ABSTRACT
The aim of this study was to investigate the effects of thermal processing on physicochemical properties, steviol glycosides, bioactive compounds, and antioxidant capacity degradation of a beverage based on exotic fruit juices, orange juice, açaí, and oat and sweetened with Stevia rebaudiana water extracts at different concentrations. The experimental design comprised a response surface methodology according to a central composite face-centered design. The variable ranges were 60–99°C, 0.25–15 min, 0–2.5% Stevia percentage. This design was used to determine the optimal thermal processing-Stevia concentration in order to obtain the best retention of bioactive compounds and physicochemical properties in the beverage following thermal processing. Thermal processing conducted in the sample containing 2.5% Stevia at 82.4°C for 3.8 min led to the beverage with the greatest presence of antioxidant compounds. The evaluation of the thermal processing critical parameters on antioxidant compounds can contribute to achieve optimal processing conditions in order to obtain new beverages with high antioxidant potential.
Introduction
The combination of two or more fruit juices can be a possible strategy in order to prepare new ready-to-serve beverages.[Citation1] Recently, there has been an increasing interest in the use of natural sweeteners, known as steviol, obtained from the leaves of Stevia rebaudiana. These compounds can be a nutritional strategy in order to replace or substitute sugar energy content with one or more ingredients of low-calorie content.[Citation2]
Stevia has attracted economic and scientific interests due to the sweetness and the supposed therapeutic benefits of its leaf. FDA approved Stevia for commercialization in 2008 and more recently, in November 2011, the European Commission has approved steviol glycosides as a new food additive (E 960). Food industry is developing an array of new products based on Stevia plant extracts in order to satisfy the demand of consumers concerned with healthier eating. Many of these new low-sugar products are not just the old standbys like diet sodas and sugarless gum, but foods and drinks like cereals, fruit juices, cookies, bread, ice cream, flavored milk, pasta sauce, and even bottled water.[Citation3] In Europe, the recent green light will probably lead to wide-scale use.[Citation4] So far, little data have been available regarding the practical applications in foods and the effects of processing on steviol glycosides stability in real food matrices.[Citation5]
Thermal processing causes irreversible losses of nutritional compounds, undesirable changes in physicochemical properties, and alteration of their antioxidants.[Citation6,Citation7] A few inadequate conditions during thermal treatment of the juice reflect on an increase of the concentration of the different derivatives of furfural, and changes in color. Moreover, in the presence of high temperatures substantial degradation of steviol glycosides might take place.
In previous studies, the stability of steviol glycosides in several food matrices was investigated.[Citation8] These authors did not find any sign of decomposition of steviol glycosides after processing and subsequent storage. However, more recently, it was investigated the degradation kinetics of rebaudioside A in various buffer solutions, observing an important degradation of this compound when neutral pH was used.[Citation9]
Fruits such as mango, papaya, and açaí contain a large quantity of bioactive compounds such as ascorbic acid, phenolic compounds, and carotenoids that have been shown to be good contributors to the total antioxidant capacity of foods.[Citation10,Citation11] The aims of the present study were to investigate the effects of the thermal treatment conditions and the influence of Stevia concentrations on physicochemical properties, steviol glycosides, bioactive compounds, and antioxidant capacity of a beverage based on fruit juices (papaya-mango-orange) mixed with açaí and sweetened with Stevia and to determine optimum conditions in order to obtain a fruit juice mixture with the highest levels of health-related compounds.
Materials and methods
Samples
Papaya (Carica papaya), mango (Mangifera indica), oranges (Citrus sinensis L.), and oat beverage (Santiveri, Lérida, Spain) were purchased from a local supermarket (Valencia, Spain).
Stevia rebaudiana Bertoni leaves were supplied by company Anagalide, S.A. (Barbastro, Huesca, Spain) and stored at room temperature. A stock solution (8.33%, w/v) of Stevia rebaudiana was prepared: 100 mL of bottled water at 100°C was added on the dried leaves (8.33 g) and were kept for 30 min. The infusion was vacuum-filtered using filter paper. With this stock solution, different concentrations of Stevia leaves infusion (1.25% and 2.5%, v/v) were prepared.
The beverage was prepared by mixing 32.5%, 10%, and 7.5% (v/v) of papaya, mango, and orange juices, respectively, with the pulp removed, 20% (v/v) of oat beverage, and 30% (v/v) of water (0% Stevia) or the different Stevia leaves infusion (1.25% and 2.5%, v/v). The maximum Stevia concentration (2.5%) was selected taking into account the sucrose concentration of commercial fruit-based beverages and the sweetness equivalence Stevia/sucrose.[Citation12] Finally, açaí (1% w/v) provided by Nature’s Way Products Inc., Springville, Utah, USA, containing 450 mg of açaí berries extract, with 10% of polyphenols was added to the beverage. The beverages were prepared by triplicate just before use.
