?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Rumex hastatus being used for medicinal and nutritional purposes as a functional food in various countries is hereby evaluated by the gas chromatography-mass spectroscopy, proximate analysis, physicochemical (fluorescence) analysis, quantitative analysis of secondary metabolites and sensory evaluations studies. Various samples of R. hastatus were also evaluated for in vitro anti-diabetic potential. The investigational study demonstrated that R. hastatus is a rich source of carbohydrate, i.e., 432.4 mg/g. Moisture content, protein, fiber, ash content and fats were recorded as 22.8%, 133.9 mg/g, 124.4 mg/g, 54.5 mg/g and 25.6 mg/g, respectively. In the same way, the secondary metabolite displayed a relatively greater amount of flavonoids (84.5 mg/g) followed by saponins (65.5 mg/g) and alkaloids (49.5 mg/g). Similarly, the GC (FID-MS) analysis of R. hastatus revealed the detection of 120 compounds. Out of those identified compounds, selected anti-diabetic compounds were sorted out, viz butyl phthalate, phytol, ethylthreonine, dihydrobenzofuran, indoline, guanidine, nerolidol, myristic acid, palmitic acid, caryophyllene, anozol. In physicochemical fluorescence analysis and the sensory evaluation, data were also recorded along with the anti-diabetic with IC50 value of 42.09 µg/ml. The overall investigational analysis of R. hastatus obviously demonstrated that this plant was a rich source of primary and secondary metabolites. It may be concluded from the GC (FID-MS) analysis that R. hastatus is a potential source of anti-diabetic constituents, which may confer hypoglycemic potential. Based on the recorded data it may also be inferred that R. hastatus is among safe and nutritious herbs, which can be used in lieu of green vegetables and functional food with anti-diabetic potential.
Introduction
Globally the advanced researchers are in continuous struggle to combat with various challenging diseases, to fabricate more and more nutritional commodities, to figure out novel resources and to facilitate the mankind.[Citation1,Citation2] One of the most critical and focused issue is the nutritional crunch throughout the world. A wholesome portion of population goes into the famine, malnutrition and drought calamities each year. In the same way people get various physiological anomalies due to starvation and nutritional deficiency.[Citation3] To cope with such calamities, the agronomists are getting focused on various procedures to enhance the agricultural outcome.[Citation4] Plants are considered as staple by majority of world’s population. The people of those areas wholly and solely depend on herbs, where the access of sophisticated techniques of getting multiple nutritional products is not developed.[Citation5] As reported by several investigators, the plants consist of primary metabolites, secondary metabolites and micronutrients.[Citation6–Citation10] But the difference is that each plant species differs in the amount and concentration of these substances. The plants rich in primary metabolites are usually used for nutritional purposes, while secondary metabolites are preferred for medicinal purposes.[Citation6,Citation11,Citation12] The plants may possess fats, protein, carbohydrates, vitamins and minerals in such quantity that are considered sufficient for our daily requirements. One can use specific species of plant as food that possess required amount of primary metabolites and low amount of secondary metabolites. But the plants that possess high amount of secondary metabolites than the primary metabolites can cause serious health complications.[Citation13,Citation14] So this is necessary to figure out the amount of nutritional and medicinal elements in those plants, which are being used as food by people. The nutritional values of various plants have been reported by different investigators.[Citation15,Citation16]
Diabetes mellitus is a metabolic disorder characterized by increase in blood glucose level. It can affect individuals at any stage of life but the frequency of diabetes is considerably high among the obese and aged people.[Citation17] Various therapeutic measures are employed to alleviate the symptoms of this disease. One of the effective therapeutic measures is to decrease the absorption of glucose from the intestine. Therefore, the absorption of glucose from the intestine can be decreased effectively by α_glucosidase inhibition. Various plants have been reported to possess the α_glucosidase inhibition potential.[Citation18]
Rumex hastatus belongs to the family Polygonaceae that consist of 48 genera and 1,200 species.[Citation19] The Rumex genus consists of about 200 species, among which a wide variety has been used for medicinal purposes.[Citation20] The R. dentatus, R. maritimus, R. bucephalophorus, R. nervosus, R. abyssinicus and many more have been used for medicinal purposes including diabetes mellitus.[Citation18,Citation21–Citation25] The R. vesicarius has been analyzed for nutritional value and recommended good and safe for nutritional purposes.[Citation26] R. crispus, R. obtusifolius and R. acetosa has been used as vegetable and fodder since long.[Citation27–Citation29] In the same way R. hastatus has been used ethnomedicinally for various ailments including GIT ailments, cuts, wounds, bleedings, as appetizer, as anthelmintic, in snake bites, blood pressure, tonsillitis, sore throat, as flavoring agent, carminative and diuretic.[Citation30–Citation35] R. hastatus has been reported by several investigators to possess high quantity of flavonoids.[Citation36] Flavonoids consist of wide variety of compounds with excellent anti-diabetic potential in vivo as well as in vitro.[Citation37–Citation39] R. hastatus has also been reported to possess anthelmintic, cytotoxic, phytotoxic, antibacterial, anticholinesterase and antioxidant potentials.[Citation36,Citation40] The R. hastatus has also been used as fodder for animals. In Pakistan R. hastatus has been used as vegetable in the northern Himalayan areas.[Citation41]
Going to the detailed literature survey of R. hastatus, it is obvious that this plant has been used for multiple purposes. So the current study was arranged to evaluate the R. hastatus for its nutritional value and sort out bioactive compounds and provide an implication regarding the use of this plant for nutritional purpose.
Materials and methods
Plant’s collection
The aerial part of R. hastatus was collected from the hilly area of Gorha Gat in the proximity of University of Malakand, Chakdara, Dir (L), KPK, Pakistan. The plant identification was performed by Dr. Ali Hazrat (plant taxonomist) and deposited in the Department of Botany, Shaheed Benazir Bhutto University, Sheringal, Dir upper, KPK, Pakistan, with voucher number (1015SJ). All the extra particles were carefully removed from the plant material and were spread on neat paper in a room in the shade and appropriately dried for two weeks. The paper was changed daily to avoid fungal growth on plant material. After shade drying the plant was pulverized using cutter mill.[Citation42]
Extraction and fractionation
The powdered plant sample was soaked in 80% methanol for a period of 8 days followed by filtration. The filtrate obtained was evaporated at low temperature under reduced pressure using rotary evaporator. The evaporation resulted in a semisolid mass, i.e., crude methanolic extract (Rh.Cr).
