ABSTRACT
Sesamol (1,3-benzodioxol-5-ol) is found in the oilseed product, sesame. In our study, the inhibitory effects of sesamol compound on acetylcholinesterase and α-glycosidase enzymes were evaluated. Another aim of this study was to compare the inhibitory effects of sesamol to acarbose for α-glycosidase enzyme and tacrine for acetylcholinesterase (AChE). Sesamol exhibited non-competitive inhibition for both metabolic enzymes. IC50 values for AChE and α-glycosidase enzymes were calculated as 86.63 nM and 99.00 nM, respectively. On the other hand, Ki values were calculated as 46.68 nM and 75.33 nM for AChE and α-glycosidase enzymes, respectively. According to the obtained results showed effective inhibition at low concentrations.
Introduction
Enzymes are catalyst molecules that are specific to reactions, which accelerate chemical reactions and return to their original state even if they have been physically altered during these reactions.[Citation1–Citation4] Reduce or destroy the enzymes in the event of activity both in vivo and in vitro by certain compounds called enzyme inhibition. Inhibitor is a molecule or ions that binds to an enzyme and decreases its activity.[Citation5–Citation7]
Acetylcholinesterase (AChE; E.C.3.1.1.7) is an enzyme in the family of cholinesterases. AChE is a membrane-bound enzyme. Acetylcholinesterase is responsible for the hydrolysis of choline esters [Citation8–Citation10] AChE is a type of hydrolase that hydrolyzes ACh to choline and acetic acid. In addition, it was found cholinergic, central, peripheral, cholinergic, adrenergic, muscle, nerve, placental tissue and erythrocytes in the body. The decrease in AChE activity causes many health problems including nervous system disorders.[Citation11,Citation12] The most important substrate of acetylcholinesterase is acetylcholine (ACh).[Citation13] A sudden drop in the level of acetylcholine can cause death. Alzheimer’s disease (AD) occurs with gradual decline of this level.[Citation14–Citation16] One of the most important treatments is to maintain the ACh concentration with AChE inhibitors.[Citation17–Citation19] AD is a disease caused by damage or death of these neuron cells.[Citation16] The AChE gene was in living organisms because it was vital to stop AChE nerve conduction. Therefore, it was determined that AChE could not have genetic diversity.[Citation20–Citation24]
Acetylcholine-like substance should be given or the acetylcholine-depleted AChE enzyme needs to be suppressed.[Citation25,Citation26] AChE enzyme was first obtained by the extraction of the electric organ from electric fish of Torpedo marmorata.[Citation27] Many properties of AChE have been discovered as a result of studies on the enzyme purified from electric eel (Electrophorus electricus).[Citation28]
Glucose is one of the most important food sources in living organisms and controlled via insulin in the blood.[Citation29,Citation30] Diabetes mellitus (DM) occurs when glucose concentration is above normal level.[Citation31,Citation32] Since insulin hormone cannot be released regularly, it cannot tolerate this glucose. DM is a chronic disease seriously affecting the quality of life.[Citation33–Citation35] Unfortunately, this disease cannot be completely treated.[Citation36,Citation37] Uncontrolled blood sugar is the main problem of diabetes.[Citation38,Citation39] In Type-2 diabetes, either a decrease in insulin secretion in peripheral tissues or a disorder in insulin secretion is caused.[Citation40] If hyperglycemia is not controlled long term, there may occur with dysfunction or failure of various organs. As an oral antidiabetic drug, α-glycosidase enzyme inhibitors are preferred.[Citation41–Citation43]
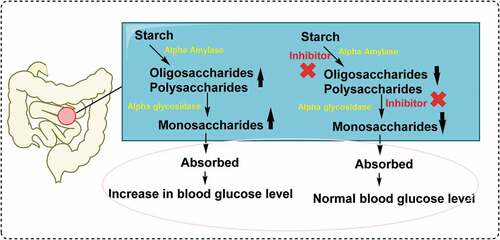
α-Glycosidase (E.C.3.2.1.20) is an enzyme found in the small intestine spicular surface. It is responsible for breaking down complex carbohydrates. α-Glycosidase inhibitors retard the absorption of carbohydrates by inhibiting α-glycosidase enzymes of the small intestine.[Citation44–Citation47] α-Glycosidase inhibitors not only inhibit carbohydrate absorption but also affect the gastrointestinal hormone axis. α-Glycosidase inhibitors are antihyperglycemic agents. The postprandial plasma glucose lowering in fasting plasma glucose in mildly decreases. It prevents the breakdown of complex carbohydrates.[Citation48–Citation50]
Sesame (Sesamum indicum L.) is one of the most important seeds used in oil. Protein and fat contents are very high.[Citation51] Sesamol (3,4-methylenedioxyphenol), a component of sesame oil used in our study, is a natural organic compound. Sesamol is a white crystalline solid with a phenol derivative (). It is slightly soluble in water but can be miscible with most fats. This compound can be produced by organic synthesis from heliotrope. Sesamol has been found to be an antioxidant and antifungal that can prevent the deterioration of fats.[Citation52,Citation53]
Materials and methods
Chemicals
α-Glycosidase from Saccharomyces cerevisiae, α-D-glycopyranoside (p-NPG), p-Nitrophenyl AChE purified from electric eel (Electrophorus electricus), Sesamol was purchased from Sigma-Aldrich (G5003; St. Louis, MO).
