facilitate oxidative reactions, and was supported by the Le Chatelier principle.[Citation23] In summary, the HPP treatments retained higher total phenols (p<0.05), total flavonoids (p>0.05), anthocyanins (p<0.05) and antioxidant activity (p<0.05) than the TP treatments.
Table 6. Pearson’s correlation analysis between different indices of fermented pomegranate juice
The effect of different treatments on the volatile flavor compounds in the FPJ
As shown in and , 35 volatile flavor compounds were present in the unfermented pomegranate juice, while 41 volatile compounds were found in the untreated FPJ. The levels of esters and alcohols displayed the most significant increase in the untreated sample (fermented but not pressure or thermal treated) when compared with the unfermented pomegranate juice. The main volatile flavor compounds in the untreated FPJ were esters (41.49%), which primarily included ethyl acetate (20.87%), ethyl butyrate (4.54%), ethyl hexanoate (3.20%), and hexyl acetate (3.36%). The second most abundant flavor compounds found in the untreated FPJ were alcohols (35.15%), which mainly include trans-2,3-hexanediol (3.19%), 1-butanol (3.21%), 2-heptanol (1.84%), 2-nonanol (6.11%), phenylethyl alcohol (3.13%), as well as traces of other compounds. These compounds have previously been identified as the major contributors to the aroma of FPJ.[Citation22] Most volatile esters can enhance the fruit flavor of a juice, in particular, ethyl acetate.[Citation4,Citation22] The increase in alcohols and esters during the fermentation process might be attributed to three reasons. Firstly, aldehydes and ketones are unstable compounds that are reduced to alcohols, or oxidized to acids in food matrices, particularly when exposed to microbial activity.[Citation22] Secondly, cerevisiae and some species in the Lactobacillus genus display the ability to produce ethanol, possessing alcohol dehydrogenase enzymes that can convert acetaldehydes into ethanol.[Citation34] Thirdly, acetate esters are formed by alcohol acetyltransferases from the reaction between acetyl-CoA and alcohols, as well as the availability of alcohol precursors.[Citation35] The results demonstrated that lactic acid fermentation through selected strains increased the flavor profile of pomegranate juice.[Citation22]
Table 7. Effects of thermal processing and high pressure processing on volatile compounds in fermented pomegranate juice
Table 8. Effects of thermal processing and high pressure processing on concentrations (mean ± SE, %) of different volatile compound groups in fermented pomegranate juice
and , indicates that a total of 41 volatile compounds were detected in untreated FPJ, and were grouped according to the following chemical class (): aldehydes and ketones (6 compounds identified), alcohols (10), esters (10), heterocyclic and aromatic compounds (4), acids (4), hydrocarbons (2) and miscellaneous (5). Furthermore, FPJ treated with TP contained 38 volatile compounds while the HPP samples displayed 39 volatile compounds. Esters were the most abundant chemical groups in the control. Their content increased following HPP and TP treatments and was highest in the FPJ treated subjected to the TP process. Compared with the control, the HPP and TP treatments significantly decreased the alcohols (p<0.05), while HPP-treated samples maintained significantly (p<0.05) higher alcohols than the TP-treated sample. Overall, the FPJ treated at 600 MPa/3 min contained the highest (p>0.05) alcohol level. Compared with the control, the concentration of ketones exhibited a significant (p<0.05) decrease in the TP-treated samples, while the ketones in all HPP-treated samples displayed only a slight increase (p>0.05). The organic acids exhibited a significant (p<0.05) decrease in all processed samples compared with the control, while the HPP preserved higher acids than the TP treatment (p<0.05). Compared with the untreated FPJ, the concentration of heterocyclic and aromatic compounds were significantly (p<0.05) increased in the HPP-treated samples (400 MPa/10 min and 500 MPa/10 min), while it showed a significant (p<0.05) decrease in the TP-treated samples. In reviewing these differences, the data revealed that HPP treatments maintained higher ketones (p<0.05), alcohols (p<0.05), and acids (p<0.05) than the TP treatment. Furthermore, the samples treated under 400 MPa/10 min and 500 MPa/10 min demonstrated a significantly (p<0.05) higher presence of heterocyclic and aromatic compounds than in the TP-treated sample. This might be attributed to the fact that these compounds were more sensitive to TP treatment.