ABSTRACT
Two hundred and seventy six mulberry juice samples were analyzed by high-performance liquid chromatography with diode-array detection quadrupole time-of-flight tandem mass spectrometry to identify the major polyphenols in the representative mulberry fruit cultivar Da10. Using FRAP, ABTS, and DPPH assays, total phenolic contents and antioxidant capacities of 276 mulberry juice samples from four provinces in China were found to be within the following ranges: 412.55–1471.86 mg/L gallic acid equivalent, 37.90–361.75 mmol/mL ferrous sulfate equivalent, 46.12–342.13 mmol/mL Trolox equivalent, and 7.30–216.41 mmol/mL ascorbic acid equivalent. Cluster analysis separated the cultivars into two groups. One group consisted of samples from Guangdong, Guangxi, and Hainan Provinces, which had high phenolic contents and antioxidant capacities. The other group consisted of samples from Xinjiang Province, which had low phenolic contents and antioxidant capacities. Antioxidant activity and phenolic content were correlated. Flavonoids were the main polyphenols of “Da10”. In addition to free phenolic compounds, various bound phenolic compounds were identified. This work provides a large dataset characterizing different mulberry cultivars for deeper research. “45–7”, “qianshan3” and “guoxuan04-129” were superior cultivars based on their high phenolic contents and antioxidant capacities. The results of this study support further research into mulberry use in human food and healthcare industries.
Introduction
Fruits make up a large horticultural group that includes many species and numerous cultivars, genotypes, accessions, etc., occurring in most parts of the world in cultivated, semi-wild, and wild states. Species within each of these three states are important genetic sources of biodiversity, which support the diverse array of life on earth.[Citation1–Citation5] Wild black mulberry is a valuable resource in medicinal chemistry, gaining importance for its potential to prevent and treat diseases.[Citation6] Mulberry (Morus, Moraceae) grows across an extensive range of climatic, topographical, and soil conditions.[Citation7] There are 24 species of Morus, and 1 subspecies. Most mulberry species were discovered in Asia, especially in China (approximately 24 mulberry species and more than 1,000 cultivars).[Citation7,Citation8] Morus atropurpurea Roxb.; a promising fruiting genotype, has been cultivated widely in southern China. In commercial production, the growing period of mulberry fruit (MorusatropurpureaRoxb.) takes only 25 to 30 d, which is relatively short compared with other fruits.[Citation9] Mulberry fruits are increasingly popular with consumers because of their unique flavor, and nutritional and medicinal values.[Citation10] Mulberry fruits are eaten in various forms, including fresh, or processed into jams and juices.[Citation7,Citation11–Citation13] Juices are a common berry product, and the pulp fractions of berries are good sources for extraction of phenolic compounds, which have positive effects on human health.[Citation14]
Polyphenols are present at high levels in mulberry fruit, and play important roles as active antioxidants.[Citation15,Citation16] Polyphenols, complex secondary metabolites containing phenolic hydroxyl groups, are widespread, and responsible for the antioxidant capacities of fresh fruits and vegetables by which they are thought to lower the risk of many chronic diseases, such as cardiovascular disease, diabetes, and cancer.[Citation17–Citation19] Thus, polyphenols are beneficial for human health. Humans consume, on average, 1 g of phenolic compounds per day.[Citation20] The main groups of phenolic compounds are phenolic acids, flavonoids, coumarins, tannins, lignans, stilbenes, and quinonoids.[Citation21,Citation22]
A knowledge of the phenolic contents of mulberries is of great theoretical value, and is therefore worthy of research. Interest has grown rapidly in investigating the health benefits of phenolic compounds, and their abilities to mitigate oxidative stress in humans.[Citation23,Citation24] The characteristics of the phenolic compounds in mulberries have been previously studied, but only in a few cultivars, including “Da10”, “Taiwan 1 hao”, and “Yue74”.[Citation9,Citation25,Citation26] There are at least 1,000 mulberry cultivars.[Citation8] Little attention has been given to other mulberry cultivars. Different mulberry cultivars are significantly different in chemical composition, nutritional value, and antioxidant activity.[Citation11]
Hence, in this study, 276 mulberry cultivars were evaluated to determine the variability in their total phenolic content (TPC) values and antioxidant capacities, to inform future development and utilization of mulberry-based products, and help farmers choose cultivars and better understand their mulberries’ harvest time. A representative mulberry fruit cultivar, Da10, was analyzed by high-performance liquid chromatography with diode-array detection quadrupole time-of-flight tandem mass spectrometry (HPLC-DAD-Q-TOF-MS/MS) to determine its polyphenol profile, and quantitate individual phenolic compounds in both free and bound forms.
