?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In order to investigate the taste compounds of 18 strong fragrance spices, high performance liquid chromatography was used to determine the content of free amino acids, nucleotides and organic acids. Cluster analysis was used to classify on the basis of similar data. The results showed that the content of free amino acid in tarragon was the highest, 44.97 g/kg, followed thyme, 11.88 g/kg. The contents of organic acids in 3 spice samples were significantly higher compared with others, including thyme (54.67 g/kg), sweet basil (42.28 g/kg) and small cardamon (40.72 g/kg). Nucleotides’ contents were significantly higher in star anise (3.54 g/kg) than the other 17 spice samples. The taste active value (TAV) of glutamic acid (22.01) was the highest. In addition, the TAVs of lactic acid, acetic acid, ascorbic acid, tartaric acid and malic acid in 18 spice samples were also more than 1, which indicated that these compounds contribute greatly to spices taste. The equivalent umami concentration (EUC) of tarragon was the highest, with 26.15 g monosodium glutamate (MSG)/100 g. The 18 strong fragrance spices were divided into 2 categories according to the cluster analysis of EUC. The tarragon and dill were one category, other 16 spices were the other category. When the Euclidean distance increased to 25, the tarragon and dill were incorporated into the category of other 16 spices.
Introduction
Spices refer to the specific parts of plants, such as seeds, buds, leaves, stems, roots, or their extracts. Spices could be added to food in their natural state or as powder or extract not only in the formulation of nutraceutical and functional food, but also in a series of food condiments or seasonings.[Citation1] At present, there were many kinds of spices, which can be divided into strong fragrance spices, pungent spices and elegant spices according to the characteristics of flavor of the natural spices. As edible plant spices, they could make food fragrant, spicy, bitter, sweet and other characteristics.[Citation2,Citation3] Among them, volatile and nonvolatile components played critical roles in flavor characteristics. They were of great significance to determine and evaluate spices.
Spices are widely used in dish processing. Different spices have different effects on food. Chang et al.[Citation4] compared the effects of different cooking time on products of stewed chicken in soy sauce. The results showed that the content of amino acids in chicken soup increased with extension of cooking time, and the chicken soup would be more delicious and nutritious. This is not only because more substances migrate from the chicken into the soup, but also due to the release of the effective flavor components in the spices which were promoted as the stewing time increases. Liu et al.[Citation5] compared seasoned duck with the unseasoned duck, the free amino acids content of Nanjing cooked duck was significant higher. Furthermore, the desirable amino acids which possess umami (Asp and Glu) and sweet (Ala, Gly and Ser) taste of Nanjing cooked duck were significantly greater than the unseasoned duck, which is possibly because spices contribute to the delicious taste of the cooked Nanjing duck. Jongberg et al.[Citation6] using a traditional recipe (control) or the same recipe but added green tea extract or rosemary extract to prepared sausages. It showed that in contrast to green tea extract, rosemary extract contains relatively high concentrations of di-terpenes, volatile oils, and phenolic acids, which all may add to the organoleptic experienced by the consumers. Accordingly, the result of the sensory evaluation showed that the taste of the sausages is significantly different.
The volatile compounds of spices were reported in recent years. Ryu et al.[Citation7] repoted that a large number of volatile oils (aromatic compounds) and flavonoids were detected in the clove bud extraction by the 1D- and 2D-NMR. Diaz-Maroto et al.[Citation8] identified thymol and carvacrol as key compounds contributing to the aroma of thyme leaves. Other components with similar odor descriptions were methyl thymol, which to a lesser extent also contributed to the characteristic aroma of thyme.[Citation9,Citation10] Shan et al.[Citation11] investigated the total equivalent antioxidant capacity and phenolic content of 26 common spice extracts from 12 botanical families. The results showed that rosmarinic acid was the dominant phenolic compound in the six spices of the family Labiatae. Phenolic volatile oils were the principal active ingredients in most spices. Even though intensive studies on the volatile components and their total content in many spices had been carried out, the nonvolatile components identification data were insufficient and incomplete. The nonvolatile components have an important contribution to the taste presentation of food, which needs to be further studied.