Some of the Rh.Cr was kept for activities and the remaining was suspended in sufficient amount of water followed by fractionation with various solvents in separating funnel. The fractionation was started with less polar n-hexane (500 mL × 3), then chloroform (500 mL × 3), ethyl acetate (500 mL × 3) and final fraction obtained was aqueous.[Citation9] Similarly, the fractions obtained were n-hexane (Rh.Hex), ethyl acetate (Rh.EtAc), chloroform (Rh.Cf) and aqueous fraction (Rh.Aq).
Gas chromatography-flame ionization detector (GC-FID) analysis
The GC-FID analysis of Rh.Cr was carried out with the help of gas chromatograph Agilent USB-393752 (Agilent Technologies, Palo Alto, CA, USA) via HHP-5MS (5%) phenylmethylsiloxane capillary column (30 m × 0.25 mm × 0.25 μm film thickness; Restek, Bellefonte, PA) attached with FID detector. The oven was allowed to set at temperature of 70°C for a minute and then augmented to 180°C at the rate of 6°C/min for the period of five minutes and lastly to 280°C at the rate of 5°C/min for a period of 20 min. The temperature of detector and injector were maintained at 290°C and 220°C correspondingly. The flow rate of carrier gas (Helium) was maintained as 1 mL/min and the diluted samples (1/1,000 in n-pentane, v/v) of 1 μL were injected manually in the split-less mode.
Gas chromatography–mass spectrometry (GC-MS) analysis
The -F/MS of Rh.Cr was performed via USB-393752 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) with a HHP-5MS 5% phenylmethylsiloxane capillary column (30 m × 0.25 mm × 0.25 μm film thickness; Restek, Bellefonte, PA) outfitted with an Agilent HP-5973 mass selective detector in the electron impact mode (Ionization energy: 70 eV) working under the experimental conditions as those maintained for GC.
Identification of components
All the major constituents of Rh.Cr were identified by the comparison of their retention time with the literature of genuine compounds. The identification of compounds was further carried out with the help of the spectral data obtained from the libraries of Wiley and NIST as well as their fragmentation patterns and comparisons of the mass spectra with data reported in literature or with those of mass spectra from literature.[Citation43,Citation44] Each process was carried twice.
Proximate analysis
Proximate analysis of powdered plant sample was carried out following the standard procedure of Association of Official Analytical Chemist.[Citation45]
Moisture content
Loss on drying (LOD) method was followed for the determination of moisture content of the plant sample. A weighed quantity of powdered plant sample was taken in a suitable container and allowed to dry at 105°C in oven till the achievement of constant weight. Thus the amount of moisture present in the powdered plant sample was figured out from the difference of dried weight of sample and the total weight of the sample.
Ash content
Incineration procedure was followed for determination of ash content of powdered plant sample. A weighed amount of sample was put in a crucible and transferred into the muffle furnace and allowed to incinerate at 550°C for 24 h. Similarly, total ash content was figured out after conversion of dried mass of powdered plant sample into ashes.
Crude fats
Soxhlet method was followed for the determination of total fats in the sample. Briefly, 2 g of dried powdered plant sample was transferred into a Soxhlet extractor and petroleum ether was added to the flask of the extractor. The extraction was carried out for 6 h till the exhaustion of sample from fat content. The obtained petroleum ether was filtered and the filtrate obtained was allowed to be evaporated in a weighed beaker. The total fats were calculated as the total increase in weight of the beaker.
Crude fibers
The value of crude fiber was figured out from the data of loss in weight after the ignition of dried samples remaining after digestion of fat-free samples with 1.25% each of sulfuric acid and sodium hydroxide solution under specified conditions.
Crude protein
For the determination of crude proteins, the method of micro Kjeldahl nitrogen method was followed. This method involved the digestion of plant sample with concentrated sulfuric acid and catalyst for the conversion of organic nitrogen into ammonium sulfate in the solution. After which the decomposition of ammonium sulfate was carried out via NaOH. The liberated ammonia was distilled into 5% boric acid. After this the titration of trapped ammonia was carried out with 0.05 N HCl for the deduction of nitrogen from ammonia. The indicators used were methylene red and blue both. The percent proteins were calculated from the value of nitrogen obtained multiplied by 6.25.
Carbohydrate content
The total crude carbohydrate content was determined by the subtraction formula. In short, the total protein, total fiber, ash content, moisture content and total lipids were subtracted from the dried mass and the total carbohydrates were calculated.
Secondary metabolites
Alkaloids
The alkaline precipitation gravimetric method was followed to find out alkaloids. A weighed powdered plant sample was taken in a beaker and 10% acetic acid solution in ethanol was transferred into the beaker. The mixture was incubated at 28°C for 4 h. Then it was filtered through Whatman No. 42 filter paper. The filtrate was allowed to be evaporated to one quarter of its original volume followed by the addition of drop-wise NH4OH for the precipitation of alkaloids. The precipitated alkaloids were received on filter paper and washed with 1% ammonia solution and then dried in an oven at 80°C. So the amount of alkaloids was calculated per gram of the dried powdered sample of R. hastatus.
Flavonoids
For the extraction of flavonoids, the procedure of Harborne was followed.[Citation46] Plant sample (5 g) was taken and boiled in 50 mL of 2 M HCl under reflux for 30 min. It was cooled and filtered using Whatman No. 42 filter paper. The extract was treated with equal volume of ethyl acetate. The flavonoids present in the extract were precipitated which were recovered with the help of weighed filter paper. The amount of flavonoids recovered on filtered paper was calculated.
Saponins
For the determination of crude saponins in powdered sample of R. hastatus, 20 g of powdered sample was put in a conical flask and 100 mL of 20% ethanol was added to the conical flask. The sample allowed to heat at 55°C in the water bath for 4 h with continuous stirring. After 4 h the sample was filtered and the residue was re-extracted with 200 mL of 20% ethanol. The sample after extraction was allowed to heat until a concentrated volume of 40 mL was obtained. The sample obtained was shifted into a separating funnel and 20 mL of diethyl ether was added to it. After vigorous shaking, the separating funnel was put in a stand to get two layers. The lower aqueous layer was collected while the upper diethyl ether layer was discarded. The aqueous layer obtained was diluted with 60 mL of n-butanol and the combined n-butanol extract was washed with 10 mL of 5% saline. The final solution obtained was kept in a hot water bath until complete evaporation and the saponins obtained were dried in an oven and the saponins per gram of powdered plant sample was calculated.[Citation40]
Fluorescence analysis
The powdered plant sample was treated with different chemical reagents for the determination of fluorescence characters. The plant sample was put in small quantity on glass slide and treated with various reagents followed by the determination of color under the visible light and ultraviolet light.
