ABSTRACT
Current evidence about the feasible effects of probiotic consumption on the metabolic status of athletes including lipid profile, fecal zonulin, and cortisol is debatable. Therefore, the present systematic reviewed and meta-analysis was aimed to clarify this inconclusiveness. Online databases (PubMed, Scopus, ISI Web of Science, and Cochrane’s library) were searched until May 2020. The overall effect was presented as weighted mean difference (WMD) or standard mean difference (SMD) and 95% confidence interval (CI) in a random-effects meta-analysis model. Publication bias was assessed using Egger’s and Begg’s statistics. A p-value <0.05 was considered as statistically significant. Cochrane Collaboration Risk of Bias Tool was used for quality assessment. Eleven RCTs (n = 398 participants) were considered as eligible. Probiotic consumption significantly decreased fecal zonulin (SMD = −0.63; 95% CI, −1.17 to −0.08; P = .02) with significant heterogeneity (I2 = 79.4%, P = .008). Also, serum triglyceride (WMD = −32.26 mg/dL; 95% CI, −60.44 to −4.08; P = .02) was reduced with high heterogeneity across the studies (I2 = 93.0%, P < .001). Subgroup analysis revealed that probiotic consumption did increase HDL-C among non-Asian studies. Also, serum triglyceride was higher when probiotics were consumed as food, among non-Asian population, and for less than 4 weeks. Probiotics may be beneficial to improve metabolic markers in athletes including fecal zonulin and serum triglyceride.
Introduction
Frequent exhausting trainings lead to incremental quantities of physical stress in athletes.[1] However, despite positive effects of daily moderate strength training on the overall health, immune dysregulation, [Citation2] and elevated levels of inflammatory markers and oxidative stress[Citation3,Citation4] due to prolonged periods of intense training and competition are likely. Furthermore, there is a high prevalence of gastrointestinal (GI) complaints among athletes specially those engaging in endurance trainings.[Citation5] These GI problems could be attributed to factors such as lower intestinal blood flow, impaired intestinal barrier, and increased gut permeability.[Citation6] Besides, endotoxemia due to increased intestinal wall permeability leads to higher susceptibility to auto-immune and infectious diseases due to elevated levels of inflammatory markers and oxidative stress.[Citation6]
Among dietary components indicated for diminishing exercise-induced GI permeability and its related symptoms and illnesses are probiotics, defined as live microorganisms with positive regulatory effects on microbiota and health of the host.[Citation7] There is an increasing body of evidence which support the potential health benefits of probiotics, such as reduction of oxidative stress, [Citation8] promotion of antitumor activity, [Citation9] stimulation of immunity, [Citation10] and improvement of gastrointestinal barrier.[Citation11]
Considering the inconsistencies between studies about the impact of probiotics on metabolic markers in athletes, the current study aimed to explore the effects of probiotics on selected metabolic markers including total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), serum cortisol, and fecal zonulin among adult athletes using systematic review and meta-analysis of randomized controlled trials (RCTs).
Methods
Data source and search strategy
Based upon Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement, [Citation12] articles published up to September 2019 were selected for inclusion in this review from PubMed, Scopus, Cochrane Library, and ISI Web of Sciences without any restrictions on language, publication time, or study design. Additional papers were retrieved from the reference lists of the included studies and/or relevant reviews. The following search strategy was run in PubMed and tailored to each database as necessary: (”probiotics” OR “Fermented Foods” OR “Lactobacillus” OR “Bifidobacterium” OR “Streptococcus” OR “Saccharomyces” OR “Enterococcus”) AND (”Lipid” OR “Cholesterol” OR ”Chol” OR “Hypercholesterolemia” OR “Triglyceride” OR “Hypertriglyceridemia” OR “TG” OR “Lipoprotein” OR “Hyperlipoproteinemia” OR “LDL” OR “LDL-C” OR “HDL” OR “HDL-C” OR “Cortisol” OR “Zonulin”) AND (“Athlete” OR “Athletes” OR “exercise” OR “training” OR “sport”) AND (“Intervention studies” OR “Intervention” OR “Controlled trial” OR “Randomized” OR “Randomized” OR “Random” OR “Randomly” OR “Placebo” OR “Assignment”)
Inclusion criteria
To be included in the study, publications investigating the effects of probiotics on inflammatory markers had to meet the following criteria: (1) original RCTs with parallel or crossover design; (2) which employed probiotics (supplement or food) for intervention; (3) examined the effect of probiotics on at least one of the metabolic markers including TC, TG, LDL-C, HDL-C, serum cortisol, and fecal zonulin; (4) English publications; and (5) studies recruiting athletes as the main population. Studies were excluded if they: (1) administrated synbiotics or prebiotics; (2) included participants younger than 18 years of age, or pregnant or lactating women; (3) had an intervention duration of less than 2 weeks; (4) reported duplicate data (in this case, the ones with complete follow-up and outcome measures were included); or (5) were not peer-reviewed articles (protocol or conference proceeding). To minimize the potential error, two independent investigators guided the overall selection process. In the case of any disagreement, it was resolved by consensus or involving a third researcher.
