?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Present study reports metabolites profiling, antioxidant, and antidiabetic effects of Cuscuta reflexa grown on Casearia tomentosa. The UHPLC-QTOF-MS/MS method was used to characterize phytochemicals in most potent extract. Thirteen bioactive compounds/metabolites including (2S)-3-(β-D-galactopyranosyloxy)-2-(hexanoyloxy)propyl nonanoate, 4-O-β-D-glucosyl-4-coumaric acid, isorhamnetin-3-O-glucoside, 7-(α-D-glucopyranosyloxy)-2,3,4,5,6-pentahydroxyheptanoic acid, 13S-hydroxyoctadecadienoic acid, astragaloside derivative, salicylic acid, oleanolic acid, citric acid, pinellic acid, quinic acid, caffeic acid derivative and caffeic acid-3-glucoside were detected in 80% hydroethanolic (HE) extract. Among different extracts (aqueous, 20–80% HE and pure ethanolic), 80% HE extract showed maximum TPC (186.19 ± 2.15 mg GAE/g DE) and TFC (106.50 ± 1.68 mg RE/g DE). A strong correlation was observed between TPC, TFC, DPPH radical scavenging (IC50 = 60.64 ± 1.74 µg/mL) and TAP activity (235.96 ± 1.33 mg AAE/g DE) of 80% HE extract among others. The same extract also depicted highest α-amylase and α-glucosidase enzyme inhibition with IC50 of 71.84 ± 1.06 and 57.25 ± 1.40 µg/mL, respectively. Molecular docking was performed to explore possible role of identified phytochemicals. Binding affinity data as well as interaction patterns have revealed possible interaction of the identified bioactives against α-glucosidase and α-amylase. Moreover, intra-molecular charge transfer, energies of the FMOs, HOMO-LUMO-Egaps, MEP and IP were also explained. The present findings suggested that, C. reflexa is a promising natural antioxidant and antidiabetic agent, thus supporting the use of this plant for designing related functional foods and nutra-pharmaceuticals.
Introduction
Diabetes Type-2 is a complex and widespread chronic disease characterized by hyperglycemia. According to a current estimate, this disease has adversely affected the life of more than 100 million people world over, causing socio-ecological challenges for the suffering families.[Citation1] Diabetic pathogenesis leads to several long-term adverse health effects for example retinopathy, neuronal damage, cardiovascular disease, impaired wound healing, nephropathy[Citation2] and in some chronic cases it also causes death.[Citation3] It has already been ascertained that reactive oxygen species (ROS) and oxidative stress are the crucial mediator, leading to diabetic complications. In hyperglycemia, unnecessary glucose loading stimulates the production of ROS within the mitochondria, which results in impairement of mitochondrial functions.[Citation4] The medical treatments for controlling diabetes mellitus involve the use of selected pharmacological agents having diverse modes of action. Although, an effective approach for management of this disease is hormone therapy but due to limited actions and adverse effects (i.e., diarrhea, liver damage, abdominal pain, loss of appetite, hypoglycemia, weight loss, etc.) its usage is restricted.[Citation2,Citation5] The second approach is to lower glucose absorption by simply inhibiting α-amylase and/or α-glucosidase activities in digestive system.[Citation6] The α-amylase breaks long chain carbohydrate while α-glucosidase catalyzes hydrolysis of starch and disaccharide into glucose.[Citation7] The use of natural or synthetic carbohydrate digestive enzyme inhibitors might be an emphatic option to limit digestion rate of carbohydrates, leading to reduction in postprandial hyperglycemia.[Citation8] So far, limited number of α-amylase and α-glucosidase enzyme inhibitors are commercially available due to their complex synthesis and severe side complications.[Citation9]
On the other hand, fruits, medicinal herbs and vegetables containing high level of polyphenols with therapeutic potential but limited side effects are now gaining interest for the treatment of diabetic patients. Numerous edible and non-edible medicinal herbs have been investigated for their potent antioxidant, α-amylase and α-glucosidase inhibitory effects.[Citation10,Citation11] Some plant-based bioactives are even more effective than commonly used synthetic drug.[Citation12] With the resurgence of interest in the utilization of plants/herbs as medicine and food source coupled with recent trends of optimal nutrition, the development of several plant based food supplements or therapeutic products is an emerging area of scientific research since past two decades. Thus, exploring biochemical composition of traditionally used medicinal plants toward development of functional foods and nutraceuticals is of vital importance.[Citation13]
Cuscuta reflexa Roxb., commonly recognized as akash bail/amar bail, is an unusual, wild parasitic plant belonging to family Convolvulaceae. C. reflexa can parasitize by wrapping itself around hosts and absorb water and nutrients from them. Therefore, the phytochemical constituents of this parasitic plant may depend upon nature of the host plant. C. reflexa, native to India, China, Afghanistan, Pakistan and Thailand grows wildly in tropical and subtropical areas.[Citation14,Citation15] C. reflexa has extensively been used by traditional medical practitioners for treatment of numerous bilious disorders such as diabetes,[Citation1] cancer, bone fracture, night blindness, rickets, eczema, stomachache, diaphoretic,[Citation16] jaundice and hypertension.[Citation17] It has also been applied for preventing aging, abortion, migraine, amnesia, constipation and chronic catarrh clinically.[Citation14]
To the best of author’s knowledge, limited comprehensive report is available on polyphenolic composition and active principles of C. reflexa in particularly grown on Casearia tomentosa (Salicaceae). Therefore, current work was planned to assess antioxidant and antidiabetic attributes of various HE stem extracts from C. reflexa grown on C. tomentosa. Furthermore, metabolites profiling of most potent HE extract has been carried out using a novel and sensitive UHPLC-QTOF-MS/MS method, which led to the detection of promising antioxidant and antidiabetic bioactive constituents. Molecular docking and computational studies were also performed to explore possible role of identified phytochemicals as antioxidant and antidiabetic agents.
Materials and methods
Reagents and chemicals
All reagents and/or chemicals used were of analytical research grade. Folin Ciocalteu (FC) reagent, α-amylase and α-glucosidase enzymes, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, p-nitrophenyl glucopyranoside (p-NPG), aluminum chloride (AlCl3), ammonium molybdate (NH4)2MoO4), sodium nitrite (NaNO2), sodium phosphate (Na3PO4), sodium hydroxide (NaOH), anhydrous sodium carbonate (Na2CO3), sulfuric acid (H2SO4), ethanol, methanol as well as reference standards including acarbose, rutin, ascorbic acid (AA), gallic acid, butylated hydroxyanisole (BHA) were acquired from Merck and Sigma Aldrich.
Plant materials and extraction
The fresh aerial parts of C. reflexa grown on C. tomentosa were manually collected from AJK, Pakistan. Plant identification and authentication was performed by a taxonomist at Department of Botany, University of Gujrat, Hafiz Hayat Campus, Gujrat, Pakistan and a voucher specimen was deposited under number, UOG/CHEM/19/2018 for reference purposes. Sample material was washed initially, dried, quenched with liquid N2 for metabolite preservation and freeze dried using a freeze dryer for 2 days at −68°C (Christ Alpha 1–4, LD, Germany). The sample was then ground to fine powder, sieved (by a 60-mesh sieve) and stored in Ziplock bag at −80°C. Ten grams of powder was extracted by means of 100 mL HE solvent systems of different concentrations i.e., aqueous, 20, 40, 60, 80 and 100% ethyl alcohol under constant temperature 35 ± 2 °C and humidity 25 ± 5% for 2 days. Afterward, each sample mixture was vortexed by using mixer for 2 h (Wise Mix SHO-1D, DAIHAN Scientific, Korea), ultrasonicated for 1h at 35 ± 2°C (Soniprep 150 Ultrasonicator MSE, UK), centrifuged (13,000 rpm) for 10 min, filtered by automated porcelain assembly fitted with Whatman grade-42 filter paper and vacuum pump (Today’s Rocker-300) and evaporated at 35°C by a rotary vacuum evaporator till viscous extract. The crude C. reflexa extracts were freeze-dried again and finally stored at −80°C for future experiments.
Total phenolic contents
The assessment of TPC was executed using an already described method with minor modifications.[Citation18] For this, each sample extract (0.10 mL) was dissolved in 1 mL of FC reagent, followed by the addition of 3 mL of Na2CO3 (10%, w/v) solution. The sample mixture was incubated at 23°C for 90 min. Absorbance was recorded at 750 nm, spectrophotometrically (UV1700, Schimadzu Japan). Gallic acid was taken as reference standard. Findings were articulated as milligram gallic acid equivalent/gram dried extract (mg GAE/g DE). The experiment was carried out in triplicate.
Total flavonoid contents
Determination of TFC in different HE extracts was performed using a spectrophotometric method.[Citation19] For this purpose, sample (0.20 mL) was dissolved in a mixture comprising, 0.30 M AlCl3.6H2O (0.15 mL), 30% CH3OH (3.4 mL) and 0.50 M NaNO2 (0.10 mL). After 5 min, 1 M solution of NaOH (1 mL) was dissolved into sample mixture. Absorbance was noted at 510 nm. Appraisal of TPC was carried out with rutin as reference/control. Findings were recorded as milligram of rutin equivalent/gram dried extract (mg RE/g DE). The analysis was done in triplicate.
DPPH radical scavenging activity
The scavenging effect of various C. reflexa extracts toward DPPH radical was evaluated according to a previously reported DPPH radical scavenging assay with modifications.[Citation20] Briefly, each extract was added in 500 µL DPPH solution in methanol. Sample mixtures were then incubated for 15 min at 30°C and absorbance was noted at 517 nm spectrophotometrically. The BHA was taken as reference antioxidant. The antioxidant effect was expressed as µg/mL. The percent inhibition was computed by given formula:
Furthermore, half maximal inhibitory concentrations (IC50) were computed by plotting extract compositions versus percent inhibitions. The experiment was executed in triplicate.
