?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Grewia optiva Drummond ex Burret (Tiliceae) is extensively applied for the treatment of typhoid, diarrhea, malaria, etc., in traditional medication. In this study, four bioactive compounds I (β- sitosterol), II (1, 2, 3 benzene triol), III (7-O-methyl cathachin) and VI (betulinic acid) were isolated from the root of G. optiva. The compounds were characterized through UV, FTIR, NMR, and mass spectroscopic techniques. The isolated compounds were tested for the inhibition of synthetic-free radicals: ABTS and DPPH, and anticholinesterases: AChE and BChE. Compound III demonstrated highest percent free radicals scavenging activities against DPPH and ABTS (86.11 ± 2.20, 85.23 ± 1.61, respectively, for 1000 µg/ml concentrations) with IC50 of 63 µg/ml which were comparable to the IC50 value (35 µg/ml) of ascorbic acid. All isolated compounds also showed excellent inhibitory potential against AChE and BChE. However, β-sitosterol amongst them exhibited highest percent inhibition (87.66 ± 1.93, 85.71 ± 1.17, respectively, at 1000 µg/ml concentration) with IC50 of 62 µg/ml, which was close to the standard galanthamine IC50 values (40 µg/ml). To support the findings of the study silico docking model was applied that predicted possible binding modes of β-sitosterol with AChE and BChE. The para hydroxyl group of the phenolic moiety interacted with the active site water molecule and the side chain carbonyl residues through H-bonding. The remaining part of the active compound packed in a shallow pocket through H-bond.
Introduction
Grewia optiva Drummond ex Burret belongs to family Tiliaceae (comprising 150 species). Most of the species of this family are restricted to tropical and subtropical regions of the world and have been reported from northern part of Pakistan, South Africa, India, Bangladesh, Madagascar, Malaysia, China, Northern Australia, and Thailand.[Citation1] Fruits of several species of genus Grewia are edible, e.g. phalsa and are commercially important. In summer time, the astringent and refreshing Grewia drupes are popular in temperate regions of the world.[Citation2] The Grewia optiva ripe fruit is also edible which has a pleasant acid taste. In winter, when there is scarcity of green leaves to be used as fodder, the green leaves Grewia which contain large amount of protein and other nutrients, plays an important role as fodder.[Citation2] From Pakistan, 10 species of this plant have been reported. Most of them are used as folk medicines in treatment of dysentery, typhoid, diarrhea, fever, cough, small pox, eczema and malaria in folk medicine.[Citation2] Anti-bacterial, anti-malarial and antioxidant potentials of several species of genus Grewia have already been reported.[Citation1,Citation2]
Oxidative stress is a condition arising due to excessive production of free radicals inside the body that leads to oxidation of biologically important molecules. To prevent them from doing so, certain substances having the capability of stopping free radicals called antioxidants present in fruit and vegetable are utilized as food supplements.[Citation3,Citation4] Certain antioxidants of synthetic origin used as preservatives in food industries.[Citation5] However, they have shown toxic properties (carcinogen).[Citation3–Citation7] As plant product have limited side effects, therefore, scientist around the globe are trying to isolate natural antioxidants from plant origin.[Citation6,Citation7]
A number of neurodegenerative diseases like amnesia, Alzheimer’s disease (AD), dementia, etc., are common these days. In old age people (above 60 years), their frequencies are high.[Citation8] Acetylcholine (ACh) and Butyrylcholine (BCh) are important neurotransmitters that are involved in acquisition and storage of memories, and in the transmission of impulses across the synapse. Usually in the neurodegenerative processes the level of neurotransmitters: ACh and BCh exhausted.[Citation9] To restore the level of ACh and BCh at the synapse, anticholinesterase inhibitors are used. Two compounds of natural origin, galantine and rivastigmine are amongst the clinically approved cholinesterase inhibitors.[Citation10,Citation11] Although these two compounds are used as standard inhibitors of the mentioned enzyme but still they are not 100% potent, therefore, effort have been made to isolate efficient inhibitors of these enzymes from plant origin.
