ABSTRACT
Two triterpenoid compounds, 3β-hydroxy-Olean-12-en- 28-oic acid (1) and 3,19-Dihydroxyurs-12-en-28-oic acid (2) were isolated from the leaves of Saurauia vulcani Korth. (Actinidiaceae). The chemical structures of compounds 1–2 were elucidated with spectroscopic data (UV, IR, 1H, 13C, DEPT 135º, HMQC, HMBC, 1H-1H COSY NMR) and MS as well as compared with previously reported spectra data. All the compounds were evaluated for their anti-cholesterol activity by the Liebermann-Burchard (LB) colorimetric assay. Compound 1–2 showed a remarkable anti-cholesterol activity. Most importantly, the raised concentration of 1–2 exhibited a dose-dependent manner. The discoveries of anti-cholesterol compounds 1–2 were first reported.
Introduction
The genus Saurauia vulcani Korth. (Actinidiaceae) is known as pirdot plant and mainly distributed in a tropical climate.[Citation1] This plant has not been researched well and less information about its phytochemical studies; however, its biological screening exhibited interesting pharmacological properties, including antihyperglycemic, antihyperlipidemic,[Citation1] anti-diabetes,[Citation2,Citation3] anti-cancer,[Citation4] antihypoglycemic,[Citation5] and antioxidant.[Citation6]
Recently, our group screened the presence of triterpenoid compound from the leaves of Saurauia vulcani Korth.[Citation7] As a part of our continuing search for active compounds from this plant, we isolated and described the triterpenoid compounds, 3β-hydroxy-Olean-12-en- 28-oic acid (1) and 3,19-Dihydroxyurs-12-en-28-oic acid (2). These compounds were first reported from this plant. Compound 1–2 were evaluated for their anti-cholesterol activity by the Liebermann-Burchard (LB) colorimetric assay. As a result, the isolated compounds 1–2 showed a remarkable an anti-cholesterol activity.
Materials and methods
UV spectra were measured by using shimazu UV-8452A in methanol. The IR spectra were recorded on a Shimazu FT-IR Spectrometer in KBr. Mass spectra were recorded with Agilent 5977B single quadrupole GC/MS. NMR spectra were obtained with a JOEL JNM A-500 (500 MHz). Chemical shift is donated in δ (ppm) relative to residual solvent peak as internal standard (CDCl3: 1H δ 7.26, 13C δ 77.0). Coupling constant (J) are reported in Hertz (Hz). Multiplicity abbreviation: s = singlet, d = doublet, dd = double doublet, t = triplet, m = multiplet. Chromatography separations were carried out on silica gel 60 (70–230 mesh, Merck), octa desyl silane (200–400 mesh, Fuji Silysia). TLC plates were precoated with silica gel GF254 (Merck, 0.25 mm). The target compounds were detected by spraying with 10% H2SO4 in ethanol, followed by heating. All other commercially available reagents and solvents were used as purchased.
Plant material
The leaves of Saurauia vulcani Korth. (Actinidiaceae) were collected in Silangkitang Village, North Tapanuli, North Sumatera Province, Indonesia in July 2017. The plant was identified by the staff of the LIPI, Serpong, Indonesia and a voucher specimen (No. FR-SP 0101) was deposited at the herbarium.
Extraction and isolation
Dried ground leaves (1 kg) of Saurauia vulcani Korth. (Actinidiaceae) were extracted exhaustively with n-hexane, ethyl acetate (EtOAc), and methanol (MeOH) at room temperature and then filtered. The evaporation resulted in the crude extracts of n-hexane (15.5 g), EtOAc (12.1 g) and MeOH (10.9 g), respectively. The EtOAc extract (12.1 g) was subjected to column chromatography over silica gel (70–320 mesh) using a gradient elution of mixture of n-hexane/EtOAc (9/1 to 1/9) as eluting solvents yielded 11 fractions (E01-E11). Fraction E07 (192 mg) was subjected to reverse-phase column chromatography over ODS RP-18 using a gradient elution of mixture of MeOH/H2O (7/3 to 10/0) as eluting solvents yielded 24 fractions (E07-1- E07-24). Further purification of fractions E07-11- E07-15 (23 mg) and E07-7- E07-10 (28 mg) by reverse-phase column chromatography using an isocratic elution of mixture of MeOH/H2O (3/1) gave compounds 1 (13.7 mg) and 2 (11.3 mg), respectively.