Data extraction
For all the included studies, two reviewers independently extracted information, including first author’s name, year of publication, country, sample size, participants’ age, gender, and body mass index, RCT design, duration of intervention, dose and type of intervention in experimental and comparison groups, probiotics’ strains, and mean and standard deviation (SD) of outcome measures at baseline, post-intervention, and if possible, their change from baseline. In case of any additional or missing information, corresponding authors were contacted.
In current systematic review and meta-analysis, serum cortisol and fecal zonulin were considered as primary outcomes, and lipid profile (TC, TG, LDL-C, and HDL-C) was analyzed as secondary outcome.
Quality assessment
Two authors performed quality assessment using Cochrane Collaboration Risk of Bias Tool.[Citation13] The domains assessed using this tool were as follows: sequence generation, allocation concealment, blinding, outcome assessment, drop-outs and incomplete outcome data, selective outcome reporting, and other potential sources of bias. Disagreements between reviewers were resolved by involving a third author.
Statistical analysis
STATA statistical program version 11.2 (Stata Corporation, College Station, TX, USA) was used for statistical analysis. In case of standard error (SE) for variation of mean in a study, SD was calculated by the following formula: SE×√n. Effect sizes for the meta-analysis were defined as the weighted mean difference (WMD; measurement at the end of the trial minus the measurement at baseline) and 95% confidence interval (CI) for TC, TG, LDL-C, and HDL-C. Since the unit used for cortisol and zonulin differed among included studies and it was not possible to perform unit conversion, standard mean difference (SMD) with 95% CI was used.[Citation14] When SD of difference was missing, it was imputed following the method of Follmann et al.[Citation15] using a correlation coefficient of 0.5. Heterogeneity was assessed using the I2 index. I2 index <40, 40–75 and >75% indicated Low, moderate, and high heterogeneity, respectively.[Citation16] Subgroup analyses based on the duration of the intervention (<4 weeks or ≥4 weeks), geographical location (Asian or non-Asian), and number of bacteria strains (single or multi) were carried out to check the sources of heterogeneity. To determine the influence of individual RCTs on the overall meta-analysis results, sensitivity analyses using leave-one-out method were carried out. Egger’s and Begg’s statistics were used for assessment of Publication bias. A P-value <0.05 was considered as statistically significant.
Results
Search results
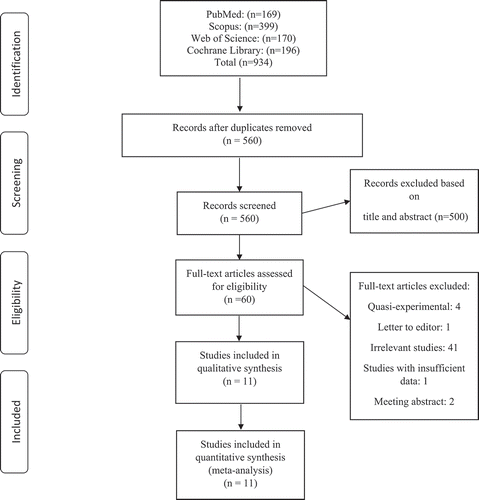
A total of 4207 papers were initially retrieved as shown in . 3103 remained after duplicates were excluded. Next, based on title and abstract, 3043 studies were deemed ineligible as well. Full texts of 60 papers were reviewed, and finally, 11 articles were used in this systematic and meta-analysis. Overview of the included studies
The general characteristics of the included articles are shown in . A total of 11 studies (398 participants) entered our systematic review and meta-analysis with sample sizes ranging from 7 to 129 subjects. Studies were published between 2007 and 2018. Mean age of the participants ranged between 20.1 and 37.9 years. Two studies recruited both gender, [Citation21,Citation22] eight were conducted in men, [Citation1,Citation3,Citation6,Citation17-Citation20,Citation23] and one used female subjects only.[Citation4] Majority of the included studies used probiotic supplements except for two [,Citation4,Citation23] which used probiotic foods. Two studies were conducted in the United States, [Citation1,Citation21] two in Iran, [Citation4,Citation20] two in turkey [,Citation19,Citation23] and the others were established from Australia, [Citation24] Serbia, [Citation18] Austria, [Citation6] Japan, [Citation17] and France.[Citation3] Nine studies used aerobic exercise[Citation3,Citation4,Citation6,Citation17-Citation19,Citation21-Citation23] while others administered anaerobic[Citation1,Citation20] training in combination with probiotic consumption. Using the Cochrane Risk of Bias Assessment Tool, 3,[Citation6,Citation17,Citation18] 5, [Citation1,Citation3,Citation4,Citation20,Citation21] and 3[Citation19,Citation22,Citation23] studies showed good, fair, and poor quality, respectively.