Thermal treatment
The experiments were carried out in a plate and frame heat exchanger equipped with nominal 66-s hold time tube (FT74X/HTST/UHT, Armfield, Inc.). The beverage, placed in a feed tank, was pumped through the heat exchanger to achieve the treatment conditions. The temperature of 60°C was chosen because it is the temperature usually associated with the blanching of fruits and vegetables. Similarly, heating liquid foods to 88–99°C for 15–30 s is normal in commercial practice in order to pasteurize this kind of product.[Citation13,Citation14] All the treatments were applied in duplicate. The samples were cooled in an ice/water bath (FT61, Armfield, Inc.), packed, and then stored under refrigeration (4 ± 1°C) until analysis.
Physicochemical properties
The pH was determined in a Crison GLP 21 pH-meter (Barcelona, Spain). Brix was determined with an Atago RX-1000 digital refractometer (Atago Company Ltd., Tokyo, Japan). To measure the turbidity index (TI), a sample was centrifuged, the supernatant was taken, and the absorbance at 660 nm was measured.[Citation15] To determine the browning index (BI), a sample was centrifuged, and the supernatant was taken and diluted with ethanol (1:1, v/v). The mixture was filtered and the absorbance was measured at 420 nm.[Citation16] The color analysis was performed using a Hunter Labscan II spectrophotometric colorimeter (Hunter Associates Laboratory Inc., Reston, VA., USA). The results were expressed in accordance with the Commission International d′Eclairage LAB (CIELAB) system with reference to illuminant D65 and with a visual angle of 10°. Three consecutive measurements of each sample were taken. The CIE L*, a*, and b* values were used to calculate the total color differences (ΔE* = [(ΔL*)2+ (Δa*)2+ (Δb*)2]1/2), where ΔL*, Δa*, and Δb* are differences between the untreated thermally treated beverages.[Citation17]
Liquid chromatographic analysis of steviol glycosides
The method of JECFA[Citation18] with various modifications was used. Samples were filtered through a Sep-Pak® cartridge (a reverse-phase C-18 cartridge; Millipore, MA, USA). The cartridges were previously activated with 10 ml of methanol and 10 ml of water. Every 10 ml of sample was eluted with 2 ml of methanol, and filtered through a 0.45 µm membrane filter (Millex-HV13, Millipore) and then analyzed by liquid chromatography. Kromasil 100 C18 precolumn (5 µm, 150 × 4.6 mm) and Kromasil 100 C18 column (5 µm, 150 × 4.6 mm) were used. The mobile phase was Solvent A, acetonitrile, and Solvent B, 10 mmol/L sodium phosphate buffer (pH = 2.6, 32:68, v/v). Steviol glycosides were eluted under 1 mL/min flow rate and the temperature was set at 40°C. Triplicate analyses were performed for each sample. Chromatograms were recorded at 210 nm. The identification of steviol glycosides was carried out by using authentic standards and by comparing the retention times, while quantification was performed by external calibration.
Polarographic determination of ascorbic acid
Beverage (5 mL) was diluted to 25 ml with the extraction solution (oxalic acid 1%, w/v, trichloroacetic acid 2%, w/v, sodium sulfate 1%, w/v). After vigorous shaking, the solution was filtered through a folded filter (Whatman no. 1). Oxalic acid (9.5 ml) 1% (w/v) and 2 ml of acetic acid/sodium acetate 2 M buffer (pH = 4.8) were added to an aliquot of 0.5 ml of filtrate and the solution was transferred to the polarographic cell. A Metrohm 746 VA Trace Analyzer (Herisau, Switzerland) equipped with a Metrohm 747 VA stand was used for the polarographic determination.[Citation19]
Total carotenoids
Two milliliters of the sample was homogenized with 5 mL of extracting solvent (hexane/acetone/ethanol, 50:25:25, v/v/v) and centrifuged for 5 min at 4000 rpm at 5°C. The top layer of hexane was recovered and transferred to a 25 mL volumetric flask. The volume of recovered hexane was then adjusted to 25 mL with hexane. Total carotenoid determination was carried out on the hexane extract by measuring the absorbance at 450 nm. Total carotenoids were calculated using an extinction coefficient of β-carotene, E1% = 2505.[Citation10]
Total anthocyanins
Total anthocyanins were determined using a modified method of Mazza et al.[Citation20] A 10-fold diluted sample of 100 μL was mixed with 1700 μL of distilled water and 200 µL of 5% (v/v) HCl. The sample was held at room temperature for 20 min before measuring the absorbance at 520 nm. Calculations of total anthocyanins were based on cyanidin-3-glucoside (molar absorptivity 25740). All spectrophotometric analyses were performed using a UV–visible spectrophotometer Lambda 20 (Perkin-Elmer, Überlingen, Germany).