Several alkaloids including 6-methoxyharman, 6-hydroxyharman, lignans like nitidanin and grewin from genus Grewia have been reported.[Citation12] Flavone glucosides including vitexin and isovitexin, gulonic acid, dihydroxy contanoic acid have also been purified from this genus.[Citation13] From G. biloba six compounds have been isolated by Liu and his team in 2008: heneicosanoic acid, friedelin, epi-friedelan-3-ol, β-sitosterol, catechin, and propyl palmitate.[Citation14] In 2009, Ahamed and his team reported gulconic acid γ-lactone from G. tiliafolia, subsequently he also isolated and characterize gulonic acid gamma lactone and D-erythro-2-hexenoic acid gamma lactone from G. tiliaefolia stem.[Citation15,Citation16] Abirami and his coworker isolated an antidiabetic compound: 4Z, 12Z-cyclopentadeca-4,12-dienone from Grewia hirsute in 2014.[Citation17] In 2017, Mital and his team isolated octanoic acid, tridecanoic acid, octadecatrienoic acid and eicosanoic acid from Grewia tenax.[Citation18] These isolated compounds have been investigated for a number of biological application and some of them have shown remarkable biological activities.[Citation12–Citation18] As far as we are aware, no phytochemical studies have been reported on the roots of G. optiva.
This study was undertaken on the roots of G. optiva, and resulted in the isolation of betulinic acid, 1, 2, 3 benzene triol, and 7-O-methyl Cathachin for the first time from this genus. β-Sitosterol has already been reported from this genus. The spectroscopic techniques 1D, 2D NMR, and available spectroscopic analyses data in literature were used to elucidate structures of isolated compounds. The in-silico molecular docking of the isolated compounds was performed against AChE and BChE crystal structure (PDB ID: 1ACL, 4BOP) to ascertain the role of different functionalities in the formation of ligand-protein complex.
Materials and methods
Silica gel 60 (mesh size = 70–230 purchased from E. Merck, 0.063–0.200 mm) were used as adsorbent in column separation of the compounds. Preparative TLC was done with the help of silica gel 60 PF254 (E. Merck). Detection of compounds at 254 and 266 nm visualized with the help of CeSO4 spray and solid iodine. 1H and 13C NMR, COSY, NOESY, J-resolved, HSQC and HMBC spectra through Bruker Spectrometer, Avance Av 500, 600/150 MHz), chemical shifts (δ) in ppm, coupling constants (J) in Hz were measured.
Plant materials
Roots of the Grewia optiva were collected from Sia Top Mountain village Khanpoor, Dir Lower KPK Pakistan in March 2016 and were identified by expert in the department of Botany University of Malakand. The voucher specimen (1022HU) have been deposited in Herbarium University of Malakand.
Extraction and fractionation
To eliminate soil contaminants and dust the roots of G. optiva were cleaned with tape water. After drying in shade, the root samples were grounded to fine powder mechanically. The powder sample was soaked in 95% methanol/water for 72 h to prepare crude extract. After 72 h, the mixture was filtered and the residue left was again contacted with methanol for additional 72 h. The filtrates obtained from the two steps were mixed together and evaporated at 40ºC using Rota vapor R-200 Rotary evaporator. The crude extract was obtained in semisolid form which was then fractionated to obtain ethyl acetate, chloroform, aqueous and n-hexane subfractions. About 300 g crude extract was mixed with 1500 ml of distilled water and subjected to fractionation. The subfractions were evaporated into semisolid mass using Rota vapor R-200 and stored in refrigerator at 4°C which were later on subjected to the isolation of natural product.
Based on our unpublished results of HPLC analysis, the ethyl acetate fraction was found rich in bioactive compounds and thus was selected for the isolation of bioactive compounds. The ethyl acetate soluble fraction was separated through filtration, evaporated under vacuum to get powder residue (23 g) which was then loaded to silica gel (using 450 g) gravity column which was eluted with n-Hexane/ethyl acetate solvent system from low to high polarity. The effluents containing bioactive compounds were collected in glass vails. The contents of vails were recombined on the basis of TLC profiling. The fractions obtained were then subjected to pencil column isolation. Fraction 5 gave β- sitosterol (50 mg) eluted from the pencil column with the ratio of ethyl acetate/n-hexane = 3:7. Fraction 6 (large column fraction) provided 1, 2, 3 benzene triol (25 mg) and 7-O-methyl cathachin (20 mg) as white amorphous solid (ethyl acetate/n-hexane = 4:6) when subjected to pencil column isolation. Betulinic acid (08 mg) was purified from fraction 9 in the ratio of ethyl acetate/n-hexane = 5:5.