The objective of this work was to investigate the taste compounds of 18 strong fragrance spices. The contents of taste compounds were compared, including free amino acid, organic acid and nucleotide. At the same time, the important indexes of taste were calculated, including the taste active value (TAV) and equivalent umami concentration (EUC). The cluster analysis of 18 strong fragrance spices was carried out. This work could provide theoretical bases for the quality improvement of processing spices, and promote the application of spices in food processing and improve their value and meet the consumers’ needs.
Materials and methods
Materials and chemicals
L (+) -tartaric acid, formic acid, lactic acid, acetic acid, citric acid, fumaric acid, succinic acid, L (+) -ascorbic acid, propionic acid, potassium dihydrogen phosphate dodecahydrate phosphate (all AR grade) and malic acid (BR grade) were obtained from Sinopharm Chemical Reagent Co. (Shanghai, China). Potassium dihydrogen phosphate (KH2PO4), phosphoric acid (H3PO4), hydrochloric acid (HCl) and disodium hydrogen phosphate dodecahydrate (Na2HPO4•12H2O) (all AR grade) were purchased from Sinopharm Chemical Reagent Company (Shanghai, China). Inosine 5´-monophosphate (5´-IMP), adenosine 5´-monophosphate (5´-AMP), guanosine 5´-monophosphate (5´-GMP), and cytidine 5´-monophosphate (5´-CMP) were purchased from Sigma-Aldrich (St. Louis, Mo., U.S.A.). Durashell AA analysis kit, including an internal standard solution, which was purchased from Tianjin Bona Agel Technology Co., Ltd. Methanol, trifluoroacetic acid (TFA), and acetonitrile (ACN) (all HPLC grade) were purchased from Fisher Scientific (Shanghai, China). Sulfosalicylic acid (AR grade) was obtained from Biochemical Technology Co., Ltd. (Shanghai, China). The ultra-pure water was purchased from Hangzhou Wahaha Group Co., Ltd. (Hangzhou, China).
Preparation of sample
The sample information of strong fragrance spices was shown in . The treatment of spices sample referred to the method of Kong et al.[Citation12] with some modification. The spices were powdered, then weighed 5.00 g and mixed with 45 mL of 50% ethanol (50:50, V/V), whirlpool oscillated for 20 s, ultrasound kept under the 40 W for 15 minutes, then centrifuged for 10 min at 10000 g (RCF) under 4 °C. The supernatant was filtered with No. 4 qualitative filter paper (Ge Biotechnology (Hangzhou) Co., Ltd), the residue was re-extracted in the same way for three times, the filtrate was combined, and fixed volume with ethanol to 50 mL to get the sample solution, then stored at 4 °C before use.
Table 1. Information of 18 strong fragrance spices
Free amino acid analysis
The content of free amino acids in the samples was determined by high performance liquid chromatography (Agilent 1260, Agilent Technology Co., Ltd, China). The 2 mL sample solution was added to 1 mL 10% (V/V) sulfosalicylic acid, then diluted with 0.1 mol/L HCl to a total amino acid concentration of 1 ~ 2 mg/mL, then mixed with internal standard solution and filtrated through 0.22 µm nylon filter membrane before analysis. The detection process was identical to that of Kong et al.[Citation13] The quantitative analysis of amino acids was carried out by an internal standard method. The standard concentration of the amino acid solution was Cys-Cys: 0.014 ~ 0.341 mol/L, and other amino acids: 0.027 ~ 0.628 mol/L. Each sample was analyzed three times. The amino acid concentration was calculated using the following equation.
According to the formula: K is the coefficient; M1 is the peak area of internal standard in the 17 amino acids mixed standard; M2 is the peak area of internal standard in the sample; C1 is the amino acid concentration in the sample; C2 is the amino acid concentration in the 17 amino acids mixed standard; A1 is the peak area of amino acid in the sample; A2 is the peak area of amino acid in the standard.
