ABSTRACT
The aim of this study was to evaluate the preservation effect of the pomegranate peel extract (PPE)-lysozyme (Lys) combination addition on gelatin (G)–polycaprolactone (PCL) composite film on the quality of mackerel Scomberomorus commerson via physicochemical analysis (pH, total viable bases nitrogen (TVB-N), thiobarbituric acid reactive substances (TBARS), free fatty acid (FFA), weight loss, color), microbiological (total mesophilic count (TMC) and psychrotrophic (PTC) count) and sensorial assessments during refrigerated storage. The results showed that incorporating PPE and Lys into G/PCL solution could significantly (P < .05) improve the quality of fish fillets. Among all treatments, TMC and PTC of mackerel fish with the control treatment increased rapidly and were generally higher than other treatments (P < .05). Thus, G-PCL film containing both of Lys and PPE can remarkably delay the growth of bacteria of mackerel fillets. The results indicated that the Lys in combination with PPE could be effective in the reduction of TBARS and FFA values of mackerel wrapped. In addition, the synergistic effect between Lys and PPE improved the antibacterial activity of the gelatin solution. G/PCL-Lys-PPE had the best effect on reducing the weight loss and color properties of the samples. It was seen that the addition of Lys/PPE improved the sensory quality of mackerel fish and these results were supported by the outcomes of bacterial and physicochemical analysis. The increase (>9 days) in the shelf-life was attributed to the antioxidant and antimicrobial characteristics of G/PCL film containing both of PPE and Lys. These results confirmed that G/PCL-Lys-PPE film was effective for the preservation of mackerel.
Introduction
The mackerel (Scomberomorus commerson; Scombridae) also known as “Sheer fish” in Persian is the most popular fish species landed in the tropics and sub-tropics including south of Iran with the highest economic value.[Citation1] S. commerson is one of the seafood resources, due to its low cholesterol content and high protein content. This fish is mainly offered on the Iranian market as a whole (ungutted) fish or eviscerated fish. Fish and fishery products are more perishable due to high water content, free amino acids, and other non-protein nitrogenous compounds. After catching, initial loss of freshness results from changes brought about by oxidation of lipids and enzymatic activity[Citation2] even under chilled storage, whiles subsequent obvious spoilage occurs as a result of microbiological activity and produce metabolites. Therefore, various novel techniques to delay the spoilage of fish quality and to extend the shelf-life of seafood products are necessary. Previous studies concluded that natural additives and various biopolymers as packaging materials such as carbohydrates and proteins were effective in extending the shelf-life of seafood.[Citation3,Citation4] Among these biopolymers, gelatin resulting from partial hydrolysis of collagen is hydrophilic one with good affinity and compatibility, to form coatings with good properties including excellent film-forming ability, biodegradability, abundancy, good oxygen barrier capacity, low gelling and melting points and potential ability to be used as a carrier for functional agents.[Citation5–Citation7] Whereas, gelatin has some problems such as poor water vapor barrier properties and mechanical properties. To overcome these difficulties, some chemical treatments or polymeric materials can be applied to improve mechanical and barrier properties.[Citation8] Saki et al.[Citation9] and Nowzari et al.[Citation10] showed that chitosan-gelatin composite and bi-layer coating and/films extended the shelf life of seafood during storage. According to Rescek et al.,[Citation11] polyethylene/polycaprolactone film improved on the physical properties and barrier properties of packaging. Sogut and Seydim[Citation12] showed that edible coating of chitosan and polycaprolactone bilayer films showed better functional properties and mechanical resistance than chitosan monolayer films. Sogut and Seydim[Citation13] found that chitosan- and polycaprolactone-based bilayer films have been applied to augment the shelf life of chicken breast fillets. Polycaprolactone (PCL), a thermoplastic aliphatic polyester, shows flexible and biodegradable properties. However, no published data exist on the associated usage of gelatin coatings in combination with PCL for fishery products. In the last decade, many studies have been demonstrated the development of active packaging by incorporating natural food additives to improve the shelf life of fish products. Lysozyme, as a type of glycanhydrolase, has attracted significant attention in recent years in the field of food packaging because of antibacterial activity, especially against Gram-positive bacteria.[Citation14] Edible coating-based protein or chitosan incorporated with lysozyme were developed by Wang et al.[Citation15] and Wu et al.[Citation16] Moreover, the antibacterial activity of pomegranate peel extract (PPE) against Gram-positive (Staphylococcus aureus, Listeria monocytogenes) and Gram-negative bacteria (Pseudomonas stutzeri, Escherichia coli, Yersinia enterocolitica) was observed by Akhtar et al.[Citation17] and Devatkai et al.[Citation18] The antioxidant activity of PPE delays the lipid oxidation due to inhibiting the initiation or propagation step of the oxidative chain reactions or forming stable radicals,[Citation19] metal chelation and singlet oxygen quenching.[Citation20] The combination of a bio-based polymer such as gelatin and PCL with Lys and PPE, where gelatin, lysozyme, and PPE include the antioxidant agent and antimicrobial activity in contact with the food, respectively, can be an interesting way to increase active properties that increase the shelf life and safety of food products like seafood. Therefore, the aim of the present study is to investigate the effectiveness of gelatin–PCL composite films incorporated with Lys and PPE on the quality of mackerel Scomberomorus commerson fillet in refrigerated condition (4 ± 1°C).
Material and methods
Materials
The dry cold water fish skin gelatin was purchased from Sigma Chemical Company, UK. Glycerol was purchased from Duksan Pure Chemical Co. (Sungkok-Dong, Korea). Lysozyme (from eggs, 40,000 U·mg−1) was obtained from Macklin Reagent Co., Ltd. (Shanghai, China). Polycaprolactone (PCL, CAPA 6800) was kindly supplied by Perstorp Holding AB, Sweden. Plate count agar (PCA) culture medium was purchased from Merck (PCA, Merck, Darmstadt, Germany). All other chemicals were of analytical grade or of the highest grade available.