Total antioxidant power assay
The total antioxidant capacity of extracts was investigated as per previously described phosphomolybdenum assay with small modifications.[Citation21] This method involves reduction of Mo (VI) to Mo (V) by sample extract, followed by the formation of phosphate Mo (V) green colored complex at acidic pH. For this purpose, plant samples (0.10 mL each) were added in reagent solution separately, consisting of Na3PO4 (28 mM), H2 SO4 (0.60 M) and (NH4)2MoO4 (4 mM). Reaction mixtures were incubated for 90 min at 90°C and then cooled to 30°C. Absorbance was noted at 765 nm, using a spectrophotometer. Ascorbic acid was taken as positive control. Antioxidant capacity was presented as milligram ascorbic acid equivalent/gram dried extract (mg AAE/g DE). All tests were performed in triplicate.
α-glucosidase inhibitory assay
The inhibitory effects of all the tested extracts against α-glucosidase were assessed as described by Jabeen et al.[Citation22] with minor changes. For this purpose, sample extracts (10 µL each) were mixed with 30 mM potassium phosphate buffer (70 µL) separately, adjusted to pH of 6.80. After that the enzyme α-glucosidase (10 µL) was mixed followed by the addition of 5 mM p-NPG (10 µL), as substrate. The sample mixtures were reincubated for 30 min. To terminate the reaction, 0.10 M Na2CO3 (2 mL) was mixed. Absorbance was computed at 405 nm, spectrophotometrically. Acarbose was taken as reference inhibitor. The analysis was performed three times and IC50 (μg/mL) values were determined by plotting extract compositions versus percent inhibitions.
α-amylase inhibitory assay
The α-amylase inhibitory effects of all understudy extracts were assessed using a modified method explained by Shai et al.[Citation23] Briefly, the mixtures containing different amounts of C. reflexa extracts and α-amylase enzyme solution (2 units/mL) in Na3PO4 (0.10 M) buffer (pH of 6.80) were incubated for 20 min at 37°C, followed by the addition of 1% starch solution and heated again for 1 h at 37°C. At the end, absorbance was noted at 540 nm by means of a spectrophotometer. Acarbose was employed as standard inhibitor. All measurements were carried out in triplicate. The IC50 (μg/mL) values were calculated by plotting extract/sample compositions versus percent inhibitions.
UHPLC-QTOF-MS/MS analysis
High resolution full scan with MS/MS fragmentations was executed using Sciex 5600 Triple TOF High resolution Mass Spectrometer with Eksigent UHPLC. The system was equipped with Thermo Hypersil Gold, 2.10 mm × 3 µm × 100 mm column. A mobile phase comprising H2O (A) and CH3CN (B) with HCOOH (0.10%) and HCOONH4 (5 mM) was utilized in gradient mode. The sample was extracted by dissolving in 5 mL of 80% methanol, vortexed/shaked and filtered using nylon syringe filter (0.45 µm). Data analysis was performed with ACD Labs-MS/MS fragmenter software and Sciex-Peak View (2.10) software. Identification and characterization were carried out based upon online (Chemspider) database as well as matching with possible fragmentation patterns, accurate masses and molecular ion peaks.
Docking studies
Molecular docking calculations were executed by Molecular-Operating-Environment version 2016.08 (MOE-2016.08). Docking studies for the enzyme, α-glucosidase were performed on a reported homology-modeled α-glucosidase.[Citation24] Three-dimensional (3-D) structure of porcine pancreatic-α-amylase (PPA) in complex with acarbose was taken from Protein-Data-Bank (PDB, ID-1OSE). Preparation of ligand’s downloaded enzymes such as determination of binding sites, energy minimization and 3-D protonation was performed by previously reported methods.[Citation24] After the identification of bioactive compounds, their structures were drawn by Builder option in MOE. Simplified Molecular Input Line Entry Specification, representing a 2-D chemical drawing as a string, from literature was also generated from 2-D structural models and employed as input for docking. A data base of identified phytoconstituents was built as ligand, MDB. Energy minimization of all structures was carried out up to 0.01 Gradient (in MMFF94X forcefield). Structure of enzyme was opened in MOE window. The H2O molecules (if present) were detached. The protonation of 3-D structure was carried out in implicit solvated environment at salt concentration of 0.10 mM, pH of 7 and temperature of 300 K. The energy minimization of complete structure was then carried out using MMFF94X forcefield. At last, docking of all identified bioactives was carried out into binding site of prepared enzyme. Default docking parameters were adjusted, and 10 various conformations were generated for every compound. Docking results were interpreted, and their surfaces were analyzed with graphical representation utilizing MOE as well as discovery-studio visualizer.[Citation25]
Computational studies
Presently, first principles calculations are gaining much importance in chemistry because these are very useful in dedicating and explaining various properties of the compounds. Several studies revealed that Density-Functional-Theory (DFT) is an excellent method to investigate electronic nature of materials that effectively reproduced the experimental data.[Citation26] Moreover, DFT has been used successfully for the optimization of ground state (S0) geometries of the diverse bioactive constituents.[Citation27] The B3LYP has been found rational among various DFT functionals.[Citation28]In current work, the S0 geometry optimization has been accomplished by functional B3LYP and Triple-Zeta with Polarization (TZP) functions basis set utilizing ADF (Amsterdam-Density-Functional) package.[Citation28]
Statistical analysis
The findings of the experiments were analyzed using MINITAB (version-17) software. Each experiment was executed in triplicate. Findings of all experiments are expressed as mean value ± standard deviation. Differences among data were considered statistically significant with p < .05.
Results and discussion
Percent extract yield
Extracts of C. reflexa were prepared using different solvents such as; aqueous, aqueous:ethanol (20–80%, v/v) and ethanol (100%). The effect of solvents, on percent extract yield is depicted in . Maximum yield of C. reflexa extract (21.89 ± 0.18%) was procured by 80% ethanol, whereas lowest yield (16.02 ± 0.09%) was revealed by aqueous extract. The yields of C. reflexa extracts using 20%, 40%, 60% HE and 100% ethanol were depicted to be 16.98 ± 0.11%, 18.31 ± 0.20%, 20.52 ± 0.44% and 19.25 ± 0.31%, respectively. As revealed statistically, maximum yield obtained by 80% HE extract was different considerably (p < .05) from rest of understudy extracts. Previously, extract yield of C. reflexa grown on different hosts has been reported by many researchers viz., in one study, 80% methanolic extract of C. reflexa grown on Azdirecta indica showed highest yield (26.14 ± 0.81%). Extract yield (22.63 ± 0.51%) has been reported for C. reflexa grown on Zizyphus jojoba using 80% ethanol. In another previous report, highest extract yield (8 ± 0.60%) has been described for C. reflexa grown on Lycium barberum using methanol as extracting solvent. Similarly, extract yield (8.26 ± 0.33%) has been documented for C. reflexa grown on Acacia nilotica using hexane as extraction solvent.[Citation29,Citation30] Such variations in percent yields of C. reflexa extracts could be ascribed to different composition of solvents as well as extraction procedures/techniques used, in addition to other factors such as plant maturity, host plants, harvest season and climatic conditions.[Citation31]
Table 1. Extract yield, TFC and TPC in different extracts from C. reflexa.
Total phenolic contents (TPC) and total flavonoid contents (TFC)
Polyphenols (phenolics and flavonoids), are well-known phytochemicals in Cuscuta species, that have remarkable health promoting properties including immune-stimulatory, antioxidant, antidiabetic and anticancer effects.[Citation29,Citation32] Previously, it is reported that levels of extracted phenolic compounds depend on different parameters such as pH, solvent polarity, extraction temperature and time.[Citation4] In the current work, we determined total phenolic and flavonoid contents of C. reflexa hosted on C. tomentosa. Data regarding TFC and TPC determined in different extracts from C. reflexa is shown as .
Highest values of TPC and TFC observed in 80% HE extract were 186.19 ± 2.15 mg GAE/g DE and 106.50 ± 1.68 mg RE/g DE, respectively. Meanwhile, minimum values of TPC and TFC were found for aqueous extract i.e., 108.46 ± 2.92 mg GAE/g DE and 79.31 ± 1.28 mg RE/g DE, respectively. Higher TPC and TFC associated with 80% HE may be linked to the solvent polarity. Ethanol is relatively a safe and/or green solvent for the extraction of plant bioactives. Addition of water in ethanol may possibly be a significant factor to improve the extraction efficiency, which was reflected in the form of maximum levels of TPC and TFC in present case. The TFC and TPC are renowned phytochemicals for their antioxidant effect. They can cause significant decrease in the oxidative stress.[Citation33] A statistically significant difference (p < .05) was indicated among TPC and TFC of different extraction solvents. Present results were comparable with a previous study where TPC (146.32 ± 0.83 mg GAE/100 g) and TFC (59.45 ± 0.41 mg/100 g QE) in methanolic extract of C. reflexa grown on A. nilotica were reported.[Citation30] The difference in amounts of TPC and TFC contents could be due to different host plant, solvent and method used for extraction.
Antioxidant activity
Antioxidants, commonly known as free radical scavenger, peroxide decomposer, metal chelator, singlet oxygen quencher or electron donor are necessary for protecting the body against several chronic health diseases/disorders such as cardiovascular diseases, diabetes, cancers, Alzheimer’s disease, etc. Although synthetic antioxidants can prevent or attenuate risk of oxidative stress both in vitro and in vivo[Citation34,Citation35] but exploration of natural antioxidants is an emerging trend to potentiate antioxidative defense mechanism with minimum side effects.[Citation35] There are several different methods that can be used to investigate antioxidant potential of natural antioxidants including beverages, foods and plant extracts, but the most commonly used methods are DPPH radical scavenging and TAP assay.[Citation36] In our present study, in vitro antioxidant potential of C. reflexa was determined using DPPH and TAP assays.
