ABSTRACT
Much attention has been recently given to the effect of diet compounds on physical and mental health. Gallic acid is a phenolic compound with antioxidant activity. This compound is widely presented in black tea leaves, green tea, apples, grapes, strawberries, and pineapples. During the past years, it has been reported that gallic acid is effective against nervous system’s disorders including Alzheimer’s disease, Parkinson’s disease, ischemia, and reperfusion, depression and anxiety. These indicate that gallic acid can be considered as a valuable agent for nutraceutical interventions. In this study, several clinical studies suggested that gallic acid can improve human health by preventing or delaying the onset of neurological diseases. The present study indicated the neuroprotective features of gallic acid including the pre-clinical evidence for its effects in AD and PD and other diseases related to the nervous system. Significant efforts are required to confirm the neuroprotective effects of gallic acid in treating the diseases related to the nervous system.
Introduction
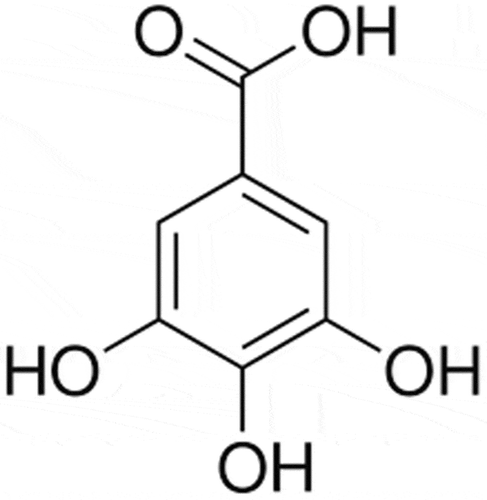
Gallic acid (3,4,5-trihydroxybenzoic acid) is widely presented in different plants as free or an ester compound. In fact, polyphenols are the most significant secondary metabolite groups in the plant defense system. Gallic acid is available in different foods such as grapes, pomegranates, nuts, berries, etc. Apart from plant sources, gallic acid also exists in some beverages such as wine and tea.[Citation1] The amount of gallic acid in plants is significantly affected by external stimuli such as storage, exposure to radiation, and microbial contamination. For this reason, the content of phenol in grape juice and wine is mainly variable.[Citation2]
Today, researchers focus on bioactive compounds extracted from different plants and semi-synthetic derivatives of natural compounds as a tool for progressing in the treatment of different diseases. Nowadays, flavonoids are highly considered because of various medicinal properties including anti-tumor, antioxidant, anti-inflammatory, and antiviral properties.[Citation3,Citation4] Among them, gallic acid, which is a natural vegetable tri-phenol, has been studied for its pharmacological effects in vivo and in vitro models.[Citation5]
Gallic acid, as a low molecular weight polyphenol, has strong antioxidant activity. Gallic acid has sufficient protection against oxidative damage caused by reactive species which are often involved in pathologic states.[Citation1,Citation6,Citation7] It was reported that gallic acid effective against a wide range of diseases like cancer,[Citation8,Citation9] cardiovascular diseases,[Citation10,Citation11] liver diseases,[Citation12,Citation13] inflammation,[Citation14,Citation15] and neurodegenerative diseases.[Citation16,Citation18]
Neurodegenerative diseases have increased in parallel with the aging population in the western countries. It was estimated that there will be about 14 million Alzheimer’s patients in the USA by 2050 with an expected death rate of one million people per year.[Citation19] A similar trend was also predicted for many neurodegenerative diseases around the world. Such a global concern creates a huge social and economic burden for the current and future generations. Current therapeutic interventions for neurodegenerative diseases limit the therapeutic interests. Therefore, there is a basic need for developing effective pharmacological agents that prevent Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative diseases.[Citation20]
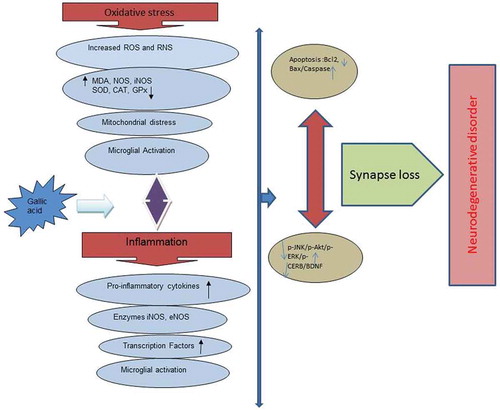
Recent studies have also indicated some promising results on the effect of plants and their compounds in treating or preventing the diseases related to the nervous system like Alzheimer’s disease,[Citation21,Citation22] stroke,[Citation23,Citation24] depression,[Citation25–Citation28] and anxiety.[Citation29] Neurodegenerative diseases primarily are related to oxidative stress and inflammation conditions.[Citation30,Citation31] Therefore, oral polyphenols like gallic acid with anti-oxidant and anti-inflammatory properties can be considered as preventive and therapeutic agents for neurodegenerative diseases.[Citation1,Citation32] In this review, the suggested effects of gallic acid in the treatment of nervous system disorders were evaluated. .
Gallic acid: chemical properties
The chemical formula of gallic acid is C6H2(OH)3COOH. It is found both as free and as part of hydrolyzable tannins. A variety of substituents in the gallic acid allow the obtainment of esters with a number of analogues with distinct pharmacological properties. The difference among the ester derivatives is only in the atom carbon number of the aliphatic side chain, giving them different physicochemical characteristics, especially lipophilicity evaluated by the value of partition coefficient chemical changes in the gallic acid molecule can modify their pharmacokinetic and pharmacodynamic properties, altering the solubility and the degree of ionization[Citation33] ().