Preparation of pomegranate peel extract
Pomegranates were manually washed, peeled and dried by hot air for 48 h at 50°C.[Citation21] The dried pomegranate peel was powdered using a mixer grinder and 200 g portions of finely powdered pomegranate peel was blended with 50% ethanol for 2 h at 40°C in a shaking water bath. The ratio pomegranate peel powder: solvent was 1:10 (w/v). The extracts were through Waterman No. 1 filter paper and concentrated under vacuum with a rotary evaporator. The concentrate was dried overnight in an oven at 40°C to form a powder which was stored at 4°C until further use. The 1.5% of pomegranate peel extract (PPE) dipping solution was prepared by dissolving PPE in distilled water.[Citation9]
Preparation of coating samples and treated fillets
The dry cold water fish skin gelatin was dissolved in distilled water (3% w/v), first being allowed to swell at 7°C for 15 min and then warmed to 55°C for 30 min.[Citation22] The film was obtained by casting 100 ml film-forming solution on a horizontal glass plate (16 × 27 cm). To prepare composite film, the gelatin solution and PCL solution (5% w/w in chloroform) were blended using an Ultra-Turrax (Ika-Werke, model T25, Germany) at 20,000 rpm for 5 min, then lysozyme (0.1 g w/v) and PPE (1.5% w/v) were added to the gelatin/PCL solution to a final solution. Solution poured on the surface and dried at 40°C in an air-forced oven (Tecnal, TE-394/2, Brazil) for about 24 h. Glycerol (0.75 ml/g gelatin) was added as a plasticizer and stirred for 10 min. The G/PCL-Lys-PPE film was stirred until the mixture became clear, and stored at 4 ± 1°C. Fillet samples were randomly assigned into five treatment lots consisting of:
a control lot (un-wrapped)
wrapped with G/PCL (G/PCL)
treated with G/PCL film and PPE (G/PCL -PPE)
and wrapped with G/PCL in combination with Lys (G/PCL-Lys)
wrapped with film (G/PCL) in combination with Lys and PPE (G/PCL-Lys-PPE)
All samples were placed in polyethylene bags, stored at 4 ± 1°C for 12 days. Physicochemical, microbiological and sensorial analyses were performed at 3-day intervals to determine the overall quality of fish.
Microbiological analysis
The samples (25 g) were placed in a stomacher bag containing 225 ml of 0.85% saline water. After mixing for 1 min in a stomacher blender, further serial dilution was done using the same diluent. Thereafter, 0.1 mL of appropriate dilution was used for microbiological analysis by spread plate method. The media and condition used were: Plate Count Agar (PCA, Merck, Denmark, Germany) incubated for psychrotrophic (PTC) bacteria count at 4°C for 10 days and for total mesophilic bacteria at 30°C for 24–48 h.[Citation23] The microorganism value was expressed as log10 CFU/g.
pH
The measurement of pH was carried out on 5 g of sample homogenized in 45 mL distilled water. The pH values of the samples were determined using a digital pH meter (913 pH meter, Metrohm, Herisaw, Switzerland).[Citation24]
Total volatile base nitrogen (TVB-N)
Total volatile basic nitrogen (TVB-N) values were determined using the method of Goulas and Kontominas.[Citation25] TVB-N was determined by distillation after the addition of MgO (2 g) to 10 g of homogenized fish sample mixed with 50 mL distilled water. The distillate was collected in a flask containing 25 mL of aqueous solution of boric acid (2%) and methyl red as an indicator. Afterward, the boric acid solution was titrated with sulfuric acid (H2SO4) solution (0.1 N). The TVB-N value (mg N/100 g of fish) was determined according to the consumption of sulfuric acid. The constant 14 was used to calculate the TVB-N number using EquationEquation (1)(1) .
V = mL of sulfuric acid (H2SO4) solution for titration
2-Thiobarbituric acid reactive substances (TBARS)
Thiobarbituric acid reactive substances (TBARS) were determined following Siripatrawan and Noipha[Citation26] with some modifications. Ten grams of homogenized sample were added with 97.5 mL of distilled water and 2.5 mL of 4 M HCl. The mixture was heated with steam distillation. Five milliliters of distillate were added to 5 mL of thiobarbituric reactive reagent containing 0.02 M TBA in 90% glacial acetic acid and incubated in boiling water for 35 min. After cooling, the absorbance of the pink solution was measured at 532 nm using a spectrophotometer (Analytik Jena US, SPECORD, Upland, CA, USA). The constant 7.8 was used to calculate the TBA number using EquationEquation (2)(2) . The TBA value is expressed as mg malonaldehyde equivalents/kg sample.
Free fatty acid (FFA)
The free fatty acid value was determined in the lipid extract by the procedures of Woyewoda et al.[Citation27] according to EquationEquation (3)(3) . The lipid extract was collected in a 250 mL volumetric flask containing 10 g of samples, 50 mL chloroform, 50 mL methanol and 45 mL distilled water. Results were expressed in % of oleic acid.
N = normality of NaOH, V2 = mL NaOH for samples, V1 = mL NaOH for blank, W = weight (g) of lipid.
Weight loss
The weight loss was determined as described by Lu et al.[Citation28] The percentage weight loss relative to the initial weight was calculated by weighing the samples in triplicate.
where Wo is the initial weight of the sample and Wi is the weight of the same sample after 3, 6 and 12 days of refrigerated storage.
Color measurements
A Minolta Chroma Meter (CR400, Minolta, Osaka, Japan) was used for color measurement. Colors were expressed as CIE Lab coordinates. In this system, L* represents the color lightness on a 0–100 point scale from black to white; a* is the position between red (+) and green (-); and b* is the position between yellow (+) and blue (-).
Sensory analysis
Sensory analysis of raw mackerel fillets was carried out using the quality index method (QIM) by 10 panelists.[Citation29] Scores were given for each quality attributes according to descriptions, ranging from 0 to 5. On the first and last days of storage (day 0 and day 12), mackerel received a freshly: QI (quality index) = 0 and completely deteriorate: QI = 5. A preference score of 3 was indicated as the threshold for acceptable quality.
Statistical analysis
All experiments were performed in triplicate and a completely randomized design was used. Analysis of variance (ANOVA) was performed and mean comparisons were done by Duncan̕s multiple range tests. Analysis was performed using a SPSS package (SPSS 11.0 for windows, SPSS Inc, Chicago, II, USA). P values less than 0.05 were considered statistically significant.
