ABSTRACT
In order to increase use of soybean protein in meat products, the objective of this research was to investigate the effects of k-carrageenan on the gel properties of the myofibrillar protein (MP)–soybean protein isolate (SPI) composite with various salt levels. The results showed that the addition of SPI in a small amount improved the gelation and WHC of MP-SPI composite gel, and incorporation of k-carrageenan enhanced WHC at low NaCl concentration (0.1 mol/L and 0.3 mol/L). The observation results of the environmental scanning electron microscope (ESEM) suggested that the strong WHC of k-carrageenan and the filling of soybean protein promoted the gelation forming. It was related to the positive effects on the elastic modulus, gel strength and WHC of MP mixing with a small amount of soybean protein and larger content of k-carrageenan. However, at a high salt concentration (0.6 mol/L), SPI showed negative effects on the gel strength and WHC of composite MP-SPI. The gel strength and WHC of mixed MP-SPI were reinforced with the addition of k-carrageenan. In conclusion, the results of this research showed that the interactions in the system of the k-carrageenan-MP-SPI composite were significantly influenced by salt concentration. Low concentration of soybean protein and high level of k-carrageenan at low-salt concentration improved composite gel properties.
Introduction
The quality of meat is mainly determined by the properties of the myofibrillar protein (MP). MP plays an important role in the water-holding capacity, elastic properties, and gel strength of meat products. Up to 2–3% NaCl is often added to meat products, and this enables MP to exhibit a relatively high solubility and produces a high-quality product.[Citation1] However, a study by Chang has found that the dissolution of MP is not necessary to produce high-quality meat.[Citation2,Citation3] Chin et al. found that the gel strength of MP formed when the salt ion intensity was lower than 0.3 mol/L was weak, and when it was less than 0.1 mol/L, almost no gel was formed.[Citation4]
It is difficult to reduce the salt content in meat products that rely on MP gel properties, such as salted ham, roasted sausage, and Taiwanese-style sausages, and the existing improvement methods include the addition of soy protein, polysaccharides, etc. The gel interaction modes between MP and soy protein isolate (SPI) can be divided into incompatible, semi-compatible and compatible systems according to whether the two are immiscible, partially miscible, and miscible;[Citation5] when fully miscible, the two can intersperse with each other to form a polymer network.[Citation6] Feng et al. reported that after preheating treatment, the SPI could enhance the gel strength and elastic modulus of MP and found that SPI without preheating showed a destructive effect.[Citation7] Studies have shown that the addition of a small amount of nonmeat protein, such as soy protein, to the meat product recipe, can improve the gel-forming capacity, water-holding capacity, and oil holding capacity of the meat product as well as increase the yield of the product. However, the addition of a high amount of soy protein and other nonmeat proteins will often cause decreases in the gel strength, elasticity, and water-holding capacity of the meat product, and the quality of the meat product is lowered.[Citation8,Citation9] Chin found that the use of 2.2% commercial SPI instead of meat protein did not affect the gel strength of bologna sausages, but when the amount was 4.4%, the texture of the sausages was softened,[Citation10] while Matulis reported that the addition of 3% commercial SPI could increase the hardness of Frankfurter-type sausage.[Citation11]
In the simulated system, great progress has been made in using polysaccharides to improve the gel-forming capacity of MP under low-salt conditions.[Citation4,Citation10,Citation12,Citation13] However, in SPI-containing meat product systems, especially in those with a high SPI content and low-salt content, the application of polysaccharides has only produced very limited results. Most of the current research considers the MP-SPI system, MP-polysaccharide system[Citation14–Citation17] and SPI-polysaccharide composite gel system. However, for different salt ion concentration conditions, few studies have focused on the MP-SPI-polysaccharide ternary composite system, and there is a lack of systematization. For example, Hasanpour studied the effects of xanthan gum and soy protein concentrate on the emulsifying properties of products during the storage of meat products.[Citation18] Lin studied the effects of i-carrageenan and sodium alginate on the emulsifying properties of SPI-containing low-fat meat products.[Citation19] However, the two studies did not systematically study the gel properties of the composite system. Therefore, this study investigated the effect of hydrophilic colloid on the gel properties and gel structure of soy protein-containing MP at different salt ion concentrations by establishing a ternary composite system consisting of k-carrageenan, MP, and SPI in order to provide a basis for improving the quality of meat products that contain a high proportion of soy protein.