[Citation6] Moreover, various other researchers have also investigated the effect of HPP and TP on volatile compounds in fruit and vegetable juices.[Citation6,Citation7] Wang et al.[Citation7] reported that the HPP treatment (500 MPa/10 min) preserved higher volatile compounds in mulberry juice than the TP treatment (85 °C/15 min). Chen et al.[Citation6] indicated that the HPP treatments (200 MPa, 400 MPa, and 600 MPa for 10 min and 20 min) maintained significantly higher concentrations of aldehydes, alcohols, and ketones in green asparagus juice than the TP treatment (121 °C/3 min). The data in this study showed that changes in the types and content of volatile compounds in processed samples indicate the decomposition and oxidation of compounds during processing. Other published studies have shown that the aroma of pomegranate juice is closely related to operating temperature and processing time.[Citation11] When the juice is heated during pasteurization, a complex series of chemical reactions occur, leading to losses of volatile compounds, which can affect the aroma of the product.[Citation6,Citation12] The HPP treatment could enhance or retard enzymatic and chemical reactions, which could lead to changes in the overall aroma profile.[Citation7,Citation12] In summary, the esters and alcohols were the main aroma compounds in the FPJ, while HPP treatments could better preserve the flavor quality of the FPJ than the TP treatment.
Effect of TP and HPP treatments on the sensory quality of the FPJ
The average scores of the sensory attributes such as color, flavor, mouthfeel, and acceptability of the FPJ when exposed to different HPP and TP treatments are depicted in . The sensory results showed acceptance means for color, mouthfeel, aroma, flavor, and acceptability, while the sensory evaluation revealed no significant change (p >0.05) in the color of all the treated FPJ samples. Compared to TP, the sensory scores of aroma, flavor, mouthfeel, and acceptability of the FPJ treated with HPP were either lower or at an equal level. As the treatment pressure and time increased, the flavor, mouthfeel, and aroma of the FPJ presented no significant changes (p>0.05) at an appropriate pressure and time. The sensory scores of the aroma and flavor of the TP-treated sample were higher (p>0.05) than in the HPP-treated samples. This might be due to higher (p<0.05) ester content in the TP processed sample, which might provide an aroma that contain both fruity and floral elements. Although the HPP treatments showed a similar effect on the sensory scores as TP, the volatile compounds emphasized distinct differences in flavor. Finally, HPP should be considered as a promising processing technique, even though the original fresh sensory properties were not always fully retained or were lower than those of the TP sample.
Conclusion
Results show that the inactivation of microorganisms by HHP treatment in FPJ fit the Weibull model and positively correlate with pressure and holding time. The pH, TA, TSS, and ΔE in the FPJ remain stable following the application of the HPP and TP treatments. Moreover, the HPP treatment maintains significantly higher levels of total phenolic content, anthocyanins, and total antioxidant activity in the FPJ than does the TP treatment. Furthermore, treatment of the FPJ at 600 MPa/3 min exhibits the highest retention of total flavonoids, total phenols, anthocyanins, and antioxidant activity. Lastly, HPP treatment maintains higher volatile compounds in the FPJ, of which esters, ketones, alcohols, acids, heterocyclic, and aromatic compounds are the main contributors. The results suggest that HPP treatment can facilitate higher levels of bioactive compounds, antioxidant activity, and flavor quality in the FPJ compared to the TP treatment. Therefore, HPP at 600 MPa/3 min is the optimal treatment option.
Additional information
Funding
References
- Putnik, P.; Kresoja, Ž.; Bosiljkov, T.; Režek Jambrak, A.; Barba, F. J.; Lorenzo, J. M.; Roohinejad, S.; Granato, D.; Žuntar, I.; Bursać Kovačević, D. Comparing the Effects of Thermal and Non-thermal Technologies on Pomegranate Juice Quality: A Review. Food Chem. 2019, 279, 150–161. DOI: 10.1016/j.foodchem.2018.11.131.