Measurement of acetylcholinesterase activity
Acetylcholinesterase activity was spectroscopically performed according to Ellman’s method[Citation54] as described previously.[Citation55,Citation56] In this method, acetylthiocholine is used as substrate. 5,5ʹ-Dithio-bis(2-nitrobenzoic) acid (DTNB) and acetylcholine iodide (AChI) were used as substrates of AChE activities. Tris-HCl buffer (1.0 M, pH 8.0) and sample solutions with different concentrations were dissolved in pure water. The resulting solution 30 µL AChE solution was added. The mixture was incubated for 10 min at 25°C. Then, DTNB (0.5 mM, 100 µL) was supplemented. After this procedure, the reaction mixture was added 100 mL AChI (10 mM) and activity studies was complete. Absorbances at 412 nm were evaluated in the enzyme.[Citation57]
Measurement of α-glycosidase inhibitory activity
For determination of α-glycosidase activity, p-NPG was used as the substrate.[Citation58] For this purpose, sesamol was prepared by dissolving ethanol (1 mg mL−1). Firstly, 0.2 mL of NaH2PO4 with pH 7.4 and 20 μL of enzyme solution were mixed. And then, different quantities of sesamol were added into the current solution and these were mixed. After, it was incubated at 35°C for 10 min by adding the p-NPG to initiate the reaction. In addition, 100 μL of p-NPG at pH 7.4, 5 mM of sodium phosphate buffer after preincubation was added and the incubation was again carried out at 35°C. IC50 values were evaluated with the obtained data. Absorbance values were measured at 405 nm.
Results and discussion
Today, enzymes are widely used in medicine, food industry, and environmental industries. The elucidation of metabolic mechanisms of inhibitors is a guide for biochemists.[Citation59,Citation60] The chemical structure of sesamol is given in . It was known that sesamol could cross the blood–brain barrier. In this case, sesamol can affect many tissues, especially kidneys, liver, and brain. It was first came the liver by spreading into other tissues such as kidney, lung, and brain.[Citation61] As shown in and , sesamol had marked inhibition effects against α-glycosidase enzyme at nanomolar level. Agents identified as α-glycosidase inhibitors reduce postprandial plasma glucose and moderate fasting plasma glucose. These agents prevent the breakdown of complex carbohydrates. Malabsorption is not observed with α-glycosidase inhibition. Thus, the agents can reduce glucose levels and postprandial hyperglycemia by 35–45%.[Citation62]
Table 1. IC50 and Ki values, inhibition types of the sesamol on acetylcholinesterase (AChE) and α-glycosidase
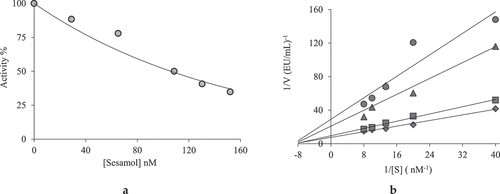
Figure 2. Determination of IC50 (a) and Ki (b) values of the Sesamol on acetylcholinesterase (AChE) enzyme
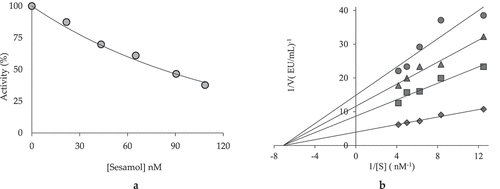
Also, it was determined that sesamol inhibited AChE and α-glycosidase enzymes as noncompetitive ( and ). Accordingly, the inhibitor was bound to a different domain than the active site of the enzyme.[Citation63] AD is a neurodegenerative disease that demonstrates behavioral disorders such as loss of cognitive activity, lack of self-care, and neuromuscular damage as a result of synapse and neuron cell damage affecting the central nervous system.[Citation63,Citation64] Today, AChE inhibitors are the most important and the only group of drugs used to treat AD.[Citation65,Citation66] If the activity of the AChE enzyme is reduced or stopped, acetylcholine molecules will be less degraded and thus the acetylcholine concentration will remain high and the solution for the disease may be present.[Citation7,Citation67]
Preferred AChE inhibitors for treating AD are inhibitors that increase the activity of cholinergic neurotransmission by increasing the amount of ACh.[Citation68,Citation69] As the most potent inhibitor, the long-acting tacrine (IC50: 8.18 nM; Ki: 8.59 ± 5.38 nM) compound has a hepatotoxic effect. In particular, it was found that the liver enzyme increases alanine transaminase level. Because of these reasons, its usage has been restricted.[Citation70–Citation72] Galantamine, donepezil, and rivastigmine are the current drugs used for the treatment of AD.[Citation73,Citation74] As can be seen in Figure 1, the sesamol contains benzodioxol and phenolic groups. Both groups are responsible for biological activities. In recent studies, sesamol has been shown to be anticancer and inhibits growth in heart cells. It was thought that there was a relationship between neurodegenerative diseases and monoamine oxidase activity. Sesamol may play a protective role in such diseases.[Citation75] It is thought that the sesamol used in this study can also be used for this purpose. In particular, it has been shown that this compound obtained from the sesame plant may be more effective for our natural metabolism. The IC50 of the AChE was calculated as 86.63 nM and the Ki value was 46.68 nM. Sesamol showed non-competitive inhibition. So, the molecule by showing the non-competitive inhibition, it binds to outside active site of the enzyme. In this way, it reduces the catalytic activity of the enzyme and causes non-competitive inhibition. Thus, the amount of ACh is thought to remain in high level. Comparing current results with previous results, it was found that novel bromophenols (Ki: 159.6–924.2 nM)[Citation4], oxazolidinone (Ki: 16.5–35.6 nM)[Citation48], pyrazolines (Ki: 48.2–84.1 nM)[Citation76], hydrazones (Ki: 66–128 nM)[Citation77], tacrine derivatives (Ki: 68–8480 nM)[Citation78], sulfamides (Ki: 0.027–0.076 nM)[Citation79], olivetol (Ki: 3.40 nM)[Citation73], and benzenesulfonamides (Ki: 22.7–109.1 nM)[Citation80] effectively inhibited AChE enzyme.
