 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Jengkol (Pithecellobium jiringa) is a typical plant in the tropical region of Southeast Asia that is mostly liked by indonesian people because of savory taste. Jengkol seeds having high protein and healthy effects need processing for keeping their nutrient quality. This study aims to determine the effect of steam blanching on the non-volatile taste components of jengkol seeds. The results showed that steam blanching during 5 min decreased the total content of soluble sugars from 998.10 to 903.59 ppm, organic acid from 442.80 to 362.89 ppm and 5ʹ-nucleotides from 2.92 to 2.57 mg/g dry weight. However, steam blanching could be able to increase the total content of free amino acids from 40.99 to 44.98 mg/g dry weight. The EUC (equivalent umami concentration) value in jengkol seeds also increased from 38.27 to 42.22 g MSG/100 g dry weight. The total content of amino acids also increased slightly from 9.81% to 10.08% (w/w) on jengkol seed flour after blanching treatment. Jengkol seed flour consists of hydrophobic amino acids (36.00–34.41%). The E/T value decreased from 35.54% to 33.92% but the PER (protein efficiency ratio) value increased from 2.43 to 2.63 on jengkol seed flour after steam blanching treatment. The steam blanching treatment increased the MSG-like components in jengkol seed flour and also gave the sweet taste and prevented the formation of the bitter taste. Hence, jengkol seed flour showed a high potential to serve as a functional food with a umami taste.
Introduction
Pithecellobium jiringa or jengkol belongs to the family of Fabaceae and classified as a subfamily of Mimosaceae that is consumed raw or cooked in many tropical countries. Jengkol is traditionally used for treating diabetes, high blood pressures, overcoming dysentery, stomach disorders, and bladder stones.[Citation1] Pithecellobium jiringa are popular as jering seeds in Malaysia, jengkol in Indonesia, krakos in Cambodia, and niang-yai in Thailand. Jengkol seeds had a high potential for further use, processed, and modified for the development of new food products.[Citation2]
Jengkol was a seed that had flavor and could grow well in Indonesia.[Citation3] Jengkol also had high protein and amino acids.[Citation4] The flavor was influenced by the presence of non-volatile flavor components such as organic acids, free amino acids, 5-nucleotides, and dissolved sugars that provide a sensation called umami.[Citation5] Umami was the fifth taste sensation received by the senses of human taste, besides four other traditional taste sensations (sweet, sour, salty, bitter).[Citation6] The umami component is found mostly in the form of amino acids L-glutamate and L-aspartate.[Citation7] It is also known that several compounds have contributed to the appearing of umami taste, namely monosodium glutamate (MSG), inosine monophosphate (IMP), and guanosine monophosphate (GMP).[Citation8] The mixture of the three compounds produces synergistic properties at certain comparisons.[Citation9]
Jengkol seeds that are high in nutrients and have health effects require processing due to perishable with high nutrient content. This processing aims to maintain the quality of jengkol seeds and extend shelf life. In the processing stage, the pre-process treatment needs to be carried out to determine the chemical characteristics of the jengkol before further processing such as packaging, marketing, and storage. One of the pre-process treatments that is often used is blanching. Blanching is a preheating treatment carried out on fresh vegetables or fruit before the process of freezing, drying, or canning. In addition, blanching is an effective way to simplify the process of peeling the skin on green legumes and can maintain nutrition in beans, carrots, and broccoli.[Citation10]
Blanching method that is often used is steam blanching. Steam blanching is the best method to maintain the nutritional quality of broccoli.[Citation7,Citation11] Steam blanching is the best method for maintaining the quality of nutrients such as carotenoids, glucosinolates, sulphorane, folate, and phytochemicals.[Citation12] Though literature reported the beneficial effect of steam and other processing methods like fermentation in reducing the anti-nutritional factors and improving the digestibility of food grains.[Citation13,Citation14] The blanching method also affects the sensory properties of food. One sensory attribute that is significantly affected by the blanching method is the flavor attribute. Blanching steam of vegetables was reported to be more flavorsome than vegetables using the boiling method. Steam blanching as a method for cooking vegetables was believed to be very suitable for vegetables such as broccoli. Steam blanching also reduced the washing of sugar into boiled water.[Citation15]
So far, the research on jengkol seeds is still limited and the lack of information about the non-volatile taste component and amino acid profile is the basis of this research. However, the aims of this research were to know the effect of steam blanching to the changes of non-volatile taste components and amino acid profile of jengkol seed flour. The non-volatile taste components consisting of soluble sugars, organic acids, 5ʹ-nucleotides, and free amino acids were also evaluated and compared. The data obtained from this study are considered useful to provide information on the potential of local plants in Indonesia.