Determination of free radical scavenging activities of compounds using DPPH assay
The DPPH (2,2- diphenyl-1-picrylhydrazyl) free radical scavenging assay was used to find out free radical scavenging abilities of the isolated compounds.[Citation19] The DPPH control solution was prepared by dissolving 20 mg DPPH in methanol (100 ml) which was then diluted to adjust its absorbance to 0.70 (at 515 nm). To generate free radical this stock solution was then kept in dark place for 24 h. Sample solutions were prepared by dissolving 5 mg from each isolated compounds methanol (5 ml). Working standards: 62.5, 125, 250, 500, 1000 μg/ml were prepared using dilution formula. About 2 ml from each of them were thoroughly mixed with 2 ml DPPH solutions and kept in dark for 15 min. To calculate the percent scavenging of DPPH radical by isolated compounds following equation was used.
where A is absorbance of DPPH radical in oxidized form and B is the absorbance after reacting with sample.
ABTS assay of the isolated compounds
ABTS (2,2-azinobis [3-ethylbenzthiazoline]-6-sulfonic acid) assay of the isolated compounds was performed following standard protocol.[Citation19] About 7 mM (383 mg) of ABTS and 2.45 mM (66.2 mg) of K2S2O8 were prepared in methanol (100 ml) separately and mixed well. Working dilutions (2 ml) prepared in above step were added with 2 ml ABTS. After 25 min of incubation, absorbance was measured at 745 nm. EquationEquation 1(1)
(1) is used to calculate scavenging potentials of the compounds.
Cholinesterase inhibition assay
To evaluate the enzyme inhibitory potential (against AChE and BChE) of compounds Ellman’s assay was used.[Citation20] Sample solutions (1000 μg/ml) were prepared by dissolving 5 mg of compounds in methanol. Working dilutions of compounds as mentioned in above step were used as testing substances. For the preparation of 0.1 M phosphate buffer (8.0 ± 0.1 pH), KH2PO4 (13.6 g/L) and K2HPO4 (17.4 g/L) solutions were prepared, using KOH solution the final pH of mixture was adjusted. Enzymes solutions were prepared by dissolving AChE (518 U/mg solid) and BChE (7–16 U/mg) in phosphate buffer and diluted to final concentrations of 0.03 U/ml and 0.01 U/ml, respectively. Other solutions including ATchI, BTchI (0.0005 M) and DTNB (0.0002273 M) were prepared using distilled water. The positive control (galanthamine) solution of same concentrations as that of compounds was prepared in methanol. This assay is based on the hydrolysis of Acetylthiocholine iodide or butyrylthiocholine iodide by respective enzymes and the resulting products then complexed with 5-thio-2-nitrobenzoate anion provided by DTNB resulting in yellow coloration which can be observed after 15 min of incubation by spectrophotometer.
where V is the rate of reaction in the presence of inhibitor while Vmax is without inhibitor.
Molecular docking simulations
Isolated compounds (I-IV) were studied for possible binding with the crystal structure of the AChE and BChE using molecular docking simulations. For this purpose, we used the crystal structure of the AChE and BChE (Pdb: 1ACL, 4BOP) from the RCSB Protein Data Bank RCSB. All ligands were prepared by LigPrep (Schrödinger) in their neutral form and optimizing their conformation in the OPLS-3 force field. The protein structure was prepared by using the Protein Preparation (Schrödinger) for adding hydrogens and setting protonation’s states appropriate for pH 7. The receptor grid box was defined as 20 Å with the active site containing water molecule in its center. Docking was performed with Glide (Schrödinger) using XP precision with default settings and glide scoring function, reporting the 10 top-ranked poses for each ligand. Visual inspection of the binding pose and generation of figure was done with Maestro (Schrödinger).[Citation21]
Results and discussion
Isolation of bioactive compounds
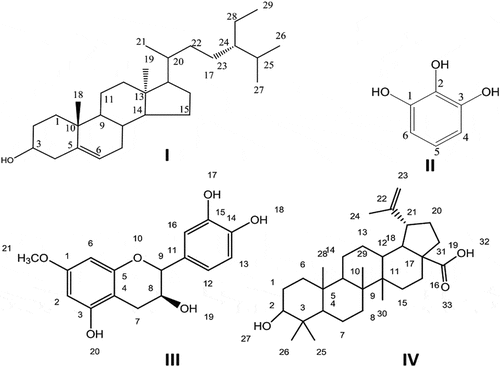
The silica gel column isolation led to the isolation of four compounds from the ethyl acetate fraction of the roots of G. optiva. The isolated compounds were identified as β- sitosterol, 1, 2, 3 benzene triol, 7-O-methyl cathachin, and betulinic acid. The structural formulae of the isolated compounds are presented in .