As seen in , α-glycosidase enzyme, which released from intestine cells, hydrolyzes the oligosaccharides and polysaccharide to monosaccharide units, such as glucose and fructose in small intestine. It is not able to use glucose as a result of a metabolic disorder in Type 2-diabetes mellitus (T2DM). After the meal, cellular glucose uptake increases with insulin secretion.[Citation81] Thus, the production of glucose stops in the liver. As a result, the blood sugar concentration increases. In this way, a condition called hyperglycemia occurs. The osmotic balance in dehydrated cells is impaired and causes symptoms of diabetes. These symptoms include thirst and excessive urination.[Citation82] The treatment of DM includes physical activity, diet, education, and medicine.[Citation83,Citation84] Oral antidiabetics and insulin therapy are used if other routes are unsuccessful or insufficient to control blood sugar in T2DM.[Citation83] Acarbose and voglibose are α-glycosidase inhibitors and orally used. Highly effective but they can cause adverse effects such as bloating, diarrhea and abdominal distention.[Citation73] As given in , sesamol showed nanomolar level inhibition against α-glycosidase enzyme. The IC50 value was calculated as 99.00 nM (r2: 0.9567). On the other hand, the Ki value was calculated as 75.33 nM. Whereas, IC50 value of acarbose, which used as the α-glycosidase inhibitor, was found to be 22.80 μM. It is thought that sesamol obtained from natural product will guide oral diabetic drug groups according to these values.[Citation85–Citation87]
Conclusion
It is clearly seen that sesamol had efficient inhibition properties on AChE and α-glycosidase enzymes. So this phenolic compound can be considered for treatment of nervous system and T2DM. Besides consumption, it was believed that its biological activity would inspire for drug designers in the treatment of many diseases including myasthenia gravis, postural tachycardia syndrome, AD (as cholinergic enzymes inhibitors) and treatment of T2DM (as α-glycosidase inhibitors).
References
- Akbaba, Y.; Bastem, E.; Topal, F.; Gülçin, İ.; Maraş, A.; Göksu, S. Synthesis and Carbonic Anhydrase Inhibitory Effects of Novel Sulfamides Derived from L-aminoindanes and Anilines. Arch. Pharm. 2014, 347(12), 950–957. DOI: 10.1002/ardp.201400257.
- Ozbey, F.; Taslimi, P.; Gulcin, İ.; Maraş, A.; Goksu, S.; Supuran, C. T. Synthesis, Acetylcholinesterase, Butyrilcholinesterase, Carbonic Anhydrase Inhibitory and Metal Chelating Properties of Some Novel Diaryl Ether. J. Enzyme Inhib. Med. Chem. 2016, 31(S2), 79–85. DOI: 10.1080/14756366.2016.1189422.
- Oztaşkın, N.; Taslimi, P.; Maraş, A.; Göksu, S.; Gülçin, İ. Novel Antioxidant Bromophenols with Acetylcholinesterase, Butyrylcholinesterase and Carbonic Anhydrase Inhibitory Actions. Bioorg. Chem. 2017, 74, 104–114. DOI: 10.1016/j.bioorg.2017.07.010.
- Boztas, M.; Taslimi, P.; Yavari, M. A.; Gülçin, İ.; Sahin, E.; Menzek, A. Synthesis and Biological Evaluation of Bromophenol Derivatives with Cyclopropyl Moiety: Ring Opening of Cyclopropane with Monoester. Bioorg. Chem. 2019, 89, 103017. DOI: 10.1016/j.bioorg.2019.103017.
- Taslimi, P.; Gulcin, I.; Oztaskın, N.; Cetinkaya, Y.; Goksu, S.; Alwasel, S. H.; Supuran, C. T. The Effects of Some Bromophenol Derivatives on Human Carbonic Anhydrase Isoenzymes. J. Enzyme Inhib. Med. Chem. 2016, 31, 603–607. DOI: 10.3109/14756366.2015.1054820.
- Koksal, Z.; Alım, Z.; Çetin, S.; Gülçin, İ.; Özdemir, H. Investigation of the Effects of Some Sulfonamides on Acetylcholinesterase and Carbonic Anhydrase Enzymes. J. Biochem. Mol. Toxicol. 2019, 33(5), e22300. DOI: 10.1002/jbt.22300.
- Genc Bilgicli, H.; Kestane, A.; Taslimi, P.; Karabay, O.; Bytyqi-Damoni, A.; Zengin, M.; Gulçin, İ. Novel Eugenol Bearing Oxypropanolamines: Synthesis, Characterization, Antibacterial, Antidiabetic, and Anticholinergic Potentials. Bioorg. Chem. 2019, 88, 102931. DOI: 10.1016/j.bioorg.2019.102931.
- Turkan, F.; Huyut, Z.; Taslimi, P.; Gulçin, I. The Effects of Some Antibiotics from Cephalosporin Groups on the Acetylcholinesterase and Butyrylcholinesterase Enzymes Activities in Different Tissues of Rats. Arch. Physiol. Biochem. 2019, 125(1), 12–18. DOI: 10.1080/13813455.2018.1427766.
- Öztaskin, N.; Kaya, R.; Maraş, A.; Sahin, E.; Gülçin, İ.; Göksu, S. Synthesis and Characterization of Novel Bromophenols: Determination of Their Anticholinergic, Antidiabetic and Antioxidant Activities. Bioorg. Chem. 2019, 87, 91–102. DOI: 10.1016/j.bioorg.2019.03.010.
- Cetin, A.; Turkan, F.; Taslimi, P.; Gulçin, İ. Synthesis and Characterization of Novel Substituted Thiophene Derivatives and Discovery of Their Carbonic Anhydrase and Acetylcholinesterase Inhibition Effects. J. Biochem. Mol. Toxicol. 2019, 33(3), e22261.
- Soreq, H.; Seidman, S. Acetylcholinesterase-new Roles for an Old Actor. Nat. Rev. Neurosci. 2001, 2(9), 670. DOI: 10.1038/35067589.