Table 1. Characteristics of included trials.
Findings from meta-analysis
Effect of probiotic consumption on cortisol
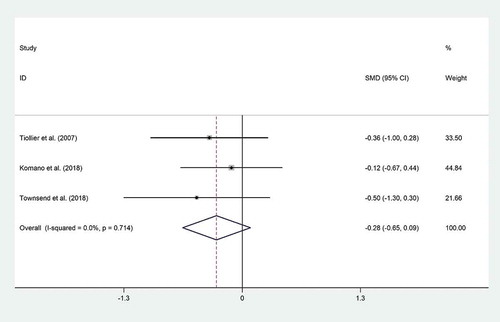
Three trials[Citation1,Citation3,Citation17] reported data on cortisol. Pooled effect size of these studies revealed that probiotic consumption did not reduce cortisol (SMD = −0.28; 95% CI: −0.65 to 0.09; P = .13). There was no evidence of heterogeneity (I2 = 0%, P = .71) (). Exclusion of individual studies from analysis did not alter the overall effect. No evidence of publication bias was found (P = .11, Begg’s test and P = .2, Egger’s test) ().
Effect of probiotic consumption on fecal zonulin
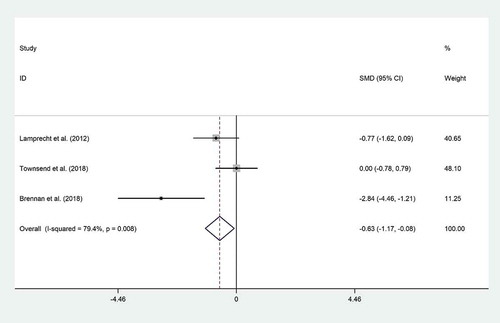
Pooled effect size of 3 datasets[Citation1,Citation6,Citation21] indicated a significant effect of probiotic consumption on zonulin (SMD = −0.63; 95% CI, −1.17 to −0.08; P = .02), with significant heterogeneity (I2 = 79.4%, P = .008) (). We did not run subgroup analysis to assess the source of heterogeneity due to lack of sufficient studies for comparison. Sensitivity analysis revealed that these outcomes were dependent on Lamprecht’s study (SMD = −0.53; 95% CI, −1.24 to 0.17), and Brennan’s study (SMD = −0.34; 95% CI, −0.92 to 0.22). No evidence of publication bias was found for zonulin (P = .11, Begg’s test and P = .19, Egger’s test).
Effects of probiotic consumption on lipid profile (TC, TG, LDLC and HDL-C)
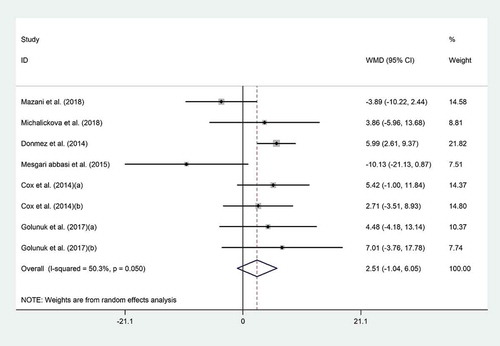
Pooled mean difference of six datasets[Citation4,Citation18-Citation20,Citation22,Citation23] showed no significant effects of probiotics on HDL-C (WMD = 2.51 mg/dL; 95% CI, −1.04 to 6.05; P = .16) (); however, trials were moderately heterogeneous (I2 = 50.3%, P = .05). Subgroup analysis revealed that probiotic consumption did increase HDL-C in non-Asian studies (WMD = 5.71 mg/dL; 95% CI, 2.81 to 8.60; I2 = 0.0%) (). Based on sensitivity analysis, Mazani’s study (WMD = 3.95 mg/dL; 95% CI, 0.85 to 7.04) and Abbasi’s study (WMD = 3.68 mg/dL; 95% CI, 0.84 to 6.52) significantly affected the summary effects.