Toxicity
Gallic acid is bound to key proteins and minerals like iron, zinc, and calcium and affects its bioavailable complex through an insoluble complex. Gallic acid has a negative effect on the mutagenic potential of TA98, TA100, TA1535 salmonella typhimurium.[Citation34] Edible LD50 for gallic acid in rabbits is 5000 mg/kg. Nevertheless, there is little information on the long-term toxicity of gallic acid.[Citation35] Based on the study of Bhavesh et al., the LD50 of gallic acid is above 2,000 mg/kg in mice. Hematological studies showed no change in transaminases and other parameters of blood homeostasis. Histopathological findings indicated no significant change in the histology of bone marrow tissue except the small amounts of fat cells inhibiting with no bone marrow suppression. In addition, no clear evidence of hematological parameters was observed. A high dose of gallic acid (900 mg/kg/day) had no significant changes in morphological and behavioral parameters for 28 days. Histopathologic findings confirmed the safety of gallic acid in mice.[Citation36]
Gallic acid (GA) toxicity was evaluated in F344 rats with a diet containing 0, 0.2, 0.6%, 1.7% gallic acid for 13 weeks. The results indicated that body weight gain in 5% of GA-treated animals in both males and females was significantly lower than the control group from week one to the end of the trial. The toxic effects of gallic acid were 0.6% or more in male rats after infusion and 5% in female rats including decreased hemoglobin concentration, hematocrit, red blood cell count, and increased reticulocytes. Histologically, the modular hemorrhage, hemosiderin obstruction, and congestion appear in the spleen of the glial glycine receptor 5% indicating hemolytic anemia. The centrilobular liver cell hypertrophy, reflecting the liver weight gain was observed in both genders.[Citation37]
Gallic acid and its derivatives have cytotoxic effects on concentration-dependent L1210 cells. The major difference between these molecules is perhaps due to the cytotoxic potential changes due to the length of the carbon chain. It was suggested that the lipophilicity of these compounds rises with the length of the alkaline chain and enhances their dependence on the two layers of the membrane of the cell membrane. As a result, it affects the interaction and entry into the cell. Gallic acid can produce apoptosis in tumor cells and can have an impact on cellular toxicity by creating chemical changes in the gallic acid molecule. Such simple structural changes in the gallic acid molecule led to a change in its physical and chemical properties involving solubility and its dispersion coefficient. Moreover, the change in the propagation potential through the membrane of fat can influence the interaction of molecules with their intracellular targets. Since the orientation of the head group, as well as total lipophilicity, determines the pharmacological activity, the drug reaction with the cell membrane activates caspases which may start the apoptotic process and lead to DNA fragmentation.[Citation38]
Antioxidant properties
Oxidative stress is involved in the pathophysiology of chronic diseases like neoplastic and neurodegenerative diseases. Thus, the antioxidant properties of dietary polyphenols play a role in improving the pathology of free radicals and have therapeutic effects in many chronic diseases. Although polyphenols are derived from different food and non-food sources, all of them have a bipartite bond, several hydroxyl groups, and more than one phenolic group.[Citation39]
Oxidative stress, the result of excessive production and the accumulation of free radicals, is the major cause of many degenerative diseases like cancer, atherosclerosis, cardiovascular disease, aging, and inflammation. Among the various polyphenols, gallic acid (3,4,5-trihydroxybenzoic acid), a low molecular weight molecule, is a strong antioxidant and an apoptotic agent.[Citation1]
GA, at 1.65 mM, accelerates the deoxyribose oxidation caused by Fe3 + -EDTAJH2O2. The reduction in the strength of this compound increases with increasing concentrations. Gallic acid at a concentration of 4.17 mM has no ability to decrease iron (II) but can neutralize DPPH-free radicals by 43.9%. In addition, gallic acid at a concentration of 4.7 mM can neutralize 60% of hydrogen peroxide radicals.[Citation40]
GA, propyl gallate (PG), and gallic acid methyl ester (GM) can reduce hypochlorous acid to protect α-1-protease against the inactivation by this molecule. When GA, lauryl ester (GL), PG and GM are dissolved in ethanol, they decreased the peroxidation of the phospholipids in the brain.[Citation41]
Anti-inflammatory properties
Inflammation is a key mediator in the pathogenesis of many chronic diseases like autoimmune diseases, cardiovascular, endocrine, neoplastic, and neuropathic diseases. Gallic acid cause the acetylation of the kappa nuclear factor B (NF-kB) to stabilize and decreases the production of cytokines in Microglia cells and protect neurons from neurotoxicity caused by Aβ.[Citation14]
Gallic acid has anti-inflammatory activity against acute inflammation caused by zymosan in mice foot. In vitro studies on gallic acid function showed that this compound by interacting with the multi-nucleotide leukocytes imposes its effects. The elimination of superoxide anions, inhibiting the release and activity of myeloperoxidase (MPO) and the possible involvement in the accumulation of active NADPH-oxidase can be a factor in inhibiting the beginning of the inflammatory process by gallic acid.[Citation15]
Oxidative stress, protein aggregation, and cell death has been proposed as a vicious cycle in the pathophysiology of CNS neurodegenerative diseases, including Alzheimer’s disease, Parkinsonian disease (PD), stroke, and spinal cord injury; neuroinflammation appears to be the center of this vicious cycle.[Citation42] The systemic administration of GA (100 mg/kg) significantly attenuated LPS-induced increases in glial fibrillary acidic protein (a biomarker of activated astrocytes) and ED-1 (a biomarker of activated microglia), as well as inducible nitric oxide synthase (iNOS, a proinflammatory enzyme) and interleukin-1β (a proinflammatory cytokine), in the LPS-infused substantia nigra (SN) of rat brain. GA attenuated LPS-induced elevation in heme oxygenase-1 level (a redox-regulated protein) and α-synuclein aggregation (a hallmark of CNS neurodegeneration), suggesting that GA is capable of inhibiting LPS-induced oxidative stress and protein conjugation. GA prevented LPS-induced caspase 3 activation (a biomarker of programmed cell death) and LPS – induced increases in receptor-interacting protein kinase (RIPK)-1 and RIPK-3 levels (biomarkers of necroptosis), indicating that GA inhibited LPS-induced apoptosis and necroptosis in the nigrostriatal dopaminergic system of rat brain[43Citation43]. GA reduced the expression of cyclooxygenase-2, NFκB, tenascin-C, chondroitin sulfate proteoglycans and glial fibrillary acidic protein in astrocytes in the LPC-induced model of inflammation. The level of myelin protein in neurites and oligodendrocyte cell bodies was significantly upregulated by GA.[Citation44]
Gallic acid and Alzheimer’s disease
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder which is characterized by progressive cognitive deterioration, loss of quality of life, and behavioral impairment. AD is the most common type of dementia among the elderly and about 5% of the world’s population aged over 60 have AD. Demographic changes and population aging have significantly increased the risk of AD to 25 million in 2000 and it is predicted to increase to 114 million by 2050.[Citation45]
Amyloid cascade hypothesis
The amyloid hypothesis of AD began to gain traction in the 1990s, and centres on abnormal processing of the amyloid precursor protein (APP), leading to production of amyloid-beta (Aβ).[Citation45] Secretase enzymes cleave APP and aberrancy of this process, specifically mutations in gamma and beta-secretases, can lead to the abnormal production of Aβ. Aβ can then trigger a cascade leading to synaptic damage and neuron loss, and ultimately to the pathological hallmarks of AD: amyloid plaques and neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein, with resulting neurodegeneration.[Citation46] Beta-amyloid causes oxidative stress and inflammatory response that results neuronal damage and neurological disorders .[Citation47] It has come to light that metal dyshomeostasis is associated with neurodegenerative amyloid diseases .[Citation48] Cu (II) ions can bind to amyloid β fibrils and generate reactive oxygen species.[Citation48] Recent studies have shown that certain compounds can act as a metal chelator as well as interact with amyloid aggregates, which has revealed the possible development of more multifunctional small molecules with improved activity and selectivity against metal-induced amyloid formation and toxicity.[Citation49] Metal ions play an important role in prognosis of neurodegenerative diseases. Thus, metal chelation therapy could provide a valuable therapeutic approach in the treatment of such diseases. Gallic acid has a bifunctional inhibitory role, i.e., apart from its established anti-amyloidogenic nature, it can also inhibit metal-induced aggregation. Gallic acid chelates Mg2+ ion as concluded from UV–Vis spectroscopy and fluorescence spectroscopy thereby inhibiting the metal induced aggregation.[Citation50]
Tau hypothesis
Tau is a protein expressed in neurons that normally functions in the stabilization of microtubules in the cell cytoskeleton.[Citation7] Hyperphosphorylation causes it to accumulate into these NFT masses inside nerve cell bodies. These tangles then aberrantly interact with cellular proteins, preventing them from executing their normal functions. Hyperphosphorylation occurs downstream of Aβ, with research suggesting that accumulation of Aβ may initiate this process. Additionally, there is evidence that toxic tau can enhance Aβ production via a feedback loop mechanism.[Citation51]
Cholinergic hypothesis
An initial breakthrough in AD came in the 1970s with the demonstration of a cholinergic deficit in the brains of patients with AD, mediated by deficits in the enzyme choline acetyltransferase. This, along with the recognition of the role of acetylcholine in memory and learning, led to the cholinergic hypothesis of AD and stimulated attempts to therapeutically increase cholinergic activity. Cholinergic depletion is a late feature of the neurodegenerative cascade. Cholinesterase inhibitors block the cholinesterase enzyme, which breaks down acetyl choline at the synaptic cleft, potentiating cholinergic transmission.[Citation52]
Excitotoxicity
Excitotoxicity, defined as overexposure to the neurotransmitter glutamate or overstimulation of its N-methyl-D-aspartate (NMDA) receptor, plays an important role in the progressive neuronal loss of AD. It is thought that loss of cholinergic neurons is affected by this process, resulting inexcessive influx of calcium into cells.[Citation53]
Much of the research in AD in the last decade has been directed towards disease-modifying therapy that will alter the course of the disease rather than act on symptoms alone, however the lack of effective disease-modifying drugs arising from these studies reflects the challenges involved in developing a therapeutic agent with potential to modify the course of a disease as complex as AD.[Citation54] Cholinesterase inhibitors Tacrine was the first-generation cholinesterase inhibitor but was limited by hepatotoxic side effects. Donepezil, rivastigmine and galantamine then followed, with the former probably the most widely used agent. Efficacy appears similar between these different agents so choice should be based on cost, individual patient tolerance and physician experience.[Citation55]
Anti-amyloid therapy
Until recently, several high-profile clinical trials of pharmacological agents targeted at modifying this amyloid cascade have been undertaken, with largely disappointing results. These agents generally had three different target sites: directly targeting Aβ, and either the gamma or beta-secretase enzymes involved in APP cleavage.[Citation56]
Immunization
The initial human clinical trial of active immunisation against Aβ with the agent AN 1792 was stopped because of cases of meningoencephalitis in 6% of subjects.[Citation57]
Monoclonal antibodies
Bapineuzumab, a monoclonal antibody to Aβ,[Citation58] underwent a phase III clinical trial from 2007 to 2012 in patients with mild to moderate AD. It was shown to reduce the rate of amyloid accumulation in Apoe4 carriers but did not demonstrate any treatment effect on either cognitive or functional outcomes despite engaging its target.[Citation59]
Tau-targeted therapy
Tau-targeted strategies that are currently in clinical trials include agents to prevent hyperphosphorylation, as well as those targeting microtubule stability and aggregation .[Citation60] Diet phytochemicals and particularly the polyphenols like gallic acid have potentials for Alzheimer's disease.[Citation61,Citation62] The neuroprotective effects of gallic acid on the treatment of Alzheimer’s disease have been of great significance in recent decades. The primary effects of gallic acid, which is responsible for its neuroprotection potential involve decreased accumulation and density of the beta-amyloid peptide. In vivo and in vitro studies as indicated in are expressed the effects of gallic acid in different studies on Alzheimer’s disease.