- Taslimi, P.; Osmanova, S.; Caglayan, C.; Turkan, F.; Sardarova, S.; Farzaliyev, V.; Sujayev, A.; Sadeghian, N.; Gulçin, I. Novel Amides of 1,1-bis-(carboxymethylthio)-1-arylethanes: Synthesis, Characterization, and Acetylcholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Inhibitory Properties. J. Biochem. Mol. Toxicol. 2018, 32(9), e22191. DOI: 10.1002/jbt.22191.
- Sujayev, A.; Garibov, E.; Taslimi, P.; Gulcin, I.; Gojayeva, S.; Farzaliyev, V.; Alwasel, S. H.; Supuran, C. T. Synthesis of Some Tetrahydropyrimidine-5-carboxylates, Determination of Their Metal Chelating Effects and Inhibition Profiles against Acetylcholinesterase, Butyrylcholinesterase and Carbonic Anhydrase. J. Enzyme Inhib. Med. Chem. 2016, 31, 1531–1539. DOI: 10.3109/14756366.2016.1156104.
- Wright, R. M.; Repine, T.; Repine, J. E. Reversible Pseudohyphal Growth in Haploid Saccharomyces Cerevisiae Is an Aerobic Process. Curr. Genet. 1993, 23, 388–391.
- Ohno, K.; Engel, A. G.; Brengman, J. M.; Shen, X. M.; Heidenreich, F.; Vincent, A.; Milone, M.; Tan, E.; Demirci, M.; Walsh, P.; et al. The Spectrum of Mutations Causing End-plate Acetylcholinesterase Deficiency. Ann. Neurol. 2000, 47, 162–170.
- Turkan, F.; Cetin, A.; Taslimi, P.; Karaman, M.; Gülçin, İ. Synthesis, Biological Evaluation and Molecular Docking of Novel Pyrazole Derivatives as Potent Carbonic Anhydrase and Acetylcholinesterase Inhibitors. Bioorg. Chem. 2019, 86, 420–427. DOI: 10.1016/j.bioorg.2019.02.013.
- Çağlayan, C.; Demir, Y.; Küçükler, S.; Taslimi, P.; Kandemir, F. M.; Gulçin, İ. The Effects of Hesperidin on Sodium Arsenite-induced Different Organ Toxicity in Rats on Metabolic Enzymes as Antidiabetic and Anticholinergics Potentials: A Biochemical Approach. J. Food Biochem. 2019, 43(2), e12720. DOI: 10.1111/jfbc.12720.
- Topal, F.;. Anticholinergic and Antidiabetic Effects of Isoeugenol from Clove (Eugenia caryophylata) Oil. Int. J. Food Propert. 2019, 22(1), 583–592. DOI: 10.1080/10942912.2019.1597882.
- Kuzu, B.; Tan, M.; Taslimi, P.; Gülçin, İ.; Taspınar, M.; Menges, N. Mono- or Di-substituted Imidazole Derivatives for Inhibiton of Acetylcholine and Butyrylcholine Esterases. Bioorg. Chem. 2019, 86, 187–196.
- Azevedo, R. S.; Avila, C. L. D. S.; Dias, E. S.; Bertechini, A. G.; Schwan, R. F. Utilization of the Spent Substrate of Pleurotus Sajor Caju Mushroom in Broiler Chicks Ration and the Effect on Broiler Chicken Performance. Acta Sci. Anim. Sci. 2009, 31, 139–144.
- Getman, D.; Eubanks, J.; Camp, S.; Evans, G.; Taylor, P. The Human Gene Encoding Acetylcholinesterase Is Located on the Long Arm of Chromosome 7. Am. J. Hum. Genet. 1992, 51(1), 170.
- Pisanti, S.; Picardi, P.; Ciaglia, E.; D’Alessandro, A.; Bifulco, M. Novel Prospects of Statins as Therapeutic Agents in Cancer. Pharmacol. Res. 2014, 88, 84–98. DOI: 10.1016/j.phrs.2014.06.013.
- Koçyiğit, U. M.; Taşkıran, A.; Taslimi, P.; Yokuş, A.; Temel, Y.; Gulcin, I. Inhibitory Effects of Oxytocin and Oxytocin Receptor Antagonist Atosiban on the Activities of Carbonic Anhydrase and Acetylcholinesterase Enzymes in the Liver and Kidney Tissues of Rats. J. Biochem. Mol. Toxicol. 2017, 31, e21972. DOI: 10.1002/jbt.21972.
- Gulcin, I.; Scozzafava, A.; Supuran, C. T.; Akıncıoğlu, H.; Koksal, Z.; Turkan, F.; Alwasel, S. The Effect of Caffeic Acid Phenethyl Ester (CAPE) Metabolic Enzymes Including Acetylcholinesterase, Butyrylcholinesterase, Glutathione S-transferase, Lactoperoxidase and Carbonic Anhydrase Isoenzymes I, II, IX and XII. J. Enzyme Inhib. Med. Chem. 2016, 31(6), 1095–1101. DOI: 10.3109/14756366.2015.1094470.
- Gulcin, I.; Scozzafava, A.; Supuran, C. T.; Koksal, Z.; Turkan, F.; Çetinkaya, S.; Bingol, Z.; Huyut, Z.; Alwasel, S. H. Rosmarinic Acid Inhibits Some Metabolic Enzymes Including Glutathione S-transferase, Lactoperoxidase, Acetylcholinesterase, Butyrylcholinesterase and Carbonic Anhydrase Isoenzymes. J. Enzyme Inhib. Med. Chem. 2016, 31(6), 1698–1702. DOI: 10.3109/14756366.2015.1135914.