Result and discussion
Changes in microbial counts
Variations in the total mesophilic counts (TMC) and psychrotrophic (PTC) bacteria counts of mackerel fillets during the refrigerated storage are shown in . The initial bacterial counts for TMC in the mackerel fillets varied from 2.07 log10 CFU/g to 2.10 log10 CFU/g. The initial PTC of all samples ranged from 1.12 log10 CFU/g to 1.16 log10 CFU/g. According to Sikorski et al.,[Citation30] the number of bacteria in fresh fillet with high-quality vary from 3 to 4 log10 CFU/g. TMC and PTC of all samples increased gradually as the storage time increased (P < .05). In this study, we revealed that all of the treatments (G/PCL, G/PCL-Lys, G/PCL-PPE, G/PCL-PPE-Lys) led to a dramatic reduction in TMC and PTC in mackerel fillet compared to the control samples, while there was no significant (P > .05) difference in TMC and PTC between G/PCL-Lys and G/PCL -PPE. The sample treated with G/PCL-Lys-PPE had the lowest TMC and PTC compared with other treatments (P < .05), which might be attributed to the antimicrobial activity of Lys and PPE. Treatment of fish coated with G/PCL could retard the growth of total mesophilic bacteria more effectively, compared with control due to the antimicrobial effect of gelatin due to the presence of oligopeptide chains from the hydrolysis of gelatin, which is suspected of having antimicrobial activity due to the presence of side-chain amino groups.[Citation6] On mackerel fillet, a mixture of Lys and PPE would be more effective antimicrobials than either Lys or PPE alone. The antimicrobial activity of PPE has been ascribed to the presence of many bioactive compounds such as alkaloids and tannins, and phytochemical groups (e.g. gallotannins, catechins, flavonols, procyanidins and derivatives of ellagic acid), which show antibacterial effect.[Citation17,Citation31] PPE has been reported to be effective as antimicrobial agents on inhibition of the growth of spoilage bacteria that affect the cellular membranes and retard microbial growth.[Citation21,Citation32] Blending natural polymer with plant extract has been reported in several studies.[Citation3,Citation33,Citation34] Yuan et al.[Citation35] showed that bacterial growth in chitosan-coated pacific white shrimp in combination with PPE reached less than that treated by chitosan coating or PPE alone. Saki et al.[Citation9] showed that chitosan-gelatin composite and bilayer coating containing PPE could extend the shelf life of Belanger’s Croaker (Johnius Belangerii) during storage. In addition, lysozyme delayed the deterioration of food due to the muramidase activity, leading to the degradation of the murein-containing layer of the bacterial cell wall and eventually resulting in bacterial lysis.[Citation16,Citation36] Wu et al.[Citation16] stated how bacterial growth (such as S. aureus, E.coli, Salmonella, P. aeruginosa) in chitosan-coated large yellow croaker in combination with lysozyme reached less than that treated by chitosan coating or lysozyme alone. Lysozyme is effective at inhibiting the growth of Gram-positive bacteria such as Listeria monocytogenes.[Citation37] Wang et al.[Citation15] reported that collagen coating containing lysozyme could extend the shelf life of salmon fillet during storage at the refrigerator. Moreover, the in vitro antimicrobial activity of gelatin containing lysozyme against bacteria has been reported by Rawdkuen et al.[Citation38] It was indicated that the coating consisting of a blend of gelatin dissolved in acetic acid and lysozyme, exerted an inhibitory effect on the Gram-negative flora of food product.[Citation14] Boyac et al.[Citation39] found that smoked salmon slices treated by whey proteins/oleic acid-lysozyme composite films can effectively inhibit the growth of Listeria innocua. Rao et al.[Citation40] stated the synergistic effect of chitooligosaccharides and lysozyme for meat preservation. This result indicates that G/PCL of the Lys-PPE mixture did not constrain the synergistic effect of the antimicrobials in mackerel fillet, allowing the lysozyme to hydrolyze the peptidoglycan layer surrounding the cytoplasmic membrane of bacteria, improving the inhibitory effect of pomegranate peel extract. By day 6 of storage, TMC in mackerel fillet reached to about 7 log10 CFU/g for control samples, which is higher than the maximal recommended limit in raw fish[Citation23] while that of all treatments did not achieve this count to the end of 12-day storage time.
Table 1. Bacterial changes of Scomberomorus commerson fillets of unwrapped and wrapped with G/PCL incorporated with Lys and PPE
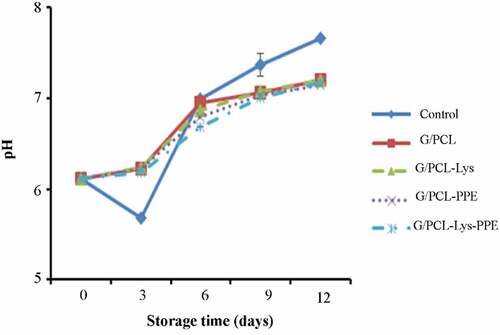
pH
shows the changes in the pH value of mackerel fillet during the storage at the refrigerator with different treatments. The pH of the mackerel muscle on day 0 was 6.11, which was similar to the results of Tzikas et al.[Citation41] The pH value of fresh fish fillet varies from 6 to 7.[Citation42] pH values of the untreated fish were decreased up to day 3 (P > .05) and then increased (P < .05). The pH value of the treated samples increased continuously. The reduction of pH is related to the generation of lactic acid through lactic acid bacteria (LAB) metabolism and the release of inorganic phosphate by the degradation of adenosine triphosphate (ATP) during storage. The accumulation of basic compounds generated from both autolytic processed by endogenous enzymes and microbial enzymatic actions leads to an increase in pH value.[Citation43] At day 12 of storage, pH values of treated samples (G/PCL-Lys, G/PCL-PPE, and G/PCL-Lys -PPE) were lower than control (P < .05), due to the inhibition of the growth of bacteria, yeasts and molds.[Citation44] No significant effect was observed between G/PCL-Lys, G/PCL-PPE and G/PCL-Lys-PPE during storage at refrigerator (P > .05). Saki et al.[Citation9] and Mohebi and Shahbazi[Citation33] reported that chitosan-gelatin incorporated with PPE significantly inhibited the increase of pH in seafood products due to the antioxidant activity of PPE. Wang et al.[Citation15] and Wu et al.[Citation16] noted that the combination of Lys and packaging could clearly prevent a decrease in pH values. According to the result of pH value, it can be observed that G/PCL composite film incorporated with Lys and PPE showed no higher antibacterial activity compared to G/PCL-PPE/or G/PCL-Lys alone. The pH value is positively correlated with TVB-N value due to the increase in volatile amine led to an increase of pH.