Cholesterol determination
Liebermann–Burchard test was applied to measure cholesterol, which gives a deep green color.[Citation8] Reductions of cholesterol levels of isolated compounds 1–2 were determined by comparison with the blank absorbance (cholesterol solution). The stock cholesterol solution was made by mixing cholesterol salt (100 mg) in 100 mL ethanol (1000 ppm) at 45°C in water bath. To 0.025, 0.05, 0.1, 0.2, 0.3, and 0.4 mL of cholesterol solution in a cuvette (BRAN® UV cuvette) were added acetic anhydride (2 mL) and H2SO4 (0.1 mL) and diluted with ethanol (EtOH) to a final volume of 5 mL, respectively (blank solutions). To 0.5 mL of cholesterol solutions were added 0.025, 0.05, 0.1, 0.2, 0.3, and 0.4 mL of solution 1 and 2 (1000 ppm), acetic anhydride (2 mL) and H2SO4 (0.1 mL) and diluted with EtOH to a final volume of 5 mL, respectively. After being incubated in the dark for 15 min at room temperature, the absorbance of the solutions was measured at 423 nm.
Statistical analysis
Experiments were carried out independently three times and averages are presented. Statistical analysis was performed by unpaired two-tailed t-test (Excel). Differences were considered significant at *p< .05, **p< .01 and ***p< .001.
Result and discussion
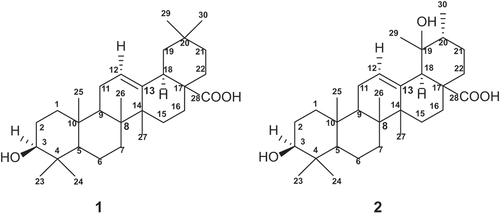
The leaves of Saurauia vulcani Korth. (Actinidiaceae) (1 kg) were grounded and successfully extracted with n-hexane, EtOAc and MeOH at room temperature. The EtOAc extract was chromatographed over column chromatography packed with silica gel (70–320 mesh) by gradient elution. The fractions were repeatedly subjected to reverse-phase column chromatography to give two anti-cholesterol compounds 1–2 ().
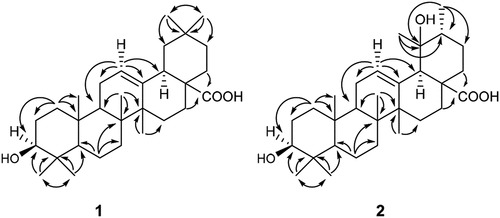
Compound 1 was obtained as a white amorphous powder. The molecular formula of 1 was established to be C30H48O3 from GC-MS Agilent MS which showed a [M+ Na]+ pseudo-molecular peak m/z 479.3496, found 479.3466, together with nuclear magnetic resonance (NMR) data, thus requiring seven degrees of unsaturation. The ultraviolet (UV) spectrum exhibited absorption peak at λmax nm (log ε): 202 nm explain no conjugated bond in compound. The Infrared (IR) spectrum showed absorption of hydroxyl group (3400 cm−1), C-H aliphatic (2987 cm−1), C-H bending (1456 cm−1), gem dimethyl stretch (1374 cm−1), a strain stretching of the C-OH group (1040 cm−1), carbonyl group (1688 cm−1), olefinic group C = C (656 cm−1). The 1H-NMR of 1 showed the presence of a triplet signal of olefinic proton resonating at δH 5.21 ppm (J= 3.9 Hz) as α position, an oxygenated methine resonating at δH 3.17 ppm (1H; dt, J= 11.0, 3.9 Hz) indicates as an axial position, double doublet signal of methine resonating at δH 2.90 ppm (1H; dd, J= 9.7, 3.9 Hz) indicated as α orientation, seven overlap signals of methyl groups, resonating at δH 1.17–0.77 ppm. These resonances suggesting the presence of a triterpene with olefin and alcohol functionalities of 1. A total 30 carbon resonances were observed in the 13C NMR. The distortionless enhancement by polarization transfer (DEPT 135º) and Heteronuclear Multiple-Quantum Correlation (HMQC) experiments showed the peaks corresponding to two olefinic carbons at δc 122.2 and 144.2, one carbonyl carbon at δc 178.2, one sp3 oxygenated methine, ten methylenes, three methines sp3, seven methyl, and six quaternary carbons. The Heteronuclear Multiple Bond Correlation (HMBC) spectrum confirmed the position of the C3 (δC 77.7 ppm) oxygenated methine carbon by showing correlations of H3-23 and H3-24. In addition, the signal of gem dimethyl showed mutual correlation between H-23 with C-24, H-24 with C-23, H-29 with C-30 and H-30 with C-29. The HMBC spectrum exhibited the correlation of H-11 with C-9, C-12 and C-13 and H-12 with C-9, C-11 C13 and C-18 thus confirming the position of the double bond at C12 and C13, respectively (). Furthermore, the location of carboxylic acid at C28 was established by the HMBC correlation of H22 and H16 with the C28 carbonyl. The Correlation Spectroscopy (1H-1H COSY) experiment showed a correlation between H-11 with H-12, H-3 with H-1 and H-18 with H-19. A comparison of the NMR data of 1 with 3β-hidroxy-12(13)-en, 28-oic acid revealed that the structures of two compounds are very similar.[Citation9,Citation10] Therefore, compound 1 was identified as a 3β-hydroxy-Olean-12-en- 28-oic acid.
Compound 2 was obtained as a white amorphous powder. The molecular formula of 2 was established to be C30H48O4 from GC-MS Agilent MS which showed a [M+ Na]+ pseudo-molecular peak m/z 495.3445, found 495.3447, together with NMR data (), thus requiring seven degrees of unsaturation. The UV spectrum exhibited absorption peak at λmax nm (log ε): 204 nm explain no conjugated bond in compound. The IR spectrum showed absorption of hydroxyl group, C-H aliphatic, carbonyl group, C-H stretching, gem dimethyl stretch, and carbon sp2 at νmax 3437, 2981, 1700, 1460, 1385, 727 cm−1, respectively. The 1H and 13C NMR spectra of 2 were resembled with compound 1 except for the signal of gem dimethyl at C20. The methine carbon C-19 of 1 was resonated downfield as quaternary carbon at δc 73.2 ppm since it is attached to a hydroxyl group. Furthermore, the location of hydroxyl at C-19 was also established by the HMBC correlation of H3-30 and H3-29 with C-19 and C20, respectively. A comparison of the NMR data of 2 with 3,19-Dihydroxy-12-ursen-28-oic acid revealed that the structures of two compounds are very similar.[Citation11] Therefore, compound 2 was identified as a 3,19-Dihydroxyurs-12-en-28-oic acid (2). The isolated compounds 1–2 were first reported from this plant.
Table 1. NMR data (500 MHz for 1H and 125 MHz for 13C in CDCl3) for 1 and 2.
3β-hydroxy-Olean-12-en- 28-oic acid (1)
A white amorphous powder; UV MeOH λmax nm (log ε); 202 (5.25); IR (KBr) νmax cm−1: 3400, 2987, 1456, 1374, 1040, 1688, 656; 1H NMR (CDCl3, 500 MHz) see ; 13C NMR (CDCl3 125 MHz), see ; GC-MS Agilent MS: m/z 479.3466 [M+ Na]+ calculated 479.3496.
3,19-Dihydroxyurs-12-en-28-oic acid (2)
A white amorphous powder; UV MeOH λmax nm (log ε); 204 (5.23); IR (KBr) νmax cm−1: 3437, 2981, 1700, 1460, 1385, 727; 1H NMR (CDCl3, 500 MHz) see ; 13C NMR (CDCl3 125 MHz), see ; GC-MS Agilent MS: m/z 495.3447 [M+ Na]+ calculated 495.3445.