Organic acid analysis
Organic acid was determined as described by the method of Kong et al.[Citation14] The 2 mL sample solution through 0.22 µm nylon filter membrane, detected by Thermo 3000 high performance liquid chromatography (Thermo Fisher Scientific Inc., USA). The detection process was similar to that of Wang et al.[Citation15] Quantitative analysis of organic acid was carried out by an external standard method. Twelve standard substances, malic acid, citric acid, lactic acid, succinic acid, oxalic acid, tartaric acid, formic acid, acetic acid, propionic acid, ascorbic acid and pyroglutamic acid were weighed 0.200 g respectively. They were dissolved in ultrapure water and fixed in a capacity bottle of 50 mL, then obtained the standard reserve solutions of 1.000 mg/mL and stored at 4 °C in dark. The reserve solution was diluted to 4.000, 2.000, 0.800, 0.400, 0.200, 0.080 and 0.040 mg/mL, respectively. With the concentration of organic acids as abscissa and the peak area as ordinate, the standard curves of organic acids were obtained. Each sample was analyzed three times.
5ʹ-Nucleotides analysis
The 5´-nucleotide was determined by Thermo 3000 high performance liquid chromatography with software (version 7.1, Thermo Fisher Scientific Inc., USA). It was the same as that for organic acids. Quantitative analysis of nucleotides was carried out by external standard method. 5´-AMP, 5´-IMP, 5´-GMP and 5´-CMP were weighed at 0.010 g respectively, dissolved in 100 g ultrapure water, then obtained the standard reserve solutions of 0.100 mg/mL. They were stored at 4 °C in dark. The reserve solution was diluted to 0.100, 0.050, 0.020, 0.010, 0.005, 0.002 and 0.001 mg/mL, respectively. The standard curves of nucleotides were obtained by plotting the concentration of nucleotides as abscissa and the peak area of chromatogram as ordinate. Each sample was analyzed three times.
Equivalent umami concentration
The EUC was calculated using the equation according to the reference of Yamaguchi et al. and Krishnan et al.[Citation16,Citation17]
Taste activity value calculation
Referring to the method of Kato et al.[Citation18] TAV reflects the contribution of a single compound to the overall taste. When TAV is more than 1, it is considered that the substance contributes to the overall taste. The larger the TAV, the greater the contribution. When TAV is less than 1, it is considered that the substance does not contribute to the overall taste. The TAV could be calculated using the following equation.[Citation19,Citation20]
where C1 is the concentration and C2 is the threshold value of taste compounds.
Statistical analysis
Statistical analysis was performed by SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA). Based on the square of Euclidean distance, the similarity of EUC was analyzed by cluster analysis using SPSS 19.0. The Euclidean distance coefficients between 18 samples were between 0 and 25. Significance analysis between samples was performed by one-way ANOVA and Duncan’s multilevel test (p < 0.05). All statistical analyzes were performed in triplicate and the results were expressed as (mean ± SD).