DPPH radical scavenging activity
The DPPH antiradical assay was executed to explore scavenging effect of understudy C. reflexa extracts. The IC50 values of all samples and positive control (BHA) at various concentrations are shown in . Among tested extracts, 80% HE extract demonstrated strongest antiradical effect with IC50 value 60.64 ± 1.74 µg/mL, that might be linked to high levels of phenolics and flavonoids in respective extracts, while aqueous extract exhibited lowest antioxidant activity (IC50 value 113.43 ± 3.35 µg/mL) among others. As revealed by statistical analysis, antioxidant effect of 80% HE extract was higher and significantly different (p < .05) from those of 60%, 100%, 40%, 20% and pure aqueous extracts but lower than BHA (IC50 of 37.33 ± 1.20 µg/mL). However, differences were not significant statistically (p > .05) among 40%, 60% and100% extracts. DPPH radical scavenging ability (IC50) for C. reflexa grown on several different host plants, ranged from 88.85 ± 0.07 to 669.37 ± 2.04 µg/mL.[Citation15,Citation30,Citation37] The difference in antiradical effect could be attributed to different host plants, extracting methods and solvents used to dissolve endogenous compounds.
Table 2. DPPH and TAP results for different extracts from C. reflexa extracts.
Total antioxidant power assay
The TAP is an imperative in vitro antioxidant assay. It helps to monitor antioxidant capability of both water and fat-soluble antioxidants.[Citation36] The results obtained for the studied extracts are expressed in . As revealed from results, 80% C. reflexa extract showed maximum TAP (235.96 ± 1.33 mg AAE/g DE) followed by 60% (199.56 ± 2.18 mg AAE/g DE), 40% (182.41 ± 2.43 mg AAE/g DE), 100% (178.85 ± 1.53 mg AAE/g DE), 20% (168.06 ± 1.95 mg AAE/g DE) and aqueous (156.51 ± 0.75 mg AAE/g DE) extract. The analysis revealed statistically significant difference of means among the 80% HE and other extracts with p < .05, however, in case of 40% and 100% HE extracts, the differences were non-significant statistically (p > .05). The difference in antioxidant activities of the sample extracts could be ascribed to various amounts of antioxidant compounds. Previously reported TAP values of methanol/chloroform and aqueous extracts of C. reflexa were 34.70 ± 4.40 mg AAE/g DE and 31.90 ± 0.90 mg AAE/g DE, respectively.[Citation38] The outcomes of the current work revealed that C. reflexa hosed on C. tomentosa unveiled strong TAP and thus can be valued as a promising natural source of antioxidants.
α-amylase and α-glucosidase inhibitory activities
Consumption of antioxidant rich herbal extracts that can decrease oxidative stress and abnormal hyperglycemia can be regarded as one of most promising options to treat Type 2 diabetes.[Citation6] The α-glucosidase or α-amylase enzyme inhibitory activity assay is the most studied and reliable test to assess antidiabetic potential of food and pharmaceutical products. Also, the enzyme inhibitors are used clinically to cure or control post-prandial hyperglycemia.[Citation39] Due to severe complications of some synthetic enzyme inhibitors, the search for novel and natural inhibitors with minimum side effects is gaining greater interest. The α-amylase catalyzes hydrolysis of long chain carbohydrates, on the other hand α-glucosidase digests disaccharides/starch into simple sugars (glucose), thus helps in glucose absorption from dietary polysaccharides into small intestine.[Citation4,Citation39] Therefore, inhibition of these enzymes is being effectual for controlling diabetes by delaying glucose absorption.[Citation40]
In current work, different HE extracts from C. reflexa were estimated for their inhibitory properties toward α-amylase and α-glucosidase enzymes and the outcomes are given in . Maximal α-amylase inhibition was depicted by 80% HE extract with IC50 of 71.84 ± 1.06 µg/mL followed by 60% (IC50 = 81.07 ± 1.71 µg/mL), 40% (IC50 = 85.35 ± 0.75 µg/mL), 100% (IC50 = 86.46 ± 0.98 µg/mL) and 20% HE extracts (IC50 = 91.77 ± 1.53 µg/mL). The lowest α-amylase inhibition was shown by aqueous extract (IC50 of 97.11 ± 0.33 µg/mL). The α-amylase inhibitory effect of 80% extract was different significantly (p < .05) compared with other extracts and acarbose (IC50 = 38.95 ± 0.13 µg/mL). However, the differences were not significant statistically (p > .05) between 40% and 100% extracts.
Table 3. The α-glucosidase and α-amylase inhibition activities for different extracts from C. reflexa.
The same 80% HE C. reflexa extract was revealed to be the most active α-glucosidase enzyme inhibitor (IC50 of 57.25 ± 1.40 µg/mL) among others, while the minimum inhibitory effect was shown by aqueous extract with IC50 value, 87.65 ± 1.67 µg/mL. It was observed that α-glucosidase inhibitory effect of 80% HE extract differed significantly (p < .05) from other extracts and acarbose (IC50 = 34.52 ± 0.26 µg/mL). However, the difference was not significant statistically (p > .05) between 40% and 100% extracts.
The findings demonstrated that HE extracts of C. reflexa possessed significant inhibitory activity toward key clinical enzymes (carbohydrate digestive enzymes), revealing the presence of biologically active phytomolecules. Our results were in agreement with some previous studies, which indicated antidiabetic potential of C. reflexa hosted on various plants such as Coccinia grandis, Dimocarpus longan, Streblus asper, Zollingeria dongnaiensis, Samanea saman and Ficus racemosa.[Citation15,Citation41] However, current investigation is probably the first report on antidiabetic effect of C. reflexa grown on C. tomentosa by exploring possible phytochemicals role via molecular docking and computational studies.
UHPLC-QTOF-MS/MS based metabolite profile of C. reflexa
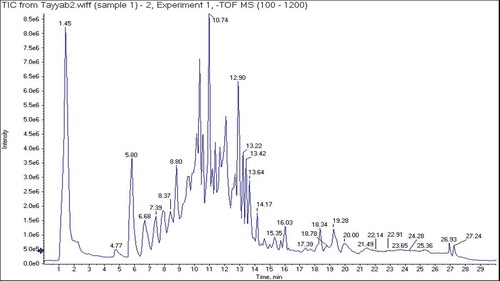
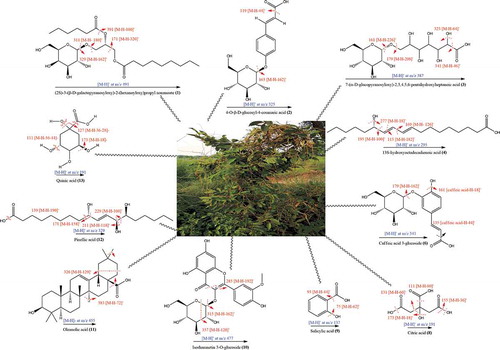
The characterization of bioactive constituents in most potent (80% HE) extract was carried out with UHPLC-QTOF-MS/MS. Chromatogram of test sample is shown as . Bioactive constituents were identified by explicating their retention times (min), [M-H]− ions, molecular formulae, MS-fragmentation patterns and by comparing data with reference or verified with literature. Complete information of all the identified compounds is provided in and . Notably 13 compounds were identified including phenolic acids, phenolic glycoside, flavonoid, fatty acid and triterpene.
Table 4. Phytoconstituents identified in 80% C. reflexa HE extract by UHPLC-QTOF-MS/MS.