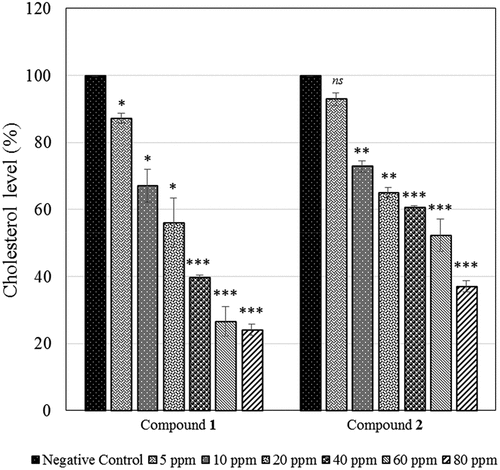
The anti-cholesterol effects of the two isolated compounds were carried out according to the described methods in the previous paper.[Citation12–Citation14] With the isolated pure compound 1–2 in hand, we investigated an anti-cholesterol activity by them. Cholesterol level was determined by the LB colorimetric assay using UV Vis spectrophotometer.[Citation15,Citation16] The cholesterol rate decreased when the isolated compounds 1–2 were treated, indicating that the anti-cholesterol induced by them. At a diluted concentration (5 ppm), the anti-cholesterol activity of 1–2 was limited (86% for 1 and 94% for 2). However, dose dependence of anti-cholesterol activity of 1–2 concentration was observed. The cholesterol level was dramatically reduced over a range of 10–80 ppm. The cholesterol levels were approximately 67%, 56%, 40%, 27% and 24% for 1 and 73%, 65%, 61%, 52% and 37% for 2 at the concentration of 10, 20, 40, 60 and 80 ppm, respectively (). This result clearly showed the anti-cholesterol effect of isolated compound 1–2. It is also worth noting that an anti-cholesterol of compounds 1–2 were influenced by the presence of hydroxyl, double bond, and carboxylic acid groups. Orientation of the hydroxyl group of 2 showed less effective at reducing the cholesterol level. These results suggested that the additional of hydroxyl group into structure 2 may be a critical pharmacophore for its anti-cholesterol activity. The investigation of the in vivo using isolated compounds 1–2 is ongoing.
Figure 3. The anti-cholesterol effects of the isolated compounds 1–2. The level of cholesterol was reduced in a dose-dependent manner. Cholesterol level of the untreated compound or negative control was calculated as 100%. All experiments were conducted in triplicate (n = 3) and compared with negative control. Standard deviation (SD) is indicated by error bars. ns: not significant, *p < .05, **p < .01, ***p < .001
Conclusion
Two triterpenoids, 3β-hydroxy-Olean-12-en- 28-oic acid (1) and 3,19-Dihydroxyurs-12-en-28-oic acid (2) were isolated from the leaves of Saurauia Vulcani. Korth (Actinidiaceae). The isolated compounds 1–2 showed an anti-cholesterol effect by the LB colorimetric assay. The discoveries of anti-cholesterol 1–2 were first reported. Insignificant effect of 2 than 1 caused by orientation of the hydroxyl group that probably interacts with other functional group through hydrogen bonding formation and therefore the activity was reduced. Unlike compound 2, intramolecular reaction of 1 does not occur. These results suggested that the additional of hydroxyl group of 2 at the position C-19 is a critical point for important structural feature for anti-cholesterol activity in triterpenoid structures.
Acknowledgments
We thank Dr. Sofa Fajriah and Dr. Ahmad in the research center for chemistry, Indonesian Science Institute for NMR measurements. We are grateful to Mr. Irfan Junaedi in the center laboratory of Sekolah Tinggi Analis Kimia Cilegon for the biological assay.
References
- Hutahean, S.; Tanjung, M.; Sari, D. P.; Ningsih, V. E. Antihyperglycemic and Antihyperlipidemic Effects of Pirdot (saurauia Vulcani Korth.) Leaves Extract in Mice. IOP Conf. Ser. Earth Environ. Sci. 2018, 130(1), 012042. DOI: 10.1088/1755-1315/130/1/012042.