Table 2. Subgroup analysis to assess the effect of probiotic consumption on metabolic markers.
Table 3. Risk of bias assessment for included randomized controlled clinical trails.
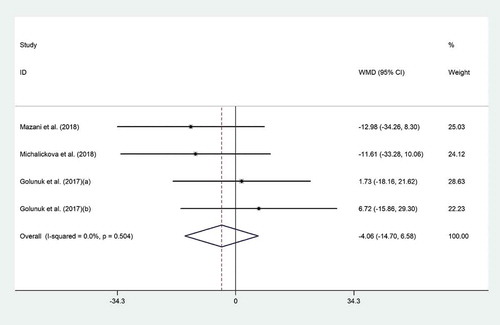
The effect of probiotic consumption on LDL-C was examined in three clinical trials.[Citation4,Citation18,Citation23] Meta-analysis revealed no significant effect for probiotic supplementation on LDL-C (WMD = −4.06 mg/dL; 95% CI, −14.70 to 6.58; P = .45) (), with no evidence of heterogeneity (I2 = 0.0%, p ≤ 0.50). Sensitivity analysis showed that no particular study prominently affected the overall effects.
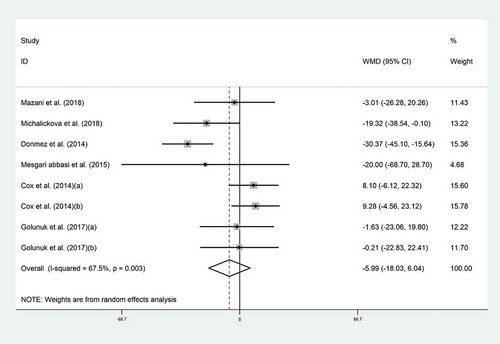
Pooled analysis indicated no effect of probiotic consumption on TC (WMD = −5.99 mg/dL; 95% CI: −18.03 to −6.04; P = .32). Between six trials, [Citation4,Citation18-Citation20,Citation22,Citation23] heterogeneity was moderate (I2 = 67.5%, P = .003) (). In our subgroup analysis, heterogeneity was removed in non-Asian studies in which probiotics marginally reduced TC (WMD = −14.48 mg/dL; 95% CI, −29.48 to 0.52; P = .5; I2 = 59%) (). Sensitivity analysis showed that no individual study influenced the overall effects.
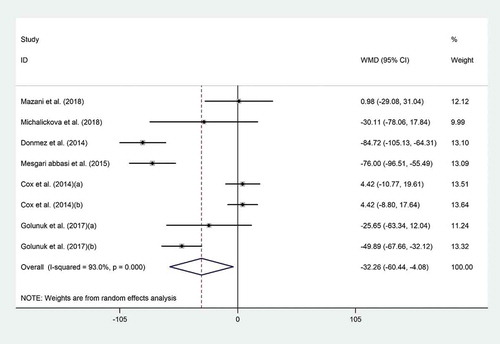
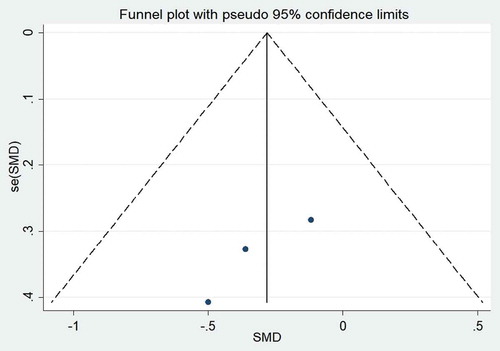
The effect of probiotic consumption on TG was explored in six datasets.[Citation4,Citation18-Citation20,Citation22,Citation23] Pooled effect size revealed that probiotics had a noticeable effect on TG (WMD = −32.26 mg/dL; 95% CI, −60.44 to −4.08; P = .02) (), with high heterogeneity across the studies (I2 = 93.0%, P < .001). To explore the source of heterogeneity, subgroup analyses were performed which revealed a significant reduction in TG levels when probiotics were consumed as food (WMD = −41.61 mg/dL; 95% CI, −76.50 to −6.73; I2 = 87.4%), by non-Asian populations (WMD = −51.87 mg/dL; 95% CI, −78.89 to −24.85; I2 = 73.9%), and with duration of less than 4 weeks (WMD = −49.24 mg/dL; 95% CI, −77.17 to −21.32; I2 = 85.7%) (). The findings from the sensitivity analysis indicated that exclusion of Donmez’s study (WMD = −24.29 mg/dL; 95% CI, −50.78 to 2.19), Abbasi’s study (WMD = −25.65 mg/dL; 95% CI, −54.05 to 2.74), and Golnuk’s study (b) (WMD = −29.54 mg/dL; 95% CI, −61.77 to 2.67) altered the overall effect. No evidence of publication bias was found for HDL-C (P = .45, Begg’s test and P = .25, Egger’s test), LDL-C (P = .49, Begg’s test and P = .98, Egger’s test), TC (P = .32, Begg’s test and P = .7, Egger’s test), and TG (P = .32, Begg’s test and P = .48, Egger’s test).