Materials and methods
Material
Jengkol seeds were obtained from Kranggan Traditional Market, Yogyakarta. The chemicals used were analytical grade purchased from Sigma-Aldrich and Merck for analytical purposes. Sugar standards, total amino acid standards, and organic acid standards were HPLC grade from Sigma Aldrich. Nucleotide (IMP, GMP, and AMP) standards and free amino acid standards were HPLC grade obtained from Sigma Aldrich. Mobile phases for HPLC analysis were HPLC grade quality and purchased from Merck.
Steam blanching methods
Jengkol seeds were first blanched for 5 min with steam blanching which refers to Opikasari[Citation16] method. Fresh jegkol seeds were washed and then drained. As much as ± 1500 g of drained jengkol seeds were placed on the steaming stimulator below which contains boiling water (6 l) for 5 min. The steamed jengkol was then immediately cooled with running tap water before slicing and drying. Steamed jengkol seeds and jengkol seeds without steam blanching were sliced to be smaller parts (reducing size) for helping in the proccess of drying and grinding. The small parts of jengkol seeds without steam blanching and steamed jengkol seeds were dried using a cabinet dryer at 50°C for 18–20 h. The dried seeds were then milled and sieved with a 50 mesh sieve for obtaining the jengkol seed powder.
Assay of soluble sugars
Soluble sugar in jengkol seed flour was extracted and analyzed according to Li et al.[Citation17] Suspensions of powdered jengkol (0.25 g) in 50 ml 80% ethanol were shaken at 80 rpm for 45 min at room temperature. After filtration through Whatman No. 3 filter paper, the filtrate was evaporated to dry at 40°C on a rotary evaporator. The extract was redissolved in deionized water to a final volume of 10 ml and the solution was filtered through (0.45 millex filter, Millipore) prior to analysis.
Soluble sugar was determined by a Knauer HPLC system (autosampler, Knauer smartline 3950, German) equipped with a Refractive Index Detector (RID). The assay was performed on a Metacharb 87C column (300 × 7.8 mm) and the mobile phase used was H2O (LC grade) with an injection volume of 20 μL at a flow rate of 0.5 mL/min. The oven temperature was maintained at 85°C, and isocratic elution. The identification and quantification of sugar compounds were compared to calibration curve of authentic standards.
Assay of organic acids
Organic acids were extracted as described by Li et al.[Citation17] Powdered jengkol samples (5.0 mg) were suspended in 20 ml deionized water and subjected to ultrasound (400 W, 30 min, ambient temperature) using a 1500 W High-Intensity Ultrasonic Processor. The suspension was centrifuged at 4000 rpm for 30 min and the supernatant was filtered through a 0.45 µM cellulose membrane (Millipore) prior to analysis by HPLC.
The assay was performed on a MethaCarb H Plus column (7.8 × 300 mm) with the Knauer HPLC system (autosampler, Knauer smartline 3950, |German). The injection volume was 20 μL and the mobile phase was H2SO4 0,005 M at a flow rate of 0.5 mL/min. The oven temperature was maintained at 70°C. In addition, the detection wavelength of organic acid was 215 nm by UV and each organic acid was identified by an authentic standard. Lastly, all the organic acids were quantified the use of a calibration curve prepared from the external standards.
Free amino acid assay
Free amino acids were extracted as the method that was described by Wang et al.[Citation18] Sample (0.25 g) is ground and dissolved in 10 ml of distilled water. The suspension is then heated at 90° for 20 min, cooled till ambient temperature. The extract was centrifuged at 4500 g and the supernatant was made up to 10 mL with distilled water and filtered with 0.45 µm nylon membrane filter. The SPE-PAKC18 cartridges were conditioned with 30 mL of methanol and 10 mL of distilled water. Sample infusion (1 mL) was loaded into the conditioned cartridges, and the retained compounds were eluted with 5.0 mL of 10% ethanol. The resulted elute was collected and filtered through a 0.45 µm nylon filter membrane before its pre-column derivatization with o-phthalaldehyde (OPA).
The OPA derivatization solution was freshly prepared as follows: 0.01 g of OPA was dissolved in 1 mL of methanol, and then added with 4 mL of 0.4 M borate buffer (pH = 9) and 25 µL of mercaptoethanol. The 10 µL of sample infusion or standard amino acid was mixed with 300 µL OPA solution, incubated at ambient temperature (27°C) for 2 min, and used directly for HPLC analysis.