- Cetin, A.; Turkan, F.; Taslimi, P.; Gulcin, I. Synthesis and Characterization of Novel Substituted Thiophene Derivatives and Discovery of Their Carbonic Anhydrase and Acetylcholinesterase Inhibition Effects. J. Biochem. Mol. Toxicol. 2018, 33(3), e22261. DOI: 10.1002/jbt.22261.
- Nachmansohn, D.;. Action of Ions on Choline Esterase. Nature. 1940, 145, 513–514. DOI: 10.1038/145513b0.
- Ecobichon, D. J.; Israel, Y. Characterization of the Esterases from Electric Tissue of Electrophorus by Starch-gel Electrophoresis. Canadian J. Biochem. 1967, 45(7), 1099–1105. DOI: 10.1139/o67-127.
- Yigit, B.; Kaya, R.; Taslimi, P.; Isık, Y.; Karaman, M.; Yigit, M.; Ozdemir, I.; Gulcin, I. Imidazolinium Chloride Salts Bearing Wing Tip Groups: Synthesis, Molecular Docking and Metabolic Enzymes Inhibition. J. Mol. Struct. 2019, 1179, 709–718. DOI: 10.1016/j.molstruc.2018.11.038.
- Turker, F.; Barut Celepci, D.; Aktaş, A.; Taslimi, P.; Gok, Y.; Aygun, M.; Gulcin, I. Meta-cyanobenzyl Substituted Benzimidazole: Synthesis, Characterization, Crystal Structure and Carbonic Anhydrase, α-glycosidase, Butyrylcholinesterase, Acetylcholinesterase Inhibitory Properties. Arch. Pharm. 2018, 351, e201800029. DOI: 10.1002/ardp.201800029.
- Myers, L. B.; Midence, K. Adherence and Diabetes. Adherence to Treatment in Medical; OPA: Netherlands, 1998; pp 423–453.
- Turkan, F.; Çetin, A.; Taslimi, P.; Gulcin, I. Some Pyrazoles Derivatives: Potent Carbonic Anhydrase, α-glycosidase and Cholinesterase Enzymes Inhibitors. Arch. Pharm. 2018, 351, e1800200. DOI: 10.1002/ardp.201800200.
- Ruggiero, L.; Glasgow, R.; Dryfoos, J. M.; Rossi, S. R.; Prochaska, J. O.; Orleans, C. T.; Prokhorov, A. V.; Rossi, J. S.; Greene, G. W.; Reed, G. R.; et al. Diabetes Self-management: Self-reported Recommendations and Patterns in a Large Population. Diabetes Care. 1997, 20, 568–576. DOI: 10.2337/diacare.20.4.568.
- Shobhana, R.; Begum, R.; Snehalatha, C.; Vijay, V.; Ramachandran, A. Patients’ Adherence to Diabetes Treatment. J. Assoc. Physicians India. 1997, 47, 1173–1175.
- Cramer, J. A.;. A Systematic Review of Adherence with Medications for Diabetes. Diabetes Care. 2004, 27, 1218–1224. DOI: 10.2337/diacare.27.5.1218.
- Aktaş, A.; Barut Celepci, D.; Kaya, R.; Taslimi, P.; Gök, Y.; Aygün, M.; Gulcin, I. Novel Morpholine Liganded Pd-based N-heterocyclic Carbene Complexes: Synthesis, Characterization, Crystal Structure, Antidiabetic and Anticholinergic. Polyhedron. 2019, 159, 345–354. DOI: 10.1016/j.poly.2018.11.048.
- Taslimi, P.; Aslan, H. E.; Demir, Y.; Oztaskin, N.; Maraş, A.; Gulcin, I.; Beydemir, S.; Goksu, S. Diarilmethanon, Bromophenols and Diarilmetan Compounds: Discovery of Potent Aldose Reductase, α-amylase and α-glycosidase Inhibitors as New Therapeutic Approach in Diabetes and Functional Hyperglycemia. Int. J. Biol. Macromol. 2018, 119, 857–863. DOI: 10.1016/j.ijbiomac.2018.08.004.
- Gulcin, I.; Taslimi, P.; Aygün, A.; Sadeghian, N.; Bastem, E.; Kufrevioglu, O. I.; Turkan, F.; Şen, F. Antidiabetic and Antiparasitic Potentials: Inhibition Effects of Some Natural Antioxidant Compounds on α-glycosidase, α-amylase and Human Glutathione S-transferase Enzymes. Int. J. Biol. Macromol. 2018, 119, 741–746. DOI: 10.1016/j.ijbiomac.2018.08.001.
- Taslimi, P.; Caglayan, C.; Farzaliyev, V.; Nabiyev, O.; Sujayev, A.; Turkan, F.; Kaya, R.; Gulçin, I. Synthesis and Discovery of Potent Carbonic Anhydrase, Acetylcholinesterase, Butyrylcholinesterase, and α-glycosidase Enzymes Inhibitors: the Novel N, N′-bis-cyanomethylamine and Alkoxymethylamine Derivatives. J. Biochem. Mol. Toxicol. 2018, 32(4), e22042. DOI: 10.1002/jbt.22042.
- Teng, H.; Chen, L.; Fang, T.; Yuan, B.; Lin, Q. Rb2 Inhibits α-glucosidase and Regulates Glucose Metabolism by Activating AMPK Pathways in HepG2 Cells. J. Funct. Foods. 2017, 28, 306–313. DOI: 10.1016/j.jff.2016.10.033.
- Bursal, E.; Aras, A.; Kılıç, O.; Gören, A. C.; Gulcin, I. Phytochemical Content, Antioxidant Activity, and Enzyme Inhibition Effect of Salvia eriophora Boiss. & Kotschy against Acetylcholinesterase, α-amylase, Butyrylcholinesterase, and α-glycosidase Enzymes. J. Food Biochem. 2019, 43(3), e12776. DOI: 10.1111/jfbc.12776.