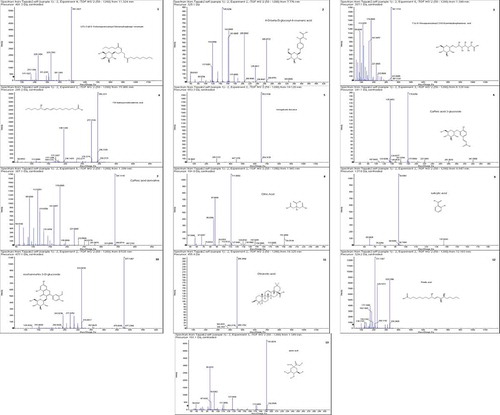
Negative ionization mode of UHPLC-QTOF-MS/MS formed precursor ion [M-H]−, which was examined further by product ion mode to produce their fragments. In total 13 metabolite peaks were analyzed ( and ), while remaining peaks in the chromatogram remained unidentified. Compound (1) recorded at Rt, 11.3 min displayed precursor ion [M-H]− at 491. Product ion mode for this compound produced other fragments at 391 [M-H-100]−, 329 [M-H-162]− and 311 [M-H-180]−, may be by the elimination of C6H11O (hexanoyl group), C6H11O5 (galactopyranosyl) and (C6H11O6) glucopyranoside from precursor ion, while the fragment at 171 was typical for C10H19O2 (methyl nonanoate). Compound (1) was therefore tentatively identified as (2S)-3-(β-D-galactopyranosyloxy)-2-(hexanoyloxy) propyl nonanoate ( and ). Compound (2) generated a molecular ion peak [M-H]− at 325 (Rt, 7.7 min) and a prominent fragment [M-H-162]− at 163, resembling the cleavage of hexosyl moiety, which produced further fragment at 119 [M-H-44]−, indicating loss of CO2, and was therefore recognized as 4-O-β-D-glucosyl-4-coumaric acid ( and ), matching to literature data.[Citation42] Antioxidant efficacy assessed by ABTS radical scavenging assay in vitro disclosed that the SC50 value of compound (2) was 31.44 ± 2.06 µg/mL.[Citation43] As shown in and , the deprotonated [M-H]− ion for compound (3) was detected at 387 with Rt, 1.3 min. The other characteristic fragments in MS/MS-spectrum for compound (3) at 341, 323, 179 and 161 might be appeared by successive removal of HCOOH, H2O, C7H12O7 and C7H14O8, respectively, from precursor ion. Thus, this compound was identified as 7-(α-D-glucopyranosyloxy)-2,3,4,5,6-pentahydroxyheptanoic acid.[Citation44] Compound (4) with Rt of 15.9 min and a precursor ion [M-H]− at 295 was suggested to be 13S-hydroxyoctadecadienoic acid (13-HODE) ( and ). A strong peak was appeared at 277 [M-H-H2O]− indicating loss of H2O molecule. Furthermore, cleavage of carbon-carbon single bond attached to -OH group displayed fragment ion at 195 [M-H-C6H12O]−, characteristic for 13-HODE, while peak at 113 [M-H-C11H18O2] − was generated by breakage of double bond adjacent to the -OH group.[Citation45,Citation46] QTOF-MS/MS analysis ( and ) led to the detection of astragaloside derivative (Rt = 19.1 min) giving molecular ion [M-H]− at 653 and other fragments at 447 and 285, which are characteristic for astragaloside, agreed to reported data,[Citation47] however structure of compound (5) remained unresolved might be due to insufficient information. Previously, astragaloside derivatives are reported to have antidiabetic effect.[Citation48] As per QTOF-MS/MS analysis compound (6) (Rt of 6.1 min) generated the [M-H]− at 341 and a characteristic fragment at 179 [M-H-162]− corresponded to caffeic acid, appeared by the cleavage of glucose residue whereas the ions at 161 [caffeic acid-H-H2O]− and 135 [caffeic acid-H-CO2]− were typical for caffeic acid.[Citation49] Therefore, compound (6) was characterized as caffeic acid 3-glucoside ( and ) and confirmed by comparing with data reported in prior studies.[Citation50] This compound has been reported to inhibit α-glucosidase, human aldose reductase and stable DPPH radical with IC50 of >100 µM, >100 µM and 0.55 mM, respectively.[Citation51,Citation52] Compound (7) at Rt, 1.3 min yielded a deprotonated ion [M-H]− at 387 and further MS-fragment ions at 341, 323 and 233 were also identified in spectrum. A characteristic fragment at 179 revealed that the compound contains caffeic acid,[Citation53] while the fragments at 161 and 131 indicated the cleavage of H2O and CH2O from the fragment at 179. However, compound (7) was identified tentatively as caffeic acid derivative ( and ). Caffeic acid derivatives are reported to have good potential for controlling hypertension and hyperglycemia.[Citation54] Compound (8) detected at Rt = 1.9 min with molecular ion [M-H]− at 191, corresponded to formula of C6H8O7. This compound presented prominent fragments at 173, 155, 131, 129, 127, 111 through the loss of neutral molecules such as CO2, H2O and -OH from the fragment at 191. As per reported data, the base peak for citric acid was recorded at 111.[Citation55] Finally, compound (8) was assigned to be citric acid ( and ). Previously, this compound showed weak antioxidant activity based on DPPH antiradical (10%) and ferrous ion-chelating assay with IC50 of 9764 µg/mL.[Citation55] Compound (9) at Rt of 9.8 min, revealed a diagnostic deprotonated ion in negative mode [M-H]− at 137. The fragments existing at 93 [M-H-44]− and 75 [M-H-44-18]−, ascribed to the loss of CO2 and H2O molecules.[Citation56] Thus, compound (9) was identified as salicylic acid ( and ), consistent with data searching from on-line database. Compound (9) has been reported as DPPH radical scavenger with EC50 > 800 µmol and weak α-glucosidase a well as α-amylase inhibitor with IC50 of 99.98 ± 0.12 µM and 4.72 ± 0.18 mg/mL, respectively.[Citation57,Citation58] Metabolite (10) was characterized as isorhamnetin-3-O-glucoside based on QTOF-MS/MS experiment with Rt at 9.5 min and formula of C22H22O12. As shown in MS spectrum ( and ), the precursor ion [M-H]− at 477 supported an aglycone isorhamnetin. The fragment at 315 was probably produced by elimination of glucose moiety. The fragment at 151 might be yielded from fragment at 315 after retro Diels–Alder cleavage of C9H8O3. Furthermore, the fragment at 357 was probably shown by removal of C4H8O4 group. Another diagnostic peak at 285 was indicative for the elimination of C7H12O6 from precursor ion. Previously, it is reported that compound (10) could alleviate oxidative stress by inhibiting DPPH radical with IC50 values of 31.3 ± 0.70 µM[Citation59] and 131.3 µmol/L.[Citation60] It can also prevent diabetes by inhibiting α-amylase with IC50 value of 0.61 mM.[Citation61] Compound (11), a triterpene detected at Rt of 18.3 min was identified as oleanolic acid. In MS/MS spectrum it yielded deprotonated [M-H]− ion at 455[Citation62] while other fragments at 383 [M-H-72]− and 326 [M-H-129]−, could be due to cleavage of CO2 + 2CH2 and C7H13O2 moieties ( and ). Oleanolic acid is reported to be a good scavenger of DPPH, superoxide anion, hydroxyl, and NO radicals with IC50values of 32.46, 37.69, 4.46 and 1.36 µg/mL, respectively.[Citation63] It is also reported as good α-amylase inhibitor (IC50 of 91.72 ± 1.63 µM and 2.01 μg/mL) and α-glucosidase inhibitor (IC50 values of 17.35 ± 0.88 µM and 6.29 ± 0.37 μg/mL).[Citation64,Citation65] Compound (12) eluting at Rt of 12.1 min, indicated deprotonated ion [M-H]− at 329. In its MS-spectrum the characteristic fragment ions were [M-H-100]− at 229 (C12H21O4), [M-H-118]− at 211 (C12H19O3), [M-H-158]− at 171 (C9H15O3) and [M-H-190]− at 139 (C9H15O). It was identified as pinellic acid ( and ) and is consistent with reported data.[Citation66] This compound is reported to exhibit mild antioxidant effect based on DPPH antiradical assay with IC50 value >500 μM.[Citation67] Compound (13) eluting at Rt of 1.3 min exhibited precursor ion [M-H]− at 191. In its MS-spectrum parent ion further fragmented into daughter ions at 173 [M-H-H2O]−, 127 [M-H-2H2O-CO]−, 111 [M-H-2H2O-CO2]− and 85 [M-H-C3H6O4]−, while the fragment at 93 was attributable to phenol moiety. These fragmentations were consistent with literature data.[Citation49,Citation55] Thus compound (13) was identified as quininic acid ( and ). Quinic acid is reported as potent inhibitor of α-amylase (IC50 value of 1.57 μM) and α-glucosidase (IC50 of 4.95 μM). It has also been reported to exhibit antioxidant effect due to its free radical scavenging capacity with IC50 value of 663.10 µg/mL.[Citation48]
Majority of these compounds are reported as antioxidant, α-glucosidase and α-amylase inhibitors. Therefore, we believe that bioactive rich 80% HE C. reflexa extract with potential antidiabetic functionality may contribute significantly for possible development of functional foods or nutra-pharmaceuticals. Medicinal plants are promising source of polyphenolic compounds (flavonoids, phenolics, terpenoids, etc.) which possess antidiabetic effects due to their potent antioxidant activities. They can act as hydrogen atom donators, reducing agents, ROS scavengers and aldose reductase inhibitors. They can also regulate lipid and glucose metabolism and prevent hyperlipidemia/hyperglycemia.[Citation4] Previously, GC-MS analysis of crude methanolic extracts from C. reflexa grown on 3 different host plants (D. longan, S. asper and Z. dongnaiensis) has resulted in identification of 30 constituents with hydroxyquinone, 4-vinylphenol, syringol, 4-vinylguaiacol, methyl 4-hydroxycinnamate and methyl cinnamate as the dominant phenolic compounds.[Citation41] In another previous study, HPLC analysis has indicated the presence of quercetin, vanillic acid and rutin in acetone extracts from C. reflexa hosted on three different hosts including C. grandis, F. racemose and S. saman.[Citation15] Likewise, HPLC profiling of hydro-methanolic stem and flower extracts from C. reflexa hosted on Conocarpus erectus have resulted in identification of 16 bioactive constituents including 13 phenolic acids and 3 aldehydes, with caffeic acid and p-coumaric acid as dominant bioactives in both flower and the stem extracts.[Citation68] Based on the result of our current study and some previous literature reports, it can be revealed that phytochemicals composition of C. reflexa parasite might be dependent on the nature of the host plant.
Docking studies
To elucidate the possible role of each constituent, we performed docking simulations against both antidiabetic molecular targets i.e., α-amylase and α-glucosidase. In literature, the synergistic role of identified bioactive constituents of the plant extracts via enzyme inhibition has been explored by using docking studies.[Citation34,Citation69] Hence, we also performed docking studies by using MOE. Role of the constituent was determined by computing binding affinities. In the current study, binding affinity data (kcal/mol) as well as interaction pattern of the phytochemicals was determined to explore the synergistic inhibition of α-glucosidase and α-amylase to avoid hyperglycemia.