Total antioxidant capacity
ABTS·+ test: The Trolox Equivalent Antioxidant Capacity (TEAC) test was adapted from Re et al.[Citation21], based on the capacity of antioxidants to inhibit the radical cation2,2-azino-bis(3-ethylbenzothiazoline6-sulfonate) (ABTS), showing a maximal peak at 734 nm. The ABTS radical cation is formed by the interaction of ABTS (7 mM) with K2S2O8 (2.45 mM).
Oxygen radical absorbance capacity assay (ORAC): The oxygen radical absorbance capacity (ORAC) assay used, with fluorescein, was that described by Ou et al.[Citation22] The automated ORAC assay was carried out on a Wallac 1420 VICTOR2 multilabel counter (Perkin-Elmer, USA), for an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The measurements were made in plates with 96 white flat bottom wells (Sero-Wel, BibbySterilin Ltd., Stone, UK). The reaction was performed at 37°C, as the reaction was started by thermal decomposition of AAPH in 75 mM phosphate buffer (pH 7.0). The final reaction tested and the concentrations of the different reagents were determined following Zulueta et al.[Citation11]
Statistical analysis
A face-centered central composite response surface analysis was used to determine the effect of temperature (T, °C) time (t, min) and Stevia rebaudiana concentration (Stevia, %, v/v) on the steviol glycosides, and physicochemical properties (pH, °Brix, color, turbidity, and browning index) of the beverage. The independent variables of the RSM were temperature (from 60 to 99°C), time (from 0.5 to 15 min), and Stevia concentration (from 0% to 2.50%, v/v). Three (maximum, minimum, and central) values of each factor were considered, leading to 16 experiments. The experimental design was performed twice. The combinations included thermal processing-Stevia conditions with an intermediate level (central point) of the three variables replicated 4 times, which was used to determine the inherent variance in the technique. Experiments were randomized to minimize the systematic bias in the observed responses due to extraneous factors and to increase precision. Experimental data were fitted to a polynomial response surface. The non-significant terms were deleted from the second-order polynomial model after an ANOVA test, and a new ANOVA was performed to obtain the coefficients of the final equation for better accuracy. In the present study, desirability functions were developed in order to obtain the beverage with the highest levels of steviol glycosides and the best physicochemical properties.
An ANOVA of three factors (temperature, time, and Stevia rebaudiana concentration) was applied, and in the parameters for which significant differences were obtained with more than two levels, Tukey’s test was applied to ascertain the range of values in which the differences were located. Finally, a study was conducted with the aim of determining whether there were correlations between a pair of variables (Pearson’s test). All statistical analyses were performed using SPSS® (Statistical Package for the Social Sciences) v.20.0 for Windows (SPSS Inc., Chicago, USA).
Results and discussion
The value of pH in the untreated beverage sweetened with 0, 1.25, and 2.50% (v/v) was 4.45 ± 0.10 in all cases. After the application of the different thermal treatments, non-significant changes in pH values were observed (data not shown). Moreover, the values of °Brix were 7.7 ± 0.1, 8.7 ± 0.1, and 9.7 ± 0.1 for the untreated beverages with 0, 1.25% (v/v), and 2.50% (v/v) of Stevia, respectively. Nonstatistically significant modifications (p > .05) were obtained for °Brix values when the various thermal treatments were applied in these beverages (data not shown).
The results obtained for turbidity index (TI), browning index (BI), and color parameters in the untreated and thermally treated samples are shown in and and the reduced regression model for each studied parameter is presented in . As can be expected, among the studied parameters, Stevia percentage had the greatest effect on the quality parameters of the beverages. The turbidity index of the beverages increased significantly (p < .05) in all the thermally treated samples with 1.25% (v/v) Stevia and in the thermally processed (60°C/0.5–15 min) samples without Stevia added. In addition, the opposite trend was obtained after applying thermal processing when Stevia percentage was 2.50% (v/v).
Table 1. Steviol glycosides and physicochemical properties of untreated exotic fruit juice mixture sweetened with Stevia rebaudiana (Stevia) Bertoni
Table 2. Experimental design matrix in terms of actual variables and the average values of the response for experiments on the effect of combined thermal processing-Stevia rebaudiana concentration on the physicochemical parameters of an exotic fruit juice mixture sweetened with Stevia rebaudiana (Stevia) Bertoni
Table 3. Reduced regression models used to express the parameters analyzed (Y) as a function of independent variables
Compared to the untreated samples, higher browning index values were obtained for the thermally treated samples when 0% and 1.25% (v/v) Stevia percentages were used; however, a significant decrease in BI was observed after applying thermal processing (90°C, 0.5–15 min) in the samples with 2.50% (v/v) Stevia. With regard to the CIELAB parameters, statistical analysis of the results obtained immediately after processing showed significant differences (p < .05) for a*, b*, and L* values among the untreated and thermally treated beverages. Compared to the untreated beverages, non-significant changes in L* values were found in the samples without Stevia and treated at 60°C. However, higher L* values, which indicated a lightening of juice surface color, were found for the samples without Stevia thermally treated (80–99°C) and for the samples with 1.25% and 2.50% (v/v) of Stevia.