Materials and methods
Mulberry fruit preparation
This study selected 276 mulberry varieties found in China for characterization of their polyphenol content. Of these varieties, 240 were collected from Guangdong Province (22°06′N, 113°3′E), 21 from Guangxi Province (22°13′N, 107°45′E), 9 from Hainan Province (20°05′N, 110°20′E) and 6 from Xinjiang Province (43°50′N, 87°37′E). All mulberries tested in this study were germplasm resources of M. atropurpurea Roxb., except for six from Xinjiang Province that were hybrids of M. atropurpurea Roxb. and M. alba L. These are all preserved in the South China Branch of the National Mulberry Germplasm Resources Bank, and were grown under the same conditions in Guangzhou, Guangdong Province, and were harvested in May, 2014–2015. Approximately 200 g of mature disease-free mulberries were selected and milled through a 100-mesh double-layered sieve. Filtrates were stored at −40°C for later use. Then, 1.0 g of the aforementioned filtrates was mixed with 9.00 mL of 50% alcohol solvent. Each mixture was centrifuged at 3,000 × g for 30 min. Supernatants were filtered, and filtrates were preserved at −40°C until use.
Chemicals and reagents
2,4,6-Tri(2-pyridyl)-s-triazine(TPTZ), Trolox, 2,2ʹ-amino-di(2-ethyl-benzothiazoline sulfonic acid-6)ammonium salt (ABTS), 2,2ʹ-diphenyl-1-picryl-hydrazyl(DPPH), potassium persulfate,2 mol/L Folin–Ciocalteu reagent, and gallic acid were purchased from Sigma-Aldrich Chemical Co. (Shanghai, China). HPLC-grade acetic acid and acetonitrile were purchased from Thermo Fisher Scientific Co. (Shanghai, China). Water was purified using a Milli-Q system. Other chemicals used were of analytical grade.
Determination of total polyphenol content (TPC)
The TPC of mulberry juice was analyzed using the Folin–Ciocalteu colorimetric method described previously by Fu.[Citation27] Briefly, 500 µL of mulberry extracts were mixed with 2.50 mL of 0.2 mol/L Folin–Ciocalteu reagent. Four minutes later, 2.00 mL of aqueous sodium carbonate solution was added to each sample. The mixtures were incubated at room temperature for 2 h, and absorbance was measured at 760 nm. Known concentrations of gallic acid were used to establish a standard curve. The results were expressed as mg/L gallic acid equivalent (GAE).
Determination of antioxidant capacities by ABTS assay
Antioxidant capacities were determined using an ABTS assay according to the method described by Fu.[Citation27] Five mL of 7 mM ABTS solution (0.0192 g of ABTS in 5.00 mL distilled water) was mixed with 5 mL of 2.45 mM potassium persulfate (K2S2O8) solution (0.0662 g K2S2O8 in 100.00 mL distilled water). This solution was stored in the dark for 16 h. Before use, it was diluted with water to produce an absorbance of 0.7 ± 0.05 at 734 nm. Then, 100 µL of each diluted mulberry extract was mixed with a 3.80 mL aliquot of diluted ABTS radical solution. After 6 min, absorbance of each sample at 734 nm was recorded and compared to that from the calibrated Trolox standard. Results were expressed as mmol/g Trolox equivalents.
Antioxidant capacities determined by DPPH radical scavenging activity assay
Free radical scavenging activity of the mulberry extracts was determined using a 1,1-biphenyl-2-picrylhydrazyl radical (DPPH) scavenging protocol described by Ren[Citation28] and Lai.[Citation29] Three milliliters of 0.2 mM freshly prepared DPPH solution was mixed with 3 mL of mulberry extract, and 3 mL of methanol, and stored in the dark for 30 min. Then, absorbance at 517 nm was measured in triplicate. Known concentrations of ascorbic acid were used to establish a standard curve, and DPPH scavenging capacity was quantified with ascorbic acid.
Antioxidant capacities determined by the ferric reducing antioxidant power (FRAP) assay
Total antioxidant capacities were assessed using the FRAP assay as described by Fu.[Citation27] Briefly, 100 mL of 300 mM acetate buffer (0.1870 g of CH3COONa, 1.60 mL of CH3COOH, and distilled water, pH 3.6), 50.00 mL of 10 mM TPTZ solution (0.1562 g TPTZ in 40 mM HCl), and 50.00 mL of 20 mM FeCl3·6H2O solution (0.2705 g FeCl3·6H2O and 50.00 mL distilled water) were mixed sequentially to produce a working solution of 10:1:1 (v/v/v). The working solution was incubated in a 37°C water bath before use. One hundred microliter aliquots of diluted mulberry extracts were mixed with 3.00 mL of working solution. After 4 min, absorbance was measured at 593 nm. Known concentrations of FeSO4·7H2O were used to establish a standard curve, and FRAP antioxidant activity was expressed as mmol/mL ferrous sulfate equivalent (FSE).