Docking studies on yeast α-glucosidase were executed in our previously described homology modeled-α-glucosidase.[Citation24] The binding affinity data computed for each constituent is presented in . The active sites of the model included catalytic triad residues such as Glu276, Asp214 and Asp349 (red spheres as ), while other significant amino acid residues (AARs) in the active sites were indicated as yellow. The significant AARs in stick form are indicated as . Initially, we carried out docking of acarbose (reference inhibitor) into the binding sites of α-glucosidase. Minimum energy binding-pose of acarbose is presented as . A ribbon diagram of superposed compounds of current study onto binding sites of homology modeled α-glucosidase is shown as . While, 3-D interaction plot of all constituents is indicated as –. Most of the compounds showed hydrogen bond interactions (HBIs) with key AARs of catalytic triad Asp214, Glu 276 and Asp349. Other important residues, Lys155, Phe157, Arg312, also forms HBIs with these compounds (). Compound (1) forms HBIs with Asp68, Asp214, Glu276, Arg312, Asp349, Arg439, whereas Asp214 with hydroxyl group of the compound at a distance of 3.05 Å. Similarly, hydroxy groups also form HBIs with Asp68, Asp349 and Arg439 at the distance of 2.07, 2.77 and 2.93 Å, respectively. Compound (2) having binding affinity −7.6887 kcal/mol forms HBIs with Phe157, Asp214, His348, Arg439. Phe157 establishes HBIs with hydroxyl group of the compound 2 at a distance of 1.92 Å (). Compound (12) with lowest binding affinity value of −7.7870 kcal/mol forms five hydrogen bonds. Asp214 forms hydrogen bond with carboxylic group (distance 1.92 Å) while, Glu304 forms HBIs at a distance of 1.94 Å (). Apart from conventional HBIs, π–π stacking interactions were also found to stabilize the ligand-enzyme complexes in compounds (3) and (8) (purple-dotted lines and , respectively). The binding affinity data presented in showed that compound (1) with the binding affinity value of −9.1927 kcal/mol is possibly the main contributor of α-glucosidase inhibition. Other important compounds with lowest binding affinity values are (4) (−7.1164 kcal/mol), (10) (−7.6978 kcal/mol) and (12) (−7.7870 kcal/mol).
Figure 4. (a). Ribbon diagram for α-glucosidase (homology modeled). The 3 AARs shown as red sphere (Asp349, Glu276 and Asp214) are catalytic triad residues. The possible active sites are indicated in the form of yellow spheres (b) A ribbon diagram indicating eminent residue in stick form (c) 3-D docking pose of standard drug (acarbose) into the active sites of enzyme (d) A ribbon diagram of superposed compounds of current study into the binding sites.
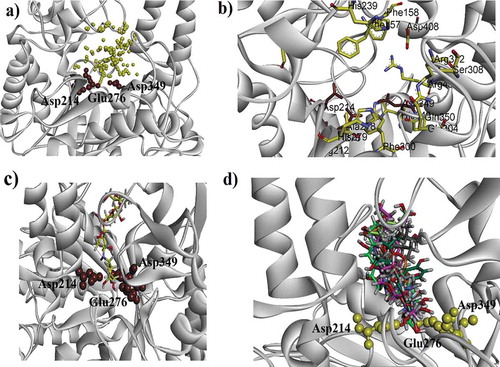
Figure 5. (a–d). The 3-D interaction plot of identified phytochemicals (1–4) into the active sites of α-glucosidase, respectively. Green dotted lines showed hydrogen bonds and purple dotted lines presented π–π stacking interactions. Distances (in Å) of hydrogen bonds are written in blue. While, for π–π stacking interactions it is written in green.
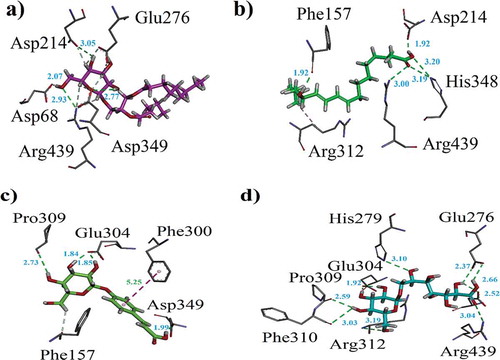
Figure 6. (a–d). The 3-D interaction plot of identified phytochemicals (6, 8, 9–10), respectively, into the active sites of α-glucosidase.
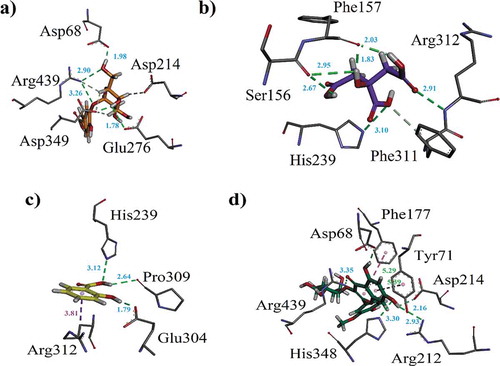
Figure 7. (a–c). The 3-D interaction plot of identified phytochemicals (11–13) into the active sites of α-glucosidase, respectively (d) 3-D interaction plot of acarbose.
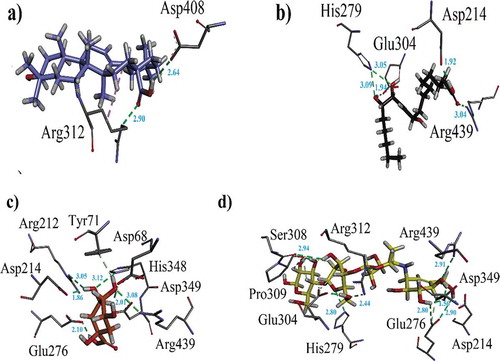
Table 5. Binding affinity values (kcal/mol) and ligand interaction pattern revealed by identified phytoconstituents and acarbose toward yeast α-glucosidase.
Docking studies of the identified phytochemicals were also carried out against α-amylase. The 3-D structure of PPA in complex with acarbose was obtained from PDB. The binding affinity data computed for each constituent is presented in . While, 3-D interaction plot of all constituents is shown as –k. The binding-cleft of α-amylase lie deep near its center and contains Asp300, Glu233, Asp197. Whereas, active sites contain numerous aromatic residue and side chains. The aromatic residues present are as follows: Ala307, His305, His299, Tyr258, Ile235, Pro163, His101, Tyr62, Trp59 and Trp58. The side chains like Asp236, Lys200, Asp165 and Arg61 are also valuable. Here, compound (1) also exhibited the lowest binding affinity value (−7.9445 kcal/mol). A bifurcated hydrogen bond was found at distance of 2.60 and 2.61 Å between -NH of His201 and oxygen atoms of the compound. Another hydrogen bond was found between Asp300 and hydroxyl group (Distance = 2.24 Å). Standard drug acarbose showed the lowest binding affinity value (−9.5683 kcal/mol). The interacting AARs are shown in .
Figure 8. (a-k). The 3-D interaction plot for identified phytochemicals (1–4, 6, 8–13), respectively. (l) The 3-D interaction plot of acarbose into active sites of PPA.
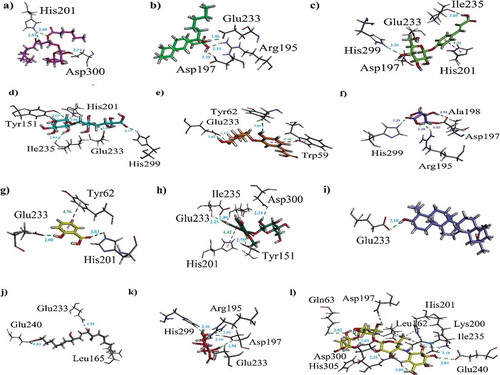
Table 6. Binding affinity values (kcal/mol) and ligand interaction patterns computed for possible isolated phytoconstituents and acarbose toward yeast α-amylase.
Current work provides deep understanding of molecular modulation amongst targeted enzymes and the potent bioactives recognized from C. reflexa. The binding affinity data mentioned above clearly indicates that metabolites (1–4, 6, 8–13) can inhibit synergistically α-glucosidase and α-amylase to treat or avoid hyperglycemia.
Electronic properties and single electron transfer mechanism
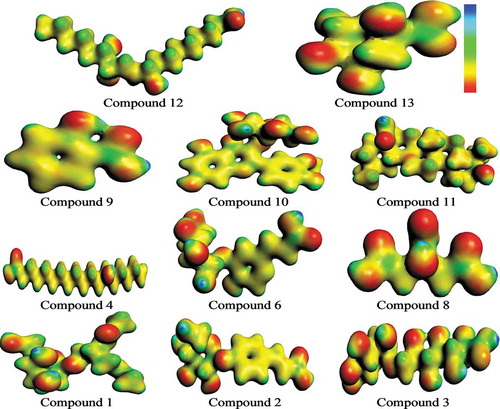
The plant extracts exhibit multiple biological, antioxidant and pharmacological activities and have immense interest of researchers on account of their beneficial health effects. Therefore, in compounds displaying fascinating activities, electronic properties and molecular electrostatic potential (MEP) exploration is essential to comprehend active sites. The charge density distribution of frontier molecular orbitals (FMOs), namely highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) is illustrated in . The spatial distribution revealed comprehensible intramolecular charge transfer from HOMOs to LUMOs. The antioxidant capability of identified phytocompounds is also strongly associated with spatial distribution of HOMO, indicative of most probable sites that can be attacked by free radicals. In most of the studied bioactives, FMOs overlapped to considerable extent indicating that compounds have extremely reactive nature to be used as antioxidants and for diabetes mellitus. The energies of FMOs and HOMO-LUMO-Energy gaps (Egaps) are important parameters to explore electronic nature of biological active metabolites. The energies of the HOMOs (EHOMOs, EHOMOs-1), LUMOs (ELUMOs, ELUMOs+1) and Egaps of various compounds at B3LYP and TZP levels are shown as . The EHOMO values are good indicator of antioxidant activities of the compounds and those with lesser EHOMO values usually display lower electron donating ability and vice versa.