The increase in CIE L* values were similar to the results found by Genovese et al.[Citation23] when they studied the effects of thermal pasteurization (65–70°C/15–20 s) in cloudy apple juice and Lee and Coates[Citation24] for thermally treated (91°C, 10 s) grapefruit juice. They attributed it to partial precipitation of unstable, suspended particles in the juice particles in the juices. With regard to b* values, the behavior was different depending on temperature applied. The b* values of the samples treated at 60–80°C were similar or even lower to those found in the untreated samples. However, a significant increase in b* values was observed in the samples treated at 99°C, independently of the Stevia percentage used in the formulation of the beverage. These results were in line to those reported by Lee and Coates[Citation25] after thermal pasteurization of orange juice (90°C, 30 s), Cortes et al.[Citation6] for pasteurized orange juice (90°C, 20 s), Barba et al.[Citation26] for thermally treated (90°C for 15, 21 s and 98°C for 15, 21 s) vegetable beverage and Barba et al.[Citation27] after applying thermal treatment (90°C for 15, 21 s and 98°C for 15, 21 s) in an orange juice-milk beverage.
The a* values after thermal processing were lower to those obtained in untreated samples independently of the Stevia percentage used. This can be explained by the isomeric changes in carotenoids. In this line, Lee and Coates[Citation25] and Gama and de Sylos[Citation28] reported that the highest thermal losses in orange juice were from 5,6-epoxide carotenoids. Nevertheless, β-carotene, which is mainly responsible for the bright orange color of orange juice and total carotenoid pigment content loss, was not significantly altered after thermal treatments. The results obtained in this study are in close agreement to those found by Patras et al.[Citation29] for thermally processed (70°C, 120 s) strawberry and blackberry purées and Barba et al.[Citation27] after applying thermal processing (90°C for 15–21 s and 98°C for 15–21 s) in an orange juice-milk beverage. Total color change (ΔE) was significantly (p < .05) different in thermally processeda sample at (99°C, 0.5–15 min) from unprocessed samples when Stevia percentage used in the formulation was 1.25% and 2.50% (v/v).
With regard to hydroxymethylfurfural (HMF), the results obtained for the three-way ANOVA showed that treatment temperature applied and Stevia percentage had a significant influence (p < .05) on the HMF content of the beverage analyzed in the present study, obtaining a higher increase in HMF after applying higher temperatures (). But in all cases, the values did not reach the tolerable limit after processing (5 mg/L of HMF).[Citation30] These results were consistent with the findings in the literature.[Citation6,Citation31]
Four different steviol glycosides (rebaudioside A (Reb A), rebaudioside C (Reb C), rebaudioside F (Reb F), and stevioside (Ste)) were detected in the samples analyzed in the present work (, ). Reb A content in the untreated beverage sweetened with 1.25% and 2.50% (v/v) of Stevia was 171.5 ± 0.8, and 286.9 ± 8.4 mg/100 mL, respectively. Three-way ANOVA showed a significant influence (p < .05) of temperature processing on Reb A contents. The analysis of variance showed that the regression model was accurate enough (R2 = 99.71, p < .05, standard error = 6.519). The relationship between the independent variables and Reb A can be seen in .
Figure 1. Chromatogram HPLC analysis of steviol glycosides 1: Rebaudioside A, 2: Stevioside hydrate, 3: Steviol hydrate, 4: Rebaudioside F, 5: Rebaudioside C in a beverage mixture of exotic fruit juice and sweetened with Stevia rebaudiana (Stevia) Bertoni
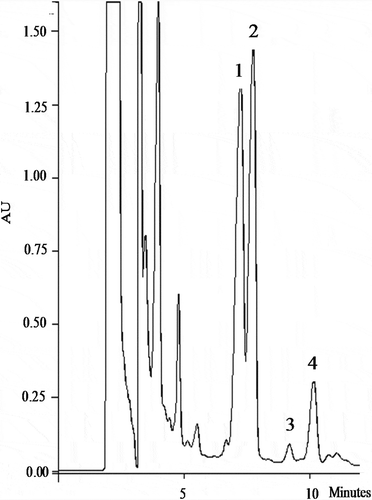
The analysis of variance indicated a decrease (>14%) in Reb A values after thermal processing when the temperature was higher than 60°C, independently of the Stevia percentage used. These findings were in accordance with several other publications that reported a significant effect of thermal processing on steviol glycosides degradation.