- Varela-Santos, E.; Ochoa-Martinez, A.; Tabilo-Munizaga, G.; Reyes, J. E.; Pérez-Won, M.; Briones-Labarca, V.; Morales-Castro, J. Effect of High Hydrostatic Pressure (HHP) Processing on Physicochemical Properties, Bioactive Compounds and Shelf-life of Pomegranate Juice. Innovative Food Sci. Emerg. Technol. 2012, 13, 13–22. DOI: 10.1016/j.ifset.2011.10.009.
- Patras, A.; Brunton, N. P.; Da Pieve, S.; Butler, F. Impact of High Pressure Processing on Total Antioxidant Activity, Phenolic, Ascorbic Acid, Anthocyanin Content and Colour of Strawberry and Blackberry Purees. Innovative Food Sci. Emerg. Technol. 2009, 10(3), 308–313. DOI: 10.1016/j.ifset.2008.12.004.
- Lan, Y.; Wu, J.; Wang, X.; Sun, X.; Hackman, R. M.; Li, Z.; Feng, X. Evaluation of Antioxidant Capacity and Flavor Profile Change of Pomegranate Wine during Fermentation and Aging Process. Food Chem. 2017, 232, 777–787. DOI: 10.1016/j.foodchem.2017.04.030.
- Chen, D.; Xi, H.; Guo, X.; Qin, Z.; Pang, X.; Hu, X.; Liao, X.; Wu, J. Comparative Study of Quality of Cloudy Pomegranate Juice Treated by High Hydrostatic Pressure and High Temperature Short Time. Innovative Food Sci. Emerg. Technol. 2013, 19, 85–94. DOI: 10.1016/j.ifset.2013.03.003.
- Chen, X. H.; Qin, W.; Ma, L.; Xu, F.; Jin, P.; Zheng, Y. Effect of High Pressure Processing and Thermal Treatment on Physicochemical Parameters, Antioxidant Activity and Volatile Compounds of Green Asparagus Juice. LWT Food Sci. Technol. 2015, 62(1), 927–933. DOI: 10.1016/j.lwt.2014.10.068.
- Wang, F.; Du, B.-L.; Cui, Z.-W.; Xu, L.-P.; Li, C.-Y. Effects of High Hydrostatic Pressure and Thermal Processing on Bioactive Compounds, Antioxidant Activity, and Volatile Profile of Mulberry Juice. Food Sci. Technol. Int. 2017, 23(2), 119–127. DOI: 10.1177/1082013216659610.
- Vegara, S.; Martí, N.; Mena, P.; Saura, D.; Valero, M. Effect of Pasteurization Process and Storage on Color and Shelf-life of Pomegranate Juices. LWT Food Sci. Technol. 2013, 54(2), 592–596. DOI: 10.1016/j.lwt.2013.06.022.
- Barba, F. J.; Esteve, M. J.; Frigola, A. Physicochemical and Nutritional Characteristics of Blueberry Juice after High Pressure Processing. Food Res. Int. 2013, 50(2), 545–549. DOI: 10.1016/j.foodres.2011.02.038.
- Wang, C. Y.; Huang, H- W.; Hsu, C- P.; Yang, B- B. Recent Advances in Food Processing Using High Hydrostatic Pressure Technology. Crit. Rev. Food Sci. Nutr. 2016, 56(4), 527–540.
- Ferrari, G.; Maresca, P.; Ciccarone, R. The Application of High Hydrostatic Pressure for the Stabilization of Functional Foods: Pomegranate Juice. J. Food Eng. 2010, 100(2), 245–253. DOI: 10.1016/j.jfoodeng.2010.04.006.
- Mastello, R. B.; Janzantti, N. S.; Bisconsin, A.; Monteiro, M. Impact of HHP Processing on Volatile Profile and Sensory Acceptance of Pera-Rio Orange Juice. Innovative Food Sci. Emerg. Technol. 2018, 45, 106–114. DOI: 10.1016/j.ifset.2017.10.008.