Extraction of free phenolic compounds
Free phenolic compounds were extracted using methods described by Sun et al.[Citation30] with minor modifications. Mixtures of 200 g of fresh mulberry fruits in chilled (4°C) 80% aqueous acetone (1:2, w/v) were homogenized in a Philips blender for 5 min in an ice water bath. Mixtures were then centrifuged at 6,000 µg for 8 min to separate solid and liquid phases. After filtering, residues were extracted again under the same conditions. Subsequently, supernatant fractions were collected and concentrated under vacuum at 45°C until almost dry. Concentrated extracts were reconstituted in 85:15 (v/v) methanol: H2O to a final volume of 50 mL, and stored at – 80°C until analyzed.
Extraction of bound phenolic compounds
Bound phenolic compounds were extracted using methods described by Hartzfeld et al.[Citation31] After hydrolyzing with 90:10 (v/v) methanol: sulfuric acid (1:3, w/v) for 20 h at 85°C, the residue mentioned above was neutralized with 10 M NaOH, and extracted six times with ethyl acetate. Organic solvents were collected and concentrated under vacuum at 35°C to dry. Dried extracts were reconstituted with 85:15 (v/v) methanol:H2O to a final volume of 50 mL, and stored at – 80°C until analyzed.
HPLC-DAD-Q-TOF-MS/MS analysis
HPLC analysis was performed according to a method proposed by Zhang et al.[Citation32] The HPLC system was an Agilent 1260 series coupled to a Q-TOF MS with binary pump, hip sampler, column compartment, and DAD. Separation was performed at 30°C on a Zorbox SB-C18 column with a flow rate of 1.0 mL/min, and an injection volume of 20 µL. Acidified water (0.4% acetic acid, v/v) and acetonitrile were used as mobile phases A and B, respectively. The DAD detector wavelengths were 260, 280, and 320 nm. A linear gradient was programmed as follows: 0–40 min, 5%–25% B; 40–45 min, 25%–35% B; 45–50 min, 35%–50% B, and finally, initial conditions were held for 5 min as a re-equilibration step. Compounds were identified using a combination of MS and MS/MS data.
Results and discussion
TPC and antioxidant properties as assessed by FRAP, ABTS, and DPPH assays
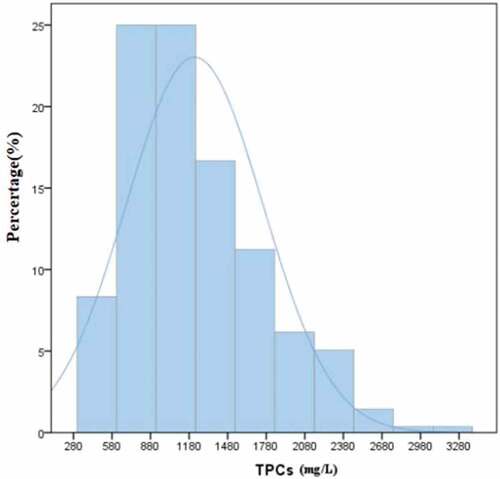
The TPC of 276 mulberry juices ranged from 412.55 to 1,471.86 mg/L GAE in the original data. Frequency distributions of the TPC of 276 mulberry juices are presented in . Distribution was partially normal, ranging from 746.54 to 1,120.56 mg/L GAE in 68.12% of the total cultivars. “Tunche1” (Guangxi Province) had the highest TPC, followed by “pengzhen2” (Guangdong Province), “guoxuan04-129” (Guangdong Province) and “45–7” (Guangdong Province). “Kangxuan603” (Guangdong Province) had the lowest TPC among all mulberry cultivars tested.
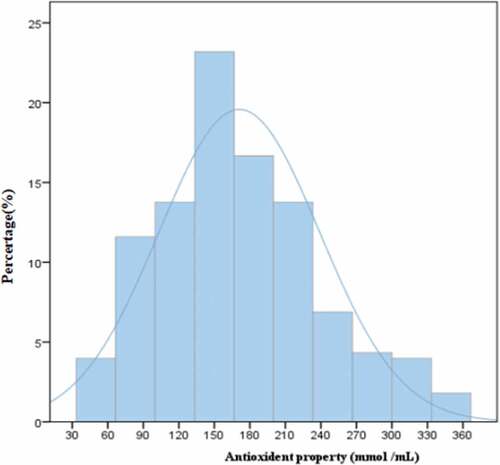
The antioxidant capacities of the 276 mulberry juices varied from 37.90 to 361.75 mmol/mL FSE in the original data. The distribution of antioxidant capacities in the 276 mulberry juices is presented in . Distribution was almost normal. “45–7” (Guangdong Province) had the highest antioxidant capacity, followed by “qianshan3” (Guangdong Province), “98–1” (Guangdong Province) and “guoxuan04-129” (Guangdong Province). “Lunbai7” (Xinjiang Province) had lower antioxidant properties than most other mulberry cultivars tested. “Kangxuan603” ranked last, with 40.08 mmol/mL FSE.