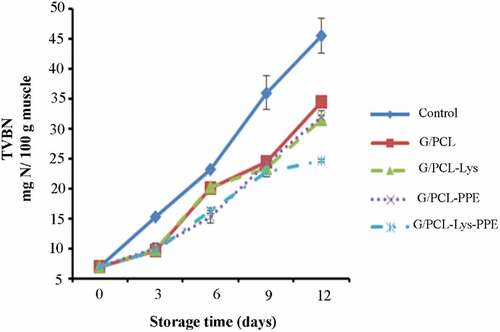
TVB-N
The variation of TVB-N value of mackerel fillet during the storage is showed in . The initial TVB-N value of mackerel fish was 6.86 mg N/100 g of fish. The TVB-N value increased gradually along with the time of storage in unwrapped and wrapped samples (P < .05), but the increasing rate varied in treatments (wrapped samples). The increase of TVB-N may have been due to the activity of endogenous enzymes, spoilage bacteria and the subsequent increase of microbial degradation products.[Citation3] The increase of TVB-N value of seafood through time was in agreement with the microorganism activity during storage.[Citation16] There was a positive correlation between TMC and TVB-N in treated samples (R2 = 0.916–0.990) and control (R2 = 0.881). There was a difference between control and treated samples (P < .05) at the end of storage. The lowest TVB-N increase in G/PLS composite film incorporated with Lys and PPE may be attributed to the presence of bioactive phenolic compounds in pomegranate peel extract and antibacterial activity of lysozyme, which could remarkably inhibit capacity of bacteria for oxidative deamination of non-protein nitrogen compounds, compared with the control and those treated with G/PCL film, or G/PCL-Lys or G/PCL-PPE. It can be concluded, G/PCL film in combination with Lys and PPE is more effective than other treatments in controlling TVB-N of fillets, suggesting that G/PCL film demonstrated synergism in retarding of TVB-N value when used in combination with Lys and PPE. Wu et al.[Citation16] observed higher amounts of TVB-N for chitosan-coated combined with lysozyme and un-coated large yellow croaker on the 15th day of refrigerated storage. Wang et al.[Citation15] reported that 4% collagen combined with lysozyme could effectively retard the increase in the TVB-N of salmon fillet during refrigerated storage compared to that of the uncoated sample. Moreover, Yuan et al.[Citation35] reported that 1% chitosan combined with 1.5% PPE reduced the formation TVB-N of Pacific white shrimp at the end of a 10-day storage period. Another study conducted by Fan et al.[Citation45] revealed polyphenols in PPE as antimicrobial action prevented protein decomposition by bacteria and hinder their capacity for oxidative deamination of nitrogenous compounds from non-protein sources. Similar results in Nile tilapia were reported by Alsaggaf et al.[Citation21] No information was found in the literature on the effect of G/PCL film incorporated with Lys and PPE on TVB-N production in seafood products. According to the highest acceptable level of TVB-N (25 mg N/100 g fish muscle), the unwrapped and wrapped fish fillets (G/PCL, G/PCL-PE, and G/PCL-Lys) were spoiled on days 6 and 9, respectively. However, the TVB-N value of mackerel wrapped with G/PCL-PPE-Lys was less 25 mg N/100 g fish sample after 12 days.
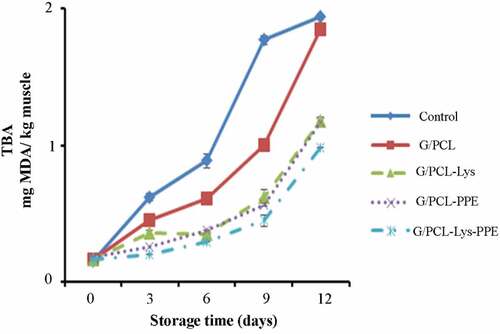
TBARS
Changes in TBARS value of mackerel fillet during storage with different treatments are shown in . At the beginning of storage, TBARS values were ranged from 0.16 mg malonaldehyde/kg sample to 0.18 mg malonaldehyde/kg sample. TBARS values were increased in all samples when the storage time increased (P < .05). Higher increase in TBARS value was observed in unwrapped fillets (P < .05), due to the partial dehydration of fish and to the increased oxidation of unsaturated fatty acids.[Citation46] We observed all G/PCL films with or without natural preservation lessened TBARS values (P < .05). On day 12, there was a difference between control and treated samples (P < .05). The addition of PPE to G/PCL-wrapped samples can increase the antioxidant activity of the gelatin. The antioxidant activity of pomegranate peel extract with antioxidant agents (polyphenolics compounds) could be through radical scavenging capacities against free radicals, peroxynitrite chelate copper and ion, preventing metal-catalyzed free radical formation.[Citation17] On the other hand, lysozyme, an antimicrobial enzyme, has antimicrobial activity against the growth of bacteria specially psychrotrophic bacteria such as Pseudomonas spp which produce lipase and phospholipase causing an increase in free fatty acid. Free fatty acids are more susceptible to oxidation and form unstable lipid hydroperoxide. The shorter chain hydrocarbons such as aldehydes are produced as a result of decomposition of hydroperoxide. These products are detected as TBARS.[Citation47] Moreover, polymers such as gelatin and chitosan showed a significant effect on delaying lipid oxidation through forming a biodegradable film (oxygen barrier properties) treated samples during storage.[Citation10] According to Antoniewski et al.,[Citation48] gelatin coating on fishery products may reduce lipid oxidation due to hydrogen bonds in gelatin as a barrier to oxygen. Wu et al.[Citation16] found that chitosan coating combined with lysozyme applied on the surface of large yellow croaker fillets acted as a barrier between the fillets and the surrounding air, slowing down the diffusion of oxygen from the surroundings via the surface into the fillet. According to Rao et al.,[Citation40] no significant difference in TBARS values was observed in untreated and treated meat. Addition of G/PCL in combination with Lys or PPE increased the antioxidant activity of gelatin. TBARS values of 1 to 2 mg malonaldehyde/kg muscle are an acceptable sensory limit.[Citation49] In the current study, TBARS values for all samples were less than 2 mg malonaldehyde/kg muscle at the end of the storage. This indicates that the G/PCL film with or without PPE and Lys is able to reduce the lipid oxidation, resulting in lower rancidity compared to controls.
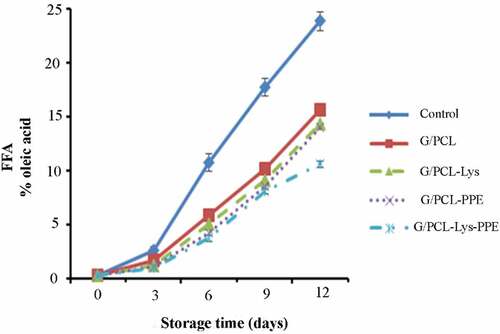
FFA
The initial FFA values varied from 0.29% to 0.34% of oleic acid (). At the end of storage, the FFA value was 15.67%, 14.34%, 14.00%, 10.67%, and 23.89% of oleic acid for G/PCL, G/PCL-Lys, G/PCL-PPE, G/PCL-Lys-PPE, and control, respectively. FFA content gradually increased in all samples, but fillet wrapped with G/PCL-Lys-PPE slowed the formation of FFA during refrigerated storage (P < .05). The production of lipase and phospholipase with bacteria can be reduced by the synergistic effect between Lys and PPE for increasing antibacterial activity of them in samples wrapped with G/PCL-Lys-PPE. Moreover, delaying the oxidative changes of lipids (TBARS) at samples G/PCL-Lys-PPE can protect mackerel fillets again increase of production of free fatty acids. This result could be confidently due to the powerful antioxidant and antibacterial activity of both Lys and PPE. However, our results showed that the lysozyme was less effective than lysozyme combined with PPE on FFA content.