Discussion
This systematic review and meta-analysis indicate that probiotic consumption may improve TG and fecal zonulin in athletes. Subgroup analyses suggested that a greater improvement in serum TG levels may be expected when probiotics are consumed as foods as compared to supplements for less than 4 weeks in non-Asian populations.
As compared to sedentary lifestyle, inflammatory status is improved by regular exercise.[Citation25] Evidence suggest that moderate-intensity exercise could alter human microbiota based on body composition and exercise duration and intensity.[Citation26] Low-intensity physical activity can increase the abundance of health-promoting micro-organisms, while low-to-moderate activity may improve immune function, inflammation, and oxidative stress with favorable upshots to intestinal health.[Citation27–Citation29] However, high-intensity training, as part of athletes’ routine program, results in the production of inflammatory metabolites and intercessors.[Citation25] Systemic release of pro-inflammatory cytokines, due to bacterial translocation into the circulation across the gastrointestinal barrier, may lead to metabolic abnormalities.[Citation30] Since athletes are often exposed to excessive levels of physical stress as a byproduct of training demands, biomarker monitoring of this phenomenon is gaining momentum in the athletic realm as an approach to detect periods of excessive negative physiological stress.[Citation31] Furthermore, an assembly of various biomarkers is required as the most effective strategy in examining complex equilibrium of anabolic and catabolic processes in athletes.[Citation31]
Cortisol, known as a hormonal parameter of anabolic status in athletes, has already been utilized in male athletes to detect and prevent overtraining.[Citation31,Citation32] To manage exercise-induced fatigue, Hypothalamus-Pituitary-Adrenal axis (HPA-Axis) management is crucial. Cortisol status is affected as a result of HPA-Axis activation. Lower levels of cortisol may be associated with better homeostatic balance for general health, recovery, and physiological adjustment. Our meta-analysis revealed no beneficial effect for probiotic consumption on cortisol level, which is in agreement with previous works.[Citation1,Citation3,Citation17] In contrast to our findings, Lalitsuradej et al.[Citation33] reported that 12 weeks of probiotic supplementation (L. paracasei HII01) decreased salivary cortisol. Similarly, Messaoudi et al.[Citation34] and Sawada et al.[Citation35] also indicated that probiotic supplementation could attenuate urine free cortisol and salivary cortisol levels. Further interpretation is unlikely due to some limitations including scant number of studies and differences in terms of study duration and probiotics administered in the included studies.