Sensory evaluation
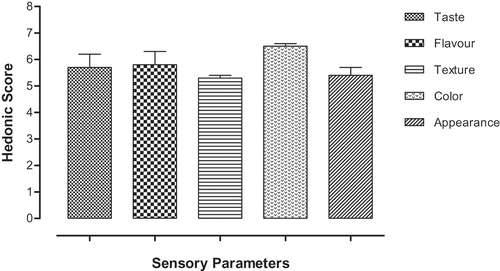
The sensory evaluation of R. hastatus was performed based on the Hedonic Scale following AOAC procedure.[Citation47] Various parameters were assayed such as color, flavor, taste, general appearance and texture. The evaluation was performed based on the questionnaire to score out of seven points of hedonic scale.
α-Glucosidase inhibition assay
α-Glucosidase inhibitory activity was carried out following chromogenic assay.[Citation48] Enzyme solution was prepared having 0.5 unit/mL and 20 μL of this solution was mixed 120 μL of phosphate buffer having pH 6.9. p-nitrophenyl- α-D-glucopyranoside solution (5 mM) was prepared in the same buffer that was employed as substrate. Briefly, 10 μL of test samples having various concentrations (31.25–1,000 μL) were added to them and kept for 15 min at 37°C. After incubation, 20 μL of substrate solution was added to all of them and incubated for further 15 min. Sodium carbonate solution (0.2 M) having volume of 80 μL was added to them to terminate the reaction. Absorption of each sample was measured at 405 nm via double beam spectrophotometer (Thermo electron corporation USA). The reaction mixture with the test sample served as control while acarbose served as positive control. The percent enzyme inhibitory potential was calculated as;
Statistical analysis
The statistical analysis was carried out by Two-way ANOVA followed by Bonferroni post test, in which the positive control was compared with the groups of test samples. P values less than or equal to 0.05 were considered as significant statistically. GraphPad Prism and XL sheet were employed to draw the graphs and IC50 values. The standard error mean (SEM) were calculated at 95% confidence intervals.
Results
Proximate composition
In the proximate analysis, the powdered sample of R. hastatus exhibited high percentage of carbohydrate as compared to the protein, fats, fibers and minerals. The proximate analysis has been summarized in . represents the percent of protein along with various parameters in the powdered sample of R. hastatus. Each of the analysis was performed in triplicates and the percentage of each ingredient is approximately the same in the results. The percent proteins were detected as 13.4 ± 0.7% (133.9 mg/g), which demonstrate that R. hastatus is rich in proteins. Similarly, in the percent ash content is summarized. The ash content shows adequate amount of minerals in the plant sample, i.e., 54.5 mg/g. In the same way, the percent fats analysis was also performed, which is summarized in . The percentage of fats is comparatively less from the other components, i.e., 25.6 mg/g. The powdered sample was also assessed for the percent moisture content, which is summarized in . Though the powdered sample was dried for 2 weeks prior to Loss on drying procedure, but still the amount of moisture trapped in the tissues of plant was considerably sufficient, i.e., 22.9 ± 1.4%. Moreover, the percent fiber and carbohydrates are summarized in , which represent a wholesome amount of carbohydrates in the sample of R. hastatus. As far as the percent fiber is concerned, it also goes parallel with the percent proteins, i.e., 133.9 mg/g. The proximate analysis shows that the carbohydrate was in high quantity, i.e., more than 40% followed by the moisture content, proteins, fibers, ashes and then fats.
Table 1. Proximate and phytochemical analysis of samples of Rumex hastatus.
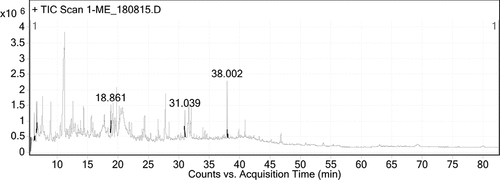
GC (FID-MS) analysis
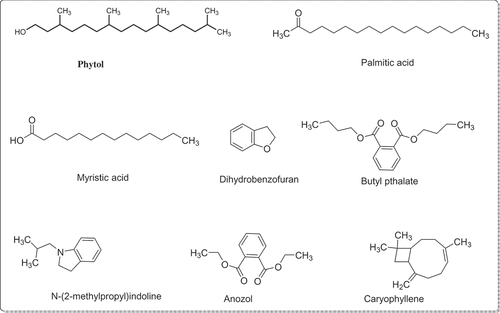
The GC (FID-MS) analysis of R. hastatus revealed the identification of 120 compounds. The literature review of the identified compounds was performed, in which several compounds previously reported to possess anti-diabetic potential were sorted out. The bioactive compounds sorted out were butyl phthalate, phytol, ethylthreonine, dihydrobenzofuran, indoline, guanidine, nerolidol, myristic acid, palmitic acid, caryophyllene, anozol. The anti-diabetic literature of these compounds is summarized in the discussion section.
Saponins, flavonoids and alkaloids
The total saponins, flavonoids and alkaloids have been summarized in . The data is represented in the terms of percentage and mg/g of the powdered sample. The table shows that the flavonoids are comparatively in greater amount, i.e., 84.3912 (8.4391%), 81.8865 (8.1886%) and 87.3657 (8.7365%) mg/g in test 1, 2 and 3, respectively. Similarly, the saponins were 6.57321%, 6.31794% and 6.75507% in test 1, 2, and 3, respectively. As far as the alkaloids are concerned, this plant possess normal amount of them, i.e., 4.7973%, 5.1443% and 4.9001% in test 1, 2 and 3, respectively. The percent of secondary metabolites present in R. hastatus is highest for flavonoids following saponins and alkaloids.
Table 2. Fluorescence analysis of powdered sample of Rumex hastatus under visible and UV lights
Fluorescence analysis
The fluorescence characteristics of powdered sample of R. hastatus were studied under ordinary visible light and ultraviolet light. The powdered sample was treated with various reagents in on a slide on bench top and studied for the color under UV and visible lamps. The powdered sample upon treatment with any reagent gave grayish brown color in visible light and brown color under UV lamp. The powdered sample treated with FeCl3 exhibited grayish black color in visible light and yellowish gray color under UV lamp. Similarly, the plant sample was treated with variety of chemical reagents, i.e., HCl, HNO3, K2Cr2O7, H2SO4, Br2, H2O2, CCl4, CH3OH, CH3COOH, xylene, NH3 and I2. All the reagents displayed different colors in different environments. All the results obtained have been summarized in .