Materials and methods
Chemicals and reagents
Pork loin was purchased from Oushang supermarket in Wuxi city. SPI (Protein content is 85%) came from Pingdingshan Tianjing Plant Protein Co., Ltd. k-carrageenan was purchased from Danisco.
The extraction of MP
MP was extracted from lean pork tenderloin according to Park’s method.[Citation20] The protein content in MP was determined by the biuret method. Bovine serum protein was used as the standard protein to make the standard curve. The MP concentration was corrected to 50 mg/mL with 0.1, 0.3, 0.6 mol/L NaCl, and pH6.2 phosphate buffer, and stored in crushed ice for later use.
Preparation of SPI and k-carrageenan solution
SPI and k-carrageenan were dissolved in PBS (pH6.2) of 0.1, 0.3, 0.6 mol/L NaCl, stirred for 4 h, and the mass fraction of SPI and k-carrageenan solution were 5% respectively.
Preparation of k-carrageenan–SPI-MP composite system
K-carrageenan concentration gradients of 0%, 0.1%, 0.5% and 1% were considered, respectively. Four concentration gradients of 0%, 0.1%, 0.5%, and 1% were considered, respectively. Under different concentrations of NaCl (0.1, 0.3, and 0.6 mol/L), MP and SPI were mixed according to the ratios of 1:0, 3:1, and 1:1 (m:m). The sample protein concentration was maintained at 40 mg/mL. In a 50 mL centrifuge tube, 25 g of each sample was added, and the sample was centrifuged at 1000 r/min to remove the bubbles from the sample. Subsequently, 5 g of sample was transferred to a flat-bottom glass tube (an inner diameter of 16.8 mm), which was sealed with aluminum foil. The circulating water bath was used for heating, the temperature was raised to 80°C, the heating rate was maintained at 1.0–1.2°C/min, and the temperature was held for 5 min. The sample was quickly cooled in ice water and placed in a refrigerator at 4°C overnight. Three parallel samples were prepared for each group.
Rheological properties of k-carrageenan–SPI-MP composite system
The viscoelasticity of the composite protein gel was determined according to Liu and Xiong ’s method.[Citation21] In the linear region of the sample deformation, the AR G2 rheometer in the small-amplitude sinusoidal oscillation mode was used to measure the change in the elastic modulus (G’) during the gel formation process of the mixed sample at salt concentrations of 0.3 and 0.6 mol/L NaCl. A 2 cm diameter plate clamp was used, the temperature was raised from 20°C to 80°C at a rate of 2°C/min, the frequency was 0.1 Hz, the maximum strain was 0.02, and the plate spacing was 1 mm. The rheometer recorded the change in the elastic modulus of the entire system when the mixed sample was heated to form gels.
Gel strength of k-carrageenan–SPI-MP complex
The protein gel was prepared at a low temperature overnight according to the method of preparation of the k-carrageenan–SPI-MP composite system, and the k-carrageenan–SPI-MP composite was allowed to stand at room temperature for 1 h before the measurement. The texture analyzer setting parameters were as follows: P/0.5 cylinder probe; pretest speed, 1.0 mm/s; test speed, 0.5 mm/s; posttest speed, 10 mm/s; measuring distance, 50% of the sample height; trigger type, auto; trigger force, 5 g; and data acquisition speed, 200 pps. The peak force that occurred during probe compression was defined as the gel strength.
Water-holding capacity of k-carrageenan–SPI-MP composite system
The K.-W.Lin et al.[Citation19] method was used for determination with slight modifications. The prepared protein gel was placed overnight at low temperature, and the sample was balanced at room temperature for 1 h before determination. In order to reduce the experimental error, the sampling position should be as consistent as possible and three parallel samples should be made each time. The sample (m0) was transferred to a centrifuge tube, and the total mass of the sample and centrifuge tube was m1. After the sample was centrifuged at 10000 g at 4°C for 10 min, the supernatant was removed, and the filter paper was used to absorb the residual water on the surface of the gel blocks. The centrifuge tube was weighed, and the mass of the sample and centrifuge tube at this moment was m2. The results were calculated according to the following equation: WHC (%) = (m0+ m2-m1)/m0 × 100%.