Table 1. The effects of gallic acid on Alzheimer’s disease in pre-clinical models
Gallic acid and Parkinson’s disease
Parkinson’s disease is an advanced neurodegenerative disease involving the selective loss of dopaminergic neurons in the substantia nigra and pars compacta. Parkinson’s is classified as a motor disorder with abnormal walking, tremor, stiffness, slow motion, and in advanced stages with cognitive impairment and dementia.[Citation68] This disease is tremor in the resting state that prevalence of which is more common in aging but also observed in young people. Its prevalence is the same around the world which means that the percentage of the prevalence does not vary according to the region. Increasing the ratio of acetylcholine to dopamine in cerebellar complexes leads to tremor symptoms, muscle stiffness, and slowness of movement.[Citation68] Oxidative stress plays a role in the pathophysiology of Parkinson’s disease. Dopamine auto-oxidation leads to the formation of quinones and the activity of monoamine oxidases leads to a defect in mitochondrial transmission, an increase in the formation of free radicals, and the oxidative damage to the nigrostriatal pathway neurons. The pro-inflammatory events caused by the activation of microglia cause dopaminergic damage in parkinson’s disease. The accumulation and formation of fibril from a-synuclein protein is considered as one of the main pathogenesis of parkinson’s disease.[Citation69]
The current treatments for Parkinson’s disease have many limitations. They often improve the motor symptoms in the short term, so that they are persistent during the first 2 years of treatment symptoms while with the advancement of the disease, they develop more disturbed motor disorders and non-motor disorders like depression and dementia.[Citation69]
This disease is not definitive but drugs like levodopa, amantadine, biperiden, and selegiline are prescribed for its treatment. The most widely used treatment is the use of Levodopa. Some studies indicated that Levodopa inhibits a mitochondrial complex and its long-term treatment decreases the activity of the protease system. Another study indicated that Levodopa causes neuronal death by disabling the Thioredoxin and Glutaredoxin systems both of which play a role in cell death. Therefore, Levodopa has significantly failed to satisfy the therapeutic expectations. As a result, many studies attempted to achieve effective preventive measures and less complicated treatment .[Citation69] In recent years, researchers used pre-clinical models to study the therapeutic effects of gallic acid for Parkinson’s disease. In vivo and in vitro studies, as indicated in stated the effects of gallic acid on Parkinson’s disease.
Table 2. The effects of gallic acid on Parkinson’s disease in pre-clinical models
Gallic acid and stroke
Brain stroke is the third cause of death and disability in industrialized countries, and if the person is survived, the stroke will cause some complications like the paralysis of the body and problems in memory, thinking, talking, and moving. Fifteen percent of brain strokes occur because of hemorrhage and 85% by ischemia.[Citation73] In other words, cerebral ischemia is considered as one of the most disabling cerebral events being one of the leading causes of mortality in the world in people above 65 years old. Different factors contribute to the development of cerebral ischemia involving cardiac arrest, atherosclerosis, thromboembolic events, vascular contraction, congenital heart disorders, low blood pressure, head injuries, chest anemia, choking, and some tumors. Cerebral ischemia leads to neurological disorders like motor, sensory, visual impairment, speech impairment and cognitive impairment, forgetfulness, and the impairment of spatial learning and memory.[Citation73]
Brain requires 25% of cardiac output for its metabolic needs. Thus, any reduction in brain blood flow can lead to ischemia and neurological disorders. During ischemia, the temporary or permanent reduction of blood flow to the brain causes decrease or not transfer the glucose and oxygen required for providing cell homeostasis. At this step, the processes like stimulation-induced cytotoxicity, acidosis, ion balance, oxidative stress, lipid peroxidation, inflammation, and apoptosis lead to the death of cells. Then, the reperfusion stage occurs, impairment in mitochondrial efficacy, glutamate release and inflammatory mediators, production of reactive oxygen species, and peroxidation of lipids will occur. The main event during cerebral ischemia is the production of free radicals and reactive species of oxygen and nitrogen, which cause damage to lipids, proteins, and DNA and cause neuronal death due to their high reactivity. Free radicals play a role in breaking the blood-brain barrier and causing cerebral edema.[Citation74,Citation75] In various studies shown in , gallic acid and some of its derivatives were displayed to decrease the size of the cerebral lesion, cerebral edema, neuronal damage, and decrease the rate of post-ischemic complications.
Table 3. The effects of gallic acid on cerebral ischemic/reperfusion models
Gallic acid and psychiatric disorders
In today’s society, the increased prevalence of neurological disorders like anxiety, personality disorders, schizophrenia, and depression has increased largely due to increased exposure to various stressful factors.[Citation79] Anxiety and depression are two mental illnesses with an increasing prevalence in the world. Based on the World Health Organization, depression and anxiety will be the second major cause of morbidity by 2030, which imposes significant economic burdens on societies.[Citation16] Monoamines play an essential role in the pathophysiology of depression and anxiety, so that their treatment is based on the selection of the medications that changing the activity of monoamine neurotransmitter system.[Citation80] Monoamine neurotransmitters such as serotonin (5-HT), noradrenaline and dopamine play a significant part in mediating depressive behaviors. Depression symptoms develop mainly due to declined activity of these neurotransmitters. Monoamine oxidase (MAOA) is a key enzyme that is dependent on neurotransmitters metabolism. MAOA activity rate has been suggested to be one of the susceptibility indices of psychological traum.[Citation81]
It has been shown that the enhanced glucocorticoid secretion results in accelerated glucose production through gluconeogenesis, glycogenesis, and lipolysis while being exposed to physical and psychological stress. Not only the increase in the rate of cellular metabolism enhances the production of free radicals and active oxygen species but also glucocorticoids play direct and indirect roles in promoting free radicals and oxidative stress which can finally damage and kill neurons.[Citation82] In addition, the increased levels of glucocorticoids lead to the increased glutamate levels in some areas of the brain, like cortex, thalamus, stratum, amygdala, and hippocampus, because of stress.[Citation83] High glutamate neurotransmitter activity is related to anxious and depressive behaviors in some limbic and cortical regions of the brain.[Citation84] Treatment with glutaminergic antagonists has anti-anxiety and anti-depressant effects in chronic stress-induced mice.[Citation85,Citation86]
Today, researchers attempt to find the pharmaceutical compounds with several mechanisms for preventing the progress and disturbance of mood disorders by affecting the mechanisms involved in the damages and death of neural cells.[Citation87] The neuroprotective property of gallic acid is because of the inhibition of free radicals and lipid peroxidation, anti-inflammatory properties, glutathione reduction, and anti-apoptosis properties. Different studies were performed to evaluate the therapeutic effects of gallic acid in the treatment of anxiety and depression, as displayed in .