Figure 4. FFA changes of Scomberomorus commerson fillets of unwrapped and wrapped with G/PCL incorporated with Lys and PPE. Mean values and standard errors from the three replicates are presented
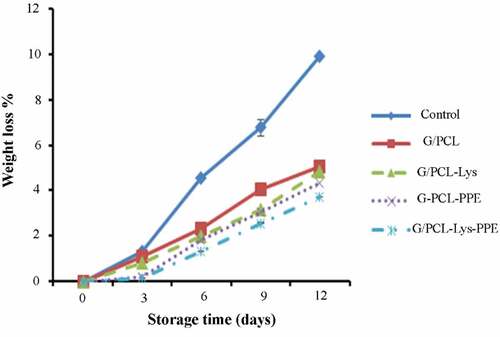
Weight loss
The changes in weight loss of unwrapped and wrapped samples are shown in . Weight loss of all samples increased as the storage times increased. Increases in weight loss with storage time could be due to the denaturation of myosin which further decreases the hydration capability of myosin fibers during the storage. This led to more tissue and cell rupture, resulting in more water and some protein loss.[Citation50] The G/PCL film on the mackerel fillets acted effectively as water vapor barriers during the whole storage period. The weight loss in wrapped samples with G/PCL-PPE-Lys was the lowest compared with G/PCL containing either Lys or PPE. This may be attributable to the addition of Lys and PPE in the film solution, preventing the evaporation of water vapors from the mackerel sections. The efficacy of active film along with plant additives or Lys in preventing water loss was also previously investigated by Nisar et al.[Citation51] Likewise, G/PCL film incorporated with Lys was proved effective in reducing weight loss and prolonging the shelf life of coated salmon fillets by Wang et al.[Citation15] The hydrophilic portions of the gelatin matrix can cause the transfer of water vapors. With the addition of PPE, the hydrophobic portion of the matrix increase and it may further limit the passage of liquid molecules through the film surface by acting as a physical barrier. Hence, less water vapor permeability of G/PCL-PPE film may explain comparatively lower moisture loss in G/PCL-PPE wrapped mackerel samples as compared to unwrapped samples. Shorter shelf life of unwrapped mackerel could also be due to higher weight loss, which increases oxidative and hydrolytic processes encouraged by microorganisms.
Color change
Color values including redness (a*) value, blueness (b*) value and lightness (L*) value of control and samples wrapped with and without Lys and PPE during storage 4°C are shown in . Mackerel fillets of control and treated samples showed a decrease in L* values with increasing storage time, which is associated with oxidation of red oxymyoglobin to metmyoglobin, yielding muscle with an unattractive brown color.[Citation52] The highest decrease in L* value was also observed in the control sample. Lightness in mackerel treated by G/PCL film alone or incorporated with Lys and PPE did not change significantly over time. Thus, treatments with G/PCL film, G/PCL-Lys, G/PCL-PPE, G/PCL-PPE-Lys could delay the change of L* value of mackerel in the present study. In all samples, a significant decrease in a* values was observed throughout the storage time, indicating the decrease in redness of samples. The highest change was observed in control samples, while wrapped fillets showed a slight decrease in a* values, compared to control samples. Thus, the application of G/PCL film either without or with Lys and PPE, on fish fillet surface could increase the stability of the red meat color to some extent. The increase in b* value was possibly associated with decomposition of primary products of lipid oxidation.[Citation53,Citation54] Samples wrapped with G/PCL-PPE, G/PCL-Lys, and G/PCL-PPE-Lys showed the lowest changes in color values, probably due to the antioxidant and antimicrobial properties of Lys and PE. Thus, G/PC film containing both Lys and PPE could retard the color changes of fillets to some extent during the storage. However, Wang al.[Citation15] observed no significant difference between collagen-lysozyme coating and control, until 15 days. In general, protein denaturation, lipid oxidation, and hydrolysis can lead to discoloration in aquatic products.[Citation53,Citation54]
Table 2. Color changes of Scomberomorus commerson fillets of unwrapped and wrapped with G/PCL incorporated with Lys and PPE
Sensory analysis
The effect of G/PCL film incorporated with Lys and PPE on the general aspect of mackerel fillet is shown in . Sensory attributes of fish were divided into three elements including color, odor and overall acceptance, whose preference levels were scored from 1 to 5, the higher preference level, the higher element score. All samples had the score of 5 at the beginning of refrigerated storage. The scores decreased significantly with extended time (P < .05). The fish with scores of below 3 were unacceptable with a sign of putrid odor, no shiny color, and overall unacceptability. For control samples, the deterioration occurred after 6 days of storage as evidenced by strong fishy and putrid odor. Odor scores of control samples declined rapidly and displayed a significantly different score compared to treated samples. The unacceptability score (3) was attained for odor after 6 days and 12 days for the untreated samples and wrapped samples, respectively, suggesting that gelatin film along with PPE or lysozyme could inhibit the growth of off-odors producing microorganisms. The untreated samples exhibited a strong off-odor linked with spoiled fishery products at the end of storage, which might be due to the release of metabolites by bacterial action.[Citation55] The deterioration of the sensory analysis was correlated with the microbial spoilage and physicochemical analysis including TBARS and TVB-N caused the change of the surface of the color of samples.[Citation56] Wang et al.[Citation15] demonstrated that collagen-lysozyme coating was a suitable treatment for long-term storage compared with control samples, considered as a potential natural antibacterial for reducing bacteria counts and stabilizing lipid-containing salmon fillets. The functional properties, e.g. antioxidant and antimicrobial effects, and also oxygen barrier of Lys and PPE has been shown to prolong the shelf life of fish by 9 days as compared to the control sample. It suggested that although Lys/PPE could inhibit bacteria of fresh mackerel effectively, G/PCL film incorporated with Lys and PPE had no significant effect on the odor and color of samples. Saki et al.[Citation9] also reported that chitosan-gelatin coatings combined with pomegranate peel extract could improve the overall acceptability of Belanger’s croaker fillet and eventually prolong the shelf life of the product.