Analysis of free amino acids was carried out with High-Performance Liquid Chromatography (HPLC-10AD Shimazu, Japan) that was equipped with a shimadzu fluorescence RF-10A detector. The column used was a lichoCART 125–4 reverse phase C-18 column 5 µm at a flow rate of 1.5 ml/min. The mobile phases used were 50 mM sodium acetate:tetrahydofuran (THF): methanol (96: 2: 2) as solvent A and methanol 65% as solvent B. The gradient elution was performed as follows: 0–2 min, 100% solvent A; 2–35 min, 100% solvent B. Each amino acid was identified and quantified by the external amino acid standards.
5ʹ-Nucleotides assay
5ʹ-Nucleotides were extracted as the method explained by Pei et al.[Citation5] 1 gram of sample is mashed and dissolved in 10 ml of distilled water. The suspension is then heated for 1 min, cooled, and centrifuged at 4500 g for 15 min. The residue from the previous process was washed 3 times with 10 ml of distilled water and then mixed with the filtrate to rotate the evaporator and diluted again with distilled water to a volume of 10 ml. The solution was filtered with a 0.45-µm micropore membrane filter before analyzing.
The analysis was carried out with High-Performance Liquid Chromatography (HPLC-10AD Shimazu, Japan). The HPLC column is Zorbax Eclipse XDB C18 (250 × 4.6 mm, 5 µm) with mobile phase aquades/methanol/acetic acid/tetrabutylammonium hydroxide (894.5/100/5/0.50). Injection volume was 20 µL with a flow rate of 0.7 mL/minute and 5ʹ nucleotides were detected by UV at 254 nm. External standard was used for quantification of each 5ʹ nucleotide.
Equivalent umami concentration (EUC)
Calculation of the equivalent umami concentration (EUC) in the jengkol seed powder was based on the equation: as described by Yamaguchi et al.[Citation9] where Y is the EUC of the sample (g MSG/100 g); ai is the concentration (g/100 g) of each umami amino acid (Asp or Glu); aj is the concentration (g/100 g) of each umami 5′-nucleotide (5′-IMP, 5′-GMP, 5′-AMP); bi is the relative umami concentration (RUC) for each umami amino acid to MSG (Glu, 1 and Asp, 0.077); bj is the RUC for each umami 5′-nucleotide (5′-IMP, 1; 5′-GMP, 2.3; 5′-AMP, 0.18) and 1218 is a synergistic constant based on the concentration (g/100 g) used.
Total amino acid assay
Amino acid profile analysis based on Fisher et al.[Citation19] was carried out by stages, namely, sample preparation and LC-MS/MS analysis which was completed with The Waters Xevo TQD (Tandem Quadrupole Detector). Sample preparation was carried out by weighing 2 g of sample and put in a 50 ml reaction tube. Hcl 6N was added as much as 20 mL and hydrolyzed in an autoclave temperature of 110°C for 12 h, and then neutralized with 6N NaOH and add up to a volume of 50.0 mL. The sample was filtered with a 0.22 µM filter and then put in a vial bottle and ready for analysis. The injection volume was 2 μl and the flow rate was 0.6 mL/min.
Analysis of total amino acids was carried out with LC-MS/MS equipment. The column used in this analysis was column C-18 (150 mm × 2.1 mm). Mobile phase A used was 0.1% Pentecafluorooctanoic Acid (PDFOA), 99.5%: 0.5% (Water/CH3CN) with 0.1% Formic acid and Mobile phase B used was 0.1% PDFOA, 10%: 90% (Water/CH3CN) with 0.1% Formic acid. Quantification was done by an external authentical standard. The mobile phase with a speed set by a gradient system in the following conditions ().
Ratio of essential amino acid to total amino acid was calculated using the following equation[Citation14]:
(1)
(1)
(b) The predicted protein efficiency ratio (PER) calculated using the following equation (Mohapatra et al., 2019):
(2)
(2)
(3)
(3)
Result and discussion
Soluble sugars
The changes of soluble sugar in jengkol after steam blanching were presented in . Fructose + Galactose and Glucose were the most major soluble sugars in without steam blanching jengkol. The content of Fructose + Galactose was around 342.70 ppm in without steam blanching jengkol and it decreased in steamed jengkol (224.53 ppm). The content of Glucose was around 271.80 ppm in without steam blanching jengkol and it was higher than in steamed jengkol (161.80 ppm) which showed a decrease caused by effecting from steam blanching treatment. There was a possibility that the short time hot water and steam blanching resulted in its decrease.[Citation20] In addition, soluble sugars also were considered as taste-active components contributed to the sweet perception so, it can be potential for flavor enhancer. Beside that, the contents of fructose, galactose, and glucose in steamed jengkol decreased compared to without steam blanching jengkol, which could be due to the decomposition of sugar and the Maillard reaction occurred during the heat treatment.[Citation20,Citation21]
Table 1. Scheme of elution gradient for amino acid total
Table 2. The changes of soluble sugar in jengkol seeds after steam blanching
The total content of soluble sugars () in without steam blanching jengkol and steam jengkol ranged from 903.59 to 998 ppm, where the total content of soluble sugars in without steam blanching jengkol was higher than the steam jengkol. According to Pei et al.[Citation5] stated that during the thermal process, due to the higher temperature of samples, thermal decomposition could have led to the decrease in sugar content. The research reported by Saldivar et al.[Citation22] stated that steam blanching effectively retained soluble sugars than another thermal treatment (hot water blanching). Hot water blanching decreased soluble sugars by leaching during the water blanching.