Table 3. α-Glucosidase inhibitory potential of various samples of Rumex hastatus.
Sensory evaluation
The results of sensory evaluations have been summarized in . The highest value was shown by the color, i.e., 6.5 followed by the 5.8 that of flavor and the least value has been shown by texture, i.e., 5.3. The overall sensory evaluation reveals the mean value of 5.74 ± 0.211.
α-Glucosidase inhibitory effect
The α-glucosidase inhibition assay revealed marked activity of various samples of R. hastatus (). Among the test samples Rh.Chf exhibited highest activity exhibiting 49.61 ± 1.70, 55.17 ± 1.40, 61.90 ± 0.52, 64.00 ± 1.15, 71.43 ± 0.90 and 77.17 ± 1.40 at the concentrations of 31.25, 62.5, 125, 250, 500 and 1,000 µg/mL, respectively with IC50 value of 42.09 µg/mL. Similarly, flavonoids and saponins also demonstrated significant enzyme inhibition with IC50 values of 88.74 and 104.96 µg/mL, respectively. The rest of sample also demonstrated enzyme inhibition potential up to considerable extent. The overall activity was recorded as dose dependent response.
Table 4. List of compounds in GC (FID-MS) of crude methanolic extract of Rumex hastatus.
Discussions
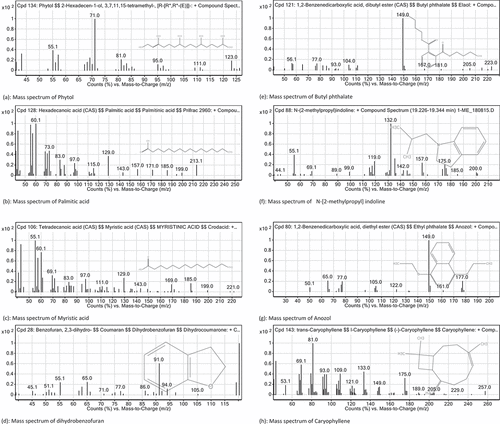
The GC (FID-MS) analysis of methanolic extract of R. hastatus revealed the presence of 120 compounds summarized in and the chromatogram in . The literature survey of these compounds showed the presence of several reported anti-diabetic compounds given in . The bioactive compounds sorted out were butyl phthalate, phytol, ethylthreonine, dihydrobenzofuran, indoline, guanidine, nerolidol, myristic acid, palmitic acid, caryophyllene, anozol. Butyl phthalate is one of the constituent identified in the sample of R. hastatus, and one of the closely similar structure, i.e., butyl iso-butyl phthalate has previously been published with significant α-glucosidase inhibition potential.[Citation49] R. hastatus also reveals the presence of medicinally important compound, i.e., phytol, which possess antidiabetic effect due activation of nuclear receptors and heterodimerization with PPARγ.[Citation50] This plant also possess guanidine derivatives that are potentially antidiabetic compounds.[Citation51] Insulinotropic polypeptides contains threonine and thylthreonine is also an identified constituent in the GC (FID-MS) analysis of R. hastatus.[Citation52] Benzoic acid derivatives have been reported with antidiabetic potential and benzamides are getting fame with good results.[Citation53] Dihydrobenzofuran is also one of the constituent of R. hastatus that possess antidiabetic properties.[Citation54] In the same way, indoline derivatives are also effective antidiabetic compounds, which have been identified in the sample of R. hastatus.[Citation55] The Momordica charantia (bitter gourd) has been demonstrated with significant antidiabetic activity and it has been reported with high percentage of Nerolidol.[Citation56] The GC (FID-MS) analysis of R. hastatus also reveals the presence of fatty acids, i.e., Myristic acid and palmitic acid and GPR-40 “fatty acid receptors” have been identified on the B-cells of pancrease that are responsible for insulin secretion.[Citation57] Caryophyllene has also been reported with notable antidiabetic activity.[Citation58] Benzoic acid derivatives have also been reported with significant hypoglycemic effect and the GC (FID-MS) of R. hastatus also contains Anozol, which is benzoic acid ester. The MS spectra of bioactive compounds of R. hastatus have been given in .
To evaluate the nutritional value of certain green vegetables, the sensory evaluation of that specific food should be performed on priority basis. The sensory evaluation can give a specific value from hedonic scale, which represents the mean acceptance and nutritional esthetics of certain vegetables. Going to the results of hedonic scale evaluation, the mean value calculated for R. hastatus was 5.74 ± 0.211 out of 7.00, which represents that this plant possesses an acceptable place in the group of green vegetables. Similarly, the proximate value of certain species can decide their use as food based on their nutritional value. Proximate analysis is performed for dietary fiber, carbohydrates, fats, protein, moisture content and ash content. Each of the parameter has its one vital function and nutritional benefits for human beings and other animals. Dietary fiber can absorb huge amount of water and make the stool soft to get easily out of alimentary canal, so in this way dietary fiber can relieve hemorrhoids and constipation. Fiber has also been reported to alleviate obesity, diabetes and cancer.[Citation59–Citation62] As far as the total fats are concerned, this plant contains 2.5% of fats and can be considered as balanced when taken in lieu of milk that contain 3.5–5% fats sufficient for daily requirements as excess cause atherosclerosis other complications.[Citation63–Citation65] In the same way, the deficiency of protein intake can cause various pathological disorders, including lung diseases, cardiac disorders, neurological disorders and cancer.[Citation66–Citation68] The carbohydrates, being the major portion of plant and the most abundant biomolecule in the universe help in the energy production and some percent of our body mass. It is obvious from the results that R. hastatus is a good source of carbohydrates, i.e., > 40% which meet the daily body requirement. In the same way, though the moisture content is high, i.e., above 20% and high moisture content degrade the bioactive compounds by increasing the microbial growth but still this plant can be protected from microbial growth by various other factors like viscosity, micronutrients, etc.[Citation69]
As far as the secondary metabolite are concerned, it is obvious from the results that R. hastatus is also a good source of secondary metabolites, especially the flavonoids, alkaloids and saponins. The percent of these secondary metabolites cannot affect the physiological processes in the human body, because this plant is also cooked as a vegetable and the majority of alkaloids and saponins are degraded by high temperature.[Citation70–Citation72] Nonetheless, these secondary metabolites have also been reported to possess antimicrobial, antioxidant and other beneficial effects.[Citation36,Citation73–Citation76] Moreover, the florescence analysis of the samples of R. hastatus revealed various colors under different wavelengths. The fluorescence analysis is a significant system of identification of powdered drugs with their particular references.[Citation77]
α-Glucosidase enzyme is responsible for the breakdown of large molecules of carbohydrate to glucose units. To target this enzyme may alleviate the blood glucose level in diabetic patients by decreasing the absorption of glucose from the intestine. Plethora of plants have been reported to possess multiple compounds responsible for α-glucosidae inhibition, which may be used to alleviate the symptoms of diabetes mellitus.[Citation39] As discussed earlier, the R. hastatus has been used by multiple communities for variety of ailments and various species of this genus has been reported to possess anti-diabetic potential.[Citation18,Citation25] The current investigational study reveals that R. hastatus contains variety of compounds responsible for in vitro inhibition of α-glucosidase.