Table 4. The effects of gallic acid in anxiety and depression pre-clinical models
Other neuroprotective effects of gallic acid
Gallic acid dose dependently has significant neuroprotective effects in the experimental model of seizure. Gallic acid reduces Ca 2+ and ROS release in PC12 cells.[Citation96] Oral administration of gallic acid improves memory avoidance and LTP in traumatic rats.[Citation97,Citation98] When calcium hemostasis disappears because of trauma, it prolongs the calcium concentration and leads to the failure of different ATPases, excessive glutamate release, activation of NMDA receptors, the activation of proteases, the formation of free radicals, the flow of potassium ions, sodium and water infiltration, mitochondrial depolarization, and the activation of cytokines including α TNF and IL-1β. Gallic acid reduces levels of TNF-α and IL-1β and IL-6 thereby decreasing the traumatic injury.[Citation97,Citation98] In several studies, the effects of gallic acid on motor activity and motor balance were studied in different models.[Citation99,Citation101] In one of these studies, the effects of 100 and 200 mg/kg gallic acid on aluminum chloride induced neurodegenerative in rats were evaluated. Gallic acid improves the ability of motor learning and motor balance, these effects occur by inhibiting Caspase 3, reducing the expression of inflammatory cytokines, and increasing the activity of antioxidant enzymes.[Citation100] In another study, the effects of gallic acid on motor activity and searching activity in 50 mg/kg lead-receiving rats were studied. The results indicated that gallic acid has improving effect on the motor activity being due to the improvement of the antioxidant defense system and the decrease of lead accumulation in the brain.[Citation101]
Neurodegeneration of the central and peripheral nervous system causes neuropathic pain. An indication of neuropathic pains is unpleasant, painful sensation and/or lack of sensation [102]. Management of neuropathic pain disorder is very complicated due to multiple complex mechanisms, and available conventional drugs produce only symptomatic relief of neuropathic pain.[Citation102] Paclitaxel (PT) causes neurodegeneration of a peripheral nerve ending, leading to progress the painful neuropathy. The primary toxic mechanism of PT has altered the production of microtubulin polymerization via synthesis of free radicals, TNF-α, and BCL2 proteins; alteration of cellular pro & anti-oxidant enzymes; calcium dyshomeostasis; and the opening of mitochondrial permeability transition pores (MPTP).[Citation103] Experimentally, administration of PT documented to produce the neuropathic pain symptoms in rodents as well as in humans. GA (20 and 40 mg/kg, i.v.) attenuated the PT induced pain behavior and biochemical changes. GA possesses potential ameliorating effect against PT induced neuropathic pain symptoms via free radical scavenging, reduction of inflammatory cytokines and cytosolic calcium ion concentration.[Citation104]
Clinical trials
The interest in using natural products in clinical trials has recently been on the increase. In 2017, the National Centre for Complementary and Integrative Health (NCCIH) introduced new funding opportunities for natural product in clinical trials. Gallic acid is a well-known secondary plant metabolite with good antioxidant activity and is under constant research for its health benefits. Gallic acid has already shown promising results in both in vitro and in vivo animal models of neurodegenerative disease. In addition, different clinical trials have been undertaken using plants contain high amounts of gallic acid as a therapy for AD. The construct mild cognitive impairment identified individuals with elevated risk for dementia, and progression from mild cognitive impairment to Alzheimer’s disease can be as high as 10 % per year.[Citation105] Grape juice contains a variety of flavonoids and antioxidants, among them anthocyanins and proanthocyanidins and comparatively high levels of total phenolics. Grape phenolics, including flavonoids and related polyphenols from grape, wine and grape seeds, are known to have free radical scavenging activities, antioxidant properties, and anti-inflammatory propertie. Supplementation with grape juice enhance cognitive function for older adults with early memory decline.[Citation106]
Discussion
During the past decades, researchers investigated the effects of gallic acid and its related compounds on the experimental models of neurodegenerative diseases like AD and PD. This review study aimed at investigating the neuroprotective effects of gallic acid as a well-known compound and a new source for drug development. Gallic acid has been highly considered due to its ability to absorb reactive oxygen species (ROS), such as superoxide anions, hydrogen peroxide, hydroxyl radicals and hypochlorous acid, and anti-mutilation and anti-tumor activity. Moreover, gallic acid has the neuroprotective effects against beta-amyloid, lead nitrate, or sodium fluoride inducing neurotoxicity and oxidative damage.[Citation64] GA improves only the synaptic failure induced by Aβ peptide and can be introduced as a promising multipotent pharmacological agent in the prevention or treatment of AD in the future.[Citation61] Gallic acid improves synaptic strength and decreases the size of plaque Aβ in the brain. In fact, in vitro analyses indicated that gallic acid disrupts Aβ1-42 accumulation and decreases neurotoxicity.[Citation18] Acetylcholine is the most significant neurotransmitter involved in the regulation of cognitive function. It was indicated that cognitive impairment is related to cholinergic transmission impairment. In addition, the selective loss of cholinergic neurons in some parts of the brain is a certain feature of dementia. Furthermore, the excessive activity of the AChE enzyme may lead to acetylcholine deficiency and memory impairment. Gallic acid can lead to improving memory and learning by reducing the activity of the AChE enzyme and enhancing the acetylcholine level.[Citation107]
Oxidative stress is one of the main factors in neurodegenerative diseases like Parkinson’s disease causing apoptosis through the production of ROS, mitochondrial dysfunction, DNA fragmentation, and finally cell death. Studies indicated that the excessive production of ROS by 6-OHDA results in oxidative stress and damage to dopaminergic cells leading to the induction of apoptosis and finally cell death. Gallic acid decreases 6-OHDA-induced cell death in SH-SY5Y cells by suppressing the Bax/Bcl2 ratio and the production of ROS.[Citation72]
Studies indicated that glutamate release following cerebral ischemia was enhanced and this neurotransmitter was shown to increase ROS production and induce neurotoxicity and cell death. Gallic acid protects the neurons against damage from ischemia and reperfusion of the brain through inhibiting the release of glutamate, Ca2+ and the producing free oxygen radicals.[Citation76] Neuroprotection can be through direct action on one or more mechanisms like anti-apoptotic effect or indirectly through anti-oxidant properties. Gallic acid inhibits neuronal damage to cerebral ischemia through inhibiting mitochondrial apoptotic signaling molecules.[Citation16]
The World Health Organization reports that depression and other mood disorders are increasing internationally and the cost of treatment for these diseases is high. Common antidepressants have many side effects and the search for drugs with less side effects is necessary. The anti-depressant activity of gallic acid through inhibitory activity on monoamine oxidase A, high antioxidant capacity and through the decrease of serum nitrate and corticosterone levels. In addition, gallic acid acts as an antidepressant by enhancing serotonin levels in the neuronal synapse through 5-HT2A/2 C and 5-HT3 receptors.[Citation91]
This review study focused on the mechanisms involved in the ability of gallic acid to cope with the neurotoxicity of oxidative stress and other neuroprotection mechanisms. However, more pathways are expected for biological activity. For example, signal transmission pathways, protease function, mitochondrial integrity, and so on. In addition, the potential role of gallic acid metabolites should be systematically evaluated because only limited information is available.
Additional information
Funding
References
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic Acid: A Versatile Antioxidant with Promising Therapeutic and Industrial Applications. RSC. Adv. 2015, 5(35), 27540–27557. DOI: 10.1039/C5RA01911G.
- Amerine, M. A.; Ough, C. S. Methods for analysis of musts and wines; 1980.