Organic acids
The content of organic acids on without steam blanching jengkol and steam jengkol is presented in . Organic acids are strongly related to the synthesis and metabolism of amino acids, aromatic compounds, esters, and phenols.[Citation17,Citation20] It could be seen that Succinic Acid was the major organic acid (1.145.87 ppm) in without steam blanching jengkol, followed by Lactic Acid (259.61 ppm), Citric Acid (182.47 ppm), malic acid (0.72 ppm) and Oxalic Acid (<0.158 ppm). The total content of organic acid in jengkol decreased from 442.80 ppm (without steam blanching jengkol) to 362.89 ppm (steam jengkol) after blanching. The organic acids loss could be ascribed to the occurrence of decarboxylation due to the higher temperature of heat treatment during steam blanching.[Citation5,Citation23] In addition, the contents of Succinic Acid, malic acid, Lactic Acid, citric acid, and Oxalic Acid also declined in different degrees, which may result in the decrease of astringency value.[Citation20,Citation24]
Table 3. The changes of organic acid in jengkol seeds after steam blanching
Free amino acids
Free amino acid profile of without steam blanching jengkol and steam jengkol could be seen in . Free amino acid total of without steam blanching jengkol was 40.99 mg/g dry weight and free amino acid total of steam jengkol was 44.98 mg/g dry weight. The total of free amino acid on steam jengkol was higher 9.73% than without steam blanching jengkol. There is a possibility that increasing free amino acids that released from the proteolysis on steam jengkol is caused by heat treatment during the steam blanching. Yoneda et al.[Citation25] reported that some free amino acids could be released from the proteolysis that occurred during heating treatment. Free amino acid total in without steam blanching jengkol and steam jengkol was higher than reported by Li et al.[Citation17] in five edible mushrooms (4.09–22.73 mg/g dry weight) and reported by Pei et al.[Citation5] in Agaricus bisporus (44.2 mg/g dry weight).
Table 4. Free amino acid levels in without steam blanching jengkol and steam jengkol.
According to Mau et al.,[Citation26]amino acids differed into several clusters on the basis of their taste characteristics that could be seen in . Aspartic and glutamic acids were MSG-like components among all the free amino acids, that contributed to the characteristic umami taste on jengkol seeds.[Citation27] MSG-like components could also contribute to the level of EUC in jengkol seeds and EUC values tended high if the concentration of MSG-like components was also high.[Citation20]
Table 5. Level of amino acid taste components in without steam blanching and steam jengkol.
The steam jengkol contained MSG-like components (3.87 mg/g dry weight) that was higher than without steam blanching jengkol (2.68 mg/g dry weight). As reported by Yang et al.[Citation28] and Biao Li et al.[Citation20], MSG-like components divided into three groups: high (>20 mg/g), middle (5–20 mg/g), and low (<5 mg/g). The level of MSG-like components in without steam blanching jengkol and steam jengkol was still under 5 mg/g. The content of sweet components on steam jengkol was 17.82 (mg/g dry weight) that was higher than without steam blanching jengkol (12.54 mg/g dry weight). The content of bitter components in without steam blanching jengkol was 16.92 (mg/g dry weight) that was slightly higher than steam jengkol (16.35 mg/g dry weight). Any bitterness in jengkol seeds would possibly be covered by the high amounts of soluble sugars, other sweet components, and MSG-like amino acids, which together appear to be responsible for their natural taste.[Citation17]
5′-Nucleotide components
Changes of 5ʹ-Nucleotide in jengkol seeds after steam blanching could be seen in . The total content of 5ʹ-Nucleotide in without steam blanching jengkol was 2.92 mg/g dry weight that was slightly higher 11.98% than steam jengkol (2.57 mg/g dry weight).
Table 6. Levels of 5′-nucleotide components in without steam blanching and steam jengkol.