Figure 2. Frequency distribution of the antioxidant properties in 276 mulberry juices. FRAP = ferric reducing antioxidant power
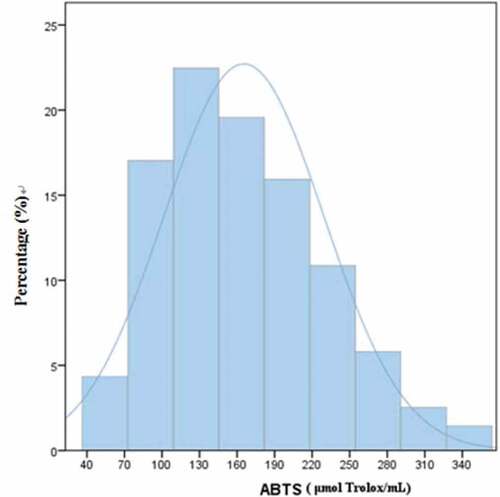
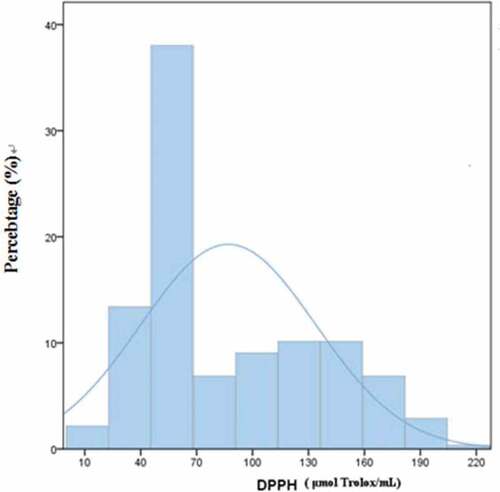
Oxygen radical inhibition capacity (ORIC) is a measure of the antioxidant activity of mulberry juice. In these experiments, DPPH and ABTS analyses were used to evaluate the radical inhibition capacity of mulberry juice. The distributions of Oxygen radical inhibition capacity by the two methods are shown in and . It can be seen in that the samples fit the normal distribution curve better when ORIC was measured using ABTS radical. ABTS radical inhibition capacity was close to 23% (near 130 μg Trolox/mL). “Anti-Selection-603” (Guangdong) was the worst (only 46.12 μg Trolox/mL) while the inhibition capacity of “42–3” (Guangdong) was the best (342.13 μgTrolox/mL). The results showed that even when geographical environment and climate conditions are the same, as well as planting technique, there remains a huge difference in ORIC between different varieties. Overall, ORIC values in the samples of Guangdong Province (<100% Trolox/mL) were no more than 10%. illustrates that the samples generally fit a normal distribution curve for capacity to inhibit distribution of DPPH radicals. Among these, DPPH free radical inhibition capacity was close to 38% (44 ~ 69 μg Trolox/mL). “Uguo 06–17” (Guangdong) had the best inhibition capacity (216.41 μg Trolox/mL), while “Lengbai 4” (Xinjiang) had the worst inhibitory capacity (7.3 μg Trolox/mL).
In previous studies, Yang et al.[Citation9] investigated mulberry fruit at different stages of ripeness, and found that fully ripened mulberry fruit extracts contained the highest TPC. Here, the mulberry fruits tested were all mature, allowing meaningful comparisons of TPC levels among different mulberry cultivars. Zang et al.[Citation33] presented the antioxidant capacities of 21 kinds of fresh fruit in terms of vitamin C equivalents. The results were as follows: mulberry ranked first, with 500.85 mg/100 g, followed by green plum (215.94 mg/100 g), black grape (152.59 mg/100 g), strawberry (144.46 mg/100 g), and black kiwi (115.27 mg/100 g). Mulberry fruit had extremely high antioxidant capacities.
In this study, we explored the TPC in 276 mulberry cultivars. TPC ranged from 746.54 to 1,120.56 mg/L GAE in 68.12% cultivars. In agreement with our results, Jiang et al.[Citation34] measured the TPC of 10 kinds of mulberry juices (11.67 ± 5.13–690.83 ± 7.38 mg GAE/g), finding variation in the TPC of different mulberry juices. The TPC of mulberry juices varied considerably among the different cultivars. Merveet al.[Citation35] observed considerable losses (25–50% loss) in the TPC of mulberry juice during the milling step of juice processing, compared with fresh fruit. In the milling step, the decrease in TPC might be related to enzymes (polyphenol oxidase, peroxidase, etc.) released by cell wall or membrane disruption, which catalyze oxidation and degradation reactions.[Citation36] These experiments also demonstrate that fresh mulberry fruits have more potential polyphenols.