Compound I (β- sitosterol)
β-Sitosterol, molecular formulae (C29H50O) was isolated from G. optiva along with other compounds.
1HNMR (DMSO, δppm)
Spectrum showed multiplet at δH 5.35 which is due to H-6 (exocyclic methylene protons). Other peaks observed were at δH 4.88 (s, 1OH, H-3), 3.43 (m) 0.83 (3H, d, J= 6.52 Hz), 0.90 (3H, d, J= 6.82 Hz), 0.78 (3H, s, Me-18), 0.86 (3H, t, J= 6.92 Hz), 1.04 δH (3H, s), 0.83 (3H, d, J= 6.52 Hz). The remaining methylene protons and methylene appeared between δH 1.11–2.25.
13C NMR (DMSO, δppm)
12.34 (C-19), 12.53 (C-29), 12.67 (C-18), 19.33 (C-11), 19.48 (C-21), 30.40 (C-16,7), 33.04 (C-2), 33.23 (C-1), 33.23 (C-26,22), 33.28 (C-27), 35.12 (C- 8), 37.44 (C-28), 41.92 (C-23), 47.30 (C-25), 49.15 (C-20), 49.29 (C-10), 49.43 (C-12), 51.75 (C-4), 51.78 (C-13), 52.81 (C-24). 57.39 (C-9), 57.48 (14,17), 72.46 (C-3), 122.45 (C-6), 139.80 (C-5). The physical data and spectroscopic of the compound agreed with those reported in literature for β-sitosterol.[Citation22]
Compound II (1, 2, 3 benzene triol)
1, 2, 3 benzene triol, molecular formulae (C6H6O3) is a white amorphous solid soluble in water with melting point of about 134°C.
1HNMR (DMSO, ppm)
Peaks were observed at δH 6.4 (t, J = 12 Hz H-5), 6.2 (d, J= 6.0 Hz, 2H, H-4, 6), 8.60 (s, 3OH, OH-1, 2, 3).
13C NMR (DMSO, ppm)
The observed peaks were at δC 146.10 (C-1, 3), 133.0 (C-2), 118.5 (C-5), 107.12 (C-4, 6).
Compound III (7-O-methyl cathachin)
7-O-methyl Cathachin, having molecular formulae C16H16O6, is a yellow amorphous solid with melting point of 176°C.
1HNMR (DMSO, δppm)
The proton NMR spectral detail are given as: 2.5 (dd, 2H H-7), 3.7 (s, 3H, H-21), 4.4 (d, 2H, H-8,9), 5.6 (s, 1OH, H-19),6.5 (d, 2H, H-2,6),6.6 (dd, J= 18.0 Hz, 3H, H-12,13,16),9.1 (s, 2OH, H-17,18), 9.3 (s, 1OH, H-20).
13C NMR (DMSO, δppm)
The spectral details are: 27.6 (CH2, C-7), 48.6 (CH3, C-21), 66.2 (C-8), 80.8 (C-9), 93.9 (C-6), 95.2 (C-2), 99.1 (C-4) 114.4 (C-14), 115.1 (C-13), 118.4 (C-12), 130.5 (C-11), 144.8 (C-15,18), 155.2 (C-3), 156.1 (C-5),156.3 (C-1). The spectral data were compared with already reported data.[Citation23]
Compound IV (betulinic acid)
The melting point of betulinic acid having molecular formulae C30H48O3 was 318°C.