Conclusion
It may be inferred from the current investigational studies that Rumex hastatus is a good source of basic nutritional components along with secondary metabolites. It can meet the basic needs of human body for energy production, growth and other vital functions. Furthermore, this plant possesses marked in vitro anti-diabetic potential, so it may be a possible remedy for the management of diabetes mellitus. R. hastatus can also be used as vegetable when conventional vegetables are scarce, unavailable or expensive.
Acknowledgments
The authors are thankful to Dr. Ali Hazrat for identification of the plant. The authors declare that they have no competing interests.
References
- Hafer, C. L.; Begue, L. Experimental Research on Just-world Theory: Problems, Developments, and Future Challenges. Psychol. Bull. 2005, 131(1), 128. DOI: 10.1037/0033-2909.131.1.128.
- Sadiq, A.; Mahmood, F.; Ullah, F.; Ayaz, M.; Ahmad, S.; Haq, F. U.; Khan, G.; Jan, M. S. Synthesis, Anticholinesterase and Antioxidant Potentials of Ketoesters Derivatives of Succinimides: A Possible Role in the Management of Alzheimer’s. Chem. Cent. J. 2015, 9(1), 31. DOI: 10.1186/s13065-015-0107-2.
- Holick, M. F.; Chen, T. C. Vitamin D Deficiency: A Worldwide Problem with Health Consequences. Am. J. Clin. Nutr. 2008, 87(4), 1080S–1086S. DOI: 10.1093/ajcn/87.4.1080S.
- Magdoff, F. The World Food Crisis: Sources and Solutions. Mon. Rev. New York. 2008, 60(1), 1. DOI: 10.14452/MR-060-01-2008-05.
- Qureshi, R.; Bhatti, G. R.; Memon, R. A. Ethnomedicinal Uses of Herbs from Northern Part of Nara Desert, Pakistan. Pak. J. Bot. 2010, 42(2), 839–851.
- Croteau, R.; Kutchan, T. M.; Lewis, N. G. Natural Products (secondary Metabolites). Biochem. Mol. Biol. Plants. 2000, 24, 1250–1319.
- Gershenzon, J.; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 2007, 3(7), 408–414. DOI: 10.1038/nchembio.2007.5.
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Khan, M. A.; Ahmad, W.; Shah, M. R.; Imran, M.; Ahmad, S. Comparative Chemical Profiling, Cholinesterase Inhibitions and Anti-radicals Properties of Essential Oils from Polygonum Hydropiper L: A Preliminary anti-Alzheimer’s Study. Lipids Health Dis. 2015, 14(1), 141. DOI: 10.1186/s12944-015-0145-8.
- Ayaz, M.; Junaid, M.; Subhan, F.; Ullah, F.; Sadiq, A.; Ahmad, S.; Imran, M.; Kamal, Z.; Hussain, S.; Shah, S. M. Heavy Metals Analysis, Phytochemical, Phytotoxic and Anthelmintic Investigations of Crude Methanolic Extract, Subsequent Fractions and Crude Saponins from Polygonum Hydropiper L. BMC Complementary Altern. Med. 2014, 14(1), 465. DOI: 10.1186/1472-6882-14-465.
- Ayaz, M.; Junaid, M.; Ahmed, J.; Ullah, F.; Sadiq, A.; Ahmad, S.; Imran, M. Phenolic Contents, Antioxidant and Anticholinesterase Potentials of Crude Extract, Subsequent Fractions and Crude Saponins from Polygonum Hydropiper L. BMC Complementary Altern. Med. 2014, 14(1), 145. DOI: 10.1186/1472-6882-14-145.
- Shah, S.; Shah, S. M. M.; Ahmad, Z.; Yaseen, M.; Shah, R.; Sadiq, A.; Khan, S.; Khan, B. Phytochemicals, in Vitro Antioxidant, Total Phenolic Contents and Phytotoxic Activity of Cornus Macrophylla Wall Bark Collected from the North-West of Pakistan. Pak. J. Pharm. Sci. 2015, 28(1), 23–28.
- Shah, S. M. M.; Sadiq, A.; Shah, S. M. H.; Khan, S. Extraction of Saponins and Toxicological Profile of Teucrium Stocksianum Boiss Extracts Collected from District Swat, Pakistan. Biol. Res. 2014, 47(1), 65. DOI: 10.1186/0717-6287-47-65.
- Wiedenfeld, H.; Edgar, J. Toxicity of Pyrrolizidine Alkaloids to Humans and Ruminants. Phytochem. Rev. 2011, 10(1), 137–151. DOI: 10.1007/s11101-010-9174-0.
- Wiedenfeld, H. Plants Containing Pyrrolizidine Alkaloids: Toxicity and Problems. Food Addit. Contam. 2011, 28(3), 282–292. DOI: 10.1080/19440049.2010.541288.
- Jaafar, R. A.; Ahmad Ridhwan, A.; Zaini, N.; Vasudevan, R. Proximate Analysis of Dragon Fruit (hylecereus Polyhizus). Am. J. Appl. Sci. 2009, 6(7), 1341–1346. DOI: 10.3844/ajassp.2009.1341.1346.
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M. L.; Mortensen, A. G.; Laursen, B. B.; Lin, L.-W.; De León-Rodríguez, A.; Fomsgaard, I. S., De La Rosa A.P.B. Proximate Composition, Phenolic Acids, and Flavonoids Characterization of Commercial and Wild Nopal (opuntia Spp.). J. Food Compost. Anal. 2010, 23(6), 525–532. DOI: 10.1016/j.jfca.2009.12.003.