5′-AMP, 5′-adenosine monophosphate; 5′-GMP, 5′-guanosine monophosphate; 5′-IMP, 5′-inosine monophosphate
Decreasing the total content of 5ʹ- Nucleotide components on steam jengkol seeds could be possible to the effect of thermal decomposition during the steam blanching treatment.[Citation29] 5′-guanosine monophosphate (5′-GMP) and 5′-inosine monophosphate (5ʹ-IMP) are considered as flavor 5ʹ-Nucleotide responsible for the umami or palatable taste and 5ʹ-GMP contributed for a meaty flavor, that is a flavor enhancer much stronger than MSG.[Citation28,Citation30] Based on Yang et al.[Citation28], Flavor 5ʹ-Nucleotide was ranged from low (<1 mg/g), medium (1–5 mg/g) and high (>5 mg/g) where Flavor 5ʹ-Nucleotide of these jengkol seeds was at the first level (low). The main content of 5ʹ-Nucleotide from these jengkol seeds was 5′-AMP, 5′-GMP, and 5′-AMP, respectively. 5ʹ-AMP could also give the sweet taste and effectively inhibit the formation of bitter taste.
Equivalent umami concentration (EUC)
Changes of Equivalent Umami Concentration (EUC) in jengkol seeds after steam blanching could be seen in . The EUC value of steam jengkol was 42.22 (g/100 g dry weight) and the EUC value of without steam blanching jengkol was 38.27 (g/100 g dry weight). The EUC value of steam jengkol was 9.36% higher than without steam blanching jengkol. According to Mau,[Citation31] the EUC values of flavor components could be ranged into four levels as: (1) >1000 g MSG/100g dry weight, (2) 100–1000 g MSG/100g dry weight, (3) 10–100 g MSG/100g, and (4). It was visible that the EUC value of both without steam blanching jengkol (38.27 g MSG/100 g dry weight) was at the first level and steam jengkol (42.22 g MSG/100 g dry weight) was also at the first level.
Figure 1. Changes of the equivalent umami concentration (EUC) in Jengkol after steam blanching treatment
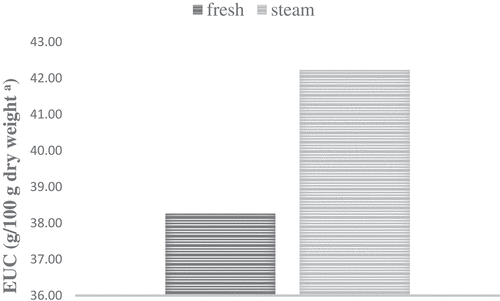
Based on Yamaguchi et al.[Citation9] stated that the umami taste could be improved by synergistic effects between flavor 5′-Nucleotides (5′-AMP, 5′-GMP and 5′–IMP) and MSG-like components (L-glutamic acid, L-aspartic acid) on jengkol seeds. The EUC value of jengkol seeds was higher than reported by Li et al.[Citation17] at those mushrooms of P. cystidiosus (13.32 g MSG/100 g dry weight) and P. eryngii (11.19 g MSG/100 g dry weight). The results have shown that jengkol seeds have a reasonably strong umami taste indicating with their equivalent umami concentration (EUC), as representing well-flavored foods and could be possible to serve as food flavoring materials and functional foods with a palatable umami taste.
Profile of amino acids
The content of amino acids in without steam blanching jengkol and steam jengkol is presented in . It could be seen that the jengkol sample contains 17 types of amino acids, where 7 of them were essential amino acids (Threonine, Valine, Methionine, Isoleucine, Leucine, Phenylalanine, and Lysine). In addition, the sample also contained eight non-essential amino acids (Aspartic acid, Alanine, Serine, Glutamine, Glycine, Cysteine, Tyrosine, and Proline) and two semi-essential amino acids (Histidine and Arginine). Lysine was one of the essential amino acids which had the highest content in the sample (1.108 − 1.310% w/w) and the lowest essential amino acid in the sample was Methionine (0.067–0.069% w/w). In semi-essential amino acids, histidine had the highest content in the sample (0.868 − 1.011% w/w), whereas in non-essential amino acids proline had the highest content (1.116 − 1.153% w/w) and the lowest was cysteine (0.019–0.031% w/w).
Table 7. The changes of amino acids in jengkol seeds after steam blanching
Generally, amino acids in jengkol before steam and after steam differed. The total amino acid content in jengkol steam was higher than in without steam blanching jengkol. Based on the research of Hadidi et al.[Citation32], Blanching steam during 4.36 min increased the protein content in alfalfa leaf from 586 to 702 g/kg dry basis. These results indicated that steam blanching could increase the amino acid content of the sample. This is thought that due to the heat present when the steam blanching, breaks down the protein sample into simple amino acids and peptides. Amino acids give important roles in both human body and animal nutrition and in the maintenance of health.