1HNMR (DMSO, δppm)
Peaks details are given as follows: 0.77 (s, 3H, H-28), 0.88 (s, 3H, H-29), 0.97 (s, 6H, H-25,26), 1.02 (s, 3H, H-30), 0.93 (s, 1H, H-4), 1.06 (s,1H, H-12), 1.42 (m, 8H, H-6,8,15,20), 1.58 (m, 4H, H-7,14), 1.35 (m, 2H, H-19), 1.71 (s, 3H, H-24), 1.94 (m, 2H, H-16), 1.56 (m, 4H, H1,13), 2.33 (m, 1H, H-21), 2.25 (m, 1H, H-18), 3.03 (m, 1H, H-2), 4.61 (s, 1OH, H-27), 3.15 (dd, 1H, H-23), 3.03 (t, 1H, H-23).
13C NMR (DMSO, δppm)
The spectral peaks obseved were: 15.12 (C-30), 16.10 (C-28), 16.67 (C-7), 16.73 (C-29), 19.46 (C-14), 19.57 (C-24), 22.11 (C-25,26) 26.93 (C-13), 28.07 (C-1), 28.62 (C-15), 30.86 (C-20), 31.75 (C-16), 33.39 (C-8), 35.63 (C-19),38.16 (C-6). 39.70 (C-9), 39.96 (C-11), 48.58 (C-21), 49.29 (C-18), 50.51 (C-10), 56.91 (C-4), 57.53 (C-17), 79.70 (C-2), 110.3 (C-23), 152.03 (C-22), 180.20 (C-31).
The spectral data were compared with already reported data.[Citation24]
DPPH and ABTS radical scavenging effect
The antioxidant potential of the bioactive compounds isolated from Grewia optiva were studied using DPPH assay. This assay is based on the ability of an antioxidant to scavenge DPPH free radicals. Amongst the tested compounds, 7-O-methyl cathachin () showed highest scavenging activity with IC50 value of 63 μg/ml with 86.11 ± 2.20% inhibition at 1000 μg/ml concentration followed by β- Sitosterol (IC50 = 68 μg/ml, % inhibition = 86.03 ± 1.75). Betulinic acid and 1, 2, 3 benzene triol also showed excellent DPPH free radical scavenging activities with IC50 values of 90 and 95 μg/ml, respectively. The most potent compound that scavenged ABTS free radicals was 7-O-methyl cathachin (percent inhibition = 85.23 ± 1.61 at 1000 μg/ml with IC 50 = 63 μg/ml). β-Sitosterol also showed very good free radical scavenging potential (IC50 = 64 μg/ml, % inhibition = 85.11 ± 2.66) against ABTS free radicals followed by betulinic acid (IC50 = 105 μg/ml). 1, 2, 3 benzene triol also showed remarkable ABTS scavenging with IC50 values of 85 μg/ml (). As positive control ascorbic acid was used and its IC50 value was 35 μg/ml. Although crude extract of Grewia optiva bark have been investigated for its antioxidant potential[Citation1], the antioxidant properties of root and isolated phytochemical has not been evaluated so far.
Table 1. Percent DPPH and ABTS radical scavenging potential of the bioactive compounds isolated from Grewia optiva.
Cholinesterase inhibition effect
In certain pathological conditions, the inhibition of AChE in the brain is desired. An attempt was made to evaluate the isolated compounds as potential inhibitors of AChE and BChE. The AChE percent inhibition and their IC50 values against the bioactive compounds from G. optiva are given . β-Sitosterol showed promising percent inhibition with IC50 value of 62 μg/ml with 87.66 ± 1.93 of percent inhibition. Amongst the other investigated compounds 7-O-methyl Cathachin showed very good percent inhibition (85.53 ± 1.40) with IC50= 65 μg/ml followed by betulinic acid and 1, 2, 3 benzene triol with the IC50 of 67 and 68 μg/ml, respectively. For positive control, Galanthamine was used in this study.
Table 2. Percent AChE and BChE inhibition of the bioactive compounds isolated from Grewia optiva.