Results and discussion
Analysis of free amino acids in strong fragrance spices
The content of free amino acids in 18 strong fragrance spices was shown in . Glutamic acid, glycine, alanine and aspartic acid in amino acids were the main factors determining the taste of food.[Citation21] The taste of amino acids could be divided into 5 kinds: umami taste (aspartic acid (Asp), glutamic acid (Glu)), sweet taste (glycine (Gly), threonine (Thr), serine (Ser), and alanine (Ala)), bitter taste (valine (Val), methionine (Met), isoleucine (Iso), leucine (Leu), tyrosine (Tyr), phenylalanine (Phe), histidine (His), lysine (Lys), and proline (Pro)) and tasteless taste (cysteine (Cys)).[Citation22] In , the main amino acids in the top 5 free amino acids of 18 strong fragrance spices are as follows: Pro, Asp, Glu, Ser, and Val. The lowest total contents of amino acids among of 18 strong fragrance spices were Indonesia cassia and the highest contents were tarragon, the values were 0.14 g/kg and 44.97 g/kg, respectively. Among them, thyme’s umami amino acid was 6.65 g/kg, while the tarragon’s sweet amino acid was 2.31 g/kg. This also explained that tarragon was a special additive in the famous French mustard, the French bearnaise soy sauce was characterized by the flavor of Artemisia dracunculus, mainly used in poultry, beef, omelets, cheese, roast fish, seafood and other meat dishes. The tarragon powder could be directly used in French salad, salad contains radish, pea, celery, green beans, carrots, lemon, onion, tomato, green vegetables, etc.[Citation23] Tyrosine, phenylalanine, valine, leucine, isoleucine, alanine and methionine were detected in 11 spice samples. The content of total free amino acid in star anise was 1.20 g/kg. This is also similar to the reports of Wang et al.[Citation24] that free amino acids of duck breast meat significantly increased with the addition of star anise in the brine soup. Compared with the nutmeg (0.04 g/kg), the umami amino acids content of tarragon (6.13 g/kg) and thyme (6.65 g/kg) was significant higher (p < 0.05). Furthermore, the sweet amino acids in dill (1.64 g/kg) were significantly greater (p < 0.05) than in greater Indian cardamom (0.03 g/kg) Chinese cassia (0.03 g/kg). In addition, the total free amino acids of tarragon (44.97 g/kg) were significantly higher when compared to the others (p < 0.05).
Table 2. The contents of amino acid, organic acid, and nucleotide acid in 18 strong fragrance spices
Analysis of organic acids in strong fragrance spices
Some organic acids and their sodium salts had umami taste, such as monosodium succinate and disodium salt, and could be added to food as flavor enhancers.[Citation25] In , the content of succinic acid in greater Indian cardamom was the highest, followed by Chinese cassia and coriander. The organic acid content of Star anise was the lowest in 18 strong fragrance spices. The organic acid content of thyme was the highest in 18 strong fragrance spices. Many European countries use thyme as an important raw material for blending spices.[Citation8] When fresh leaves and tender stems of thyme are mixed with other spices, which could add flavor to soups and dishes. At the same time, thyme has been directly eaten as a spiced vegetable in China. The delicate and elegant aroma of thyme can be used to deodorize various kinds of seafood meat, shrimp and shellfish.[Citation26–Citation28] In addition, the lactic acid of Pimento allspice (27.19 g/kg) was significantly higher when compared to the others (p < 0.05). Compared with coriander (0.26 g/kg), the oxalic acid content of sweet basil (39.57 g/kg) was significant higher (p < 0.05), moreover, the ascorbic acid content of clove (5.29 g/kg) was also significantly higher when compared to the others (p < 0.05).
Analysis of nucleotides in strong fragrance spices
5´-GMP and 5´-IMP had the umami taste, known to produce delicious and tasty flavor.[Citation29] At the same time, synergistic effects between nucleotides could also be produced, which would also have an important impact on the overall flavor enhancement. The nucleotides content in 18 strong fragrance spices were shown in . In , the lowest total amount of nucleotides was 0.08 g/kg in small cardamon, the highest content was 3.54 g/kg in star anise. Among them, the total of 5´-IMP and 5´-GMP was 1.24 g/kg in caraway. This result was also consistent with the research of Padmashree et al.[Citation30] They reported that caraway was widely used as food additives, flavoring agents and cosmetic flavors. Among of them, the 5´-GMP content of dill (0.75 g/kg) was significant higher (p < 0.05) than others. Meanwhile, the 5´-IMP content of clove (0.92 g/kg) was significant higher (p < 0.05) compared with sweet basil (0.03 g/kg).