Figure 9. The S0 charge-density distribution of FMOs in identified bioactives (contour value = 0.035).
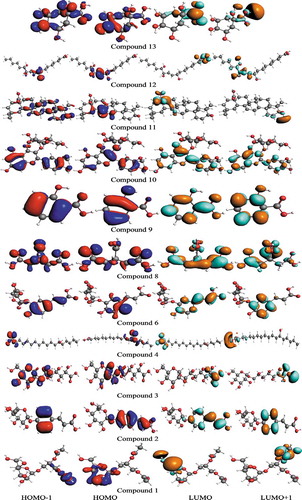
Table 7. The computed ground state HOMO energies (EHOMO, EHOMO-1), LUMO energies (ELUMO, ELUMO+1), HOMO-LUMO-Egaps and IP (-EHOMO) in eV for identified bioactives.
The MEP is an interesting property in order to determine reactivity of different materials.[Citation70] The MEP surface mapped for various constituents has been shown as . It helps to identify positive and negative charged electrostatic potential. The blue and red colors indicate maximum positive and negative potential regions in the molecule, favorable for attack by the nucleophile and electrophile, respectively. The decreasing trend in MEP is in the order: blue > green > yellow > orange > red, where blue and red colors express maximum attraction and repulsion, respectively. Here, one can see that highest negative electrostatic potential is concentrated on O atoms (-OH, keto, methoxy or carboxylic) whereas highest positive electrostatic potential is distributed on H atoms (-OH or -CH3). Findings revealed that in case of attack by nucleophile noteworthy repulsion would be detected at O atoms whereas significant attraction at H atoms. On the other hand, in case of attack by electrophile significant repulsion would be detected at H atoms while noteworthy attraction at O atoms.
In single electron transfer mechanisms, antioxidants provide electrons to free radicals and the resulted radical cations have to be stable. Antioxidant aptitudes of understudy compounds can be assessed by IP (ionization potential) values. This is an imperative physical factor illuminating electron transfer range, which can be calculated in terms of IP = -EHOMO. It is expected that antioxidant ability of those compounds would be high which have smaller IP values. In present study, we compared our computed IP values with common phenol (reference) and found that all bioactives have smaller values compared with reference i.e., 8.33 eV.[Citation71] The trend in IP value of various constituents has been found in the order: (6) < (10) < (11) < (2) < (3) < (9) < (12) < (13) < (4) < (1) < (8) < phenol. The active sites as mapped by MEP surfaces, FMOs, spatial distribution of charge density and IP revealed that the metabolites (1–4, 6, 8–13) would be reactive with good antioxidant ability as well as can have synergistic inhibition against α-amylase and α-glycosidase to treat or prevent hyperglycemia, which is in good agreement with our docking studies.
Conclusion
The present work is most likely the first investigation on phytochemicals profiling, molecular docking, computational insights and biological activities of C. reflexa grown on C. tomentosa. The results established strong antioxidant activity and inhibitory activities of C. reflexa extracts against carbohydrate hydrolyzing enzymes i.e., α-glucosidase and α-amylase. Typically, 80% HE C. reflexa extract is ascertained to be the best enzyme inhibitor and this might be in due part to the synergistic effect of diverse range of bioactive constituents present in it. The findings of the current work provide strong scientific support toward exploration of applications of C. reflexa grown on C. tomentosa for the development of functional foods and nutra-pharmaceuticals for managing diabetes and related conditions. Binding affinity data and interaction patterns of the identified phytochemicals from C. reflexa revealed that these can inhibit the enzymes, α-glucosidase and α-amylase synergistically to prevent hyperglycemia.
Acknowledgments
Dr Umer Rashid has purchased MOE license under HEC NRPU-project (5291/Federal/NRPU/R&D/HEC/2016). A. Irfan has extended his obligation to the Deanship of Scientific Research at KKU, Saudi Arabia through research group programs (R.G.P.2/91/41).
References
- Mittal, A.; Sachan, S. Antidiabetic Activity of Cuscuta reflexa. Int. J. Res. Pharm. Chem. 2017, 3, 572–576.
- Jacobsen, I. B.; Henriksen, J. E.; Beck‐Nielsen, H. The Effect of Metformin in Overweight Patients with Type 1 Diabetes and Poor Metabolic Control. Basic Clin. Pharmacol. Toxicol. 2009, 105(3), 145–149. DOI: 10.1111/j.1742-7843.2009.00380.x.
- Parasuraman, S.; Kumar, E.; Kumar, A.; Emerson, S. Free Radical Scavenging Property and Diuretic Effect of Triglize, a Polyherbal Formulation in Experimental Models. J. Pharmacol. Pharmacother. 2010, 1(1), 38. DOI: 10.4103/0976-500X.64535.
- Gulcin, I.; Kaya, R.; Goren, A. C.; Akincioglu, H.; Topal, M.; Bingol, Z.; Çakmak, K. C.; Durmaz, L.; Alwasel, S. Anticholinergic, Antidiabetic and Antioxidant Activities of Cinnamon (Cinnamomum verum) Bark Extracts: Polyphenol Contents Analysis by LC-MS/MS. Int. J. Food Prop. 2019, 22(1), 1511–1526. DOI: 10.1080/10942912.2019.1656232.
- Mohammed, A.; Kumar, D.; Rizvi, S. I. Antidiabetic Potential of Some Less Commonly Used Plants in Traditional Medicinal Systems of India and Nigeria. J. Intercult. Ethnopharmacol. 2015, 4(1), 78. DOI: 10.5455/jice.20141030015241.
- Mumtaz, M. W.; Al-Zuaidy, M. H.; Abdul Hamid, A.; Danish, M.; Akhtar, M. T.; Mukhtar, H. Metabolite Profiling and Inhibitory Properties of Leaf Extracts of Ficus benjamina Towards α-Glucosidase and α-Amylase. Int. J. Food Prop. 2018, 21(1), 1560–1574. DOI: 10.1080/10942912.2018.1499112.
- Nair, S. S.; Kavrekar, V.; Mishra, A. In Vitro Studies on α-Amylase and α-Glucosidase Inhibitory Activities of Selected Plant Extracts. Euro. J. Exp. Bio. 2013, 3(1), 128–132.
- Reka, P.; Banu, T.; Seethalakshmi, M. α-Amylase and α-Glucosidase Inhibition Activity of Selected Edible Seaweeds from South Coast Area of India. Int. J. Pharm. Pharm. Sci. 2017, 9(6), 64–68. DOI: 10.22159/ijpps.2017v9i6.17684.
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase Inhibitors Isolated from Medicinal Plants. Food Sci. Human Wellness. 2014, 3(3–4), 136–174. DOI: 10.1016/j.fshw.2014.11.003.
- Telagari, M.; Hullatti, K. In Vitro α-Amylase and α-Glucosidase Inhibitory Activity of Adiantum caudatum Linn. And Celosia argentea Linn. Extracts and Fractions. Indian J. Pharmacol. 2015, 47(4), 425.
- Yao, X.; Zhu, L.; Chen, Y.; Tian, J.; Wang, Y. In Vivo and in Vitro Antioxidant Activity and α-Glucosidase, α-Amylase Inhibitory Effects of Flavonoids from Cichorium glandulosum Seeds. Food Chem. 2013, 139(1–4), 59–66. DOI: 10.1016/j.foodchem.2012.12.045.
- Petchi, R. R.; Vijaya, C.; Parasuraman, S. Antidiabetic Activity of Polyherbal Formulation in Streptozotocin-Nicotinamide Induced Diabetic Wistar Rats. J. Tradit. Complement. Med. 2014, 4(2), 108–117. DOI: 10.4103/2225-4110.126174.
- Mollica, A.; Zengin, G.; Locatelli, M.; Stefanucci, A.; Macedonio, G.; Bellagamba, G.; Onaolapo, O.; Onaolapo, A.; Azeez, F.; Ayileka, A.; et al. An Assessment of the Nutraceutical Potential of Juglans regia L. Leaf Powder in Diabetic Rats. Food Chem. Toxicol. 2017, 107, 554–564. DOI: 10.1016/j.fct.2017.03.056.
- Raza, M. A.; Mukhtar, F.; Danish, M. Cuscuta reflexa and Carthamus oxyacantha: Potent Sources of Alternative and Complimentary Drug. Springerplus. 2015, 4(1), 76. DOI: 10.1186/s40064-015-0854-5.
- Tanruean, K.; Poolprasert, P.; Kumla, J.; Suwannarach, N.; Lumyong, S. Bioactive Compounds Content and Their Biological Properties of Acetone Extract of Cuscuta reflexa Roxb. Grown on Various Host Plants. Nat. Prod. Res. 2019, 33(4), 544–547. DOI: 10.1080/14786419.2017.1392955.
- Patel, S.; Sharma, V.; Chauhan, N. S.; Dixit, V. K. An Updated Review on the Parasitic Herb of Cuscuta reflexa Roxb. Chin. J. Integr. Med. 2012, 10(3), 249–255. DOI: 10.3736/jcim20120302.
- Sharma, S.; Hullatti, K.; Kumar, S.; Tiwari, B. Comparative Antioxidant Activity of Cuscuta reflexa and Cassytha filiformis. J. Pharm. Res. 2012, 5(1), 441–443.
- Kim, D. O.; Jeong, S. W.; Lee, C. Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81(3), 321–326. DOI: 10.1016/S0308-8146(02)00423-5.