According to previous studies found in published literature, Chang and Cook[Citation32] obtained a degradation (−32%) of the original Reb A content after heating this steviol glycoside in an aqueous solution (6.5 mg/mL) at 100°C for 48 h. Authors linked these losses to degradation products such as Reb B and glucose, indicating that the C-19 ester linkage appeared to be the most heat-labile bond in Reb A. Moreover, in a study conducted in a model solution by the Panel on Food Additives and Nutrient Sources added to Food[Citation33], they found that the stability of the Reb A was temperature- and time-dependent. The Panel also noted in this study that the extent of degradation of Reb A ranged from a few percent >63% under different storage (pH and temperature) and food production conditions.
In the present study, the Reb C content of the untreated beverages with 1.25% (v/v) and 2.50% (v/v) Stevia was 30.1 ± 0.2 and 63.6 ± 0.1 mg/100 mL, respectively. The ANOVA results indicated a decrease in Reb C values when temperature and time were increased, independently of the Stevia percentage used. Experimental data were fitted by a second-order polynomial model ().
Moreover, Reb F concentration in the beverage with 1.25% (v/v) Stevia was 7.5 ± 0.1 mg/100 mL. As can be expected, the Reb F concentration was higher when the untreated beverage was sweetened with 2.50% (v/v) Stevia (14.6 ± 0.1 mg/100 mL). In addition, the regression analysis test showed that a second-order model fits well with the Reb F content after applying thermal processing. The determination coefficient was (R2 = 98.49, p < .05, standard error = 0.718). Experimental data were fitted by a second-order polynomial model ().
On the other hand, stevioside content in the untreated beverage sweetened with 1.25% and 2.50% (v/v) was 363.8 ± 2.3, and 637.5 ± 3.0 mg/100 mL, respectively. The three-way ANOVA showed that temperature and %Stevia had a significant effect (p < .05) on stevioside content after applying the thermal treatment. The determination coefficient was (R2 = 95.48, p < .05, standard error = 58.024). The existence or nonexistence of interactions between the two factors evaluates the contribution of each factor to the dependent variable. The reduced regression model is presented in .
In the present study, stevioside was stable at 60°C. However, some losses occurred on heating to a temperature of 80°C (>9%) and 90°C (>10%), independently of the Stevia percentage used in the formulation of the beverages. Other published data[Citation34,Citation35] on the stability of stevioside in an aqueous solution (0.5 g/L) demonstrated that stevioside was stable within a pH range of 2–10 over 2 h at 60°C and losses that occurred on heating to a temperature of 80°C were >5%. Similarly, losses >5% of stevioside were observed after 4 h incubation of tea or coffee at 80°C. Chang and Cook[Citation32] also reported data on the stability of stevioside. Prolonged heating at 100°C of stevioside (purity not reported) in an aqueous solution (6.5 mg/mL) resulted in a decrease in the stevioside concentration. These authors identified steviolbioside and glucose as degradation products and the C-19 ester linkage appeared to be the most heat-labile bond in stevioside. shows the values of bioactive compounds and antioxidant capacity after the treatments. Moreover, the modifications in ascorbic acid after applying thermal treatments in the beverages analyzed in the present were also studied. Ascorbic acid content in the untreated beverage without Stevia added was 24.8 ± 0.2. Non-significant modifications were observed when the untreated samples were sweetened with 1.25% and 2.50% (v/v) of Stevia. In addition, the three-way ANOVA showed that both temperature and time had a significant effect (p < .05) on ascorbic acid content after applying the thermal treatment. The existence or nonexistence of interactions between the two factors evaluates the contribution of each factor to the dependent variable. The reduced regression model presented in allowed for the prediction of the effects of independent variables on the ascorbic acid values. These findings were in close agreement with several other publications that reported a significant (p < .05) decrease in the ascorbic acid content of fruit and vegetable juices complex mixtures when treatment temperature and time were increased. Similarly, Barba et al.[Citation27] and Barba et al.[Citation26] observed losses of ascorbic acid content ranging from 15% to 18% and 6% to 12% in thermally treated (90 and 98°C for 15 and 21 s) orange juice-milk and in a vegetable beverage, respectively, as treatment temperature increased. Moreover, Torregrosa et al.[Citation7] also found losses of 17% in thermally treated (98°C, 21 s) orange-carrot juice. In addition, Dhuique-Mayer et al.[Citation36] studied the thermal degradation of ascorbic acid at 50–100°C in citrus juices. They also established a first-order model for explaining ascorbic acid degradation during thermal pasteurization at 75–100°C until 120 min of treatment. Taking into account this model, losses of ascorbic acid content at 75–100°C during 0.5 min can be considered negligible. However, losses ranged from 1% to 6% after thermal treatment (75–100°C for 5–15 min).