- Mellitus, D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2005, 28, S37. DOI: 10.2337/diacare.28.suppl_1.S37.
- Ha, B. G.; Yonezawa, T.; Son, M. J.; Woo, J. T.; Ohba, S.; Chung, U. I.; Yagasaki, K. Antidiabetic Effect of Nepodin, a Component of Rumex Roots, and Its Modes of Action in Vitro and in Vivo. BioFactors. 2014, 40(4), 436–447. DOI: 10.1002/biof.1165.
- Yasmin, G.; Khan, M. A.; Shaheen, N. Pollen Morphology of Selected Polygonum L. Species (polygonaceae) from Pakistan and Its Taxonomic Significance. Pak. J. Bot. 2010, 42(6), 3693–3703.
- Jimoh, F.; Adedapo, A.; Aliero, A.; Afolayan, A. Polyphenolic Contents and Biological Activities of Rumex Ecklonianus. Pharm. Biol. 2008, 46(5), 333–340. DOI: 10.1080/13880200801887765.
- Rouf, A.; Islam, M.; Rahman, M. Evaluation of Antidiarrhoeal Activity Rumex Maritimus Root. J. Ethnopharmacol. 2003, 84(2), 307–310. DOI: 10.1016/s0378-8741(02)00326-4.
- Liu, S.; Sporer, F.; Wink, M.; Jourdane, J.; Henning, R.; Li, Y.; Ruppel, A. Anthraquinones in Rheum Palmatum and Rumex Dentatus (polygonaceae), and Phorbol Esters in Jatropha Curcas (euphorbiaceae) with Molluscicidal Activity against the Schistosome Vector Snails Oncomelania, Biomphalaria, and Bulinus. Trop. Med. Int. Health. 1997, 2(2), 179–188. DOI: 10.1046/j.1365-3156.1997.d01-242.x.
- Kerem, Z.; Bilkis, I.; Flaishman, M. A.; Sivan, L. Antioxidant Activity and Inhibition of α-Glucosidase by trans-Resveratrol, Piceid, and a Novel trans-Stilbene from the Roots of Israeli Rumex Bucephalophorus L. J. Agric. Food Chem. 2006, 54(4), 1243–1247. DOI: 10.1021/jf052436+.
- Getie, M.; Gebre-Mariam, T.; Rietz, R.; Höhne, C.; Huschka, C.; Schmidtke, M.; Abate, A.; Neubert, R. Evaluation of the Anti-microbial and Anti-inflammatory Activities of the Medicinal Plants Dodonaea Viscosa, Rumex Nervosus and Rumex Abyssinicus. Fitoterapia. 2003, 74(1), 139–143.
- Baluchnejadmojarad, T.; Roghani, M. Chronic Rumex Patientia Seed Feeding Improves Passive Avoidance Learning and Memory in Streptozotocin-Diabetic Rats. Basic Clin. Neurosci. 2010, 1(4), 53–56.
- Alfawaz, M. A. Chemical Composition of Hummayd (rumex Vesicarius) Grown in Saudi Arabia. J. Food Compost. Anal. 2006, 19(6), 552–555. DOI: 10.1016/j.jfca.2004.09.004.
- Yildirim, A.; Mavi, A.; Kara, A. A. Determination of Antioxidant and Antimicrobial Activities of Rumex Crispus L. Extracts. J. Agric. Food Chem. 2001, 49(8), 4083–4089. DOI: 10.1021/jf0103572.
- Pilipenko, L.; Kolesnik, A. Lipids of the Leaf Vegetables Spinacea Oleracea, Latuca Sativa, and Rumex Acetosa. Chem. Nat. Compd. 1993, 29(2), 160–166. DOI: 10.1007/BF00630106.
- Jursik, M.; Holec, J.; Zatoriova, B. Biology and Control of Another Important Weeds of the Czech Republic: Broad-leaved Dock (rumex Obtusifolius) and Curled Dock (rumex Crispus). Listy Cukrovarnicke Reparske. 2008, 124(7–8), 215–219.
- Bhatt, V.; Negi, G. Ethnomedicinal Plant Resources of Jaunsari Tribe of Garhwal Himalaya, Uttaranchal. Indian J. Tradit. Knowl. 2006, 5(3), 331–335.
- Ali, H.; Qaiser, M. The Ethnobotany of Chitral Valley, Pakistan with Particular Reference to Medicinal Plants. Pak. J. Bot. 2009, 41(4), 2009–2041.
- Rokaya, M. B.; Münzbergová, Z.; Timsina, B. Ethnobotanical Study of Medicinal Plants from the Humla District of Western Nepal. J. Ethnopharmacol. 2010, 130(3), 485–504. DOI: 10.1016/j.jep.2010.05.036.
- Manan, Z.; Razzaq, A.; Islam, M. Diversity of Medicinal Plants in Wari Subdivision District Upper Dir, Pakistan. Pak. J. Plant Sci. (Pakistan). 2007
- Taylor, R.; Hudson, J.; Manandhar, N.; Towers, G. Antiviral Activities of Medicinal Plants of Southern Nepal. J. Ethnopharmacol. 1996, 53(2), 105–110. DOI: 10.1016/0378-8741(96)01435-3.
- Ullah, A.; Rashid, A.; Parveen, S. N. Medicinal Plants Used in the Isolated Region of Bumburet, Kalash Valley, District Chitral, Pakistan. Pak. J. Weed Sci. Res. 2014, 20(3), 359–373.
- Zul, K.; Ullah, M.; Sajjad, A.; Farhat, U.; Abdul, S.; Muhammad, A.; Anwar, Z.; Muhammad, I. Ex-vivo Antibacterial, Phytotoxic and Cytotoxic, Potential in the Crude Natural Phytoconstituents of Rumex Hastatus D. Don. Pak. J. Bot. 2015, 47(SI):293–299.
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic Effects of Quercetin in Streptozocin-induced Diabetic Rats. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 135(3), 357–364.
- Bansal, P.; Paul, P.; Mudgal, J.; Nayak, P. G.; Pannakal, S. T.; Priyadarsini, K.; Unnikrishnan, M. Antidiabetic, Antihyperlipidemic and Antioxidant Effects of the Flavonoid Rich Fraction of Pilea Microphylla (L.) In High Fat Diet/streptozotocin-induced Diabetes in Mice. Exp. Toxicol. Pathol. 2012, 64(6), 651–658. DOI: 10.1016/j.etp.2010.12.009.