The ratio of essential amino acid to total amino acids (E/T) decreased from 35.54% to 33.92% on steam blanching (). During the steam process, the ratio decreased to 33.92%. This signifies the loss of essential amino acids during thermal processing and accessibility of total amino acids during steaming blanching of the jengkol seeds. The E/T (ratio of essential amino acid to total amino acids) values are considered to be adequate as per WHO guidelines FAO[Citation33] and Mohapatra et al.[Citation14] where values above 39% are acceptable for infants, 26% for children and 12% for adults, which means that essential amino acids on the jengkol seeds could be possible to fulfill protein intake for children and adults. This result shows that the jengkol seeds have a potential as alternative protein sources for children and adults.
Table 8. Percentage of amino acids and protein efficiency ratio (PER) in without steam blanching jengkol and steam jengkol.
The percentage of hydrophobic amino acids in without steam blanching jengkol and steamed jengkol seed powder was around 36.00% to 36.41%. Peptides consisting of hydrophobic amino acids such as Trp, Tyr, Phe, Ile, Leu, Val, Ala or Met are a known potential as bioactive peptides for ACE inhibitory.[Citation34] Phe and Leu are important amino acids for the ACE-inhibitory capacity.[Citation35] Various plants have been used as a source of ACE-inhibitory peptides such as wheat, peas, mushrooms, soybeans, walnuts, and bitter melon seeds.[Citation36–Citation38] It is possible that jengkol seed powder has potential as ACE-inhibitor because of their hydrophobic amino acid contents.
The calculated protein efficiency ratio (PER) by EquationEquations (1(1)
(1) )–(Equation3
(3)
(3) ) showed that PER values are definitely undergoing a major improvement during processing. The values increased from 2.12/2.44 to 2.36 during the steam blanching (). The calculated PER values were higher than the reported value (0.27) of steamed sorghum flour[Citation14] and higher than the reported value (0.27) of sorghum ogi.[Citation39] This indicated that in the present case, the products have better biologically available protein.
Conclusion
Based on the results of this study, the steam blanching treatment may affect the contents of non-volatile taste components and profile of amino acids in jengkol seed powder. The steam blanching treatment decreased the content of soluble sugar, organic acid, and 5ʹ-nucleotides but increased the content of free amino acids. The level on the EUC value (the equivalent of MSG) in steamed jengkol was higher than in without steam blanching jengkol, mainly due to the higher levels of aspartic acid and glutamic acid. It means that the steam blanching can increase the umami taste of jengkol seeds. The steam blanching treatment may affect the content of amino acids on jengkol seeds that increase slightly after blanching treatment. It suggested that steam blanching treatment could effectively preserve the amino acids. The steam blanching treatment was able to maintain the taste-active compounds of jengkol seed powder and also give the sweet taste and effectively inhibit the formation of bitter taste. The protein in jengkol could be also an alternative to fulfill the needs of amino acids for the human body. The jengkol seeds also have hydrophobic amino acids that have potential as ACE inhibitory so it needs to be explored more and further research will be the identification of ACE inhibitory.
Declaration of interest
All authors declare that there is no conflict of interest related to this paper.
Additional information
Funding
References
- Shukri, R.; Mohamed, S.; Mustapha, N. M.; Hamid, A. A. Evaluating the Toxic and Beneficial Effects of Jering Beans (Archidendron jiringa) in Normal and Diabetic Rats. J. Sci. Food Agric. 2011, 91, 2697–2706. DOI: 10.1002/jsfa.v91.14.
- Sridaran, A.; Karim, A. A.; Rajeev, B. Pithecellobium Jiringa Legume Flour for Potential Food Applications: Studies on Their Physico-chemical and Functional Properties. Food Chem. 2012, 130, 528–535.
- Handayani, S. Pengaruh Steam Blanching Terhadap Aktivitas Dan Senyawa Antioksidan Jengkol (Pithecellobium jiringa). Master’s thesis, Universitas Gadjah Mada, Yogyakarta, 2017.
- Lim, T. K. Edible Medicinal and Non-Medicinal Plants; Springer Science+Business Media B.V. 2012; 2, pp. 544. Springer Netherlands, Netherlands. DOI: 10.1007/978-94-007-1764-0.
- Pei, F.; Shi, Y.; Gao, X.; Wu, F.; Mariga, A. M.; Yang, W.; Zhao, L.; An, X.; Xin, Z.; Yang, F.; et al. Changes in Non-Volatile Taste Components of Button Mushroom (Agaricus bisporus) during Different Stages of Freeze Drying and Freeze Drying Combined with Microwave Vacuum Drying. Food Chem. 2014, 165, 547–554.