The isolated compounds also inhibited BChE which is clear from . Highest potential against BChE was shown by β- sitosterol with IC50 value of 64 μg/ml with 85.11 ± 2.66 of percent inhibition. Amongst the other compounds, 7-O-methyl Cathachin showed excellent percent inhibition with IC50 value of 65 μg/ml. The other compounds like 1, 2, 3 benzene triol and betulinic acid showed strong percent inhibition with IC50 values of 68 and 80 μg/ml, respectively. Molecular docking studies also support the binding of β-sitosterol with the target enzyme.
Molecular docking studies
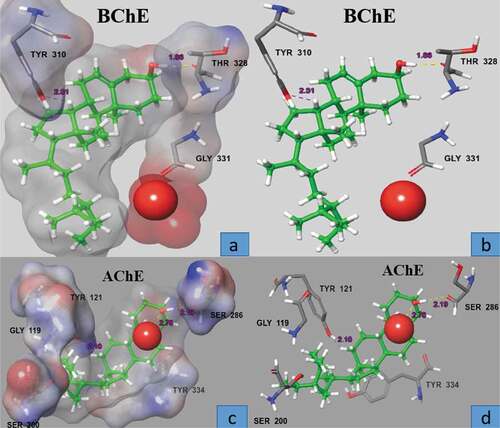
Computational docking studies were performed to predict the most favorable binding mode of β-sitosterol with AChE and BChE inside the binding pockets of the two proteins with proper orientation in term of docking score – 8.685 Kcal/mol (AChE) and −2.064 Kcal/mol (BChE). Such lower values indicates good fitting of the compound in the binding pocket of the protein showing stable β-sitosterol-protein interaction (). The selected pose of most active compound β-sitosterol was visualized in order to determine the amino acids involved in binding of the ligand to the crystal structure of the protein and while the docking scores do not reflect the IC50 values from the enzymatic assay, there are some differences in the binding poses between most active and less active compounds. For active compound (), the phenolic moiety is interacting through strong H-bond interaction (2.78 A°) between the hydroxyl and water molecule at the active site with the side chain carbonyl of Ser 286 (2.19 A°). The tail moiety of the compound occupies a distinct area of the binding site close to Gly 119 and Ser 200, picking up an H-bond interaction between the hydrogen and the hydroxyl of Tyr 121 (). In case of BChE, the docked conformation also showed a similar interaction with carbonyl of Thr 328 and bond length observed was 1.86 Å with an additional hydrogen bond with Tyr 310 (2.51A°) ().
Figure 2. Surface and ball and stick representation of the docking pose in the active site of AChE and BChE (1 ACL, 4BOP), showing the highly ranked pose for active compound β- sitosterol (green). The inhibitor predicted to bind in the active site in the space close to the water molecule. Residue, in stick representation, that forming the active site of the enzyme are labeled
Conclusion
In this study, we isolated four bioactive compounds, namely, β-sitosterol, betulinic acid, 1, 2, 3 benzene triol and 7-O-methyl cathachin first time from the roots of G. opiva. Except β-sitosterol, these compounds are reported from the genus Grewia for the first time. These compounds were evaluated for their inhibitory potential against AChE and BChE enzyme, DPPH and ABTS free radicals. In vitro studies indicated that β-sitosterol and 7-O-methyl cathachin possesses strong anticholinesterase and antioxidant potentials followed by 1, 2, 3 benzene triol and betulinic acid. Though the potency of these compounds was less as compared to standard drugs, however, their dual efficiencies (enzymes inhibition and free radicals scavenging capacity) would probably be of great interest for the scientists. The molecular docking studies support the potential use of β-sitosterol and other isolated compounds to be used as inhibitors of AChE and BChE in pathological conditions.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. RG-1440-100. The authors are grateful for the financial support (Project No: 20-2515/R&D/HEC) from the Higher Education commission of Pakistan.
References
- Anwar, J.; Shah, H.; Ali, R.; Iqbal, Z.; Khan, S. M.; Khan, S.; Rehman, S.; Shad S., Sohail; . Antioxidant Activity and Phytochemical Screening of Stem Bark Extracts of Grewia Optiva Drummond Ex Burret. J. Pharm. Phytochem. 2015, 3(6), 179–182.