Observation on microstructure of k-carrageenan–SPI-MP complex
The microstructure of the gel was observed using an XL-30 ESEM environmental scanning electron microscope (ESEM). The k-carrageenan–SPI-MP complex was cut into gel blocks of approximately 5 mm×3 mm×7 mm, adhered to a special round mold for ESEM, and placed in an ESEM sample chamber for observation. The test conditions were as follows: accelerating voltage, 20 kV; constant voltage, 119.97 kV; sample chamber temperature, 8°C; and magnification, 800 times. A metal film was coated on the surface of the sample with a TM3030 (HITACHI) ion sputter coater, and gold coating was carried out for approximately 1 min.
Statistical analysis
Results were expressed as mean±standard error of the mean of at least three independent experiments unless otherwise specified. Statistical analyses were carried out using Statistic software. The statistical significance of the data was determined using LSD and multiple comparison using two-way ANOVA followed by Tukey’s multiple-comparison test with a P < .05
Taken as the value of significance.
Results and discussion
Effect of k-carrageenan on the elastic modulus of SPI-MP
The effect of k-carrageenan on the elastic modulus of SPI-MP complex protein at different salt concentrations is shown in . From –), we can see that at a salt concentration of 0.6 mol/L NaCl, the G’ of pure MP started to rise at 40°C, reached its maximum at 50°C, and drop to its minimum at 58°C, in line with Sun and Xiong.[Citation17,Citation22] The addition of k-carrageenan hardly changed the gel temperature of MP, but the gel strength can be greatly increased. It may be that the addition of k-carrageenan increased the relative concentration of MP in the same system due to the competition of space between incompatible macromolecules. Therefore, under a certain concentration of k-carrageenan, G‘ in the mixed system increased with the increasing of k-carrageenan.[Citation23] With the addition of SPI, the rising temperature of G’ was delayed and the intensity became weaker. Studies have shown that MP G’ increased at about 50°C. It may be based on the denaturation of the myosin heavy chain in MP and the cross-linked aggregation through the disulfide bond.[Citation22] However, the addition of SPI resulted in MP heavy chain denaturation and cross-linking peak pushing toward high temperature and suggested that SPI can interfere with MP gel under the concentration of 0.6 mol/L NaCl. According to , at the concentration of 0.6 mol/L NaCl, the final value of G’ of various combinations of MP-SPI gel increased with the increase of k-carrageenan.
Figure 1. Effect of carrageenan adding levels on the G′ of MP-SPI composite gel with various salt levels
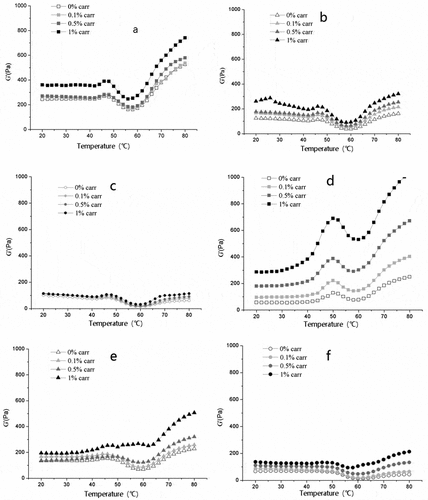
The G‘ value of MP-SPI (3:1) system with the addition of 1% k-carrageenan at the concentration of 0.3 mol/L NaCl (as shown in –) was higher than that of 0.6 mol/L NaCl. The interference effect of SPI under the condition of 0.6 mol/L NaCl suggested that the addition of a small amount of SPI and a large amount of k-carrageenan had a good gelatinizing effect on the complex protein under the condition of insufficient solubility of MP and at low-salt concentration. Figures a, b, and c and Figures d, e, and f for 0.5% and 1% k-carrageenan composite protein systems under 0.3 and 0.6 mol/L NaCl conditions were compared. It was found that the low-salt concentration is more favorable for the formation of hydrogen bonds and ionic bonds in the system.[Citation24,Citation25]
Effect of k-carrageenan on the gel strength of SPI-MP
The effect of k-carrageenan on the gel strength of the SPI-MP composite protein system at different salt concentrations is shown in . At a concentration of 0.1 mol/L NaCl and 0.3 mol/L NaCl, pure MP hardly formed a gel and the gel strength was very weak. With the addition of a small amount of SPI, the coagulant strength of the compound coagulant became stronger when the total protein concentration (40 mg/mL) remained unchanged. Especially under the concentration of 0.1 mol/L NaCl, the gel strength of 3% MP+ 1% SPI was much higher than that of pure MP glue. The gel strength weakened again when further increasing the SPI ratio. It may be that in the case of insufficient MP dissolution, the addition of low-concentration SPI molecules played a synergistic role due to hydrophobic interaction and intermolecular entanglement.[Citation4] The addition of k-carrageenan increased the strength of the composite protein gel, but the enhancement effect was not obvious when the concentration was higher than 0.5 mol/L NaCl. This was consistent with the rheological results and confirmed that the addition of a small amount of SPI and a large amount of k-carrageenan had a good gelatinizing effect on the composite protein under the condition of insoluble MP in low-salt concentration. Under the high salt concentration of 0.6 mol/L NaCl, the gel strength of the system decreased significantly with the increase of the amount of k-carrageenan added in the pure MP system.