FRAP is a reliable method for measuring the antioxidant capacities of mulberry fruit[Citation37], and the antioxidant capacities of the fruit’s TPC. Considerable variation exists in the antioxidant capacities of mulberry fruit.[Citation38,Citation39] In general, mulberry juice containing high levels of phenolic compounds also had comparatively high antioxidant capacity (r = 0.68[Citation40]). Although “tunche1” had the highest phenolic content (1,471.86 mg/L GAE), its antioxidant property was not outstanding, at only 147.21 mmol/mL FSE. “45–7”, “qianshan3” and “guoxuan04-129” not only had high phenolic contents (1,278.71, 1,185.93 and 1,280.23 mg/L GAE, respectively) but also had high antioxidant properties (361.75, 353.77, and 346.80 mmol/mL FSE, respectively).
Thus, some mulberry fruits, like those of “45–7”, “qianshan3”, and “guoxuan04-129”, may be excellent sources of phenolic compounds compared with other mulberry and fruit species mentioned by Zang et al.[Citation33] and may be used to research the correlation between phenolic compounds and antioxidant capacities.
Correlation of mulberry juice properties with their province of origin
Analyses of the phenolic content of mulberry fruits of different cultivars from four provinces in China are presented in . The coefficient of variation (CV) was highest for the TPC values of mulberry juices from Guangxi Province was (20.07%), followed by cultivars from Guangdong Province (19.42%). The CVs of the TPC values of cultivars from Xinjiang and Hainan Provinces were similar, at 16.93% and 16.41%, respectively.
Table 1. Total phenolic content and antioxidant properties of mulberries
FRAP analyses of the antioxidant properties of mulberry juices from different cultivars from each of four provinces are presented in . Mulberry fruit cultivars from Guangdong Province had the highest CV for antioxidant properties (38.76%), followed by cultivars from Hainan and Guangxi Provinces. Cultivars from Xinjiang Province had the lowest CV.
Variation in phenolic contents of mulberry fruits correlated significantly with cultivar and place of origin.[Citation11] Hierarchical clustering analysis was performed using IBM SPSS Statistics (Version 19, SPSS). A dendrogram was constructed based on TPC and antioxidant properties. 276 mulberry juice samples were divided into two main groups, Group I = samples from Guangdong, Guangxi and Hainan Provinces, and Group II = samples from Xinjiang Province (). This indicates that the characteristics of samples from Xinjiang Province were different from those of the others. Comparing TPC values and antioxidant properties between the two groups, revealed that Group I had much higher values than Group II.
Figure 5. Cluster analysis based on total phenolic content, and antioxidant properties of 276 mulberry fruit samples from four provinces in China
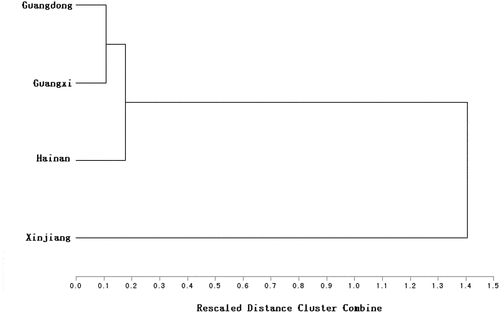
All of these data indicate that TPC and antioxidant properties vary significantly among cultivars from different regions. Genetic and environmental elements can affect phytochemical production. Group I were all M. atropurpurea, while Group II, with low TPC values and antioxidant properties, were hybrids of M. atropurpurea and Morus alba. Xinjiang Province, which has a different environment than that in most other regions in which mulberries grow, has a higher latitude and a larger difference between day and night temperatures. Mulberries may have developed drought resistance to adapt to long term cultivation in this environment.[Citation41] Because growth conditions were the same for all mulberry cultivars tested, the varietal differences may be attributed to genotype, rather than environmental factors.[Citation42,Citation43] This provides basic data for further studies on relationships between polyphenols and genotype.
Correlation analyses
The linear relationships of TPC values with FRAP, DPPH, and ABTS data are shown in . Due to the variety of species in the sample, and the differences in climate, data correlations were different in different assays. Compared with FRAP and DPPH, the relative relationship between TPC and ABTS is strongest, with an R2 of 0.6832, FRAP was second (R2 = 0.6788), and DPPH was lowest (R2 = 0.5299). Studies have shown that polyphenols make a great contribution to the antioxidant capacity of mulberries. However, the lowest correlation is between TPC and DPPH, indicating that there may be other substances in the mulberry fruit that affect DPPH free radical scavenging (inhibition). Overall, the correlation between TPC and the other three data types fluctuated more, reflecting that polyphenols are the main and most important antioxidants in mulberries.
Table 2. Correlation coefficients (R2) for relationships between TPC, and FRAP, DPPH, and ABTS assays
Characterization of phenolic compounds by HPLC-DAD-Q-TOF-MS/MS
HPLC-DAD-Q-TOF-MS/MS analysis was carried out to identify the phenolic constituents in the representative mulberry cultivar Da10. This method can not only separate and detect phenolic profiles, but also provide positive, reliable compound identifications.[Citation44] In this study, 27 individual compounds, including 9 phenolic acids (A1–A9), 14 flavonoids (B1–B14), and 4 coumarins (C1–C4) were detected in free and bound fractions of “Da10” (). Only three free phenolic compounds, 4-Methoxybenzenepropanol1-(2-sulfoglucoside), 9,10-dihydroxy-12,13-epoxyoctadecanoate, and luteolin6-C-glucoside 8-C-arabinoside, were found in “Da10”. Twenty-four bound phenolic compounds were identified. Thirteen of these (54.17%) were flavonoids. The present work revealed that “Da10” contains a great variety of phenolic compounds, and flavonoids are the most abundant. There were also more kinds of bound phenolic compounds than free phenolic compounds.