- Situmorang, R. O. P.; Harianja, A. H.; Silalahi, J. Karo’s Local Wisdom: The Use of Woody Plants for Traditional Diabetic Medicines. Indo J. Forest Res. 2015, 2(10), 121–130. DOI: 10.20886/ijfr.2015.2.2.830.121-130.
- Ginting, G. A.; Rosidah, R.; Sitorus, P.; Satria, D. Wound Healing Activity of Saurauia Vulcani Korth. Aqueous Leaves Extract Evaluation on Excision Wound in Hyperglycemia Rats. JIBS. 2018, 5(3), 52–57.
- Silalahi, M.; Supriatna, J.; Walujo, E. B.; Nisyawati, N. Local Knowledge of Medicinal Plants in Sub-ethnic Batak Simalungun of North Sumatera, Indonesia. Biodiversitas. 2015, 16, 44–54. DOI: 10.13057/biodiv/d160106.
- Sitorus, P.; Rosidah, R.; Satria, D. Hypoglycemic Activity of Ethanolic Extract of Saurauia Vulcani Korth. Leaves. Asian J. Pharm. Clin. Res. 2018, 11, 35–36. DOI: 10.22159/ajpcr.2018.v11s1.26561.
- Saragih, R. R. Phytochemical Screening, Antioxidant and Antibacterial Activities of Methanol and Etyl Acetate Extracts of the Leaves of Pirdot (saurauia Vulcani Korth.) From Tigarunggu Region; Universitas Sumatera Utara: Medan, 1991.
- Suparman, A. C.; Kadarusman, M.; Situmeang, B. Triterpenoid Compound from Pirdot Plant (sauralia Sp). J. Itekima. 2018, 3(1), 12–19.
- Sheftel, A. G. Determination of Total and Free Cholesterol. J. Lab. Clin. Med. 1944, 29(8), 875–878.
- Uddin, G.; Wairulah; Siddiqui, B. S.; Alam, S.; Sadat, A.; Ahmad, A.; Uddin, A. Chemical Constituen and Phytoxicity of Solvent Extracted Fraction of Steam Bark of Grewia Optiva Drummond Ex Burret. Middle-Eas J.Sci. Res. 2011, 8(1), 85–91.
- Martins, D.; Carrion, L. L.; Ramos, D. F.; Salome, K. S.; Da Silva, P. E. A.; Barison, A.; Nunez, C. V. Triterpenes and the Antimycobacterial Activity of Duroia Macrophylla Huber (rubiaceae). Biomed Res. Int. 2013, 2013, article ID 605831. DOI:10.1155/2013/605831.
- Ayatollahi, A. M.; Ghanadian, M.; Afsharypour, S.; Abdella, O. M.; Mirzai, M.; Askari, G. Pentacylic Triterpenes in Euphorbia Microsciadia with Their T-cell Proliferation Activity. Iran. J. Pharm. Res. 2011, 10(2), 287–294.
- Kenny, A. P. The Determination of Cholesterol by the Liebermann-burchard Reaction. Biochem. J. 1952, 52(4), 611–619.
- Xiong, Q.; Wilson, W. K.; Pang, J. The Liebermann-burchard Reaction: Sulfonation, Desaturation, and Rearrangement of Cholesterol in Acid. Lipids. 2007, 42(1), 87–96. DOI: 10.1007/s11745-006-3013-5.
- Barbour, H. M. Enzymatic Determination of Cholesterol and Triglyceride with the Abbott Bichromatic Analyser. Ann. Clin. Biochem. 1997, 14(1), 22–28. DOI: 10.1177/000456327701400104.
- Liebermann, C. Ueber das Oxychinoterpen. Dtsch. Chem. Ges. 1885, 18, 1803–1809.
- Burchard, H. Beitrage zur kenntnis des cholesterins. Inaugural-dissertation, universitat Rostock. 1889, 61-I, 25–27.