- Taslimi, P.; Gulcin, I. Antidiabetic Potential: in Vitro Inhibition Effects of Some Natural Phenolic Compounds on α-glycosidase and α-amylase Enzymes. J. Biochem. Mol. Toxicol. 2017, 31, e21956. DOI: 10.1002/jbt.21956.
- Kaneto, H.; Kajimoto, Y.; Miyagawa, J.; Matsuoka, T.; Fujitani, Y.; Umayahara, Y.; Hanafusa, T.; Matsuzawa, Y.; Yamasaki, Y.; Hori, M. Beneficial Effects of Antioxidants in Diabetes. Diabetes. 1999, 48, 2398–2406. DOI: 10.2337/diabetes.48.12.2398.
- Ecemis, G. C.; Atmaca, H. Oral Antidiabetic Agents. J. Exp. Clin. Med. 2012, 29, 23–29. DOI: 10.5835/jecm.omu.29.s1.006.
- Maharramova, G.; Taslimi, P.; Sujayev, A.; Farzaliyev, F.; Durmaz, L.; Gulçin, I. Synthesis, Characterization, Antioxidant, Antidiabetic, Anticholinergic, and Antiepileptic Properties of Novel N-substituted Tetrahydropyrimidines Based on Phenylthiourea. J. Biochem. Mol. Toxicol. 2018, 32(11), e22221. DOI: 10.1002/jbt.22221.
- Burmaoglu, S.; Yilmaz, A. O.; Taslimi, P.; Algul, O.; Kılıç, D.; Gulcin, I. Synthesis and Biological Evaluation of Phloroglucinol Derivatives Possessing α-glycosidase, Acetylcholinesterase, Butyrylcholinesterase, Carbonic Anhydrase Inhibitory Activity. Arch. Pharm. 2018, 351, e1700314. DOI: 10.1002/ardp.201700314.
- Erdemir, F.; Barut Celepci, D.; Aktaş, A.; Taslimi, P.; Gok, Y.; Karabıyık, H.; Gulcin, I. 2-hydroxyethyl Substituted NHC Precursors: Synthesis, Characterization, Crystal Structure and Carbonic Anhydrase, α-glycosidase, Butyrylcholinesterase, and Acetylcholinesterase Inhibitory Properties. J. Mol. Struct. 2018, 1155, 797–806. DOI: 10.1016/j.molstruc.2017.11.079.
- Atmaca, U.; Kaya, R.; Kahraman, H. S.; Çelik, M.; Gülçin, İ. Synthesis of Oxazolidinone from Enantiomerically Enriched Allylic Alcohols and Determination of Their Molecular Docking and Biologic Activities. Bioorg. Chem. 2019, 88, 102980. DOI: 10.1016/j.bioorg.2019.102980.
- Tohma, H.; Altay, A.; Koksal, E.; Gören, A. C.; Gülçin, İ. Measurement of Anticancer, Antidiabetic and Anticholinergic Properties of Sumac (Rhus coriaria) - Analysis of Its Phenolic Compounds by LC-MS/MS. J. Food Measure. 2019, 13(2), 1607–1619. DOI: 10.1007/s11694-019-00077-9.
- Taslimi, P.; Kandemir, F. M.; Demir, Y.; İleritürk, M.; Temel, Y.; Cağlayan, C.; Gülçin, İ. The Antidiabetic and Anticholinergic Effects of Chrysin on Cyclophosphamide-induced Multiple Organs Toxicity in Rats: Pharmacological Evaluation of Some Metabolic Enzymes Activities. J. Biochem. Mol. Toxicol. 2019, 33(6), e22313. DOI: 10.1002/jbt.22313.
- Shyu, Y. S.; Hwang, L. S. Antioxidative Activity of the Crude Extract of Lignan Glycosides from Unroasted Burma Black Sesame Meal. Food Res. Int. 2002, 35, 357–365. DOI: 10.1016/S0963-9969(01)00130-2.
- Bankole, M. A.; Shittu, L. A. J.; Ahmed, T. A.; Bankole, M. N.; Shittu, R. K.; Terkula, K.; Ashiru, O. A. Synergistic Antimicrobial Activities of Phytoestrogens in Crude Extracts of Two Sesame Species against Some Common Pathogenic Microorganisms. Afr. Trad. CAM. 2007, 4, 427–433. DOI: 10.4314/ajtcam.v4i4.31237.
- Alencar, J. S.; Pietri, S.; Culcasi, M.; Orneto, C.; Piccerelle, P.; Reynier, J. P.; Portugal, H.; Nicolay, A.; Kaloustian, J. Interactions and Antioxidant Stability of Sesamol in Dry-emulsions. J. Therm. Calorim. 2009, 98, 133–143. DOI: 10.1007/s10973-009-0102-8.
- Ellman, G. L.; Courtney, K. D.; Andres, V.; Featherston, R. M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88. DOI: 10.1016/0006-2952(61)90145-9.
- Huseynova, M.; Taslimi, P.; Medjidov, A.; Farzaliyev, V.; Aliyeva, M.; Gondolova, G.; Şahin, O.; Yalçın, B.; Sujayev, A.; Orman, E. B.; et al. Synthesis, Characterization, Crystal Structure, Electrochemical Studies and Biological Evaluation of Metal Complexes with Thiosemicarbazone of Glyoxylic Acid. Polyhedron. 2018, 155, 25–33. DOI: 10.1016/j.poly.2018.08.026.
- Zengin, M.; Genç, H.; Taslimi, P.; Kestane, A.; Güçlü, E.; Ögütlü, A.; Karabay, O.; Gülçin, İ. Novel Thymol Bearing Oxypropanolamine Derivatives as Potent Some Metabolic Enzyme Inhibitors-their Antidiabetic, Anticholinergic and Antibacterial Potentials. Bioorg. Chem. 2018, 81, 119–126. DOI: 10.1016/j.bioorg.2018.08.003.