Table 3. Characterization of polyphenols in mulberries of the “Da10” cultivar by HPLC-ESI-DAD-Q-TOF-MS/MS
Conclusion
TPC values and antioxidant capacities were determined in a significant number of mulberry varieties. To our knowledge, this is the largest study of polyphenols in mulberry juices. The TPC and antioxidant capacities of mulberry juice varied widely among cultivars. Based on hierarchical clustering analysis, the mulberry fruit cultivars tested in this study fell into two main groups. Group I, those from Guangdong, Guangxi and Hainan Provinces, which were characterized by high TPC values and antioxidant capacities, and Group II, those from Xinjiang Province, which were characterized by low TPC values and antioxidant capacities. The reasons for variation in the content of phenolic substances and antioxidants in mulberry juices were diverse. First is genetic differences. Planting conditions, climate, and latitude also affect the content of polyphenols, which in turn affects antioxidant properties. According to these results, a planting environment with sufficient sunshine, little temperature difference between day and night, and sufficient water in the soil strongly promote increased antioxidant activity. HPLC-DAD-Q-TOF-MS/MS, a powerful analytical technique, was used to analyze the phenolic compounds in “Da10”. In total, 27 individual compounds were identified. Most of these were flavonoids. Thus, mulberry fruit cultivars like “45–7”, “qianshan3” and “guoxuan04-129”, which were rich sources of phenolic compounds and had high antioxidant capacities, could be selected for industrial production of phenolic-rich juices. The results of this study support further research on mulberry fruit for human food and healthcare industries.
Disclosure statement
The authors declare no conflicts of interest
Additional information
Funding
References
- Gundogdu, M.; Canan, I.; Gece, M. K.; Kan, T.; Ercisli, S. Phenolic Compounds, Bioactive Content and Antioxidant Capacity of the Fruits of Mulberry (Morus Spp.) Germplasm in Turkey. Folia Hort. 2017, 29(2), 251–262. DOI: 10.1515/fhort-2017-0023.
- Usanmaz, S.; Öztürkler, F.; Helvaci, M.; Alas, T.; Kahramanoğlu, I.; Aşkin, M. Effects of Periods and Altitudes on the Phenolic Compounds and Oil Contents of Olives. Cv. ayvalik. Int. J. Agric. Life Sci. 2018, 2(2), 32–39.
- Yildiz, E.; Çolak, A. Resent Condition of Apple Production in Uşak Province. Int. J. Agric. Life Sci. 2018, 2(2), 189–193.
- Okatan, v.; Polat, m.; & Aşkin, m. a.; Some physico-chemical characteristics of black mulberry (Morus nigra l.) in Bitlis. Scientific Papers-series b, Horticulture 2016, 60, 27-30.
- koç, M.; çetİnkaya, H.; Yildiz, K. A Research on Recent Developments and Determination of the Potential of Olive in Kilis. Int. J. Agric. Life Sci. 2018, 2(2), 185–188.
- Okatan, V. Phenolic Compounds and Phytochemicals in Fruits of Black Mulberry (Morus Nigra L.) Genotypes from the Aegean Region in Turkey. Folia Hort. 2018, 30(1), 93–101. DOI: 10.2478/fhort-2018-0010.
- Ercisli, S.; Orhan, E. Chemical Composition of White (Morus Alba), Red (Morus Rubra) and Black (Morus Nigra) Mulberry Fruits. Food Chem. 2007, 103(4), 1380–1384. DOI: 10.1016/j.foodchem.2006.10.054.
- Huo, Y. K.; Mulberry Cultivation and Utilization in China, In Mulberry for animal production. Fao Animal Production & Health Paper 2002, 147, 11–43.
- Yang, J.; Liu, X.; Zhang, X.; Jin, Q.; Li, J. Phenolic Profiles, Antioxidant Activities, and Neuroprotective Properties of Mulberry (Morus Atropurpurea Roxb.) Fruit Extracts from Different Ripening Stages. J. Food Sci. 2016, 81(10), C2439–C2446. DOI: 10.1111/1750-3841.13426.
- Ma, Y.; Navarro, B.; Zhang, Z.; Lu, M.; Zhou, X.; Chi, S.; Di Serio, F.; Li, S. Identification and Molecular Characterization of a Novel Monopartite Geminivirus Associated with Mulberry Mosaic Dwarf Disease. J. Gen. Virol. 2015, 96(8), 2421–2434. DOI: 10.1099/vir.0.000175.