Table 1. Effect of k-carrageenan-adding levels on the gel strength (g) of MP-SPI composite with various salt levels
It was possible that the anion polysaccharide k-carrageenan and the MP with the same charge will have an antagonistic effect and destroy the gel structure.[Citation26] At the same time, the addition of SPI also weakened the gel strength of the protein system.[Citation27] In the MP-SPI (3:1) system, adding 0.1–1.0% of k-carrageenan could not significantly change the gel strength of the system and suggested that low SPI concentration at high salt concentration could reduce the damage degree of k-carrageenan to the complex protein gel system. The above result was consistent with the rheological data obtained in , demonstrating that a high salt concentration reduced the effect of k-carrageenan on enhancing the composite protein gel strength.
Figure 2. The SEM network of MP-SPI composite gel with various salt levels
Effect of k-carrageenan on water-holding capacity of SPI-MP
In the actual production process of meat products, the water-holding capacity of products is an important production indicator to be controlled. The effect of k-carrageenan on the water-holding capacity of the SPI-MP composite protein system gel is shown in . At the low-salt concentrations of 0.1 mol/L and 0.3 mol/L NaCl, vertical data comparison was conducted. When the k-carrageenan content was less than 1%, 1% SPI increased the water-holding capacity of the sample gel, and when the amount of SPI added continued to increase, the water-holding capacity of the sample decreased, indicating that 1% SPI is conducive to the formation of muscle fiber gel. The horizontal data comparison showed that with increasing amounts of k-carrageenan added, the water-holding capacity of protein samples was significantly improved. When the k-carrageenan content was 1%, the water-holding capacity of all samples was close to 100%, indicating that the water-holding capacity of polysaccharide itself was dominant when the k-carrageenan content was 1%.
Table 2. Effect of k-carrageenan-adding levels on the WHC (%) of MP-SPI composite with salt levels
When the salt concentration was 0.6 mol/L, a vertical data comparison was conducted. When the proportion of SPI increased from 0% to 2%, the water-holding capacity of the sample gel gradually decreased. The addition of k-carrageenan could reduce the destructive effect of SPI on the water-holding capacity of the composite gel; the horizontal data comparison showed that k-carrageenan could significantly improve the water-holding capacity of the MP-SPI composite gel. In summary, k-carrageenan could improve the water-holding capacity of the composite protein gel, and at low-salt concentrations, 1% SPI could also play a role in improving the water-holding capacity. A large number of studies have confirmed that k-carrageenan can improve the water-holding capacity of meat products, but some studies have shown that k-carrageenan can only increase the water-holding capacity of meat products to some extent.[Citation28,Citation29] The different effects of k-carrageenan may be due to the differences in the properties of k-carrageenan or the processing conditions.[Citation30]
Effect of k-carrageenan on gel microstructure of SPI-MP
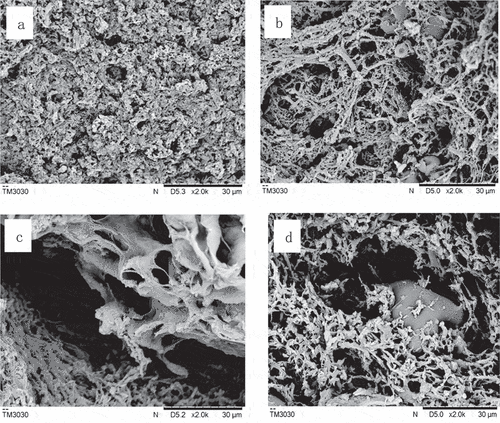
Scanning electron microscopy (SEM) was used to observe the microstructure of the composite protein gel. The four images in show the microstructures of the pure muscle fiber protein system and the composite protein system with SPI added. The four images in show the microstructures of SPI-MP composite protein gel after the addition of k-carrageenan. Since the gel formed under 0.1 mol/L NaCl was very weak, the SEM microscopic observation was only performed on the gel samples at salt concentrations of 0.3 and 0.6 mol/L NaCl. Under the 0.6 mol/L NaCl condition ()), the pure MP gel could form a uniform and microporous matrix-network structure, which is consistent with the results reported by Montero.