- Li, H.; Song, F.; Xing, J.; Tsao, R.; Liu, Z.; Liu, S. Screening and Structural Characterization of α-glucosidase Inhibitors from Hawthorn Leaf Flavonoids Extract by Ultrafiltration LC-DAD-MS N and SORI-CID FTICR MS. J. Am. Soc. Mass Spectrom. 2009, 20(8), 1496–1503. DOI: 10.1016/j.jasms.2009.04.003.
- Ahmad, S.; Ullah, F.; Ayaz, M.; Sadiq, A.; Imran, M. Antioxidant and Anticholinesterase Investigations of Rumex Hastatus D. Don: Potential Effectiveness in Oxidative Stress and Neurological Disorders. Biol. Res. 2015, 48, 20. DOI: 10.1186/s40659-015-0010-2.
- Abbasi, A. M.; Khan, M. A.; Shah, M. H.; Shah, M. M.; Pervez, A.; Ahmad, M. Ethnobotanical Appraisal and Cultural Values of Medicinally Important Wild Edible Vegetables of Lesser Himalayas-Pakistan. J. Ethnobiol. Ethnomed. 2013, 9, 84. DOI: 10.1186/1746-4269-9-84.
- Nisar, M.; Shah, S. M. M.; Khan, I.; Sheema, S. A.; Khan, S.; Shah, S. M. H. Larvicidal, Insecticidal, Brine Shrimp Cytotoxicity and Anti-oxidant Activities of Diospyros Kaki (L.) Reported from Pakistan. Pak. J. Pharm. Sci. 2015, 28, 1239–1243.
- Stein, S.; Mirokhin, D.; Tchekhovskoi, D.; Mallard, W. G. The NIST Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectra Library; Standard Reference Data Program of the National Institute of Standards and Technology; US Department of Commerce: Gaithersburg, MD, 2002.
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/mass Spectrometry; Allured Publishing: Carol Stream, IL, 2007; pp 804.
- Muhammad, N.; Saeed, M.; Khan, H.; Hassan, S.; Gul, F. Evaluation of Viola Betonicifolia for Its Nutrition Value. Pak. J. Pharm. Sci. 2012, 25(3), 639–644.
- Harborne, A. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer: Netherlands, 1998.
- Taiga, A.; Suleiman, M.; Aina, D.; Sule, W.; Alege, G. Proximate Analysis of Some Dry Season Vegetables in Anyigba, Kogi State, Nigeria. Afr. J. Biotechnol. 2008, 7(10), 1588–1590.
- Kumkrai, P.; Weeranantanapan, O.; Chudapongse, N. Antioxidant, Alpha-glucosidase Inhibitory Activity and Sub-chronic Toxicity of Derris Reticulata Extract: Its Antidiabetic Potential. BMC Complement. Altern. Med. 2015, 15, 35. DOI: 10.1186/s12906-015-0552-4.
- Bu, T.; Liu, M.; Zheng, L.; Guo, Y.; Lin, X. α‐glucosidase Inhibition and the in Vivo Hypoglycemic Effect of Butyl‐isobutyl‐phthalate Derived from the Laminaria Japonica Rhizoid. Phytotherapy Res. 2010, 24(11), 1588–1591. DOI: 10.1002/ptr.3139.
- Elmazar, M. M.; El-Abhar, H. S.; Schaalan, M. F.; Farag, N. A. Phytol/Phytanic Acid and Insulin Resistance: Potential Role of Phytanic Acid Proven by Docking Simulation and Modulation of Biochemical Alterations. PloS One. 2013, 8(1), e45638. DOI: 10.1371/journal.pone.0045638.
- Perla, V.; Jayanty, S. S. Biguanide Related Compounds in Traditional Antidiabetic Functional Foods. Food Chem. 2013, 138(2–3), 1574–1580. DOI: 10.1016/j.foodchem.2012.09.125.
- Green, B. D.; Gault, V. A.; O’Harte, F. P.; Flatt, P. R. Structurally Modified Analogues of Glucagon-like Peptide-1 (GLP-1) and Glucose-dependent Insulinotropic Polypeptide (GIP) as Future Antidiabetic Agents. Curr. Pharm. Des. 2004, 10(29), 3651–3662. DOI: 10.2174/1381612043382774.
- Singh, R.; Lather, V.; Pandita, D.; Judge, V. N Arumugam K., Singh Grewal A. Synthesis, Docking and Antidiabetic Activity of Some Newer Benzamide Derivatives as Potential Glucokinase Activators. Lett. Drug Des. Discovery. 2017, 14(5), 540–553. DOI: 10.2174/1570180813666160819125342.
- Rayanil, K.-O.; Sutassanawichanna, W.; Suntornwat, O.; Tuntiwachwuttikul, P. A New Dihydrobenzofuran Lignan and Potential α-glucosidase Inhibitory Activity of Isolated Compounds from Mitrephora Teysmannii. Nat. Prod. Res. 2016, 30(23), 2675–2681. DOI: 10.1080/14786419.2016.1143830.
- Manoharan, D.; Kulanthai, K.; Sadhasivam, G.; Raji, V.; Thayumanavan, P. Synthesis, Characterization and Evaluation of Antidiabetic Activity of Novel Indoline Derivatives. Bangladesh J. Pharmacol. 2017, 12(2), 167–172. DOI: 10.3329/bjp.v12i2.30872.
- Ahmad, Z.; Zamhuri, K. F.; Yaacob, A.; Siong, C. H.; Selvarajah, M.; Ismail, A.; Hakim, M. N. In Vitro Anti-diabetic Activities and Chemical Analysis of Polypeptide-k and Oil Isolated from Seeds of Momordica Charantia (bitter Gourd). Molecules. 2012, 17(8), 9631–9640. DOI: 10.3390/molecules17089631.
- Itoh, Y.; Hinuma, S. GPR40, a Free Fatty Acid Receptor on Pancreatic β Cells, Regulates Insulin Secretion. Hepatol. Res. 2005, 33(2), 171–173. DOI: 10.1016/j.hepres.2005.09.028.
- Basha, R. H.; Sankaranarayanan, C. β-Caryophyllene, a Natural Sesquiterpene, Modulates Carbohydrate Metabolism in Streptozotocin-induced Diabetic Rats. Acta Histochem. 2014, 116(8), 1469–1479. DOI: 10.1016/j.acthis.2014.10.001.