- Liang, L.; Wu, X.; Zhu, M. M.; Zhao, W. G.; Li, F.; Zou, Y.; Yang, L. Q. Chemical Composition, Nutritional Value, and Antioxidant Activities of Eight Mulberrycultivars from China. Pharmacognosymagazine. 2012, 8, 215–224.
- Tchabo, W.; Ma, Y.; Engmann, F. N.; Zhang, H. Ultrasound-assisted Enzymatic Extraction (UAEE) of Phytochemical Compounds from Mulberry (Morus Nigra) must and Optimization Study using Response Surface Methodology. Ind. Crops Prod. 2015, 63, 214–225. DOI: 10.1016/j.indcrop.2014.09.053.
- Zou, H.; Lin, T.; Bi, X.; Zhao, L.; Wang, Y.; Liao, X. Comparison of High Hydrostatic Pressure, High-pressure Carbon Dioxide and High-Temperature Short-Time Processing on Quality of Mulberry Juice. Food Bioprocess. Technol. 2016, 9(2), 217–231. DOI: 10.1007/s11947-015-1606-9.
- Yang, B.; Ahotupa, M.; Määttä, P.; Kallio, H. Composition and Antioxidative Activities of Supercritical CO2-extracted Oils from Seeds and Soft Parts of Northern Berries. Food Res. Int. 2011, 44(7), 2009–2017. DOI: 10.1016/j.foodres.2011.02.025.
- Hominick, C. W. I.; Maciel, G. M.; Plata-Oviedo, M. S. V.; Peralta, R. M. Phenolic Compounds in Fruits - An Overview. Int. J. Food Sci. Technol. 2012, 47(10), 2023–2044. DOI: 10.1111/j.1365-2621.2012.03067.x.
- Yu, Y.; Xu, Y.; Wu, J.; Xiao, G.; Fu, M.; Zhang, Y. Effect of Ultra-high Pressure Homogenisation Processing on Phenolic Compounds, Antioxidant Capacity and Anti-glucosidase of Mulberry Juice. Food Chem. 2014, 153, 114–120. DOI: 10.1016/j.foodchem.2013.12.038.
- Halliwell, B.;. Antioxidants in Human Health and Disease. Annu. Rev. Nutr. 1996, 16, 33–50. DOI: 10.1146/annurev.nu.16.070196.000341.
- Mihalache Arion, C.; Tabart, J.; Kevers, C.; Niculaua, M.; Filimon, R.; Beceanu, D.; Dommes, J. Antioxidant Potential of Different Plum Cultivars during Storage. Food Chem. 2014, 146, 485–491. DOI: 10.1016/j.foodchem.2013.09.072.
- Tomczak, D. W.;. Changes in Antioxidant Activity of Black Chokeberry Juice Concentrate Solutions during Storage. Acta Sci. Pol. Technol. Aliment. 2007, 6(2), 49–55.
- Manach, C. S.; Morand, A.; Rémésy, C.; Jiménez, C.; Polyphenols:, L. Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79(5), 727–747. DOI: 10.1093/ajcn/79.5.727.
- Liu, R. H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134(12), 3479S–3485S. DOI: 10.1093/jn/134.12.3479S.
- Yao, R. Q.;. Research Progress of Plant Polyphenols Classification and Biological Function. Acad. Period. Farm Produ. Proc. 2011, 241(4), 99–100.
- Pérez-Gregorio, M. R.; Regueiro, J.; Alonso-González, E.; Pastrana-Castro, L. M.; Simal-Gándara, J. Influence of Alcoholic Fermentation Process on Antioxidant Activity and Phenolic Levels from Mulberries (Morus Nigra L.). LWT - Food Sci. Technol. 2011, 44(8), 1793–1801. DOI: 10.1016/j.lwt.2011.03.007.
- Zhang, W.; Han, F.; He, J.; Duan, C. HPLC-DAD-ESI-MS/MS Analysis and Antioxidant Activities of Nonanthocyanin Phenolics in Mulberry (Morus albaL.). J. Food Sci. 2008, 73(6), C512–C518.
- Chen, L.; Zhang, X.; Jin, Q.; Yang, L.; Li, J.; Chen, F. Free and Bound Volatile Chemicals in Mulberry (Morus Atropurpurea Roxb.). J. Food Sci. 2015, 80(5), C975–82. DOI: 10.1111/1750-3841.12840.
- Han, Q.; Gao, H.; Chen, H.; Fang, X.; Wu, W. Precooling and Ozone Treatments Affects Postharvest Quality of Black Mulberry (Morus Nigra) Fruits. Food Chem. 2017, 221, 1947–1953. DOI: 10.1016/j.foodchem.2016.11.152.
- Fu, L. Antioxidant activities and total phenolic contents of fruits, herbal teas, tea drinks and wild fruits. Guangdong province, China: Master Dissertation, Sun Yat-Sen University, 2010.