[Citation31] ) shows the result after the addition of SPI. As shown in ), the quantity of gel fibrous network structures was reduced, and an uneven network structure with large pores was formed. It can be seen that the SPI particles were embedded in the MP gel network structure. Under the condition of 0.3 mol/L NaCl ()), the sample formed a poor network structure since the salt concentration was decreased and the MP was insufficiently dissolved, and the pores were large and unevenly distributed. After 1% SPI was replaced by MP, the composite protein gel structure was slightly improved, but the structure was still poor compared to that under the 0.3 mol/L NaCl condition.
Figure 3. Effect of k-carrageenan on the network (SEM) of MP-SPI composite gel with various salt levels

The effect of k-carrageenan on the microstructure of the composite protein gel is shown in . The comparison of with shows that the addition of k-carrageenan could significantly improve the gel structure of MP. Under the 0.6 mol/L NaCl condition ()), a uniform and compact gel structure was formed, probably due to the interaction of sulfate ions on the k-carrageenan molecules with MP molecules.[Citation25] In ), when both k-carrageenan and SPI were present in the MP system, a more compact gel structure could be formed, and the SPI particles were more compactly embedded in the gel structure. At the salt concentration of 0.3 mol/L NaCl ((c) and (d)), the composite protein can also form ideal gels after the addition of k-carrageenan. The above findings indicated that the strong water-holding capacity and gel-forming properties of k-carrageenan itself and the filling of the SPI played a positive role in promoting gel formation when MP was insufficiently dissolved under low-salt conditions. It also explained why SPI and k-carrageenan increased the storage modulus, gel strength, and water-holding capacity of the composite protein.
Conclusion
In this study, factors affecting the strength of the composite gel in the three-phase system of SPI-MP-k-carrageenan were discussed in detail. The effects of SPI and k-carrageenan on MP, gel properties and water-holding capacity of the mixture were clarified. For the composite protein gel system, the addition of k-carrageenan at 0.1 and 0.3 mol/L NaCl could increase the elastic modulus, gel strength, and water-holding capacity of the composite protein gel as well as improve the microstructure so that the original system (containing a high SPI content, 2% MPI + 2% SPI), which could not form gels, exhibited a higher gel-forming capacity and water-holding capacity, and the enhancement effect was significant. Under the 0.6 mol/L NaCl condition, k-carrageenan increased the elastic modulus, gel strength, and water-holding capacity of the composite protein system as well as reduced the interference of SPI on MP gel; k-carrageenan improved the gel strength and microstructure of the composite protein. The results showed that the interaction of k-carrageenan-MP-SPI in the complex system was significantly affected by the salt concentration, and under the condition of low salt, the addition of low-concentration soy protein and high concentration k-carrageenan could improve the gel properties of the compound protein.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under Grant [number 31471583]; the National Key R&D Program of China under Grant [number 2018YFD0401200]; and the Pingdingshan Science and Technology Bureau under Grant [number 2017010(10.4)]. The authors declare no conflict of interest.
Additional information
Funding
References
- Xiong, Y. L.; Brekke, C. J. Protein Extractability and Thermally Induced Gelation Properties of Myofibrils Isolated from Pre-and Post-rigor Chicken Muscles. J. Food Sci. 1991, 56(1), 210–215. DOI: 10.1111/j.1365-2621.1991.tb08013.x.