- Uddin, G.; Ullah, W.; Siddiqui, B. S.; Shah, S. Q. Grewialin and Optivanin New Constituents from the Stem Bark of Grewia Optiva Drummond Ex Burret. Nat. Prod. Res. 2013, 27(3), 215–220. DOI: 10.1080/14786419.2012.666749.
- Zhang, L.; Ravipati, A. S.; Koyyalamudi, S. R.; Jeong, S.; Reddy, N.; Smith, P. T.; Bartlett, J.; Shanmugam, K.; Munch, G.; Wu, M. J. Antioxidant and Anti-inflammatory Activities of Selected Medicinal Plants Containing Phenolic and Flavanoid Compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. DOI: 10.1021/jf203146e.
- Nacoulma, O. G.;. Mutagenic Effect, Antioxidant and Anticancer Activities of Six Medicinal Plants from Burkina Faso. Nat. Prod. Res. 2012, 26, 575–579. DOI: 10.1080/14786419.2011.562205.
- Pieme, C. A.; Penlap, V. N.; Ngogang, J.; Costache, M. In Vitro Cytotoxicity and Antioxidant Activities of Five Medicinal Plants of Malvaceae Family from Cameroon Environ. Toxicol. Pharmacol. 2010, 29, 223–228. DOI: 10.1016/j.etap.2010.01.003.
- Hamid, A.; Aiyelaagbe, O. O.; Usman, L.; Ameen, M. O.; Lawal, A. Antioxidants Its Medicinal and Pharmacological Applications. Afr. J. Pure Appl. Chem. 2010, 16(4), 142–151.
- Suryanti, V.; Marliyana, S. D.; Putri, H. E. Effect of Germination on Antioxidant Activity, Total Phenolics, β -carotene, Ascorbic Acid and α –Tocopherol Contents of Lead Tree Sprouts. Int. Food Res. J. 2016, 23, 167–172.
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A. Anti-Alzheimer ’ S Studies on beta-Sitosterol Isolated from Polygonum Hydropiper L. Front. Pharmacol. 2017, 8, 697. DOI: 10.3389/fphar.2017.00697.
- Ahmad, S.; Ullah, F.; Sadiq, A.; Ayaz, M.; Imran, M.; Ali, I.; Zeb, A.; Ullah, F.; Shah, M. R. Chemical Composition, Antioxidant and Anticholinesterase Potentials of Essential Oil of Rumex Hastatus D. Don Collected from the North West of Pakistan. BMC Complement. Altern. Med. 2016, 16(1), 1. DOI: 10.1186/s12906-016-0998-z.
- Ullah, F.; Ayaz, M.; Sadiq, A.; Hussain, A.; Ahmad, S.; Imran, M.; Zeb, A. Phenolic, Flavonoid Contents, Anticholinesterase and Antioxidant Evaluation of Iris Germanica Var Florentina. Nat. Prod. Res. 2016, 30(12), 1440–1444. DOI: 10.1080/14786419.2015.1057585.
- Hasnat, M.; Pervin, M.; Lim, B. Acetylcholinesterase Inhibition and in Vitro and in Vivo Antioxidant Activities of Ganoderma Lucidum Grown on Germinated Brown Rice. Molecules. 2013, 18, 6663–6678. DOI: 10.3390/molecules18066663.
- Ahamed, M. B. K.; Krishna, V.; Dandin, C. J.; Krishna, V.; Dandin, C. J. In Vitro Antioxidant and in Vivo Prophylactic Effects of Two Lactones Isolated from Grewia Tiliaefolia against Hepatotoxicity in Carbon Tetrachloride Intoxicated Rats. European J. Pharmacol. 2010, 31, 42–52. DOI: 10.1016/j.ejphar.2009.12.034.
- Ullah, W.; Uddin, G.; Rauf, A.; Siddiqui, B. S.; Rehman, T.; Azam, S.; Qaisar, M. Chemical Constituents and Biological Screening of Grewia Optiva Drummond Ex Burret Whole Plant. J. Agric. Environ. Sci. 2011, 11, 542–546.