- Jinap, S.; Hajeb, P. Glutamate, Its Applications in Food and Contribution to Health. J. Apetite. 2010, 55, 1–10.
- Ney, K. H.;. Flavor Enhancing Effect of L-glutamate and Similar Compounds. Z. Lebensm. Unters. Forsch. 1971, 146, 141–143. DOI: 10.1007/BF01882239.
- Kuninaka, A. Flavor Potentiators. In Symposium on Foods: the Chemistry an Physiologof Flavors; Schultz, H. W., Day, E. A., Libbey, L. M., Eds.; AVI Publishing Company: Westport, 1967; pp 515–535.
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the Relative Taste Intensity of Some L-α-amino Acids and 5ʹ-nucleotides. J. Food Sci. 1971, 36, 846–849. DOI: 10.1111/j.1365-2621.1971.tb15541.x.
- Patras, A.; Tiwari, B. K.; Dan Brunton, N. P. Influence of Blanching and Low Temperature Preservation Strategies on Antioxidant Activity and Phytochemical Content of Carrots, Green Beans, and Broccoli. J. Food Sci. Technol. 2011, 44, 299–306.
- Bongoni, R.; Verkerk, R.; Steenbekkers, B.; Dekker, M.; Dan Stieger, M. Evaluation of Different Cooking Conditions on Broccoli (brassica Oleracea Var. Italica) to Improve the Nutritional Value and Consumer Acceptance. Plant Foods Human Nutr. 2014, 69, 228–234.
- Poelman, A. A. M.; Delahunty, C. M.; DeGraaf, D. C. Cooking Time but Not Cooking Method Affect Children’s Acceptance of Brassica Vegetables. Food Qual. Preferences. 2013, 28, 441–448. DOI: 10.1016/j.foodqual.2012.12.003.
- Theurer, C. B.; Huber, J. T.; Delgado-Elorduy, A.; Wanderley, R. Invited Review: Summary of Steam-flaking Corn or Sorghum Grain for Lactating Dairy Cows. J. Dairy Sci. 1999, 82, 1950–1959. DOI: 10.3168/jds.S0022-0302(99)75431-7.
- Mohapatra, D.; Patel, A. V.; Kar, A.; Dshpande, S. S.; Tripathi, M. K. Effect of Different Processing Conditions on Proximate Composition, Antioxidants, Anti-Nutrients and Amino Acid Profile of Grain Sorghum. Food Chem. 2019, 271, 129–135. DOI: 10.1016/j.foodchem.2018.07.196.
- Rennie, C.; Wise, A. Preferences for Steaming of Vegetables. J. Hum. Nutr. Diet. 2010, 23, 108–110.
- Opikasari, P. Perubahan Aktivitas Dan Senyawa Antioksidan Petai Cina (Leucaena leucocephala) Selama Perlakuan Steam Blanching. Master’s thesis, Universitas Gadjah Mada, Yogyakarta, 2017.
- Li, W.; Gu, Z.; Yang, Y.; Zhou, S.; Liu, Y.; Zhang, J. Non-Volatile Taste Components of Several Cultivated Mushrooms. Food Chem. 2014, 143, 427–431. DOI: 10.1016/j.foodchem.2013.08.006.
- Wang, L.; Renjie, X.; Bing, H.; Wei, L.; Sun, Y.; Youying, T.; Zeng, X. Analysis of Free Amino Acids in Chinese Teas and Flower of Tea Plant by High Performance Liquid Chromatography Combined with Solid-phase Extraction. Food Chem. 2010, 123, 1259–1266. DOI: 10.1016/j.foodchem.2010.05.063.
- Fisher, G. H.; Arian, I.; Quesada, S.; Danjelo, F.; Ericco, F; Difiore, M. A Fast and Sensitive Method for Measuring Picomole Levels of Total Free Amino Acids in Very Small Amounts of Biological Tissue. Amino Acids 2001, 20, 163–173.
- Biao, L.; Kimatu, B. M.; Pei, F.; Chen, S.; Feng, X.; Hu, Q.; Zhao, L. Non-volatile Flavour Components in Lentinus Edodes after Hot Water Blanching and Microwave Blanching. Int. J. Food Prop. 2017, 20, S2532–S2542. DOI: 10.1080/10942912.2017.1373667.
- Li, Q.; Zhang, H. H.; Claver, I. P.; Zhu, K. X.; Peng, W.; Zhou, H. M. Effect of Different Cooking Methods on the Flavour Constituents of Mushroom (agaricus Bisporus (lange) Sing) Soup. Int. J. Food Sci. Technol. 2011, 46, 1100–1108. DOI: 10.1111/j.1365-2621.2011.02592.x.