EUC
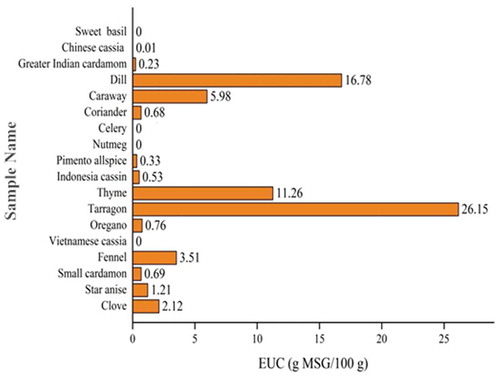
The EUC of 18 strong fragrance spices was shown in . The EUC indicated the synergistic effect between umami amino acids and nucleotides. In , the EUC of 18 strong fragrance spices ranged from 0 to 26.15 g MSG/100 g. The EUC of tarragon (26.15 g MSG/100 g) was the highest, which was mainly due to the high content of glutamic acid and aspartic acid. Then followed were dill (16.78 g MSG/100 g) and thyme (11.26 g MSG/100 g).
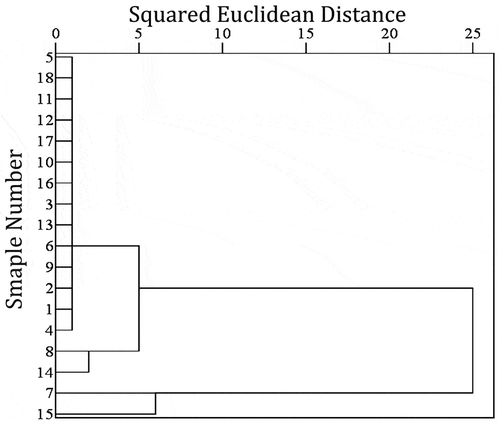
Based on the square of Euclidean distance, the similarity of EUC was analyzed by cluster analysis using SPSS 19.0. The Euclidean distance coefficients between 18 samples were between 0 and 25. Cluster analysis of 18 strong fragrance spices was shown in . When the Euclidean distance was 5, It could be divided into 4 categories, the first category included samples 1, 2, 3, 4, 5, 6, 9, 10, 11, 12, 13, 16, 17 and 18, the second category included samples 8 and 14, while the third category only included samples 7, and the fourth category only included samples 15. When the Euclidean distance increased to 7, 18 strong fragrance spices could be divided into 2 categories. The first category included samples 1, 2, 3, 4, 5, 6, 8, 9, 10, 11, 12, 13, 14, 16, 17 and 18. The second category included samples 7 and 15. When the Euclidean distance increased to 25, samples 7 and 15 could be incorporated into the first category. According to the results of cluster analysis, when the range of the Euclidean distance is between 7 ~ 25, it has high reliability to classify spices into 2 categories.[Citation31]
TAV
The TAV results of free amino acids, organic acids and nucleotides in 18 strong fragrance spices were shown in . In , the TAV range of taste components were as follows: free amino acids: 1.206 ~ 29.794; organic acids: 1.262 ~ 28.762; nucleotides: 1.629 ~ 9.870. This was consistent with the study of Zhao et al.[Citation32] They studied that 4 pork bone soups added with star anise, Chinese cassia and other spices, the TAV were 6.84 ~ 16.02, which showed that the spices had the greatest taste contribution in the soup. Among the 18 strong fragrance spices, the 5 amino acids with the highest TAV were glutamic acid, histidine, arginine, valine and aspartate. However, the TAV of umami amino acids in strong fragrant spices was higher than that of sweet and bitter amino acids. The contribution of flavor amino acids to taste was greater. Serine in sweet amino acids has a high TAV of 1.216, which contributed to the sweetness of fragrant spices. The TAVs of 5´-AMP and 5´-GMP were higher than 1. Phat et al.[Citation29] reported that the synergistic interaction between 5´-AMP and 5´-IMP could be considered in eliciting the umami taste. The 5´-IMP could combine with other constituents to improve the umami taste profile during the stewing process. The 5´-GMP provided a meaty flavor and acted as a stronger flavor enhancer than MSG.[Citation33] These taste compounds interacted to develop a different taste in chicken.