- Liu, J. Q.; Wu, J. M.; Kou, X. L.; Hong, Q. Studies on Chemical Constituents from Grewia Biloba. Zhong Yao Cai. 2008, 31, 1505–1507.
- Ullaha, W.; Uddin, G.; Siddiqui, B. Ethnic Uses, Pharmacological and Phytochemical Profile of Genus Grewia. J. Asian Nat. Prod. Res. 2012, 14(2), 186–195. DOI: 10.1080/10286020.2011.639764.
- Ahamed, M. B. K.; Krishna, V.; Dandin, C. J. In Vitro Antioxidant and in Vivo Prophylactic Effects of Two Gamma-lactones Isolated from Grewia Tiliaefolia against Hepatotoxicity in Carbon Tetrachloride Intoxicated Rats. Eur. J. Pharmacol. 2010, 631, 42–52. DOI: 10.1016/j.ejphar.2009.12.034.
- Natarajan, A.; Sugumar, S.; Bitragunta, S.; Balasubramanyan, N. Molecular Docking Studies of (4Z, 12z)-cyclopentadeca-4, 12-dienone from Grewia Hirsute with Some Targets Related to Type 2 Diabetes. BMC Complementary Altern. Med. 2015, 15, 73. DOI: 10.1186/s12906-015-0588-5.
- Aadesariya, M. K.; Ram, V. R.; Dave, P. N. Soxhtherm Extraction, Isolation and Identification of Fatty Acids Present in the Hexane Extract of Abutilon Pannosum and Grewia Tenax Using Gas Chromatography-Mass Spectrometry. IJARCS. 2017, 4(10), 26–34.
- Ovais, M.; Ayaz, M.; Khalil, A. T.; Shah, S. A.; Jan, M. S.; Raza, A.; Shahid, M.; Shinwari, Z. K. HPLC-DAD Finger Printing, Antioxidant,cholinesterase, and α -glucosidase Inhibitory Potentials of a Novel Plant Olax Nan. BMC Complement. Altern. Med. 2018, 18(1). DOI: 10.1186/s12906-018-2317-3.
- Ayaz, M.; Junaid, M.; Ahmed, J.; Ullah, F.; Sadiq, A.; Ahmad, S.; Imran, M. Phenolic Contents, Antioxidant and Anticholinesterase Potentials of Crude Extract, Subsequent Fractions and Crude Saponins from Polygonum Hydropiper. BMC Complement Altern. Med. 2014, 14, 145–153. DOI: 10.1186/1472-6882-14-145.
- Pintus, F.; Matos, M. J.; Vilar, S.; Hripcsak, G.; Varela, C.; Uriarte, E.; Santana, L.; Borges, F.; Medda, R.; Di Petrillo, A.; et al. New Insights into Highly Potent Tyrosinase Inhibitors Based on 3-hetero Aryl Coumarins: Anti-melanogenesis and Antioxidant Activities and Computational Molecular Modeling Studies. Bio. Org. Med. Chem. 2017, 25, 1687–1695. DOI: 10.1016/j.bmc.2017.01.037.
- Vitus, A.; Peter, X.; Mabiki, F.; Hamisi, M.; Robinson, H.; Fouche, G. Isolation and Identification of Euphol and β-sitosterol from the Dichloromethane Extracts of Synadenium Glaucescens. J. Pharmacol. 2016, 5(3), 100–104.
- Abdoulaye, T.; Claude, K.; Faustin, K.; Marcelline, A.; Francois, K. R.; Aminata, A.; Jacques, K.; Toussaint, G.; Barthelemy, A.; Adama, C. Isolation of (+)-catechin and (-)-epicatechin from the Leaves of Amaranthus Cruentus. Int. J. Chem. Stud. 2018, 6(2), 3697–3700.
- Haque, A.; Siddiqi, M. M. A.; Rahman, A. F. M. M.; Choudhury, M.; Sarwaruddin, A. M. Isolation of Betulinic Acid and 2,3-dihydroxyolean-12-en-28-oic Acid from the Leaves of Callistemon Linearis. Dhaka Univ. J. Sci. 2013, 61(2), 211–212. DOI: 10.3329/dujs.v61i2.17073.