- Lim, C. C.; Ferguson, L. R.; Tannock, G. W. Dietary Fibres as “prebiotics”: Implications for Colorectal Cancer. Mol. Nutr. Food Res. 2005, 49(6), 609–619. DOI: 10.1002/mnfr.200500015.
- Weickert, M. O.; Pfeiffer, A. F. Metabolic Effects of Dietary Fiber Consumption and Prevention of Diabetes. J. Nutr. 2008, 138(3), 439–442. DOI: 10.1093/jn/138.3.439.
- Liu, S.; Willett, W. C.; Manson, J. E.; Hu, F. B.; Rosner, B.; Colditz, G. Relation between Changes in Intakes of Dietary Fiber and Grain Products and Changes in Weight and Development of Obesity among Middle-aged Women. Am. J. Clin. Nutr. 2003, 78(5), 920–927. DOI: 10.1093/ajcn/78.5.920.
- Anderson, J. W.; Baird, P.; Davis, R. H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C. L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67(4), 188–205. DOI: 10.1111/j.1753-4887.2009.00189.x.
- Guggisberg, D.; Cuthbert-Steven, J.; Piccinali, P.; Bütikofer, U.; Eberhard, P. Rheological, Microstructural and Sensory Characterization of Low-fat and Whole Milk Set Yoghurt as Influenced by Inulin Addition. Int. Dairy J. 2009, 19(2), 107–115. DOI: 10.1016/j.idairyj.2008.07.009.
- Chen, C.-L.; Tetri, L. H.; Neuschwander-Tetri, B. A.; Huang, S. S.; San Huang, J. A Mechanism by Which Dietary Trans Fats Cause Atherosclerosis. J. Nutr. Biochem. 2011, 22(7), 649–655. DOI: 10.1016/j.jnutbio.2010.05.004.
- Winocur, G.; Greenwood, C. E. Studies of the Effects of High Fat Diets on Cognitive Function in a Rat Model. Neurobiol. Aging. 2005, 26(1), 46–49. DOI: 10.1016/j.neurobiolaging.2005.09.003.
- Ferdinandusse, S.; Denis, S.; Mooyer, P. A.; Dekker, C.; Duran, M.; Soorani‐Lunsing, R. J.; Boltshauser, E.; Macaya, A.; Gärtner, J.; Majoie, C. B. Clinical and Biochemical Spectrum of D‐bifunctional Protein Deficiency. Ann. Neurol. 2006, 59(1), 92–104. DOI: 10.1002/ana.20702.
- Den Boer, M. E.; Dionisi-Vici, C.; Chakrapani, A.; van Thuijl, A. O.; Wanders, R. J.; Wijburg, F. A. Mitochondrial Trifunctional Protein Deficiency: A Severe Fatty Acid Oxidation Disorder with Cardiac and Neurologic Involvement. J. Pediatr. 2003, 142(6), 684–689. DOI: 10.1067/mpd.2003.231.
- Mohaghegh, P.; Hickson, I. D. DNA Helicase Deficiencies Associated with Cancer Predisposition and Premature Ageing Disorders. Hum. Mol. Genet. 2001, 10(7), 741–746. DOI: 10.1093/hmg/10.7.741.
- Olaitan, P. B.; Adeleke, O. E.; Iyabo, O. Honey: A Reservoir for Microorganisms and an Inhibitory Agent for Microbes. Afr. Health Sci. 2007, 7(3), 159–165
- Ren, G.; Chen, F. Degradation of Ginsenosides in American Ginseng (panax Quinquefolium) Extracts during Microwave and Conventional Heating. J. Agric. Food Chem. 1999, 47(4), 1501–1505. DOI: 10.1021/jf980678m.
- Tarade, K. M.; Singhal, R. S.; Jayram, R. V.; Pandit, A. B. Kinetics of Degradation of Saponins in Soybean Flour (glycine Max.) During Food Processing. J. Food Eng. 2006, 76(3), 440–445. DOI: 10.1016/j.jfoodeng.2005.05.044.
- Casal, S.; Oliveira, M. B.; Ferreira, M. A. HPLC/diode-array Applied to the Thermal Degradation of Trigonelline, Nicotinic Acid and Caffeine in Coffee. Food Chem. 2000, 68(4), 481–485. DOI: 10.1016/S0308-8146(99)00228-9.
- Zul, K.; Farhat, U.; Muhammad, A.; Abdul, S.; Sajjad, A.; Anwar, Z.; Abid, H.; Muhammad, I. Anticholinesterse and Antioxidant Investigations of Crude Extracts, Subsequent Fractions, Saponins and Flavonoids of Atriplex Laciniata L.: Potential Effectiveness in Alzheimer’s and Other Neurological Disorders. Biol. Res. 2015, 48, 21. DOI: 10.1186/s40659-015-0011-1.
- Zeb, A.; Sadiq, A.; Ullah, F.; Ahmad, S.; Ayaz, M. Phytochemical and Toxicological Investigations of Crude Methanolic Extracts, Subsequent Fractions and Crude Saponins of Isodon Rugosus. Biol. Res. 2014, 47(1), 57. DOI: 10.1186/0717-6287-47-57.
- Zeb, A.; Sadiq, A.; Ullah, F.; Ahmad, S.; Ayaz, M. Investigations of Anticholinestrase and Antioxidant Potentials of Methanolic Extract, Subsequent Fractions, Crude Saponins and Flavonoids Isolated from Isodon Rugosus. Biol. Res. 2014, 47(1), 1–10. DOI: 10.1186/0717-6287-47-1.
- Ullah, F.; Ayaz, M.; Sadiq, A.; Hussain, A.; Ahmad, S.; Imran, M.; Zeb, A. Phenolic, Flavonoid Contents, Anticholinesterase and Antioxidant Evaluation of Iris Germanica Var; Florentina. Nat. Prod. Res. 2016, 30(12), 1440–1444. DOI: 10.1080/14786419.2015.1057585.
- Zgórka, G.; Kawka, S. Application of Conventional UV, Photodiode Array (PDA) and Fluorescence (FL) Detection to Analysis of Phenolic Acids in Plant Material and Pharmaceutical Preparations. J. Pharm. Biomed. Anal. 2001, 24(5–6), 1065–1072. DOI: 10.1016/s0731-7085(00)00541-0.