- Chang, H. S.; Feng, Y.; Hultin, H. O. Role of pH in Gel Formation of Washed Chicken Breast Muscle at Low Ionic Strength. J. Food Biochem. 2001, 25, 439–457. DOI: 10.1111/j.1745-4514.2001.tb00751.x.
- Kristinsson, H. G.; Hultin, H. O. Role of pH and Ionic Strength on Water Relationships in Washed Minced Chicken-breast Muscle Gels. J. Food Sci. 2003, 68(3), 917–922. DOI: 10.1111/j.1365-2621.2003.tb08265.x.
- Chin, K. B.; M Y, G.; Xiong, Y. L. Konjac Flour Improved Textural and Water Retention Properties of Transglutaminase-mediated, Heat-induced Porcine Myofibrillar Protein Gel: Effect of Salt Level and Transglutaminase Incubation. Meat Sci. 2009, 81(3), 565–572. DOI: 10.1016/j.meatsci.2008.10.012.
- Manson, J. A.; Sperling, L. H. Polymer Blends and Composites. 1976. Plenum Press: New York, 2003.
- Ziegler, G. R.; Foegeding, E. A. The Gelation of Proteins. Adv. Food Nut. Res. 1990, 34, 203–298. DOI: 10.1016/S1043-4526(08)60008-X.
- Feng, J.; Xiong, Y. L. Interaction of Myofibrillar and Preheated Soy Proteins. J. Food Sci. 2002, 67(8), 2851–2856. DOI: 10.1111/j.1365-2621.2002.tb08827.x.
- Foegeding, E. A.; Lanier, T. C. The Contribution of Non-muscle Proteins to Texture of Gelled Muscle Protein Foods. Cereal Foods World (USA). 1987, 13, 331–344.
- McCord, A.; Smyth, A. B.; O’Neill, E. E. Heat-induced Gelation Properties of Salt-soluble Muscle Proteins as Affected by Non-meat Proteins. J. Food Sci. 1998, 63(4), 580–583. DOI: 10.1111/j.1365-2621.1998.tb15789.x.
- Chin, K. B.; Keeton, J. T.; Longnecker, M. T., Lamkey, J.W. Utilization of Soy Protein Isolate and Konjac Blends in a Low-fat Bologna (Model System) . Meat Sci. 1999, 53(1), 45–57. DOI: 10.1016/S0309-1740(99)00035-2.
- Matulis, R. J.; Mcketh, F. K.; Sutherland, J. W., Brewer, M.S. Sensory Characteristics of Frankfurters as Affected by Salt, Fat, Soy Protein, and Carrageenan. J. Food Sci. 1995, 60(1), 48–54. DOI: 10.1111/j.1365-2621.1995.tb05604.x.
- Cofrades, S.; López-López, I.; Solas, M. T., Bravo, L.; Jiménez-Colmenero, F. Influence of Different Types and Proportions of Added Edible Seaweeds on Characteristics of Low-salt Gel/emulsion Meat Systems. Meat Sci. 2008, 79(4), 767–776. DOI: 10.1016/j.meatsci.2007.11.010.
- Hong, G. P.; Chin, K. B. Effects of Microbial Transglutaminase and Sodium Alginate on Cold-set Gelation of Porcine Myofibrillar Protein with Various Salt Levels. Food Hydrocolloids. 2010, 24(4), 444–451. DOI: 10.1016/j.foodhyd.2009.11.011.
- Montero, P.; Solas, T.; Pérez-Mateos, M. Pressure Induced Gel Properties of Fish Mince with Ionic and Non-ionic Gums Added. Food Hydrocolloids. 2001, 15, 185–194. DOI: 10.1016/S0268-005X(00)00064-3.
- Lin, K. W.; Huang, H. Y. Konjac/gellan Gum Mixed Gels Improve the Quality of Reduced-fat Frankfurters . Meat Sci. 2003, 65(2), 749–755. DOI: 10.1016/S0309-1740(02)00277-2.
- Totosaus, A.; Pérez-Chabela, M. L. Textural Properties and Microstructure of Low-fat and Sodium-reduced Meat Batters Formulated with Gellan Gum and Dicationic Salts. LWT Food Sci. Technol. 2009, 42(2), 563–569. DOI: 10.1016/j.lwt.2008.07.016.