Table 3. Sensory changes of Scomberomorus commerson fillets of unwrapped and wrapped with G/PCL incorporated with Lys and PPE
Conclusion
The result of the present study indicated that the Lys and PPE used in fresh fillet wrapped with G/PCL leads to a reduction in microbial contamination during long storage time. The results of this study revealed that incorporation of G/PCL with Lys and PPE could maintain the quality of mackerel fillets and eventually prolong the shelf life of the product up to 9 days as compared to the control samples which lost their acceptability standards approximately at 6th day of the storage. Sensory properties including color, odor and overall acceptability of wrapped samples with both of Lys and PPE were much better than those of untreated samples. The results for sensory evaluation, bacteriological examination and TVB-N were in good correlation. Generally, the use of Lys/PPE could be a suitable choice in the preserving of seafood products in the form of a solution that wrapped on the surface of fish as the natural component added into the packaging materials of fishery products.
Acknowledgments
We would like to thank Khorramshahr University of Marine Science and Technology for supporting this work under research grant contract No. 166. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. The authors declare no conflict of interest. No ethical considerations apply.
References
- Eighani, M.; Paighambari, S. Y.; Herrmann, B.; Feekings, J. Effect of Bait Type and Size on Catch Efficiency of Narrow-barred Spanish Mackerel (Scomberomorus Commerson) in the Persian Gulf Handline Fisheries. Fish. Res. 2018, 199, 32–35. DOI: 10.1016/j.fishres.2017.11.023.
- Cai, L.; Cao, A.; Li, Y.; Song, Z.; Leng, L.; Li, J. The Effects of Essential Oil Treatment on the Biogenic Amines Inhibition and Quality Preservation of Red Drum (Sciaenops Ocellatus) Fillets. Food Control. 2015, 56, 1–8. DOI: 10.1016/j.foodcont.2015.03.009.
- Kakaei, S.; Shahbazi, Y. Effect of Chitosan-gelatin Film Incorporated with Ethanolic Red Grape Seed Extract and Ziziphora Clinopodioides Essential Oil on Survival of Listeria Monocytogenes and Chemical, Microbial and Sensory Properties of Minced Trout Fillet. LWT – Food Sci. Technol. 2016, 72, 432–438. DOI: 10.1016/j.lwt.2016.05.021.
- Choulitoudi, E.; Ganiari, S.; Tsironi, T.; Ntzimani, A.; Tsimogiannis, D.; Taoukis, P.; Oreopoulou, V. Edible Coating Enriched with Rosemary Extracts to Enhance Oxidative and Microbial Stability of Smoked Eel Fillets. Food Packag. Shelf Life. 2017, 12, 107–113. DOI: 10.1016/j.fpsl.2017.04.009.
- Rivero, S.; García, M. A.; Pinotti, A. Composite and Bi-layer Films Based on Gelatin and Chitosan. Food. Eng. 2009, 90, 531–539. DOI: 10.1016/j.jfoodeng.2008.07.021.
- Pereda, M.; Ponce, A. G.; Marcovich, N. E.; Ruseckaite, R. A.; Martucci, J. F. Chitosan-gelatin Composites and Bi-layer Films with Potential Antimicrobial Activity. Food Hydrocoll. 2011, 25, 1372–1381. DOI: 10.1016/j.foodhyd.2011.01.001.
- Amjadi, S.; Emaminia, S.; Davudian, S. H.; Pourmohammad, S., .; Hamishehkar, H.; Roufegarinejad, L. Preparation and Characterization of Gelatin-based Nanocomposite Containing Chitosan Nanofiber and ZnO Nanoparticles. Carbohyd. Polym. 2019, 216, 376–384. DOI: 10.1016/j.carbpol.2019.03.062.
- Sharmin, N.; Khan, R. A.; Salmieri, S.; Dussault, D.; Fabrication, L. M. Characterization of Biodegradable Composite Films Made of Using Poly (Caprolactone) Reinforced with Chitosan. J. Polym. Environ. 2012, 20(3), 698–705. DOI: 10.1007/s10924-012-0431-8.
- Saki, J.; Khodanazary, A.; Hosseini, S. M. Effect of Chitosan-Gelatin Composite and Bi-Layer Coating Combined with Pomegranate Peel Extract on Quality Properties of Belanger’s Croaker (Johnius Belangerii) Stored in Refrigerator. J. Aquat. Food Prod.Technol. 2018, 27, 557–567. DOI: 10.1080/10498850.2018.1461161.
- Nowzari, F.; Shabanpour, B.; Ojagh, S. M. Comparison of Chitosan- Gelatin Composite and Bilayer Coating and Film Effect on the Quality of Refrigerated Rainbow Trout. Food Chem. 2013, 141, 1667–1672.
- Rescek, A.; Scetar, M.; Hrnjak-Murgic, Z.; Dimitrov, N.; Galic, K. Polyethylene/Polycaprolactone Nanocomposite Films for Food Packaging Modified with Magnetite and Casein: Oxygen Barrier, Mechanical, and Thermal Properties. Polym. Plast. Technol. Eng. 2016, 55(14), 1450–1459. DOI: 10.1080/03602559.2016.1163606.
- Sogut, E.; Seydim, A. C. Development of Chitosan and Polycaprolactone Based Active Bilayer Films Enhanced with Nanocellulose and Grape Seed Extract. Carbohyd. Polym. 2018, 195, 180–188. DOI: 10.1016/j.carbpol.2018.04.071.
- Sogut, E.; Seydim, A. C. The Effects of Chitosan- and Polycaprolactone-based Bilayer Films Incorporated with Grape Seed Extract and Nanocellulose on the Quality of Chicken Breast Fillets, LWT - Food Sci. Technol. 2019, 101, 799–805.
- Corradini, C.; Alfieri, I.; Cavazza, A.; Lantano, C.; Lorenzi, A.; Zucchetto, N.; Montenero, A. Antimicrobial Films Containing Lysozyme for Active Packaging Obtained by Sol-gel Technique. J. Food Eng. 2013, 119, 580–587. DOI: 10.1016/j.jfoodeng.2013.05.046.
- Wang, Z.; Hu, S.; Gao, Y.; Ye, C.; Wang, H. Effect of Collagen-lysozyme Coating on Fresh-salmon Fillets Preservation. LWT- Food Sci. Technol. 2017, 75, 59–64. DOI: 10.1016/j.lwt.2016.08.032.