Table 3. The TAVs of 18 strong fragrance spices
Conclusion
The taste components were detected in18 strong fragrance spices. The content of free amino acids, organic acids and nucleotides were determined by HPLC. Cluster analysis was used to classify on the basis of similar data. The total free amino acids of tarragon (44.97 g/kg) were significantly higher when compared to the others (p < 0.05). Thyme, sweet basil, and small cardamom had a higher content of organic acids, 54.67, 42.28, and 40.72 g/kg, respectively. The contents of 5´-nucleotides in star anise and clove were higher, which were 3.54 and 2.25 g/kg. The TAV of glutamic acid was the highest, which showed contributed significantly to the taste of spices. The TAV of lactic acid, acetic acid, ascorbic acid, tartaric acid and malic acid in strong fragrance spices were also more than 1, which indicated that these compounds greatly contributed to the taste of strong fragrance spices. According to the results of cluster analysis, when the range of the Euclidean distance is between 7 ~ 25, it has high reliability to classify spices into 2 categories. The tarragon and dill were one category, other 16 spices were the other category.
Additional information
Funding
References
- Ene-Obong, H.; Onuoha, N.; Aburime, L.; Mbah, O. Chemical Composition and Antioxidant Activities of Some Indigenous Spices Consumed in Nigeria. Food Chem. 2018, 238, 58–64. DOI: 10.1016/j.foodchem.2016.12.072.
- Khazdair, M. R.; Anaeigoudari, A.; Hashemzehi, M.; Mohebbati, R. Neuroprotective Potency of Some Spice Herbs, a Literature Review. J. Traditional Complementary Med. 2019, 9, 98–105. DOI: 10.1016/j.jtcme.2018.01.002.
- Nguyen, L.; Duong, L. T.; Mentreddy, R. S. The U.S. Import Demand for Spices and Herbs by Differentiated Sources. J. Appl. Res. Med. Aromat. Plants. 2019, 12, 13–20. DOI: 10.1016/j.jarmap.2018.12.001.
- Chang, Y. N.; Zhao, G. M.; Liu, Y. X.; Li, M. Y.; Huang, X. Q.; Sun, L. X. Effects of Cooking on Free Amino Acids in Chicken and Broth. Food Ind. Sci. Technol. 2014, 35, 333–337+342. DOI: 10.13386/j.issn1002-0306.2014.09.064.
- Liu, Y.; Xu, X. L.; Zhou, G. H. Changes in Taste Compounds of Duck during Processing. Food Chem. 2007, 102, 22–26. DOI: 10.1016/j.foodchem.2006.03.034.
- Jongberg, S.; Tørngren, M. A.; Gunvig, A.; Skibsted, L. H.; Lund, M. N. Effect of Green Tea or Rosemary Extract on Protein Oxidation in Bologna Type Sausages Prepared from Oxidatively Stressed Pork. Meat Sci. 2013, 93, 538–546. DOI: 10.1016/j.meatsci.2012.11.005.
- Ryu, B.; Kim, H. M.; Lee, J. S.; Lee, C. K.; Sezirahiga, J.; Woo, J. H.; Choi, J. H.; Jang, D. S. New Flavonol Glucuronides from the Flower Buds of Syzygium Aromaticum (Clove). J. Agric. Food Chem. 2016, 64, 3048–3053. DOI: 10.1021/acs.jafc.6b00337.
- Diaz-Maroto, M. C.; Diaz-Maroto, I. J.; Sanchez-Palomo, E.; Perez-Coello, M. S. Volatile Components and Key Odorants of Fennel (Foeniculum Vulgare MilL.) And Thyme (Thymus Vulgaris L.) Oil Extracts Obtained by Simultaneous Distillation-extraction and Supercritical Fluid Extraction. J. Agric. Food Chem. 2005, 53, 5385–5389. DOI: 10.1021/jf050340+.