- X D, S.; Holley, R. A. Factors Influencing Gel Formation by Myofibrillar Proteins in Muscle Food. Compr. Rev. Food Sci. Food Saf. 2011, 10(1), 33–51. DOI: 10.1111/j.1541-4337.2010.00137.x.
- Hasanpour, F.; Hoseini, E.; Motalebi, A. A.; Darvish, F. Effects of Soy Protein Concentrate and Xanthan Gum on Physical Properties of Silver Carp (Hypophthalmichthys Molitrix) Surimi. Iran. J. Fish. Sci. 2012, 11(3), 518–530.
- Lin, K.-W.; Mei., M.-Y. Influences of Gums, Soy Protein Isolate, and Heating Temperatures on Reduced-fat Meat Batters in a Model System. Food Chem Toxicol. 2000, 65(1), 45–52. DOI: 10.1111/j.1365-2621.2000.tb15954.x.
- Park, D.; Xiong, Y. L. Oxidative Modification of Amino Acids in Porcine Myofibrillar Protein Isolates Exposed to Three Oxidizing Systems. Food Chem. 2007, 103(2), 607–616. DOI: 10.1016/j.foodchem.2006.09.004.
- Liu, G.; Xiong, Y. L. Thermal Transitions and Dynamic Gelling Properties of Oxidatively Modified Myosin, β-lactoglobulin, Soy 7S Globulin and Their Mixtures. J. Sci. Food Agric. 2000, 80(12), 1728–1734. DOI: 10.1002/1097-0010(20000915)80:12<1728::AID-JSFA711>3.0.CO;2-A.
- Xiong, Y. L.; Suzanne, P. B. Myofibrillar Protein Gelation: Viscoelastic Changes Related to Heating Procedures. J. Food Sci. 1994, 59(4), 734–738. DOI: 10.1111/j.1365-2621.1994.tb08115.x.
- Ya, G. V.; Bi, I. V. Thermodynamic Incompatibility of Proteins and Polysaccharides in Solution. Food Hydrocolloids. 1997, 11(2), 145–158. DOI: 10.1016/S0268-005X(97)80022-7.
- Morita, J. I.; Choe, I. S.; Yamamoto, K.; Samejima, K.; Yasui, T. Heat-induced Gelation of Myosin from Leg and Breast Muscles of Chicken. Agric Biol Chem. 1987, 51(11), 2895–2900. DOI: 10.1080/00021369.1987.10868500.
- O’Neill, E.; Mulvihill, D. M.; Morrissey, P. A. Molecular Forces Involved in the Formation and Stabilization of Heat-induced Actomyosin Gels. Meat Sci. 1994, 36(3), 407–421. DOI: 10.1016/0309-1740(94)90136-8.
- Ramırez, J. A.; Barrera, M.; Morales, O. G.; Vázquez, M. Effect of Xanthan and Locust Bean Gums on the Gelling Properties of Myofibrillar Protein. Food Hydrocolloids. 2002, 16(1), 11–16. DOI: 10.1016/S0268-005X(01)00033-9.
- Djabourov, M.; Nishinari, K.; Ross-Murphy, S. B. Physical Gels from Biological and Synthetic Polymers; Cambridge University Press: Cambridge, 2013.
- Pietrasik, Z.; Duda, Z. Effect of Fat Content and Soy Protein/carrageenan Mix on the Quality Characteristics of Comminuted, Scalded Sausages. Meat Sci. 2000, 56(2), 181–188. DOI: 10.1016/S0309-1740(00)00038-3.
- Verbeken, D.; Neirinck, N.; Van Der Meeren, P.; Dewettinck, K. Influence of κ-carrageenan on the Thermal Gelation of Salt-soluble Meat Proteins. Meat Sci. 2005, 70(1), 161–166. DOI: 10.1016/j.meatsci.2004.12.007.
- Barbut, S.; Mittal, G. S. Use of Carrageenans and Xanthan Gum in Reduced Fat Breakfast Sausages. LWT Food Sci. Technol. 1992, 25(6), 509–513.
- Montero, P.; Hurtado, J. L.; Pérez-Mateos, M. Microstructural Behavior and Gelling Characteristics of Myosystem Protein Gels Interacting with Hydrocolloids. Food Hydrocolloids. 2000, 14(5), 455–461. DOI: 10.1016/S0268-005X(00)00025-4.