- Yiğit, B.; Yiğit, M.; Barut Celepci, D.; Gök, Y.; Aktaş, A.; Aygün, M.; Taslimi, P.; Gulçin, İ. Novel Benzylic Substituted Imidazolinium, Tetrahydropyrimidinium and Tetrahydrodiazepinium Salts-potent Carbonic Anhydrase and Acetylcholinesterase Inhibitors. ChemistrySelect. 2018, 3(27), 7976–7982. DOI: 10.1002/slct.201801019.
- Güzel, E.; Koçyiğit, U. M.; Arslan, B. S.; Ataş, M.; Taslimi, P.; Gökalp, F.; Nebioğlu, M.; Şişman, İ.; Gulçin, İ. Aminopyrazole-substituted Metallophthalocyanines: Preparation, Aggregation Behavior and Investigation of Metabolic Enzymes Inhibition Properties. Arch. Pharm. 2019, 352(2), e1800292. DOI: 10.1002/ardp.201800292.
- Atmaca, U.; Yıldırım, A.; Taslimi, P.; Tuncel Çelik, S.; Gulçin, İ.; Supuran, C. T.; Çelik, M. Intermolecular Amination of Allylic and Benzylic Alcohols Leads to Effective Inhibitions of Acetylcholinesterase Enzyme and Carbonic Anhydrase I and II Isoenzymes. J. Biochem. Mol. Toxicol. 2018, 32, e22173. DOI: 10.1002/jbt.22173.
- Bayrak, Ç.; Taslimi, P.; Kahraman, H. S.; Gulcin, I.; Menzek, A. The First Synthesis, Carbonic Anhydrase Inhibition and Anticholinergic Activities of Some Bromophenol Derivatives with Including Natural Products. Bioorg. Chem. 2019, 85, 128–139. DOI: 10.1016/j.bioorg.2018.12.012.
- Jan, K. C.; Ho, C. T.; Hwang, L. S. Bioavailability and Tissue Distribution of Sesamol in Rat. J. Agric. Food Chem. 2008, 56, 7032–7037. DOI: 10.1021/jf8012647.
- Marshall, S.; Garvey, W. T.; Traxinger, R. R. New Insights into the Metabolic Regulation of Insulin Action and Insulin Resistance: Role of Glucose and Amino Acids. Faseb J. 1991, 5, 3031–3036. DOI: 10.1096/fasebj.5.15.1743436.
- Kelley, B. J.; Petersan, R. C. Alzheimer’s Disease and Mild Cognitive Impairment. Neurol. Clin. 2007, 25, 577–609. DOI: 10.1016/j.ncl.2007.03.008.
- Ozmen Ozgun, D.; Yamali, C.; Gul, H. I.; Taslimi, P.; Gulcin, I.; Yanik, T.; Supuran, C. T. Inhibitory Effects of Isatin Mannich Bases on Carbonic Anhydrases, Acetylcholinesterase and Butyrylcholinesterase. J. Enzyme Inhib. Med. Chem. 2016, 31, 1498–1501. DOI: 10.3109/14756366.2016.1149479.
- Daryadel, S.; Atmaca, U.; Taslimi, P.; Gulcin, I.; Çelik, M. Novel Sulfamate Derivatives of Menthol: Synthesis, Characterization and Cholinesterases and Carbonic Anhydrase Enzymes Inhibition Properties. Arch. Pharm. 2018, 351, e1800209. DOI: 10.1002/ardp.201800209.
- Maharramov, A.; Kaya, R.; Taslimi, P.; Kurbanova, M.; Sadigova, A.; Farzaliyev, V.; Sujayev, A.; Gulçin, İ. Synthesis, Crystal Structure, and Biological Evaluation of Optically Active 2-amino-4-aryl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4h-chromen-3-carbonitriles: Antiepileptic, Antidiabetic, and Anticholinergics Potentials. Arch. Pharm. 2019, 352(2), e1800317. DOI: 10.1002/ardp.201800317.
- Ceylan, H.; Erdoğan, O. Cloning, Expression, and Characterization of Human Brain Acetylcholinesterase in Escherichia Coli Using a SUMO Fusion Tag. Turk. J. Biol. 2017, 41, 77–87. DOI: 10.3906/biy-1602-83.
- Ozmen, D.; Ozgün, H.; Gul, I.; Yamalı, C.; Sakagami, H.; Gulcin, I.; Sukuroglu, M. K.; Supuran, C. Synthesis and Bioactivities of Pyrazoline Benzensulfonamides as Carbonic Anhydrase and Acetylcholinesterase Inhibitors with Low Cytotoxicity. Bioorg. Chem. 2019, 84, 511–517. DOI: 10.1016/j.bioorg.2018.12.028.
- Eruygur, N.; Ataş, M.; Tekin, M.; Taslimi, P.; Koçyiğit, U. M.; Gulçin, İ. In Vitro Antioxidant, Antimicrobial, Anticholinesterase and Antidiabetic Activities of Turkish Endemic Achillea cucullata (asteraceae) from Ethanol Extract. South Afr. J. Bot. 2019, 120, 141–145. DOI: 10.1016/j.sajb.2018.04.001.
- Burmaoglu, S.; Yılmaz, A. O.; Polat, M. F.; Kaya, R.; Gulcin, I.; Algül, O. Synthesis of Novel Tris-chalcones and Determination of Their Inhibition Profiles against Some Metabolic Enzymes. Arch. Physiol. Biochem. 2019, 43(7), e12908.