Table 4. Values of physicochemical properties and antioxidant compounds in the samples analyzed
Total carotenoid content in the untreated samples without Stevia was 436.7 ± 17.6. One-way ANOVA did not show a significant influence of Stevia percentage in the carotenoid content of the untreated samples. In addition, the existence or nonexistence of interactions between the two factors evaluates the contribution of each factor to the dependent variable. Equation in describes the regression model. In addition, the total anthocyanin content of the untreated samples without Stevia was 22.0 ± 1.3. Açaí was the main contributor to anthocyanin content of the samples, because it can be considered a good source of anthocyanins compared to other known red fruits such as strawberry and raspberry.[Citation37] Moreover, a significant increase in total anthocyanin content was found when Stevia at 1.25% (27.8 ± 1.3) and 2.5% (29.7 ± 0.3) was added to the fruit complex mixture (). In the line of the results reported in the present research, Muanda et al.[Citation38] found values of 0.35 mg total anthocyanins/g dry matter when they studied the chemical composition of water extracts from Stevia rebaudiana Bertoni. The behavior of total anthocyanins after applying thermal processing can be explained by the equation in .
Table 5. Response values of the antioxidant compounds predicted under the optimized conditions and the validation experiment
Moreover, antioxidant capacity measured as TEAC values in the beverage without Stevia was 6.4 ± 0.3 mM TE. TEAC values were higher when the fruit complex mixture blended with oat was sweetened with Stevia at 1.25% (20.3 ± 2.2 mM TE) and 2.50% (30.4 ± 0.7 mM TE). With regard to antioxidant capacity measured with ORAC assay, the sample without Stevia showed an antioxidant capacity value of 5.1 ± 0.1 mM TE. The antioxidant capacity values measured with ORAC assay were significantly higher (p < .05) for the samples with Stevia at 1.25% (23.5 ± 0.1 mM TE) and 2.5% (36.1 ± 0.1 mM TE) than those obtained for the sample without Stevia (). It should be noted that antioxidant capacity measured with TEAC and ORAC methods increased significantly according to the percentage of the Stevia, in a dose-dependent manner, reaching a maximum at 2.5% Stevia. However, the results revealed significant differences between samples from different origins and were not comparable as the based chemical reactions, and the parameters being determined varied considerably.
The results obtained for the three-way ANOVA showed that temperature, time and Stevia percentage had a significant influence (p < .05) on the total antioxidant capacity of the beverages measured as ORAC values (). However, when TEAC assay was used, only Stevia percentage had a significant effect. Experimental data were fitted by a second-order polynomial model (). Optimum conditions of thermal treatment for enhancing each of the antioxidant compounds were slightly different. A number of combinations of variables produced maximum ascorbic acid, total phenolic content, antioxidant capacity, and total carotenoids while still achieving good color. As a result, emphasis was placed on optimizing the bioactive compounds (anthocyanin content, ascorbic acid, total carotenoids) and antioxidant capacity. Optimum thermal treatment conditions for maximizing bioactive constituents are depicted in . The multi-response analysis of response surface design using the desirability approach was used to optimize treatment temperature and time. The desirability function is an approach for solving the problem of optimizing several responses and is applied when various responses have to be considered at the same time. A desirability function is first constructed for each individual response, and then it is possible to obtain the overall desirability. Multiple response optimization indicated that bioactive compounds in the sample could be maximized by treating a sample containing 2.5% Stevia for 6.2 min at 80.0°C. This is a promising finding as the temperature is lower than the maximum used for thermal pasteurization. The response values predicted under these conditions by the multiple response optimization and after applying a validation experiment for confirming the values obtained are detailed in . Under these conditions, the microbiological assays performed on the treated samples show that the microbial load after the thermal treatment is always <1 log CFU/mL. The mean contents were compared by a t test, and the results showed that there were no significant differences (p > .05) between antioxidant compounds' values after applying the optimized method and the experimental values. Moreover, total color differences were lower than 3, which indicated a non-significant color change in comparison to untreated samples.
Conclusion
From the response surface plots, treatment temperature was found to have the most significant effect on the ascorbic acid content of the beverages. The high coefficients of determination of the variables at a 95% confidence level for the six mathematical models indicated that second-order polynomial models may be used to predict critical phytochemical parameters of the beverages. Nevertheless, substantial degradation of steviol glycosides might take place at high temperatures. These findings may be applied in the development of new functional foods based on fruit complex mixtures and sweetened with non-caloric sweeteners from Stevia rebaudiana.
Additional information
Funding
References
- Bhardwaj, R. L.; Pandey, S. Juice Blends–a Way of Utilization of Under-utilized Fruits, Vegetables, and Spices: A Review. Crit. Rev. Food Sci. Nutr. 2011, 51(6), 563–570. DOI:10.1080/10408391003710654.
- Chaturvedula, V. S. P.; Upreti, M.; Prakash, I. Diterpene Glycosides from Stevia Rebaudiana. Molecules. 2011, 16(5), 3552–3562. DOI:10.3390/molecules16053552.
- Food Consulting. Food and Beverage Trend Report 2010.