- Zou, H.; Lin, T.; Bi, X.; Zhao, L.; Wang, Y.; Liao, X. Comparison of High Hydrostatic Pressure, High-Pressure Carbon Dioxide and High-Temperature Short-Time Processing on Quality of Mulberry Juice. Food Bioprocess. Technol. 2016, 9(2), 217–231. DOI: 10.1007/s11947-015-1606-9.
- Buckow, R.; Kastell, A.; Terefe, N. S.; Versteeg, C. Pressure and Temperature Effects on Degradation Kinetics and Storage Stability of Total Anthocyanins in Blueberry Juice. J. Agric. Food Chem. 2010, 58(18), 10076–10084. DOI: 10.1021/jf1015347.
- Mousavi, Z. E.; Mousavi, S. M.; Razavi, S. H.; Hadinejad, M.; Emam-Djomeh, Z.; Mirzapour, M. Effect of Fermentation of Pomegranate Juice by Lactobacillus Plantarum and Lactobacillus Acidophilus on the Antioxidant Activity and Metabolism of Sugars, Organic Acids and Phenolic Compounds. Food Biotechnol. 2013, 27(1), 1–13. DOI: 10.1080/08905436.2012.724037.
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M. G. Metabolism of Phenolic Compounds by Lactobacillus Spp. During Fermentation of Cherry Juice and Broccoli Puree. Food Microbiol. 2015, 46, 272–279. DOI: 10.1016/j.fm.2014.08.018.
- Valero-Cases, E.; Nuncio-Jauregui, N.; Frutos, M. J. Influence of Fermentation with Different Lactic Acid Bacteria and in Vitro Digestion on the Biotransformation of Phenolic Compounds in Fermented Pomegranate Juices. J. Agric. Food Chem. 2017, 65(31), 6488–6496. DOI: 10.1021/acs.jafc.6b04854.
- Serment-Moreno, V.; Barbosa-Cánovas, G.; Torres, J. A.; Welti-Chanes, J. High-pressure Processing: Kinetic Models for Microbial and Enzyme Inactivation. Food Eng. Rev. 2014, 6(3), 56–88. DOI: 10.1007/s12393-014-9075-x.
- Kaur, B. P.; Rao, P. S. Modeling the Combined Effect of Pressure and Mild Heat on the Inactivation Kinetics of Escherichia Coli, Listeria Innocua, and Staphylococcus Aureus in Black Tiger Shrimp (penaeus Monodon). Front. Microbiol. 2017, 8. DOI: 10.3389/fmicb.2017.01311.
- Chen, D.; Pan, S.; Chen, J.; Pang, X.; Guo, X.; Gao, L.; Liao, X.; Wu, J. Comparing the Effects of High Hydrostatic Pressure and Ultrahigh Temperature on Quality and Shelf Life of Cloudy Ginger Juice. Food Bioprocess. Technol. 2016, 9(10), 1779–1793. DOI: 10.1007/s11947-016-1759-1.
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M. T.; Wu, M.; Sackey, A. S.; Xiao, L.; Tahir, H. E. Effect of Lactobacillus Strains on Phenolic Profile, Color Attributes and Antioxidant Activities of Lactic-acid-fermented Mulberry Juice. Food Chem. 2018, 250, 148–154. DOI: 10.1016/j.foodchem.2018.01.009.
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic Acid Fermentation Drives the Optimal Volatile Flavor-aroma Profile of Pomegranate Juice. Int. J. Food Microbiol. 2017, 248, 56–62. DOI: 10.1016/j.ijfoodmicro.2017.02.014.
- Georget, E.; Sevenich, R.; Reineke, K.; Mathys, A.; Heinz, V.; Callanan, M.; Rauh, C.; Knorr, D. Inactivation of Microorganisms by High Isostatic Pressure Processing in Complex Matrices: A Review. Innovative Food Sci. Emerg. Technol. 2015, 27, 1–14. DOI: 10.1016/j.ifset.2014.10.015.