- Alissandrakis, E.; Tarantilis, P. A.; Harizanis, P. C.; Polissiou, M. Comparison of the Volatile Composition in Thyme Honeys from Several Origins in Greece. J. Agric. Food Chem. 2007, 55, 8152–8157. DOI: 10.1021/jf071442y.
- Jiang, T. J.; Feng, L. F.; Zheng, X. L. Effect of Chitosan Coating Enriched with Thyme Oil on Postharvest Quality and Shelf Life of Shiitake Mushroom (Lentinus Edodes). J. Agric. Food Chem. 2012, 60, 188–196. DOI: 10.1021/jf202638u.
- Shan, B.; Cai, Y. Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. DOI: 10.1021/jf051513y.
- Kong, B. H.; Zhang, H. Y.; Xiong, Y. L. Antioxidant Activity of Spice Extracts in a Liposome System and in Cooked Pork Patties and the Possible Mode of Action. Meat Sci. 2010, 85, 772–778. DOI: 10.1016/j.meatsci.2010.04.003.
- Kong, Y.; Yang, X.; Ding, Q.; Zhang, Y. Y.; Sun, B. G.; Chen, H. T.; Sun, Y. Comparison of Non-volatile Umami Components in Chicken Soup and Chicken Enzymatic Hydrolysate. Food Res. Int. 2017, 102, 559–566. DOI: 10.1016/j.foodres.2017.09.038.
- Kong, Y.; Zhang, L. L.; Zhang, Y. Y.; Sun, B. G.; Sun, Y.; Zhao, J.; Chen, H. T. Evaluation of Non-volatile Taste Components in Commercial Soy Sauces. Int. J. Food Prop. 2018, 21, 1854–1866. DOI: 10.1080/10942912.2018.1497061.
- Wang, L. H.; Qiao, K. N.; Ding, Q.; Zhang, Y. Y.; Sun, B. G.; Chen, H. T. Effects of Two Cooking Methods on the Taste Components of Sanhuang Chicken and Black-bone Silky Fowl Meat. J. Food Process. Preserv. 2018, 42, 1–10. DOI: 10.1111/jfpp.13772.
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the Relative Taste Intensity of Some L-α-amino Acids and 5′-nucleotides. J. Food Sci. 1971, 36, 846–849. DOI: 10.1111/j.1365-2621.1971.tb15541.x.
- Krishnan, K. R.; Babuskin, S.; Babu, P. A. S.; Sivarajan, M.; Sukumar, M. Evaluation and Predictive Modeling the Effects of Spice Extracts on Raw Chicken Meat Stored at Different Temperatures. J. Food Eng. 2015, 166, 29–37. DOI: 10.1016/j.jfoodeng.2015.05.021.
- Kato, H.; Rhue, M. R.; Nishimura, T. Role of Free Amino Acids and Peptides in Food Taste. In Flavor Chemistrye; Teranishi, R., Buttery, R. G., Shahidi, F., Eds.; American Chemical Society: Washington DC, 1989; Vol. 388, pp 158–174. DOI:10.1021/bk-1989-0388.ch013.
- Engel, E.; Nicklaus, S.; Salles, C.; Le Quere, J. L. Relevance of Omission Tests to Determine Flavour-active Compounds in Food: Application to Cheese Taste. Food Qual. Preference. 2002, 13, 505–513. DOI: 10.1016/S0950-3293(02)00136-2.
- Schlichtherle-Cerny, H.; Grosch, W. Evaluation of Taste Compounds of Stewed Beef Juice. Food Res. Technol. 1998, 207, 369–376. DOI: 10.1007/s002170050347.
- Yang, P.; Wang, Y.; Song, H. L.; Tang, J. N.; Yu, D. H. Analysis of Non-volatile Flavor Components in Pork Broth with Different Cooking Conditions. Chin. J. Food. 2018, 18, 247–260. DOI: 10.1007/s002170050347.
- Tseng, Y. H.; Lee, Y. L.; Li, R. C.; Mau, J. L. Non-volatile Flavour Components of Ganoderma Tsugae. Food Chem. 2005, 90, 409–415. DOI: 10.1016/j.foodchem.2004.03.054.