- Stoyanova, S.; Geuns, J.; Hideg, É.; Van Den Ende, W. The Food Additives Inulin and Stevioside Counteract Oxidative Stress. Int. J. Food Sci. Nutr. 2011, 62(3), 207–214. DOI:10.3109/09637486.2010.523416.
- Nehir El, S.; Simsek, S. Food Technological Applications for Optimal Nutrition: An Overview of Opportunities for the Food Industry. Compr. Rev. Food Sci. Food Saf. 2012, 11(1), 2–12. DOI:10.1111/crf3.2012.11.issue-1.
- Cortes, C.; Esteve, M. J.; Frigola, A. Color of Orange Juice Treated by High Intensity Pulsed Electric Fields during Refrigerated Storage and Comparison with Pasteurized Juice. Food Control. 2008, 19(2), 151–158. DOI:10.1016/j.foodcont.2007.03.001.
- Torregrosa, F.; Esteve, M. J.; Frigola, A.; Cortes, C. Ascorbic Acid Stability during Refrigerated Storage of Orange-carrot Juice Treated by High Pulsed Electric Field and Comparison with Pasteurized Juice. J. Food Eng. 2006, 73(4), 339–345. DOI:10.1016/j.jfoodeng.2005.01.034.
- Jooken, E.; Amery, R.; Struyf, T.; Duquenne, B.; Geuns, J.; Meesschaert, B. Stability of Steviol Glycosides in Several Food Matrices. J. Agric. Food Chem. 2012, 60(42), 10606–10612. DOI:10.1021/jf302261j.
- Gong, Q.; Bell, L. N. Degradation Kinetics of Rebaudioside A in Various Buffer Solutions. Int. J. Food Sci. Technol. 2013, 48(12), 2500–2505. DOI:10.1111/ijfs.2013.48.issue-12.
- Barba, F. J.; Esteve, M. J.; Tedeschi, P.; Brandolini, V.; Frígola, A. A Comparative Study of the Analysis of Antioxidant Activities of Liquid Foods Employing Spectrophotometric, Fluorometric, and Chemiluminescent Methods. Food Anal. Methods. 2013, 6(1), 317–327. DOI:10.1007/s12161-012-9441-3.
- Zulueta, A.; Esteve, M. J.; Frigola, A. ORAC and TEAC Assays Comparison to Measure the Antioxidant Capacity of Food Products. Food Chem. 2009, 114(1), 310–316. DOI:10.1016/j.foodchem.2008.09.033.
- Savita, S. M.; Sheela, K.; Sunanda, S.; Shankar, A. G.; Ramakrishna, P.; Sakey, S. Health Implications of Stevia Rebaudiana. J Human Ecol.. 2004, 15, 191–194. DOI:10.1080/09709274.2004.11905691.
- Braddock, R. J. Handbook of Citrus By-products and Processing Technology (pp. 53–83). New York 1999: John Wiley & Sons, Inc.
- Irwe, S.; Olson, I. Reduction of pectinesterase activity in orange juice by high-pressure treatment. In: Singh R.P., Oliveria F.A. (eds) Minimal processing of foods and process optimization. CRC Press Florid,1994; pp 35–42.
- Krop, J. J. P.; Pilnik, W. Effect of Pectic Acid and Bivalent Cations on Cloud Loss of Citrus Juices. Lebensmittel-Wissenschaft Und-Technologie. 1974, 7, 62–63.
- Meydav, S.; Saguy, I.; Kopelman, I. J. Browning Determination in Citrus Products. J. Agric. Food Chem. 1977, 25(3), 602–604. DOI:10.1021/jf60211a030.
- Calvo, C. Optical properties. Handbook of Food Analysis. Physical Characterization and Nutrient Analysis; Nollet, L.M.L. Ed.; Marcel Dekker: New York, 2004; 1–19.
- JECFA. SteviolGlycosides. Compendium of Food Additive Specifications, 73th Meeting. In FAO JECFA Monographs 10, Rome, 2010.
- Carbonell-Capella, J. M.; Barba, F. J.; Esteve, M. J.; Frígola, A. High Pressure Processing of Fruit Juice Mixture Sweetened with Stevia Rebaudiana Bertoni: Optimal Retention of Physical and Nutritional Quality. Innovative Food Sci. Emerging Technol. 2013, 18, 48–56. DOI:10.1016/j.ifset.2013.01.011.
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, Phenolics, and Color of Cabernet Franc, Merlot, and Pinot Noir Wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biol. Med. 1999, 26(9–10), 1231–1237. DOI:10.1016/S0891-5849(98)00315-3.
- Ou, B.; Hampsch-Woodill, M.; Prior, R. L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49(10), 4619–4626. DOI:10.1021/jf010586o.