- KHAN, A. L. I.; NAZAN, M. S.; Mat Jais Am, S. Flavonoids and Anti-oxidant Activity Mediated Gastroprotective Action of Leathery Murdah, Terminalia Coriacea (Roxb.) Wight & Arn. Leaf Methanolic Extract in Rats. Arq. Gastroenterol. 2017, 54(3), 183–191. DOI: 10.1590/s0004-2803.201700000-21.
- Bakrania, A. K.; Patel, S. S. Combination Treatment for Allergic conjunctivitis–Plant Derived Histidine Decarboxylase Inhibitor and H1 Antihistaminic Drug. Exp. Eye Res. 2015, 137, 32–38. DOI: 10.1016/j.exer.2015.05.020.
- Locatelli, C.; Filippin-Monteiro, F. B.; Creczynski-Pasa, T. B. Alkyl Esters of Gallic Acid as Anticancer Agents: A Review. Eur. J. Med. Chem. 2013, 60, 233–239. DOI: 10.1016/j.ejmech.2012.10.056.
- Veluri, R.; Singh, R. P.; Liu, Z.; Thompson, J. A.; Agarwal, R.; Agarwal, C. Fractionation of Grape Seed Extract and Identification of Gallic Acid as One of the Major Active Constituents Causing Growth Inhibition and Apoptotic Death of DU145 Human Prostate Carcinoma Cells. Carcinogenesis. 2006, 27(7), 1445–1453. DOI: 10.1093/carcin/bgi347.
- Chia, Y. C.; Rajbanshi, R.; Calhoun, C.; Chiu, R. H. Anti-neoplastic Effects of Gallic Acid, a Major Component of Toona Sinensis Leaf Extract, on Oral Squamous Carcinoma Cells. Molecules. 2010, 15(11), 8377–8389. DOI: 10.3390/molecules15118377.
- Inoue, M.; Suzuki, R.; LI, Z.; Takeda, T.; Ogihara, Y et al. Selective Induction of Cell Death in Cancer Cells by Gallic Acid. Biol. Pharm. Bull. 1995, 18(11), 1526–1530.
- Ohno, Y.; Fukuda, K.; Takemura, G.; Toyota, M.; Watanabe, M.; Yasuda, N.; Xinbin, Q.; Maruyama, R.; Akao, S.; Gotou, K.;; et al. Induction of Apoptosis by Gallic Acid in Lung Cancer Cells. Anti-cancer Drugs. 1999, 10(9), 845–851.
- Priscilla, D. H.; Prince, P. S. M. Cardioprotective Effect of Gallic Acid on Cardiac troponin-T, Cardiac Marker Enzymes, Lipid Peroxidation Products and Antioxidants in Experimentally Induced Myocardial Infarction in Wistar Rats. Chem. Biol .Interact. 2009, 179(2–3), 118–124.
- Patel, S. S.; Goyal, R. K. Cardioprotective Effects of Gallic Acid in Diabetes-induced Myocardial Dysfunction in Rats. Pharmacognosy. Res. 2011, 3(4), 239. DOI: 10.4103/0974-8490.89743.
- Rasool, M. K.; Sabina, E. P.; Ramya, S. R.; Preety, P. et al. Hepatoprotective and antioxidant effects of gallic acid in paracetamol‐induced liver damage in mice. J. Pharm. Pharmacol. 2010, 62(5), 638–643.
- Ohno, T.; Inoue, M.; Ogihara, Y. Cytotoxic Activity of Gallic Acid against Liver Metastasis of Mastocytoma Cells P-815. Anticancer. Res. 2001, 21(6A), 3875–3880.
- Kim, M. J.; Seong, A. R.; Yoo, J. Y. et al. Gallic Acid, a Histone Acetyltransferase Inhibitor, Suppresses β‐amyloid Neurotoxicity by Inhibiting Microglial‐mediated Neuroinflammation. Mol. Nutr Food Res. 2011, 55(12), 1798–1808.
- Kroes, B. V.; Van den Berg, A.; Van Ufford, H. Q.; Van Dijk, H.; Labadie, R. Anti-inflammatory Activity of Gallic Acid. Planta. Med. 1992, 58(6), 499–504. DOI: 10.1055/s-2006-961535.
- Sun J, Li Y-z, Ding Y-h. et al. Neuroprotective Effects of Gallic Acid against Hypoxia/reoxygenation-induced Mitochondrial Dysfunctions in Vitro and Cerebral Ischemia/reperfusion Injury in Vivo. Brain. Res. 2014, 1589, 126–139. DOI: 10.1016/j.brainres.2014.09.039.
- Mansouri, M. T.; Farbood, Y.; Sameri, M. J.; Sarkaki, A.; Naghizadeh, B.; Rafeirad, M. Neuroprotective Effects of Oral Gallic Acid against Oxidative Stress Induced by 6-hydroxydopamine in Rats. Food. Chem. 2013, 138(2–3), 1028–1033. DOI: 10.1016/j.foodchem.2012.11.022.
- Yu, M.; Chen, X.; Liu, J, et al. Gallic acid disruption of Aβ1–42 aggregation rescues cognitive decline of APP/PS1 double transgenic mouse. Neurobiol. Dis. 2019, 124, 67–80. DOI: 10.1016/j.nbd.2018.11.009.
- Alzheimer’s, A.;. Alzheimer’s Disease Facts and Figures. Alzheimer’s Dementia. J. Alzheimers Dis. 2015, 11(3), 332.
- Scapagnini, G.; Caruso, C.; Calabrese, V. Therapeutic potential of dietary polyphenols against brain ageing and neurodegenerative disorders. Bio-Farms for Nutraceuticals. 2010, 12(2), 27–35.
- Rabiei, Z.; Rafieian-Kopaei, M.; Heidarian, E.; Saghaei, E.; Mokhtari, S. Effects of Zizyphus Jujube Extract on Memory and Learning Impairment Induced by Bilateral Electric Lesions of the Nucleus Basalis of Meynert in Rat. Neurochem. Res. 2014, 39(2), 353–360. DOI: 10.1007/s11064-013-1232-8.
- Rabiei, Z.; Setorki, M. Effect of Hydroalcoholic Echium Amoenum Extract on Scopolamine-induced Learning and Memory Impairment in Rats. Pharm. Biol. 2018, 56(1), 672–677. DOI: 10.1080/13880209.2018.1543330.
- Rabiei, Z.; Rafieian-Kopaei, M. Neuroprotective Effect of Pretreatment with Lavandula Officinalis Ethanolic Extract on Blood-brain Barrier Permeability in a Rat Stroke Model. Asian Pac. J. Trop. Med. 2014, 7, S421–S6. DOI: 10.1016/S1995-7645(14)60269-8.
- Rabiei, Z.; Bigdeli, M. R.; Rasoulian, B.; Ghassempour, A.; Mirzajani, F. The Neuroprotection Effect of Pretreatment with Olive Leaf Extract on Brain Lipidomics in Rat Stroke Model. Phytomedicine. 2012, 19(10), 940–946. DOI: 10.1016/j.phymed.2012.06.003.
- Rabiei, Z.; Gholami, M.; Rafieian-Kopaei, M. Antidepressant Effects of Mentha Pulegium in Mice. Bangladesh J. Pharmacol. 2016, 11(3), 711–715. DOI: 10.3329/bjp.v11i3.27318.
- Rabiei, Z.; Mokhtrari, S.; Babaei, F.; Rafieian, K. M. Effect of Kombucha Tea on Depression and Motor Activity in Mice. J MEDICIN PLANTS. 2017, 16(10), 156–166.
- Rabiei, Z.; Naderi, S.; Rafieian-Kopaei, M. Study of Antidepressant Effects of Grape Seed Oil in Male Mice Using Tail Suspension and Forced Swim Tests. Bangladesh J. Pharmacol. 2017 Nov 5, 12(4), 397–402. DOI: 10.3329/bjp.v12i4.33520.