- Wei, R.; Hua, P.; Guixing, R. Antioxidant Characteristics of Core Oats in Naked Oats. Acta Agronomica Sinica. 2010, 36(06), 988–994.
- Jixiang, Lai. Study on Antioxidant Activity of Black Bean Germination Water Extract and Its Mechanism. Jiangsu Province, China: Jiangnan University, 2014.
- Sun, J.; Chu, Y. F.; Wu, X.; Liu, R. H. Antioxidant and Antiproliferative Activities of Common Fruits. J. Agric. Food Chem. 2002, 50, 7449–7454.
- Hartzfeld, W. P.; Forkner, R.; Hunter, D. M.; Hagerman, E. A. Determination of Hydrolyzable Tannins (Gallotannins and Ellagitannins) after Reaction with Potassium Iodate. Food Chem. 2002, 50, 1785–1790. DOI: 10.1021/jf0111155.
- Zhang, R.; Zeng, Q.; Deng, Y.; Zhang, M.; Wei, Z.; Zhang, Y.; Tang, X. Phenolic Profiles and Antioxidant Activity of Litchi Pulp of Different Cultivars Cultivated in Southern China. Food Chem. 2013, 136(3–4), 1169–1176. DOI: 10.1016/j.foodchem.2012.09.085.
- Zang, S.; Tian, S.; Jiang, J.; Han, D.; Yu, X.; Wang, K.; Li, D.; Lu, D.; Yu, A.; Zhang, Z. Determination of Antioxidant Capacity of Diverse Fruits by Electron Spin Resonance (ESR) and UV-Vis Spectrometries. Food Chem. 2017, 221, 1221–1225. DOI: 10.1016/j.foodchem.2016.11.036.
- Jiang, D. Q.; Guo, Y.; Xu, D. H.; Huang, Y. S.; Yuan, K.; Lv, Z. Q. Antioxidant and Anti-fatigue Effects of Anthocyanins of Mulberry Juice Purification (MJP) and Mulberry Marc Purification (MMP) from Different Varieties Mulberry Fruit in China. Food Chem. Toxicol. 2013, 59, 1–7. DOI: 10.1016/j.fct.2013.05.023.
- Tomas, M.; Toydemir, G.; Boyacioglu, D.; Hall, R.; Beekwilder, J.; Capanoglu, E. The Effects of Juice Processing on Black Mulberry Antioxidants. Food Chem. 2015, 186, 277–284. DOI: 10.1016/j.foodchem.2014.11.151.
- Renard, C. M. G. C.; Le Quéré, J. M.; Bauduin, R.; Symoneaux, R.; Le Bourvellec, C.; Baron, A. Modulating Polyphenolic Composition and Organoleptic Properties of Apple Juices by Manipulating the Pressing Conditions. Food Chem. 2011, 124(1), 117–125. DOI: 10.1016/j.foodchem.2010.05.113.
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compost. Anal. 2006, 19(6–7), 669–675. DOI: 10.1016/j.jfca.2006.01.003.
- Ercisli, S.; Tosun, M.; Duralija, B.; Voca, S.; Sengul, M.; Turan, M. Phytochemical Content of Black (Morusnigra L.) and Purple (Morusrubra L.) Mulberry Genotypes. Food Technol. Biotechnol. 2010, 48, 102–106.
- Imran, M.; Khan, H.; Shah, M.; Khan, R.; Khan, F. Chemical Composition and Antioxidant Activity of Certain Morus Species. J. Zhejiang Univ. Sci. B. 2010, 11(12), 973–980. DOI: 10.1631/jzus.B1000173.
- Sim, K. S.; Nurestri, A. M.; Norhanom, A. W. Phenolic Content and Antioxidant Activity of Pereskia Grandifolia Haw. (Cactaceae) Extracts. Pharmacogn. Mag. 2010, 6(23), 248–254. DOI: 10.4103/0973-1296.66945.
- Maimaiti, Y. M.; Xia, Q. Y.; Wu, L. L.; Yin, G.; Zeng, F. J.; Yan, H. L. Study on Desert Mulberry from Xinjiang. chin. J. North Seric. 2007, 28, 1–4.
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant Genotype Affects Total Antioxidant Capacity and Phenolic Contents in Fruit. Nutrition. 2005, 21(2), 207–213. DOI: 10.1016/j.nut.2004.03.025.
- Zadernowski, R. N.; Nesterowicz, M. J. Phenolic Acid Profiles in Some Small Berries. J. Agric. Food Chem. 2005, 53, 2118–2124. DOI: 10.1021/jf040411p.
- Li, Y. L.; X, M.; Liu, J. P.; Tang, C. M.; Wang, Z. J.; Chen, Z. Y.; Xiao, G. S. Change of Non-anthocyanidinpolyphenolics Content in Mulberry Fruit from Different Varieties during Maturation Process. Can Ye Ke Xue. 2008, 34, 711–717.