- Saldivar, X.; Wang, Y.-J.; Chen, P.; Mauromoustakos, A. Effects of Blanching and Storage Conditions on Soluble Sugar Contents in Vegetable Soybean. Food Sci. Technol. 2010, 43, 1368–1372.
- Handschumacher, R.;. Orotidylic Acid Decarboxylase: Inhibition Studies With Azauridine 50 -Phosphate. J. Biol. Chem. 1960, 10, 2917–2919.
- Sowalsky, R. A.; Noble, A. C. Comparison of the Effects of Concentration, pH and Anion Species on Astringency and Sourness of Organic Acids. Chem. 1998, 23, 343–349.
- Yoneda, C.; Okubo, K.; Kasai, M.; Hatae, K. Extractive Components of Boiled-Dried Scallop Adductor Muscle and Effect on the Taste of Soup after Mixing with Chicken Leg Meat. J. Sci. Food Agric. 2005, 85, 809–816. DOI: 10.1002/(ISSN)1097-0010.
- Mau, J.-L.; Lin, H.-C.; Ma, J.-T.; Song, S.-F. Non-volatile Taste Components of Several Speciality Mushrooms. Food Chem. 2001, 73, 461–466. DOI: 10.1016/S0308-8146(00)00330-7.
- Tsai, S. Y.; Wu, T. P.; Huang, S. J.; Mau, J. L. Nonvolatile Taste Components of Agaricus Bisporus Harvested at Different Stages of Maturity. Food Chem. 2007, 103, 1457–1464. DOI: 10.1016/j.foodchem.2006.10.073.
- Yang, J.; Lin, H.; Mau, J. Non-Volatile Taste Components of Several Commercial Mushroom. Food Chem. 2001, 72, 465–471. DOI: 10.1016/S0308-8146(00)00262-4.
- Boekel, M.; Formation of Flavour Compounds in the Maillard Reaction. Biotechnol. Adv. 2006, 24, 230–233.
- Litchfield, J.; Morel Mushroom Mycelium as a Food-Flavoring Material. Biotechnol. Bioeng. 1967, 9, 289–304. DOI: 10.1002/(ISSN)1097-0290.
- Mau, J. L.; The Umami Taste of Edible and Medicinal Mushrooms. Int. J. Med. Mushrooms. 2005, 7, 119–126. DOI: 10.1615/IntJMedMushr.v7.i12.120.
- Hadadi, M.; Ibarz, A.; Conde, J.; Pagan, J. Optimisation of Steam Blanching On Enzymatic Activity, Color And Protein Degradation of Alfalfa (Medicago Sativa) to Improve Some Quality Characteristics of Its Edible Protein. Food Chem. 2019, 276, 591–598.
- FAO WHO UNU Energy and Protein Requirement. WHO Technical Report Series No. 724; WHO: Geneva, Switzerland, 1985.
- Arnold, U.; Rucknagel, K. P.; Schierhorn, A.; Ulbrich-Hofmann, R. Thermal Unfolding and Proteolytic Susceptibility of Ribonuclease A. Eur. J. Biochem. 1996, 237, 862–869. DOI: 10.1111/ejb.1996.237.issue-3.
- Sornwatana, T.; Bangphoomi, K.; Roytrakul, S.; Wetprasit, N.; Choowongkomon, K.; Ratanapo, S. Chebulin, and Terminalia Chebula Retz. Fruit-Derived Peptide with Angiotensin-I-Converting Enzyme Inhibitory Activity. Biotechnol. Appl. Biochem. 2015, 62, 746–753.
- Thewissen, B. G.; Pauly, A.; Celus, I.; Brijs, K.; Delcour, J. A. Inhibition of Angiotensin I-Converting Enzyme by Wheat Gliadin Hydrolysates. Food Chem. 2011, 127, 1653–1658.
- Qu, W.; Ma, H.; Jia, J.; He, R.; Luo, L.; Pan, Z. Enzymolysis Kinetics and Activities of ACE-Inhibitory Peptides from Wheat Germ Protein Prepared with SFP Ultrasound-Assisted Processing. Ultrason. Sonochem. 2012, 19, 1021–1026.
- Gu, Y.; Wu, J. LC-MS/MS Coupled with QSAR Modeling in Characterising of Angiotensin I-Converting Enzyme Inhibitory Peptides from Soybean Proteins. Food Chem. 2013, 141, 2682–2690.
- Oyarekua, M. A.; Eleyinmi, A. F. Comparative Evaluation of the Nutritional Quality of Corn, Sorghum and Millet Ogi Prepared by a Modified Traditional Technique. J. Food Agric. Environ. 2004, 2, 94–99.