- Rabiei, Z.; Jahanbazi, S.; Alibabaei, Z.; Rafieian-Kopaei, M. Antidepressant Effects of Oleuropein in Male Mice by Forced Swim Test and Tail Suspension Test. Middle. East J. 2018, 7(10), 132.
- Salehi, A.; Rabiei, Z.; Setorki, M. Effect of Gallic Acid on Chronic Restraint Stress-induced Anxiety and Memory Loss in Male BALB/c Mice. Iran. J. Basic Med. Sci. 2018, 21(12), 1232.
- Kelsey, N. A.; Wilkins, H. M.; Linseman, D. A. Nutraceutical Antioxidants as Novel Neuroprotective Agents. Molecules. 2010, 15(11), 7792–7814. DOI: 10.3390/molecules15117792.
- Grammas, P.;. Neurovascular Dysfunction, Inflammation and Endothelial Activation: Implications for the Pathogenesis of Alzheimer’s Disease. J. Neuroinflammation. 2011, 8(1), 26. DOI: 10.1186/1742-2094-8-26.
- Kade, I.; Rocha, J. Gallic Acid Modulates Cerebral Oxidative Stress Conditions and Activities of Enzyme-dependent Signaling Systems in Streptozotocin-treated Rats. Neurochem. Res. 2013, 38(4), 761–771. DOI: 10.1007/s11064-013-0975-6.
- Thanyacharoen, T.; Chuysinuan, P.; Techasakul, S.; Nooeaid, P.; Ummartyotin, S. Development of a Gallic Acid-loaded Chitosan and Polyvinyl Alcohol Hydrogel Composite: Release Characteristics and Antioxidant Activity. Int. J. Biol. Macromol. 2018, 107, 363–370. DOI: 10.1016/j.ijbiomac.2017.09.002.
- Rashid, K. A.; Baldwin, I. I.; Babish, J. G.; Schultz, J. C.; Mumma, R. O. Mutagenicity Tests with Gallic and Tannic Acid in the Salmonella/mammalian Microsome Assay. J. Environ. Sci. Heal. B. 1985, 20(2), 153–165. DOI: 10.1080/03601238509372473.
- Dollahite, J.; Pigeon, R.; Camp, B. The Toxicity of Gallic Acid, Pyrogallol, Tannic Acid, and Quercus Havardi in the Rabbit. Am. J. Vet. Res. 1962, 23, 1264–1267.
- Variya, B. C.; Bakrania, A. K.; Madan, P.; Patel, S. S. Acute and 28-days Repeated Dose Sub-acute Toxicity Study of Gallic Acid in Albino Mice. Regul. Toxicol Pharmacol. 2019, 101, 71–78. DOI: 10.1016/j.yrtph.2018.11.010.
- Niho, N.; Shibutani, M. et al. Subchronic Toxicity Study of Gallic Acid by Oral Administration in F344 Rats. Food. Chem. Toxicol. 2001, 39(11), 1063–1070.
- .Locatelli, C.; Rosso, R. et al. Ester Derivatives of Gallic Acid with Potential Toxicity toward L1210 Leukemia Cells. Bioorg. Med. Chem. 2008, 16(7), 3791–3799.
- Bishayee, A.; Darvesh, A. Oxidative Stress in Cancer and Neurodegenerative Diseases: Prevention and Treatment by Dietary Antioxidants. Free RadicalsFormation, Types and Effects. 2010, 34, 1–55.
- Yen, G.-C.; Duh, P.-D.; Tsai, H.-L. Antioxidant and Pro-oxidant Properties of Ascorbic Acid and Gallic Acid. Food Chem. 2002, 79(3), 307–313. DOI: 10.1016/S0308-8146(02)00145-0.
- Aruoma, O. I.; Murcia, A.; Butler, J.; Halliwell, B. Evaluation of the Antioxidant and Prooxidant Actions of Gallic Acid and Its Derivatives. J. Agric. Food Chem. 1993, 41(11), 1880–1885. DOI: 10.1021/jf00035a014.
- Manoharan, S.; Guillemin, G. J.; Abiramasundari, R. S.; Essa, M. M.; Akbar, M.; Akbar, M. D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell Longev. 2016, 2016, 1–15. DOI: 10.1155/2016/8590578.
- Liu, Y.-L.; Hsu, -C.-C.; Huang, H.-J.; Chang, C.-J.; Sun, S.-H.; M-Y, L. A. Gallic Acid Attenuated LPS-Induced Neuroinflammation: Protein Aggregation and Necroptosis. Mol. Neurobiol. 2020, 57(1), 96–104. DOI: 10.1007/s12035-019-01759-7.
- Siddiqui, S.; Kamal, A.; Khan, F.; Jamali, K. S.; Saify, Z. S. Gallic and Vanillic Acid Suppress Inflammation and Promote Myelination in an in Vitro Mouse Model of Neurodegeneration. Mol. Biol. Rep. 2019, 46(1), 997–1011. DOI: 10.1007/s11033-018-4557-1.
- Selkoe, D. J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Molecular Med. 2016, 8(6), 595–608. DOI: 10.15252/emmm.201606210.
- .Ghochikyan, A.; Petrushina, I.; Lees, A et al. A β-Immunotherapy for Alzheimer’s Disease Using Mannan–Amyloid-Beta Peptide Immunoconjugates. DNA Cell Biol. 2006, 25(10), 571–580.
- Van Marum, R. J.;. Current and Future Therapy in Alzheimer’s Disease. Fund. Clin. Pharmacol. 2008, 22(3), 265–274. DOI: 10.1111/j.1472-8206.2008.00578.x.
- Tõugu, V.; Tiiman, A.; Palumaa, P. Interactions of Zn (II) and Cu (II) Ions with Alzheimer’s Amyloid-beta Peptide. Metal Ion Binding, Contribution to Fibrillization and Toxicity. Metallomics. 2011, 3(3), 250–261. DOI: 10.1039/c0mt00073f.
- Savelieff, M. G.; DeToma, A. S.; Derrick, J. S.; Lim, M. H. The Ongoing Search for Small Molecules to Study Metal-associated amyloid-β Species in Alzheimer’s Disease. Acc. Chem. Res. 2014, 47(8), 2475–2482. DOI: 10.1021/ar500152x.
- Khan, A. N.; Hassan, M. N.; Khan, R. H. Gallic Acid: A Naturally Occurring Bifunctional Inhibitor of Amyloid and Metal Induced Aggregation with Possible Implication in Metal-based Therapy. J. Mol. Liq. 2019, 285, 27–37. DOI: 10.1016/j.molliq.2019.04.059.
- Bloom, G. S.;. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA neurol. 2014, 71(4), 505–508. DOI: 10.1001/jamaneurol.2013.5847.
- Ferreira-Vieira, H.; Guimaraes, T. M.; Silva, I. R.; Ribeiro, F. M. Alzheimer’s Disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14(1), 101–115. DOI: 10.2174/1570159X13666150716165726.
- Lipton, S. A.;. The Molecular Basis of Memantine Action in Alzheimer’s Disease and Other Neurologic Disorders: Low-affinity, Uncompetitive Antagonism. Curr. Alzheimer Res. 2005, 2(2), 155–165. DOI: 10.2174/1567205053585846.
- Salomone, S.; Caraci, F.; Leggio, G. M.; Fedotova, J.; Drago, F. New Pharmacological Strategies for Treatment of Alzheimer’s Disease: Focus on Disease Modifying Drugs. Br. J. Clin. Pharmacol. 2012, 73(4), 504–517. DOI: 10.1111/j.1365-2125.2011.04134.x.
- Bartolini, M.; Marco‐Contelles, J. Tacrines as Therapeutic Agents for Alzheimer’s Disease. IV. The Tacripyrines and Related Annulated Tacrines. Chem. Rec. 2019, 19(5), 927–937. DOI: 10.1002/tcr.201800155.
- Ghezzi, L.; Scarpini, E.; Galimberti, D. Disease-modifying Drugs in Alzheimer’s Disease. Drug Des. Dev. Ther. 2013, 7, 1471.