- Burmaoğlu, S.; Yılmaz, A. O.; Polat, M. F.; Algül, Ö.; Kaya, R.; Gülçin, İ. Synthesis and Biological Evaluation of Novel Tris-chalcones as Potent Carbonic Anhydrase, Acetylcholinesterase, Butyrylcholinesterase, and α-glycosidase Inhibitors. Bioorg. Chem. 2019, 85, 191–197. DOI: 10.1016/j.bioorg.2018.12.035.
- Thacker, P. D.;. Surpising Discovery with Alzheimer’s Medication. Drug Discov. Today. 2003, 1, 8–9.
- Taslimi, P.; Gulcin, I. Antioxidant and Anticholinergic Properties of Olivetol. J. Food Biochem. 2018, 42, e12516. DOI: 10.1111/jfbc.12516.
- Bayrak, Ç.; Taslimi, P.; Kahraman, H. S.; Gülçin, İ.; Menzek, A. The First Synthesis, Carbonic Anhydrase Inhibition and Anticholinergic Activities of Some Bromophenol Derivatives with Including Natural Products. Bioorg. Chem. 2019, 85, 128–139. DOI: 10.1016/j.bioorg.2018.12.012.
- Kohler, H. P.; Grant, P. J. Plasminogen Activator Inhibitor Type-1 and Coronary Artery Disease. N. Engl. J. Med. 2000, 342, 1792–1801.
- Türkan, F.; Çetin, A.; Taslimi, P.; Karaman, H. S.; Gulçin, İ. Synthesis, Characterization, Molecular Docking and Biological Activities of Novel Pyrazoline Derivatives. Arch. Pharm. 2019, 352(6), e1800359. DOI: 10.1002/ardp.201800359.
- Küçükoğlu, K.; Gül, H. İ.; Taslimi, P.; Gülçin, İ.; Supuran, C. T. Investigation of Inhibitory Properties of Some Hydrazone Compounds on hCA I, hCA II and AChE Enzymes. Bioorg. Chem. 2019, 86, 316–321. DOI: 10.1016/j.bioorg.2019.02.008.
- Ökten, S.; Ekiz, M.; Tutar, A.; Bütün, B.; Koçyiğit, U. M.; Topçu, G.; Gulçin, İ. SAR Evaluation of Disubstituted Tacrine Analogues as Promising Cholinesterase and Carbonic Anhydrase Inhibitors. Indian J. Pharm. Educ. 2019, 53(2), 268–275. DOI: 10.5530/ijper.53.2.35.
- Aksu, K.; Akıncıoğlu, H.; Akıncıoğlu, A.; Göksu, S.; Tümer, F.; Gulçin, İ. Synthesis of Novel Sulfamides Incorporating Phenethylamines and Determination of Their Inhibition Profiles against Some Metabolic Enzymes. Arch. Pharm. 2018, 351(9), e1800150. DOI: 10.1002/ardp.201800150.
- Yamali, C.; Gül, H. İ.; Ece, A.; Taslimi, P.; Gulçin, İ. Synthesis, Molecular Modeling and Biological Evaluation of 4-[5-aryl-3-(thiophen-2-yl)-4,5-dihydro-1h-pyrazol-1-yl]benzenesulfonamides Towards Acetylcholinesterase, Carbonic Anhydrase I and II Enzymes. Chem. Biol. Drug Des. 2018, 91(4), 854–866. DOI: 10.1111/cbdd.13149.
- Biçer, A.; Taslimi, P.; Yakali, G.; Gülçin, İ.; Gültekin, M. S.; Turgut Cin, G. Synthesis, Characterization, Crystal Structure of Novel Bis-thiomethylcyclohexanone Derivatives and Their Inhibitory Properties against Some Metabolic Enzymes. Bioorg. Chem. 2019, 82, 393–404. DOI: 10.1016/j.bioorg.2018.11.001.
- Harris, M. I.;. Undiagnosed NIDDM: Clinical and Public Health Issues. Diabetes Care. 1993, 16, 642–652. DOI: 10.2337/diacare.16.4.642.
- Edwards, C. M.; Stanley, S. A.; Davis, R.; Brynes, A. E.; Frost, G. S.; Seal, L. J.; Ghatei, M. A.; Bloom, S. R. vExendin-4 Reduces Fasting and Postprandial Glucose and Decreases Energy Intake in Healthy Volunteers. Am. J. Phsiol. Endocrinol. Metab. 2001, 281, E155–61. DOI: 10.1152/ajpendo.2001.281.1.E155.
- Demir, Y.; Taslimi, P.; Ozaslan, M. S.; Oztaskın, N.; Çetinkaya, Y.; Gulçin, İ.; Beydemir, S.; Göksu, P. Antidiabetic Potential: in Vitro Inhibition Effects of Bromophenol and Diarylmethanones Derivatives on Metabolic Enzymes. Arch. Pharm. 2018, 351(12), e1800263. DOI: 10.1002/ardp.201800263.
- Torres-Naranjo, M.; Suarez, A.; Gilardoni, G.; Cartuche, L.; Flores, P.; Morocho, V. Chemical Constituents of Muehlenbeckia tamnifolia (kunth) Meisn (polygonaceae) and Its in Vitro α-amilase and α-glucosidase Inhibitory Activities. Molecules. 2016, 21, 1461–1465. DOI: 10.3390/molecules21111461.
- Turkan, F.; Çetin, A.; Taslimi, P.; Gulçin, İ. Some Pyrazoles Derivatives: Potent Carbonic Anhydrase, α-glycosidase and Cholinesterase Enzymes Inhibitors. Arch. Pharm. 2018, 351(10), e1800200. DOI: 10.1002/ardp.201800200.
- Teng, H.; Chen, L.; Fang, T.; Yuan, B.; Lin, Q. Rb2 Inhibits α-glucosidase and Regulates Glucose Metabolism by Activating AMPK Pathways in HepG2 Cells. J. Funct. Foods. 2017, 28, 306–313. DOI: 10.1016/j.jff.2016.10.033.