- Genovese, D. B.; Elustondo, M. P.; Lozano, J. E. Color and Cloud Stabilization in Cloudy Apple Juice by Steam Heating during Crushing. J. Food Sci. 1997, 62(6), 1171–1175. DOI:10.1111/jfds.1997.62.issue-6.
- Lee, H. S.; Coates, G. A. Thermal Pasteurization Effects on Color of Red Grapefruit Juices. J. Food Sci. 1999, 64(4), 663–666. DOI:10.1111/jfds.1999.64.issue-4.
- Lee, H. S.; Coates, G. A. Effect of Thermal Pasteurization on Valencia Orange Juice Color and Pigments. LWT - Food Sci. Technol. 2003, 36(1), 153–156. DOI:10.1016/S0023-6438(02)00087-7.
- Barba, F. J.; Esteve, M. J.; Frigola, A. Ascorbic Acid Is the Only Bioactive that Is Better Preserved by High Hydrostatic Pressure than by Thermal Treatment of a Vegetable Beverage. J. Agric. Food Chem. 2010, 58(18), 10070–10075. DOI:10.1021/jf1019483.
- Barba, F. J.; Cortés, C.; Esteve, M. J.; Frígola, A. Study of Antioxidant Capacity and Quality Parameters in an Orange Juice-milk Beverage after High-pressure Processing Treatment. Food Bioprocess. Technol. 2012, 5(6), 2222–2232. DOI:10.1007/s11947-011-0570-2.
- Gama, J. J. T.; de Sylos, C. M. Effect of Thermal Pasteurization and Concentration on Carotenoid Composition of Brazilian Valencia Orange Juice. Food Chem. 2007, 100(4), 1686–1690. DOI:10.1016/j.foodchem.2005.01.062.
- Patras, A.; Brunton, N. P.; Da Pieve, S.; Butler, F. Impact of High Pressure Processing on Total Antioxidant Activity, Phenolic, Ascorbic Acid, Anthocyanin Content and Colour of Strawberry and Blackberry Purées. Innovative Food Sci. Emerging Technol. 2009, 10(3), 308–313. DOI:10.1016/j.ifset.2008.12.004.
- AIJN. Code of Practice for Evaluation of Fruit and Vegetable Juices; Association of the industry of Juices and Nectars of the European Economic Community Code of Practice for Evaluation of Fruit and Vegetable Juices, Brussels, 1996.
- Zulueta, A.; Barba, F. J.; Esteve, M. J.; Frígola, A. Changes in Quality and Nutritional Parameters during Refrigerated Storage of an Orange Juice-Milk Beverage Treated by Equivalent Thermal and Non-thermal Processes for Mild Pasteurization. Food Bioprocess. Technol. 2013, 6(8), 2018–2030. DOI: 10.1007/s11947-012-0858-x.
- Chang, S. S.; Cook, J. M. Stability Studies of Stevioside and Rebaudioside a in Carbonated Beverages. J. Agric. Food Chem. 1983, 31(2), 409–412. DOI:10.1021/jf00116a056.
- EFSA. Scientific Opinion on the Safety of Steviol Glycosides for the Proposed Uses as a Food Additive. EFSA J. 2010, 8(4), 1537. DOI:10.2903/j.efsa.2010.1537.
- Kroyer, G. T.;. The Low Calorie Sweetener Stevioside: Stability and Interaction with Food Ingredients. LWT - Food Sci. Technol. 1999, 32(8), 509–512. DOI: 10.1006/fstl.1999.0585.
- Kroyer, G. Stevioside and Stevia-sweetener in Food: Application, Stability and Interaction with Food Ingredients. Journal für Verbraucherschutz und Lebensmittelsicherheit. 2010, 5(2), 225–229. DOI:10.1007/s00003-010-0557-3.
- Dhuique-Mayer, C.; Tbatou, M.; Carail, M.; Caris-Veyrat, C.; Dornier, M.; Amiot, M. J. Thermal Degradation of Antioxidant Micronutrients in Citrus Juice: Kinetics and Newly Formed Compounds. J. Agric. Food Chem. 2007, 55(10), 4209–4216. DOI:10.1021/jf0700529.
- De Rosso, V.; Hillebrand, S.; Cuevas Montilla, E.; Bobbio, F. O.; Winterhalter, P.; Mercadante, A. Z. Determination of Anthocyanins from Acerola (Malpighia Emarginata DC.) And Açai (Euterpe Oleracea Mart.) By HPLC-PDA-MS/MS. J. Food Compost. Anal. 2008, 21(4), 291–299. DOI:10.1016/j.jfca.2008.01.001.
- Muanda, F. N.; Soulimani, R.; Diop, B.; Dicko, A. Study on Chemical Composition and Biological Activities of Essential Oil and Extracts from Stevia Rebaudiana Bertoni Leaves. LWT - Food Sci. Technol. 2011, 44(9), 1865–1872. DOI:10.1016/j.lwt.2010.12.002.