- Kumar, P.; Kalita, P.; Barman, T.; Chatterjee, T. Quantification of Total Flavonoid Content and Antioxidant Activity in Comparison to a Reference Flavonoid as in Vitro Quality Evaluation Parameter for Assessing Bioactivity of Biomarkers in Herbal Extracts or Formulations. J. Pharm. Res. 2013, 1, 27–35.
- Mensor, L. L.; Menezes, F. S.; Leitão, G. G.; Reis, A. S.; Santos, T. C. D.; Coube, C. S.; Leitão, S. G. Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method. Phytother. Res. 2001, 15(2), 127–130. DOI: 10.1002/ptr.687.
- Umamaheswari, M.; Chatterjee, T. In Vitro Antioxidant Activities of the Fractions of Coccinia grandis L. Leaf Extract. Afr. J. Tradit. Complement. Altern. Med. 2008, 5(1), 61–73. DOI: 10.4314/ajtcam.v5i1.31258.
- Jabeen, B.; Riaz, N.; Saleem, M.; Naveed, M. A.; Ashraf, M.; Alam, U.; Rafiq, H. M.; Tareen, R. B.; Jabbar, A. Isolation of Natural Compounds from Phlomis stewartii Showing α-Glucosidase Inhibitory Activity. Phytochemistry. 2013, 96, 443–448. DOI: 10.1016/j.phytochem.2013.09.015.
- Shai, L. J.; Masoko, P.; Mokgotho, M. P.; Magano, S. R.; Mogale, A.; Boaduo, N.; Eloff, J. N. Yeast α-Glucosidase Inhibitory and Antioxidant Activities of Six Medicinal Plants Collected in Phalaborwa, South Africa. S. Afr. J. Bot. 2010, 76(3), 465–470. DOI: 10.1016/j.sajb.2010.03.002.
- Ali, M.; Ali, S.; Khan, M.; Rashid, U.; Ahmad, M.; Khan, A.; Al-Harrasi, A.; Ullah, F.; Latif, A. Synthesis, Biological Activities, and Molecular Docking Studies of 2-Mercaptobenzimidazole Based Derivatives. Bioorg. Chem. 2018, 80, 472–479. DOI: 10.1016/j.bioorg.2018.06.032.
- Biovia, D. S.; Discovery Studio Visualizer, 2017 R2. San Diego: Dassault Systèmes 2017.
- Irfan, A.; Al-Sehemi, A. G.; Assiri, M. A.; Mumtaz, M. W. Exploring the Electronic, Optical and Charge Transfer Properties of Acene Based Organic Semiconductor Materials. Bull. Mater. Sci. 2019, 42(4), 145. DOI: 10.1007/s12034-019-1838-9.
- Irfan, A.; Assiri, M.; Al-Sehemi, A. G. Exploring the Optoelectronic and Charge Transfer Performance of Diaza[5]helicenes at Molecular and Bulk Level. Org. Electron. 2018, 57, 211–220. DOI: 10.1016/j.orgel.2018.03.022.
- Irfan, A.; Jin, R.; Al-Sehemi, A. G.; Asiri, A. M. Quantum Chemical Study of the Donor Bridge Acceptor Triphenylamine Based Sensitizers. Spectrochim. Acta. 2013, 110, 60–66. DOI: 10.1016/j.saa.2013.02.045.
- Fozia, A.; Bukhari, S. A.; Muhammad, S.; Shakeel, A.; Muhammad, A.; Naheed, A. Comparative Evaluation of Antioxidant Potential of Parasitic Plant Collected from Different Hosts. J. Food Processing Techno. 2013, 4(5), 2–6.
- Solat, P.; Iftikhar, H.; Qurat, U.; Kousar, S.; Rehman, J. Antimicrobial, Antioxidant and Minerals Evaluation of Cuscuta europea and Cuscuta reflexa Collected from Different Hosts and Exploring Their Role as Functional Attribute. Int. Res. J. Pharm. Appl. Sci. 2013, 3(5), 43–49.
- Imran, M.; Rasool, N.; Rizwan, K.; Zubair, M.; Riaz, M.; Zia-Ul-Haq, M.; Rana, U. A.; Nafady, A.; Jaafar, H. Z. Chemical Composition and Biological Studies of Ficus benjamina. Chem. Cent. J. 2014, 8(1), 12. DOI: 10.1186/1752-153X-8-12.
- Suresh, V.; Sruthi, V.; Padmaja, B.; Asha, V. In Vitro Anti-inflammatory and Anti-cancer Activities of Cuscuta reflexa Roxb. J. Ethnopharmacol. 2011, 134(3), 872–877. DOI: 10.1016/j.jep.2011.01.043.
- Farooq, M. U.; Mumtaz, M. W.; Mukhtar, H.; Rashid, U.; Akhtar, M. T.; Raza, S. A.; Nadeem, M. UHPLC-QTOF-MS/MS Based Phytochemical Characterization and Antihyperglycemic Prospective of Hydroethanolic Leaf Extract of Butea monosperma. Sci. Rep. 2020, 10(1), 3530. DOI: 10.1038/s41598-020-60076-5.
- Sadeer, N. B.; Llorent-Martínez, E. J.; Bene, K.; Mahomoodally, M. F.; Mollica, A.; Sinan, K. I.; Stefanucci, A.; Ruiz-Riaguas, A.; Fernández-de Córdova, M. L.; Zengin, G. Chemical Profiling, Antioxidant, Enzyme Inhibitory and Molecular Modelling Studies on the Leaves and Stem Bark Extracts of Three African Medicinal Plants. J. Pharm. Biomed.Anal. 2019, 174, 19–33. DOI: 10.1016/j.jpba.2019.05.041.
- Chandran, R.; Kumar, N. S.; Manju, S.; Kader, A. S.; Kumar, D. S. B. In Vitro α-Glucosidase Inhibition, Antioxidant, Anticancer, and Antimycobacterial Properties of Ethyl Acetate Extract of Aegle tamilnadensis Abdul Kader (Rutaceae) Leaf. Appl. Biochem. Biotechnol. 2015, 175(2), 1247–1261.
- Aliyu, A. B.; Ibrahim, M. A.; Musa, A. M.; Musa, A. O.; Kiplimo, J. J.; Oyewale, A. O. Free Radical Scavenging and Total Antioxidant Capacity of Root Extracts of Anchomanes difformis Engl. (Araceae). Acta Pol. Pharm. 2013, 70(1), 115–121.
- Tanruean, K.; Kaewnarin, K.; Suwannarach, N.; Lumyong, S. Comparative Evaluation of Phytochemicals, and Antidiabetic and Antioxidant Activities of Cuscuta reflexa Grown on Different Hosts in Northern Thailand. Nat. Prod. Commun. 2017, 12(1), 51–54.
- Akhtar, N.; Mirza, B. Phytochemical Analysis and Comprehensive Evaluation of Antimicrobial and Antioxidant Properties of 61 Medicinal Plant Species. Arab. J. Chem. 2018, 11(8), 1223–1235. DOI: 10.1016/j.arabjc.2015.01.013.
- Kazeem, M.; Adamson, J.; Ogunwande, I. Modes of Inhibition of α-Amylase and α-Glucosidase by Aqueous Extract of Morinda lucida Benth Leaf. Biomed. Res. Int. 2013, 2013, 1–6. DOI: 10.1155/2013/527570.
- Thilagam, E.; Parimaladevi, B.; Kumarappan, C.; Mandal, S. C. α-Glucosidase and α-Amylase Inhibitory Activity of Senna surattensis. J. Acupunct. Meridian Stud. 2013, 6(1), 24–30. DOI: 10.1016/j.jams.2012.10.005.
- Suttiarporn, P.; Tanruean, K. GC-MS Analysis, Antioxidant and α-Glucosidase Inhibitory Activities of the Methanol Extract of Cuscuta reflexa Roxb. Grown on Different Hosts. KMUTNB Int. J. Appl. Sci. Technol. 2017, 59–65 (Special issue).
- Zhang, Q. Q.; Xin, D.; Xin-Guang, L.; Wen, G.; Ping, L.; Hua, Y. Rapid Separation and Identification of Multiple Constituents in Danhong Injection by Ultra-High-Performance Liquid Chromatography Coupled to Electrospray Ionization Quadrupole Time-of-Flight Tandem Mass Spectrometry. Chin. J. Nat. Medicines. 2016, 14(2), 147–160. DOI: 10.1016/S1875-5364(16)60008-0.
- Li, L.; Yang, Y.; Hou, X.; Gu, D.; Ba, H.; Abdulla, R.; Wu, G.; Xin, X.; Aisa, H. A. Bioassay-Guided Separation and Purification of Water-Soluble Antioxidants from Carthamus tinctorius L. By Combination of Chromatographic Techniques. Sep. Purif. Technol. 2013, 104, 200–207. DOI: 10.1016/j.seppur.2012.11.027.
- Tosato, F.; Correia, R. M.; Oliveira, B. G.; Fontes, A. M.; França, H. S.; Coltro, W. K.; Filgueiras, P. R.; Romão, W. Paper Spray Ionization Mass Spectrometry Allied to Chemometric Tools for Quantification of Whisky Adulteration with Additions of Sugarcane Spirit. Anal. Methods. 2018, 10(17), 1952–1960. DOI: 10.1039/C8AY00071A.
- Liang, Y.; Wu, J. L.; Leung, E.; Zhou, H.; Liu, Z.; Yan, G.; Liu, Y.; Liu, L.; Li, N. Identification of Oxygenated Fatty Acid as Side a Chain of Lipo-Alkaloids in Aconitum carmichaelii by UHPLC-Q-TOF-MS and a Database. Molecules. 2016, 21(4), 437. DOI: 10.3390/molecules21040437.