- Robinson, S. R.; Bishop, G. M.; Lee, H.-G.; Münch, G. Lessons from the AN 1792 Alzheimer Vaccine: Lest We Forget. Neurobiol. Aging. 2004, 25(5), 609–615. DOI: 10.1016/j.neurobiolaging.2003.12.020.
- Prins, N. D.; Scheltens, P. Treating Alzheimer’s Disease with Monoclonal Antibodies: Current Status and Outlook for the Future. Alzheimer’s Res. Ther. 2013, 5(6), 56. DOI: 10.1186/alzrt220.
- Salloway, S.; Sperling, R.; Fox, N. C. et al. Two Phase 3 Trials of Bapineuzumab in Mild-to-moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370(4), 322–333.
- Wischik, C. M.; Harrington, C. R.; Storey, J. M. Tau-aggregation Inhibitor Therapy for Alzheimer’s Disease. Biochem Pharmacol. 2014, 88(4), 529–539. DOI: 10.1016/j.bcp.2013.12.008.
- Hajipour, S.; Sarkaki, A.; Farbood, Y.; Eidi, A.; Mortazavi, P.; Valizadeh, Z. Effect of Gallic Acid on Dementia Type of Alzheimer Disease in Rats: Electrophysiological and Histological Studies. Basic. Clini Neuro. 2016, 7(2), 97.
- Ferruzzi, M. G.; Lobo, J. K.; Janle, E. M.; Cooper, B.; Simon, J. E.; Wu, Q.-L.; Welch, C.; Ho, L.; Weaver, C.; Pasinetti, G. M.;; et al. Bioavailability of Gallic Acid and Catechins from Grape Seed Polyphenol Extract Is Improved by Repeated Dosing in Rats: Implications for Treatment in Alzheimer’s Disease. J. Alzheimers Dis. 2009, 18(1), 113–124.
- Liu, Y.; Pukala, T. L.; Musgrave, I. F.; Williams, D. M.; Dehle, F. C.; Carver, J. A. Gallic Acid Is the Major Component of Grape Seed Extract that Inhibits Amyloid Fibril Formation. Bioorg. Med. Chem. Lett. 2013, 23(23), 6336–6340. DOI: 10.1016/j.bmcl.2013.09.071.
- Mansouri, M. T.; Naghizadeh, B.; Ghorbanzadeh, B.; Farbood, Y.; Sarkaki, A.; Bavarsad, K. Gallic Acid Prevents Memory Deficits and Oxidative Stress Induced by Intracerebroventricular Injection of Streptozotocin in Rats. Pharmacol .Biochem Behav. 2013, 111, 90–96. DOI: 10.1016/j.pbb.2013.09.002.
- Baziyar, Y.; Edalatmanesh, M. A.; Hosseini, S. A.; Zar, A. The Effects of Endurance Training and Gallic Acid on BDNF and TNF-a in Male Rats with Alzheimer. Int. J. Appl. Exerc. Physiol. 2016, 5(4), 45–54.
- Nagpal, K.; Singh, S.; Mishra, D. Optimization of Brain Targeted Gallic Acid Nanoparticles for Improved Antianxiety-like Activity. Int. J. Biol. Macromol. 2013, 57, 83–91. DOI: 10.1016/j.ijbiomac.2013.03.022.
- Ogunsuyi, O. B.; Oboh, G.; Oluokun, O. O, et al. Gallic Acid Protects against Neurochemical Alterations in Transgenic Drosophila Model of Alzheimer’s Disease. Orient. Pharm Exp Med. 2019, 1–10.
- Lang, A.; Lozano, A. M. Parkinson’s Disease. First of Two Parts. N. Engl. J. Med. 1998, 339(15), 1044–1053. DOI: 10.1056/NEJM199810083391506.
- De Lau, L. M.; Breteler, M. M. Epidemiology of Parkinson’s Disease. Lancet. Neurol. 2006, 5(6), 525–535. DOI: 10.1016/S1474-4422(06)70471-9.
- Reckziegel, P.; Peroza, L. R.; Schaffer, L. F. et al. Gallic Acid Decreases Vacuous Chewing Movements Induced by Reserpine in Rats. Pharmacol. Biochem. Behav. 2013, 104, 132–137. DOI: 10.1016/j.pbb.2013.01.001.
- Kasture, V. S.; Katti, S. A.; Mahajan, D.; Wagh, R.; Mohan, M.; Kasture, S. B. Antioxidant and Antiparkinson Activity of Gallic Acid Derivatives. Pharmacologyonline. 2009, 1, 385–395.
- Chandrasekhar, Y.; Kumar, G. P.; Ramya, E.; Anilakumar, K. Gallic Acid Protects 6-OHDA Induced Neurotoxicity by Attenuating Oxidative Stress in Human Dopaminergic Cell Line. Neurochem. Res. 2018, 43(6), 1150–1160. DOI: 10.1007/s11064-018-2530-y.
- Chen, H.; Yoshioka, H.; Kim, G. S. et al. Oxidative Stress in Ischemic Brain Damage: Mechanisms of Cell Death and Potential Molecular Targets for Neuroprotection. Antioxid. 2011, 14(8), 1505–1517.
- Christophe, M.; Nicolas, S. Mitochondria: A Target for Neuroprotective Interventions in Cerebral Ischemia-reperfusion. Curr. Pharm. Des. 2006, 12(6), 739–757. DOI: 10.2174/138161206775474242.
- .White, B. C.; Sullivan, J. M.; DeGracia, D. J. et al. Brain Ischemia and Reperfusion: Molecular Mechanisms of Neuronal Injury. J. Neurol. Sci. 2000, 179(1–2), 1–33.
- Korani, M. S.; Farbood, Y.; Sarkaki, A.; Moghaddam, H. F.; Mansouri, M. T. Protective Effects of Gallic Acid against Chronic Cerebral Hypoperfusion-induced Cognitive Deficit and Brain Oxidative Damage in Rats. Eur. J Pharmacol. 2014, 733, 62–67. DOI: 10.1016/j.ejphar.2014.03.044.
- Sarkaki, A.; Fathimoghaddam, H.; Mansouri, S. M. T.; Shahram Korram, M.; Saki, G.; Farbood, Y. Gallic Acid Improves Cognitive, Hippocampal Long-term Potentiation Deficits and Brain Damage Induced by Chronic Cerebral Hypoperfusion in Rats. PJBS. 2014, 17(8), 978–990. DOI: 10.3923/pjbs.2014.978.990.
- Farbood, Y.; Sarkaki, A.; Hashemi, S.; Mansouri, M. T.; Dianat, M. The Effects of Gallic Acid on Pain and Memory following Transient Global Ischemia/reperfusion in Wistar Rats. Avicenna. J Phytomed. 2013, 3(4), 329.
- Tsigos, C.; Chrousos, G. P. Hypothalamic–pituitary–adrenal Axis, Neuroendocrine Factors and Stress. J. Psychosom. Res. 2002, 53(4), 865–871. DOI: 10.1016/S0022-3999(02)00429-4.
- Ago, Y.; Arikawa, S.; Yata, M. et al. Antidepressant-like Effects of the Glucocorticoid Receptor Antagonist RU-43044 are Associated with Changes in Prefrontal Dopamine in Mouse Models of Depression. Neuropharmacol. 2008, 55(8), 1355–1363.
- Pan, Y.; Kong, L.; Xia, X.; Zhang, W.; Xia, Z.; Jiang, F. Antidepressant-like Effect of Icariin and Its Possible Mechanism in Mice. Pharmacol. Biochem. Behav. 2005, 82(4), 686–694. DOI: 10.1016/j.pbb.2005.11.010.
- Spiers, J. G.; Chen H-J, C.; Sernia, C.; Lavidis, N. A. Activation of the Hypothalamic-pituitary-adrenal Stress Axis Induces Cellular Oxidative Stress. Front. Neurosci. 2015, 8, 456. DOI: 10.3389/fnins.2014.00456.