- Wu, T.; Ge, Y.; Li, Y.; Xiang, Y.; Jiang, Y.; Hu, Y. Quality Enhancement of Large Yellow Croaker Treated with Edible Coatings Based on Chitosan and Lysozyme. Int. J. Biol. Macromol. 2018, 120, 1072–1079. DOI: 10.1016/j.ijbiomac.2018.08.188.
- Akhtar, S.; Ismail, T.; Fraternal, D.; Sestili, P. Pomegranate Peel and Peel Extracts: Chemistry and Food Features. Food Chem. 2015, 174, 417–425. DOI: 10.1016/j.foodchem.2014.11.035.
- Devatkal, S. K.; Jaiswal, P.; Jha, S. N.; Bharadwaj, R.; Viswas, K. N. Antibacterial Activity of Aqueous Extract of Pomegranate Peel against Pseudomonas Stutzeri Isolated from Poultry Meat. J. Food Sci. Technol. 2013, 50, 555–560. DOI: 10.1007/s13197-011-0351-y.
- Shah, M. A.; Bosco, S. J. D.; Mir, S. A. Plant Extracts as Natural Antioxidants in Meat 450 and Meat Products. Meat Sci. 2014, 98(1), 21–33. DOI: 10.1016/j.meatsci.2014.03.020.
- Kanatt, S. R.; Chander, R.; Sharma, A. Antioxidant and Antimicrobial Activity of Pomegranate Peel Extract Improves the Shelf Life of Chicken Products. Int. J. Food Sci. Technol. 2010, 45, 216–222. DOI: 10.1111/ifs.2010.45.issue-2.
- Alsaggaf, M. S.; Moussa, S. H.; Tayel, A. A. Application of Fungal Chitosan Incorporated with Pomegranate Peel Extract as Edible Coating for Microbiological, Chemical and Sensorial Quality Enhancement if Nile Tilapia Fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. DOI: 10.1016/j.ijbiomac.2017.03.017.
- López-Caballero, M. E.; Gómez-Guillén, M. C.; Pérez-Mateos, M.; Montero, P. A Chitosan–Gelatin Blend as A Coating for Fish Patties. Food Hydrocoll. 2005, 19, 303–311. DOI: 10.1016/j.foodhyd.2004.06.006.
- Sallam, K. I.;. Antimicrobial and Antioxidant Effects of Sodium Acetate, Sodium Lactate, and Sodium Citrate in Refrigerated Sliced Salmon. Food Control. 2007, 18, 566–575. DOI: 10.1016/j.foodcont.2006.02.002.
- Suvanich, V.; Jahncke, M. L.; Marshall, D. L. Changes Selected Chemical Quality Characteristics of Channel Catfish Frame Mince during Chill and Frozen Storage. Food Sci. 2000, 65(1), 24–29. DOI: 10.1111/j.1365-2621.2000.tb15950.x.
- Goulas, A. E.; Kontominas, M. G. Effect of Salting and Smoking-method on the Keeping Quality of Chub Mackerel (Scomber Japonicus): Biochemical and Sensory Attributes. Food Chem. 2005, 93, 511–520. DOI: 10.1016/j.foodchem.2004.09.040.
- Siripatrawan, U.; Noipha, S. Active Film from Chitosan Incorporating Green Tea Extract for Shelf Life Extension of Pork Sausages. Food Hydrocoll. 2012, 27, 102–108. DOI: 10.1016/j.foodhyd.2011.08.011.
- Woyewoda, A. D.; Shaw, S. J.; Ke, P. J.; Burns, B. G. Recommended Laboratory Methods for Assessment of Fish Quality. Canadian Technical Report of Fish and Aquatic Science, 1986. 1448.
- Zhang, L.; Luo, Y.; Hu, S.; Shen, H. Effects of Chitosan Coatings Enriched with Different Antioxidants on Preservation of Grass Carp (Ctenopharyngodon Idellus) during Cold Storage. J. Aquat. Food Prod. Technol. 2012, 21, 508–518. DOI: 10.1080/10498850.2011.621047.
- Ebadi, Z.; Khodanazary, A.; Hosseini, S. M.; Zanguei, N. The Shelf Life Extension of Refrigerated Nemipterus Japonicus Fillets by Chitosan Coating Incorporated with Propolis Extract. Int. J. Biol. Macromol. 2019, 139, 94–102. DOI: 10.1016/j.ijbiomac.2019.07.204.
- Sikorski, Z. E.; Kolakowska, A.; Burt, J. R. Postharvest Biochemical and Microbial Changes Seafood. In Seafood: Resources Nutritional Composition and Preservation; Sikorski, A. E., Ed.; CRC Press: Boca Raton, FL, 1990; pp 55–75.
- Li, J.; He, X.; Li, M.; Zhao, W.; Liu, L.; Kong, X. Chemical Fingerprint and Quantitative Analysis for Quality Control of Polyphenols Extracted from Pomegranate Peel by HPLC. Food Chem. 2015, 176, 7–11. DOI: 10.1016/j.foodchem.2014.12.040.
- Çam, M.; Cihat Içyer, N.; Erdoğan, F. Pomegranate Peel Phenolics: Microencapsulation, Storage Stability and Potential Ingredient for Functional Food Development. LWT-Food Sci. Technol. 2014, 55, 117–123. DOI: 10.1016/j.lwt.2013.09.011.
- Mohebi, E.; Shahbazi, Y. Application of Chitosan and Gelatin Based Active Packaging Films for Peeled Shrimp Preservation: A Novel Functional Wrapping Design. LWT-Food Sci. Technol. 2017, 76, 108–116. DOI: 10.1016/j.lwt.2016.10.062.
- Berizi, E.; Hosseinzadeh, S.; Shekarforoush, S. S.; Barbieri, G. Microbial, Chemical, Textural and Sensory Properties of Coated Rainbow Trout by Chitosan Combined with Pomegranate Peel Extract during Frozen Storage. Int. J. Biol. Macromol. 2018, 106, 1004–1013. DOI: 10.1016/j.ijbiomac.2017.08.099.
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of Chitosan Coating Combined with Pomegranate Peel Extract on the Quality of Pacific White Shrimp during Iced Storage. Food Control. 2016, 59, 818–823. DOI: 10.1016/j.foodcont.2015.07.011.
- Derde, M.; Nau, F.; Guérin-Dubiard, C.; Lechevalier, V.; Paboeuf, G.; Jan, S.; Baron, F.; Gautier, M.; Vié, V. Native and Dry-heated Lysozyme Interactions with Membrane Lipid Monolayers: Lipid Packing Modifications of a Phospholipid Mixture, Model of the Escherichia Coli Cytoplasmic Membrane. Biochim. Biophys. Acta. 2015, 1848, 1065–1073.