- Yuan, Z. X.; Rapoport, S. I.; Soldin, S. J.; Remaley, A. T.; Taha, A. Y.; Kellom, M.; Gu, J.; Sampson, M.; Ramsden, C. E. Identification and Profiling of Targeted Oxidized Linoleic Acid Metabolites in Rat Plasma by Quadrupole Time‐of‐Flight Mass Spectrometry. Biomed. Chromatogr. 2013, 27(4), 422–432. DOI: 10.1002/bmc.2809.
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities. Molecules. 2016, 21(10), 1275. DOI: 10.3390/molecules21101275.
- Nadeem, M.; Mumtaz, M. W.; Danish, M.; Rashid, U.; Mukhtar, H.; Anwar, F.; Raza, S. A. Calotropis procera: UHPLC-QTOF-MS/MS Based Profiling of Bioactives, Antioxidant and Anti-Diabetic Potential of Leaf Extracts and an Insight into Molecular Docking. J. Food Meas. Charact. 2019, 13(4), 3206-3220.
- Ouyang, H.; Li, T.; He, M.; Li, Z.; Tan, T.; Zhang, W.; Li, Y.; Feng, Y.; Yang, S. Identification and Quantification Analysis on the Chemical Constituents from Traditional Mongolian Medicine Flos scabiosae Using UHPLC–DAD–Q-TOF-MS Combined with UHPLC–QqQ-MS. J. Chromatogr. Sci. 2016, 54(6), 1028–1036. DOI: 10.1093/chromsci/bmw041.
- Lee, J. H.; Park, K. H.; Lee, M.-H.; Kim, H. T.; Seo, W. D.; Kim, J. Y.; Baek, I.-Y.; Jang, D. S.; Ha, T. J. Identification, Characterization, and Quantification of Phenolic Compounds in the Antioxidant Activity-Containing Fraction from the Seeds of Korean Perilla (Perilla frutescens) Cultivars. Food Chem. 2013, 136(2), 843–852. DOI: 10.1016/j.foodchem.2012.08.057.
- Ha, T. J.; Lee, J. H.; Lee, M. H.; Lee, B. W.; Kwon, H. S.; Park, C. H.; Shim, K. B.; Kim, H. T.; Baek, I. Y.; Jang, D. S. Isolation and Identification of Phenolic Compounds from the Seeds of Perilla frutescens (L.) And Their Inhibitory Activities against α-Glucosidase and Aldose Reductase. Food Chem. 2012, 135(3), 1397–1403. DOI: 10.1016/j.foodchem.2012.05.104.
- Nam, S. H.; Kim, Y. M.; Walsh, M. K.; Wee, Y. J.; Yang, K. Y.; Ko, J. A.; Han, S.; Thanh Hanh Nguyen, T.; Kim, J. Y.; Kim, D. Synthesis and Functional Characterization of Caffeic Acid Glucoside Using Leuconostoc mesenteroides Dextransucrase. J. Agr. Food Chem. 2017, 65(13), 2743–2750. DOI: 10.1021/acs.jafc.7b00344.
- Agalar, H. G.; Ciftçi, G. A.; Goger, F.; Kırımer, N. Activity Guided Fractionation of Arum italicum Miller Tubers and the LC/MS-MS Profiles. Rec. Nat. Prod. 2017, 12(1), 64–75. DOI: 10.25135/rnp.06.17.05.089.
- Chiou, S. Y.; Sung, J. M.; Huang, P. W.; Lin, S. D. Antioxidant, Antidiabetic, and Antihypertensive Properties of Echinacea purpurea Flower Extract and Caffeic Acid Derivatives Using in Vitro Models. J. Med. Food. 2017, 20(2), 171–179. DOI: 10.1089/jmf.2016.3790.
- Chang, J. B.; Lane, M. E.; Yang, M.; Heinrich, M. A Hexa-Herbal TCM Decoction Used to Treat Skin Inflammation: An LC-MS-Based Phytochemical Analysis. Planta Med. 2016, 82, 1134–1141. DOI: 10.1055/s-0042-108206.
- Du, Y.; Wang, Z.; Wang, L.; Gao, M.; Wang, L.; Gan, C.; Yang, C. Simultaneous Determination of Seven Phenolic Acids in Rat Plasma Using UHPLC-ESI-MS/MS after Oral Administration of Echinacea purpurea Extract. Molecules. 2017, 22(9), 1494. DOI: 10.3390/molecules22091494.
- Nguyen, M. T. T.; Nguyen, N. T.; Nguyen, H. X.; Huynh, T. N. N.; Min, B. S. Screening of α-Glucosidase Inhibitory Activity of Vietnamese Medicinal Plants: Isolation of Active Principles from Oroxylum indicum. Nat. Prod. Sci. 2012, 18, 47–51.
- Tan, Y.; Chang, S. K.; Zhang, Y. Comparison of α-Amylase, α-Glucosidase and Lipase Inhibitory Activity of the Phenolic Substances in Two Black Legumes of Different Genera. Food Chem. 2017, 214, 259–268. DOI: 10.1016/j.foodchem.2016.06.100.
- Salem, J. H.; Chevalot, I.; Harscoat-Schiavo, C.; Paris, C.; Fick, M.; Humeau, C. Biological Activities of Flavonoids from Nitraria retusa (Forssk.) Asch. And Their Acylated Derivatives. Food Chem. 2011, 124(2), 486–494. DOI: 10.1016/j.foodchem.2010.06.059.
- Abdel-Mageed, W. M.; Bayoumi, S. A. L. H.; Salama, A. A. R.; Salem-Bekhit, M. M.; Abd-Alrahman, S. H.; Sayed, H. M. Antioxidant Lipoxygenase Inhibitors from the Leaf Extracts of Simmondsia chinensis. Asian Pac. J. Trop. Med. 2014, 7, S521–S526. DOI: 10.1016/S1995-7645(14)60284-4.
- Tundis, R.; Loizzo, M.; Statti, G.; Menichini, F. Inhibitory Effects on the Digestive Enzyme α-Amylase of Three Salsola Species (Chenopodiaceae) in Vitro. Pharmazie. 2007, 62(6), 473–475.
- Srivastava, P.; Chaturvedi, R. Simultaneous Determination and Quantification of Three Pentacyclic Triterpenoids-Betulinic Acid, Oleanolic Acid, and Ursolic Acid in Cell Cultures of Lantana camara L. In Vitro Cell Dev. Biol. Plant. 2010, 46(6), 549–557. DOI: 10.1007/s11627-010-9298-3.
- Kandhavelu, M.; Reetha, D. Antioxidant Properties of the Oleanolic Acid Isolated from Cassia auriculata (Linn). J. Pharm. Res. Clin. Pract. 2014, 4(1), 30–36.
- Mohammed, A.; Gbonjubola, V. A.; Koorbanally, N. A.; Islam, M. S. Inhibition of Key Enzymes Linked to Type 2 Diabetes by Compounds Isolated from Aframomum melegueta Fruit. Pharm. Biol. 2017, 55(1), 1010–1016. DOI: 10.1080/13880209.2017.1286358.
- Tiwari, A. K.; Viswanadh, V.; Gowri, P. M.; Ali, A. Z.; Radhakrishnan, S.; Agawane, S. B.; Madhusudana, K.; Rao, J. M. Oleanolic Acid-an α-Glucosidase Inhibitory and Antihyperglycemic Active Compound from the Fruits of Sonneratia caseolaris. Open Access J. Med. Aromat. Plants. 2010, 1(1), 19.
- Wojtanowski, K. K.; Mroczek, T. Study of a Complex Secondary Metabolites with Potent Anti-Radical Activity by Two-Dimensional TLC/HPLC Coupled to Electrospray Ionization Time-of-Flight Mass Spectrometry and Bioautography. Anal. Chim. Acta. 2018, 1029, 104–115. DOI: 10.1016/j.aca.2018.03.066.
- Sim, Y.; Choi, J. G.; Gu, P. S.; Ryu, B.; Kim, J. H.; Kang, I.; Jang, D. S.; Oh, M. S. Identification of Neuroactive Constituents of the Ethyl Acetate Fraction from Cyperi rhizoma Using Bioactivity-Guided Fractionation. Biomol. Ther. 2016, 24(4), 438. DOI: 10.4062/biomolther.2016.091.
- Siddiqui, M. S.; Memon, A. A.; Memon, S.; Baloch, S. G. Cuscuta reflexa as a Rich Source of Bioactive Phenolic Compounds. J. Herbs Spices Med. Plants. 2017, 23(2), 157–168. DOI: 10.1080/10496475.2017.1280867.
- Mahomoodally, M. F.; Picot-Allain, C.; Hosenally, M.; Ugurlu, A.; Mollica, A.; Stefanucci, A.; Llorent-Martínez, E. J.; Baloglu, M. C.; Zengin, G. Multi-targeted Potential of Pittosporum senacia Putt.: HPLC-ESI-MSn Analysis, Insilico Docking, DNA Protection, Antimicrobial, Enzyme Inhibition, Anti-cancer and Apoptotic Activity. Comput. Biol. Chem. 2019, 83, 107114. DOI: 10.1016/j.compbiolchem.2019.107114.
- Murray, J. S.; Politzer, P. The Electrostatic Potential: An Overview. Comput. Mol. Sci. 2011, 1(2), 153–163. DOI: 10.1002/wcms.19.
- Wright, J. S.; Johnson, E. R.; Dilabio, G. A. Predicting the Activity of Phenolic Antioxidants: Theoretical Method, Analysis of Substituent Effects, and Application to Major Families of Antioxidants. J. Am. Chem. Soc. 2001, 123(6), 1173–1183. DOI: 10.1021/ja002455u.