- Lowy, M., . T.; Gault, L.; Yamamoto, B. K. Rapid Communication: Adrenalectomy Attenuates Stress‐induced Elevations in Extracellular Glutamate Concentrations in the Hippocampus. J. Neurochem. 1993, 61(5), 1957–1960. DOI: 10.1111/j.1471-4159.1993.tb09839.x.
- Amani, M.; Samadi, H.; Doosti, M-H. et al. Neonatal NMDA Receptor Blockade Alters Anxiety-and Depression-related Behaviors in a Sex-dependent Manner in Mice. Neuropharmacol. 2013, 73, 87–97. DOI: 10.1016/j.neuropharm.2013.04.056.
- Li, N.; Liu, R-J.; Dwyer, J. M. et al. Glutamate N-methyl-D-aspartate Receptor Antagonists Rapidly Reverse Behavioral and Synaptic Deficits Caused by Chronic Stress Exposure. Biol. Psychiatry. 2011, 69(8), 754–761.
- Dabbagh, A. J.; Mannion, T.; Lynch, S.; Frei, B. The Effect of Iron Overload on Rat Plasma and Liver Oxidant Status in Vivo. Biochem. J. 1994, 300(3), 799–803. DOI: 10.1042/bj3000799.
- Kawabata, K.; Kawai, Y.; Terao, J. Suppressive Effect of Quercetin on Acute Stress-induced Hypothalamic-pituitary-adrenal Axis Response in Wistar Rats. J. Nutr Biochem. 2010, 21(5), 374–380. DOI: 10.1016/j.jnutbio.2009.01.008.
- Pereira, M. M.; de Morais, H.; dos Santos Silva, E. et al. The Antioxidant Gallic Acid Induces Anxiolytic-, but Not Antidepressant-like Effect, in Streptozotocin-induced Diabetes. Metab. Brain Dis. 2018, 33(5), 1573–1584.
- Dhingra, D.; Chhillar, R.; Gupta, A. Antianxiety-like Activity of Gallic Acid in Unstressed and Stressed Mice: Possible Involvement of Nitriergic System. Neurochem. Res. 2012, 37(3), 487–494. DOI: 10.1007/s11064-011-0635-7.
- Mansouri, M. T.; Soltani, M.; Naghizadeh, B.; Farbood, Y.; Mashak, A.; Sarkaki, A. A Possible Mechanism for the Anxiolytic-like Effect of Gallic Acid in the Rat Elevated Plus Maze. Pharmacol. Biochem. Behav. 2014, 117, 40–46. DOI: 10.1016/j.pbb.2013.12.011.
- Can, Ö. D.; Turan, N.; Özkay, Ü. D.; Öztürk, Y. Antidepressant-like Effect of Gallic Acid in Mice: Dual Involvement of Serotonergic and Catecholaminergic Systems. Life. Sci. 2017, 190, 110–117. DOI: 10.1016/j.lfs.2017.09.023.
- Chhillar, R.; Dhingra, D. Antidepressant‐like Activity of Gallic Acid in Mice Subjected to Unpredictable Chronic Mild Stress. Fundam. Clin. Pharmacol. 2013, 27(4), 409–418. DOI: 10.1111/j.1472-8206.2012.01040.x.
- Nagpal, K.; Singh, S. K.; Mishra, D. N. Nanoparticle Mediated Brain Targeted Delivery of Gallic Acid: In Vivo Behavioral and Biochemical Studies for Improved Antioxidant and Antidepressant-like Activity. Drug. Deliv. 2012, 19(8), 378–391. DOI: 10.3109/10717544.2012.738437.
- Nabavi, S.; Habtemariam, S.; Di Lorenzo, A.; Sureda, A.; Khanjani, S.; Daglia, M. Post-stroke Depression Modulation and in Vivo Antioxidant Activity of Gallic Acid and Its Synthetic Derivatives in a Murine Model System. Nutrients. 2016, 8(5), 48. DOI: 10.3390/nu8050248.
- Samad, N.; Jabeen, S.; Imran, I.; Zulfiqar, I.; Bilal, K. Protective Effect of Gallic Acid against Arsenic-induced Anxiety−/depression-like Behaviors and Memory Impairment in Male Rats. Metab. Brain Dis. 2019, 34(4), 1091–1102. DOI: 10.1007/s11011-019-00432-1.
- Huang, H-L.; Lin, C-C.; Jeng, K-CG. et al. Fresh Green Tea and Gallic Acid Ameliorate Oxidative Stress in Kainic Acid-induced Status Epilepticus. J. Agr. Food. Chem. 2012, 60(9), 2328–2336.
- Sarkaki, A.; Farbood, Y.; Gharib-Naseri, M. K. et al. Gallic Acid Improved Behavior, Brain Electrophysiology, and Inflammation in a Rat Model of Traumatic Brain Injury. Can. J. Physiol. Pharm. 2015, 93(8), 687–694.
- Mirshekar, M.A.; Sarkaki, A.; Farbood, Y. et al. Neuroprotective Effects of Gallic Acid in a Rat Model of Traumatic Brain Injury: Behavioral, Electrophysiological, and Molecular Studies. Iran J. Basic Med. Sci. 2018, 21(10), 1056.
- Georgieva, A.; Belcheva, S.; Tashev, R.; Valcheva-Kuzmanova, S. Effects of Gallic Acid on Exploratory Behavior and Locomotor Activity in Rats. Trakia J. Sci. 2015, 13(2), 29–33. DOI: 10.15547/tjs.2015.s.02.007.
- Maya, S.; Prakash, T.; Goli, D. Evaluation of Neuroprotective Effects of Wedelolactone and Gallic Acid on Aluminium-induced Neurodegeneration: Relevance to Sporadic Amyotrophic Lateral Sclerosis. Eur J. Pharmacol. 2018, 835, 41–51. DOI: 10.1016/j.ejphar.2018.07.058.
- Reckziegel, P.; Dias, V. T.; Benvegnú, D.; Boufleur, N.; Barcelos, R. C. S.; Segat, H. J.; Pase, C. S.; Dos Santos, C. M. M.; Flores, É. M. M.; Bürger, M. E.;; et al. Locomotor Damage and Brain Oxidative Stress Induced by Lead Exposure are Attenuated by Gallic Acid Treatment. Toxicol. Lett. 2011, 203(1), 74–81.
- Backonja, -M.-M.;. Use of Anticonvulsants for Treatment of Neuropathic Pain. Neurology. 2002, 59(5suppl 2), S14–S7. DOI: 10.1212/WNL.59.5_suppl_2.S14.
- Chowdhury, M. R.; Moshikur, R. M.; Wakabayashi, R. et al. Ionic-liquid-based Paclitaxel Preparation: A New Potential Formulation for Cancer Treatment. Mol. Pharm. 2018, 15(6), 2484–2488.
- Kaur, S.; Muthuraman, A. Ameliorative Effect of Gallic Acid in Paclitaxel-induced Neuropathic Pain in Mice. Toxicol. Rep. 2019, 6, 505–513. DOI: 10.1016/j.toxrep.2019.06.001.
- Kim, J.; Lee, H. J.; Lee, K. W. Naturally Occurring Phytochemicals for the Prevention of Alzheimer’s Disease. J. Neurochem. 2010, 112(6), 1415–1430. DOI: 10.1111/j.1471-4159.2009.06562.x.
- Pasinetti, G. M.; Wang, J.; Ho, L.; Zha, W.; Dubner, L. Roles of Resveratrol and Other Grape-derived Polyphenols in Alzheimer’s Disease Prevention and Treatment. Biochim. Biophys. Acta. 2015, 1852(6), 1202–1208. DOI: 10.1016/j.bbadis.2014.10.006.
- Nagpal, K.; Singh, S.; Mishra, D. Nanoparticle Mediated Brain Targeted Delivery of Gallic Acid: In Vivo Behavioral and Biochemical Studies for Protection against Scopolamine-induced Amnesia. Drug. Deliv. 2013, 20(3–4), 112–119. DOI: 10.3109/10717544.2013.779330.