- Lopes, N. A.; Pinilla, C. M. B.; Brandelli, A. Antimicrobial Activity of Lysozyme-nisin Co-encapsulated in Liposomes Coated with Polysaccharides. Food Hydrocoll. 2019, 93, 1–9. DOI: 10.1016/j.foodhyd.2019.02.009.
- Rawdkuen, S.; Suthiluk, P.; Kamhangwong, D.; Benjakul, S. Mechanical, Physic-chemical, and Antimicrobial Properties of Gelatin-based Film Incorporated with Catechin-lysozyme. Chem. Cent. J. 2012, 6, 131. DOI: 10.1186/1752-153X-6-131.
- Boyac, D.; Korel, F.; Yemenicioğlu, A. Development of Activate-at-home-type Edible Antimicrobial Films: An Example pH-triggering Mechanism Formed for Smoked Salmon Slices Using Lysozyme in Whey Protein Films. Food Hydrocoll. 2016, 60, 170–178. DOI: 10.1016/j.foodhyd.2016.03.032.
- Rao, M. S.; Chander, R.; Sharma, A. Synergistic Effect of Chitooligosaccharides and Lysozyme for Meat Preservation. LWT- Food Sci. Technol. 2008, 41, 1995–2001.
- Tzikas, Z.; Ambrosiadis, I.; Soultos, N.; Georgakis, S. Quality Assessment of Mediterranean Horse Mackerel (Trachurus Mediterraneus) and Blue Jack Mackerel (Trachurus Picturatus) during Storage in Ice. Food Control. 2007, 18, 1172–1179. DOI: 10.1016/j.foodcont.2006.07.014.
- He, Q.; Xiao, K. The Effects of Tangerine Peel (Citri Reticulatae Pericarpium) Essential Oils as Glazing Layer on Freshness Preservation of Bream (Megalobrama Amblycephala) Duringsuperchilling Storage. Food Control. 2016, 69, 339–345. DOI: 10.1016/j.foodcont.2016.05.019.
- Nirmal, N. P.; Benjakul, S. Retardation of Quality Changes of Pacific White Shrimp by Green Tea Extract Treatment and Modified Atmosphere Packaging during Refrigerated Storage. Int. J. Food Microbiol. 2011, 149, 247–253. DOI: 10.1016/j.ijfoodmicro.2011.07.002.
- Shahidi, F.; Kamil, J.; Arachchi, V.; Jeon, Y. J. Food Applications of Chitin and Chitosans. Trends Food Sci. Tech. 1999, 10, 37–51. DOI: 10.1016/S0924-2244(99)00017-5.
- Fan, W. J.; Chi, Y. L.; Zhang, S. The Use of a Tea Polyphenol Dips to Extend the Shelf Life of Silver Carp (Hypophthalmicthys Molitrix) during Storage in Ice. Food Chem. 2008, 108, 148–153. DOI: 10.1016/j.foodchem.2007.10.057.
- Kilincceker, O.; Dogan, I. S.; Kucukoner, E. Effect of Edible Coatings on the Quality of Frozen Fish Fillets. LWT - Food Sci. Technol. 2009, 42, 868–873. DOI: 10.1016/j.lwt.2008.11.003.
- Benjakul, S.; Visessanguan, W.; Phongkanpai, V.; Tanaka, M. Antioxidative Activity of Caramelisation Products and Their Preventive Effect on Lipid Oxidation in Fish Mince. Food Chem. 2005, 90, 231–239. DOI: 10.1016/j.foodchem.2004.03.045.
- Antoniewski, M. N.; Barringer, S. A.; Knipe, C. L.; Zerby, H. N. Effect of a Gelatin Coating on the Shelf Life of Fresh Meat. J. Food Sci. 2007, 72, 382–387. DOI: 10.1111/jfds.2007.72.issue-6.
- Shakila, R.; Jeyasekaran, G.; Vijayalakshmi, S. Effect of Vacuum Packaging on the Quality Characteristics of Seerfish (Scomberomorus Commersonii) Chunks during Refrigerated Storage. J. Food Sci.Technol. 2005, 42, 438–443.
- Yin, X. F.; Luo, Y. K.; Fan, H. B.; Wu, H.; Feng, L. G. Effect of Previous Frozen Storage on Quality Changes of Grass Carp (Ctenopharyngodon Idellus) Fillets during Short-term Chilled Storage. Int. J. Food Sci. Technol. 2014, 49, 1449–1460. DOI: 10.1111/ijfs.2014.49.issue-6.
- Nisar, T.; Yang, X.; Alim, A.; Iqbal, M.; Wang, Z.; Guo, Y. Physicochemical Responses and Microbiological Changes of Bream (Megalobrama Ambycephala) to Pectin Based Coatings Enriched with Clove Essential Oil during Refrigeration. Int. J. Biol. Macromol. 2019, 124, 1156–1166. DOI: 10.1016/j.ijbiomac.2018.12.005.
- Kaewprachu, P. ;.; Osako, K.; Benjakul, S.; Suthiluk, P.; Rawdkuen, S. Shelf Life Extension for Bluefin Tuna Slices (Thunnus Thynnus) Wrapped with Myofibrillar Protein Film Incorporated with catechin-Kradon Extract. Food Control. 2017, 79, 333–343. DOI: 10.1016/j.foodcont.2017.04.014.
- Benjakul, S.; Bauer, F. Physicochemical and Enzymatic Changes of Cod Muscle Proteins Subjected to Different Freeze-thaw Cycles. J. Sci. Food Agric. 2000, 80, 1143–1150. DOI: 10.1002/(ISSN)1097-0010.
- Benjakul, S.; Sutthipan, N. Muscle Changes in Hard and Soft Shell Crabs during Frozen Storage. Lwt-Food Sci. Technol. 2009, 42, 723–729. DOI: 10.1016/j.lwt.2008.10.003.
- Mohan, C. O.; Ravishankar, C. N.; Lalitha, K. V.; Srinivasa Gopal, T. K. Effect of Chitosan Edible Coating on the Quality of Double Filleted Indian Oil Sardine (Sardinella Longiceps) during Chilled Storage. Food Hydrocoll. 2012, 26(1), 167–174. DOI: 10.1016/j.foodhyd.2011.05.005.
- Hui, G. H.; Liu, W.; Feng, H. L.; Li, J.; Gao, Y. Y. Effects of Chitosan Combined with Nisin Treatment on Storage Quality of Large Yellow Croaker (Pseudosciaena Crocea). Food Chem. 2016, 203, 276–282. DOI: 10.1016/j.foodchem.2016.01.122.