- Obolskiy, D.; Pischel, I.; Feistel, B.; Glotov, N.; Heinrich, M. Artemisia Dracunculus L. (Tarragon): A Critical Review of Its Traditional Use, Chemical Composition, Pharmacology, and Safety. J. Agric. Food Chem. 2011, 59, 11367–11384. DOI: 10.1021/jf202277w.
- Wang, Z.; Zhang, Y. W.; Qian, Y. W.; Yin, J.; Zhou, G. H.; Xu, X. L.; Peng, Z. Q. Effects of Repeated Brine Cooking on Heterocyclic Amines and Their Precursors in Duck Breast Meat and Soup. Food Ind. Sci. Technol. 2019, 40, 58–64. DOI: 10.13386/j.issn1002-0306.2019.12.010.
- El-Obeid, T.; Yehia, H. M.; Sakkas, H.; Lambrianidi, L.; Tsiraki, M. I.; Savvaidis, I. N. Shelf-life of Smoked Eel Fillets Treated with Chitosan or Thyme Oil. Int. J. Biol. Macromol. 2018, 114, 578–583. DOI: 10.1016/j.ijbiomac.2018.03.125.
- Terrab, A.; Recamales, A. F.; Hernanz, D.; Heredia, F. J. Characterisation of Spanish Thyme Honeys by Their Physicochemical Characteristics and Mineral Contents. Food Chem. 2004, 88, 537–542. DOI: 10.1016/j.foodchem.2004.01.068.
- Doymaz, İ.; Tugrul, N.; Pala, M. Drying Characteristics of Dill and Parsley Leaves. J. Food Eng. 2006, 77, 559–565. DOI: 10.1016/j.jfoodeng.2005.06.070.
- Weisany, W.; Samadi, S.; Amini, J.; Hossaini, S.; Yousefi, S.; Maggi, F. Enhancement of the Antifungal Activity of Thyme and Dill Essential Oils against Colletotrichum Nymphaeae by Nano-encapsulation with Copper NPs. Ind. Crops Prod. 2019, 132, 213–225. DOI: 10.1016/j.indcrop.2019.02.031.
- Phat, C.; Moon, B.; Lee, C. Evaluation of Umami Taste in Mushroom Extracts by Chemical Analysis, Sensory Evaluation, and an Electronic Tongue System. Food Chem. 2016, 192, 1068–1077. DOI: 10.1016/j.foodchem.2015.07.113.
- Padmashree, A.; Roopa, N.; Semwal, A. D.; Sharma, G. K.; Agathian, G.; Bawa, A. S. Star-anise (Illicium Verum) and Black Caraway (Carum Nigrum) as Natural Antioxidants. Food Chem. 2007, 104, 59–66. DOI: 10.1016/j.foodchem.2006.10.074.
- Kong, Y.; Zhang, L. L.; Sun, Y.; Zhang, Y. Y.; Sun, B. G.; Chen, H. T. Determination of the Free Amino Acid, Organic Acid, and Nucleotide in Commercial Vinegars. J. Food Sci. 2017, 5, 1116–1123. DOI: 10.1111/1750-3841.13696.
- Zhao, J.; Ding, Q.; Sun, Y.; Chen, Y. Y.; Zhang, Y. Y.; Sun, B. G.; Chen, H. T. Comparison of Free Amino Acids and Taste Charateristics in Different Kinds of Pig Bone Soup. Food Res. Dev. 2015, 36, 1–6. DOI: 10.1016/j.indcrop.2019.02.031.
- Rotzoll, N.; Dunkel, A.; Hofmann, T. Quantitative Studies, Taste Reconstitution, and Omission Experiment on the Key Taste Compounds in Morel Mushrooms (Morchella Deliciosa Fr.). J. Agric. Food Chem. 2006, 54, 2705–2711. DOI: 10.1021/jf053131y.