- Saucedo-Reyes, D.; Carrillo-Salazar, J. A.; Román-Padilla, L.; Saucedo-Veloz, C.; Reyes-Santamaría, M. I.; Ramírez-Gilly, M.; Tecante, A. Modeling the Pressure Inactivation of Escherichia Coli and Salmonella Typhimurium in Sapote Mamey (pouteria Sapota (jacq.) HE Moore & Stearn) Pulp. Food Sci. Technol. Int. 2018, 24(2), 117–131. DOI: 10.1177/1082013217735472.
- Shinwari, K. J.; Rao, P. S. Stability of Bioactive Compounds in Fruit Jam and Jelly during Processing and Storage: A Review. Trends Food Sci. Technol. 2018, 75, 181–193. DOI: 10.1016/j.tifs.2018.02.002.
- Xu, Z. Z.; Wang, Y.; Ren, P.; Ni, Y.; Liao, X. Quality of Banana Puree during Storage: A Comparison of High Pressure Processing and Thermal Pasteurization Methods. Food Bioprocess. Technol. 2016, 9(3), 407–420. DOI: 10.1007/s11947-015-1635-4.
- Zhang, Y.; Liu, X.; Wang, Y.; Zhao, F.; Sun, Z.; Liao, X. Quality Comparison of Carrot Juices Processed by High-pressure Processing and High-temperature Short-time Processing. Innovative Food Sci. Emerg. Technol. 2016, 33, 135–144. DOI: 10.1016/j.ifset.2015.10.012.
- Nayak, B.; Liu, R. H.; Tang, J. M. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains-A Review. Crit. Rev. Food Sci. Nutr. 2015, 55(7), 887–918. DOI: 10.1080/10408398.2011.654142.
- Nayak, P. K.; Rayaguru, K.; Krishnan, K. R. Quality Comparison of Elephant Apple Juices after High-pressure Processing and Thermal Treatment. J. Sci. Food Agric. 2017, 97(5), 1404–1411. DOI: 10.1002/jsfa.7878.
- Nowicka, A.; Kucharska, A. Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of Polyphenol Content and Antioxidant Capacity of Strawberry Fruit from 90 Cultivars of Fragaria X Ananassa Duch. Food Chem. 2019, 270, 32–46. DOI: 10.1016/j.foodchem.2018.07.015.
- Turfan, O.; Türkyılmaz, M.; Yemiş, O.; Özkan, M. Anthocyanin and Colour Changes during Processing of Pomegranate (punica Granatum L., Cv. Hicaznar) Juice from Sacs and Whole Fruit. Food Chem. 2011, 129(4), 1644–1651. DOI: 10.1016/j.foodchem.2011.06.024.
- Tiwari, B. K.; O’Donnell, C. P.; Cullen, P. J. Effect of Non Thermal Processing Technologies on the Anthocyanin Content of Fruit Juices. Trends Food Sci. Technol. 2009, 20(3–4), 137–145. DOI: 10.1016/j.tifs.2009.01.058.
- Castaneda-Ovando, A.; Pacheco-Hernández, M. D. L.; Páez-Hernández, M. E.; Rodríguez, J. A.; Galán-Vidal, C. A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113(4), 859–871. DOI: 10.1016/j.foodchem.2008.09.001.
- Randazzo, W.; Corona, O.; Guarcello, R.; Francesca, N.; Germanà, M. A.; Erten, H.; Moschetti, G.; Settanni, L. Development of New Non-dairy Beverages from Mediterranean Fruit Juices Fermented with Water Kefir Microorganisms. Food Microbiol. 2016, 54, 40–51. DOI: 10.1016/j.fm.2015.10.018.
- Chen, R-H.; Chen, W-X.; Chen, H-M.; Zhang, G-F.; Chen, W-J. Comparative Evaluation of the Antioxidant Capacities, Organic Acids, and Volatiles of Papaya Juices Fermented by Lactobacillus Acidophilus and Lactobacillus Plantarum. J. Food Qual. 2018, 1-12. DOI: 10.1155/2018/9490435.