Zonulin is a protein with a crucial role in modifying intercellular tight junctions of intestinal endothelium, and it has been recently proposed as a novel marker of intestinal permeability.[Citation36,Citation37] Although we showed that probiotic consumption could decrease fecal zonulin, there is inconsistency among available literature.[Citation1,Citation6,Citation21] Based upon both in vitro and in vivo studies, probiotic supplementation causes a reduction in zonulin levels.[Citation6,Citation38,Citation39] Inconclusive results might be related to administration of varied strains with possibly distinct effects on gut permeability.[Citation1] Endurance training may cause a mild upsurge in the intestinal permeability by slightly increasing zonulin levels in humans.[Citation6,Citation40] Furthermore, it has been suggested that 10-day treadmill exercise (60 min/day) could influence on zonulin mRNA expression which could be related to intestinal barrier disruption.[Citation41] Probiotic supplementation caused a reduction in fecal zonulin which suggests that probiotics can affect the intestinal barrier in a positive way.[Citation6]
Besides, the lipid-lowering mechanism of probiotics is not well specified. Beneficial impact of probiotics on serum lipid profile could be via their immunomodulatory properties[Citation42] or could be attributed to reduced inflammatory cytokines and Toll-like receptor 4 (TLR4) activation.[Citation43] Production of inflammatory cytokines due to the activation of a transmembrane protein, TLR4, leads to increased innate-immunity response, [Citation44]implicated in the etiology of insulin resistance, diabetes, and atherosclerosis.[Citation45] Also, probiotics are able to integrate cholesterol in their cellular membrane[Citation46] and convert cholesterol into coprostanol, [Citation47] leading to a reduction in cholesterol absorption and serum TC levels. In addition, hydrolases produced by some types of probiotics can reduce cholesterol absorption via higher bile salt excretion.[Citation48,Citation49] Other possible mechanisms are reduced inflammation and insulin resistance, storage of triglycerides in the liver, de novo lipogenesis derived by carbohydrate-responsive element-binding protein (ChREBP)/sterol regulatory element-binding protein (SREBP), and secretion of very low-density lipoprotein (VLDL). Due to the secretion of fasting-induced adipose factor (FIAF) following probiotic consumption, endothelial lipoprotein lipase (LPL), in control of triglycerides release from circulating chylomicrons and VLDL, is inhibited. Deactivation of hepatic lipogenic enzymes by ChREBP and SREBP-1 c is another consequence of increased secretion of FIAF, which reduces triglyceride storage in adipocytes and liver.[Citation50] Probiotic-induced increase in glucagon-like peptide-1 (GLP-1) directly hinders triglyceride absorption from the gut, potentially by inhibiting gastric lipases (42). Many other metabolic pathways are also activated. For instance, glucose-dependent insulin secretion is stimulated, postprandial glucagon release is blocked, and pancreatic beta-cell proliferation is promoted.[Citation51,52]
This is the first attempt to explore the effect of probiotics on lipid profile, cortisol, and zonulin among athletes using a systematic review and meta-analysis. However, some limitations should be taken into account when interpreting the results. Included datasets showed significant heterogeneity which undermines the validity of the results. Source of heterogeneity was explored; however, other factors such as the dosage of administered probiotic, different exercise programs, different duration and sample size, age of participants at baseline, study conditions and diverse bacteria strains may also be considered as sources of heterogeneity. Among included studies, only three were of good quality while others suffered from different sources of bias which should be also taken into consideration. Furthermore, the search protocol was not documented online in the PROSPERO which could be a limitation.
Conclusion
Altogether, this systematic review and meta-analysis supports the beneficial role of probiotics in reducing blood TG and fecal zonulin. However, the magnitude of effect was greater when probiotics were administered as food, or when used for less than 4 weeks, and/or in non-Asian countries. Future interventions using different probiotic doses and strains with longer duration are warranted to explore the exact effect and possible mechanism of action of probiotics in athletes.
Acknowledgments
This article does not contain any studies with human participants or animals performed by any of the authors. The authors declare no conflict of interest. For this type of study, formal consent is not required. H. H and A. A contributed to the conception of research. Z. F, M. N and H. H searched databases, screened articles and extracted data. H. H and A. A performed statistical analysis; and all authors contributed to the writing and revision of the manuscript.
Additional information
Funding
References
- Townsend, J. R.; Bender, D.; Vantrease, W. C.; Sapp, P. A.; Toy, A. M.; Woods, C. A.; Johnson, K. D. Effects of Probiotic (Bacillus Subtilis DE111) Supplementation on Immune Function, Hormonal Status, and Physical Performance in Division I Baseball Players. Sports (Basel, Switzerland). 2018, 6(3). 10.3390/sports6030070
- Gleeson, M.; McDonald, W. A.; Cripps, A. W.; Pyne, D. B.; Clancy, R. L.; Fricker, P. A. The Effect on Immunity of Long-term Intensive Training in Elite Swimmers. Clin. Exp. Immunol. 1995, 102(1), 210–216. DOI: 10.1111/j.1365-2249.1995.tb06658.x.
- Tiollier, E.; Chennaoui, M.; Gomez-Merino, D.; Drogou, C.; Filaire, E.; Guezennec, C. Y. Effect of a Probiotics Supplementation on Respiratory Infections and Immune and Hormonal Parameters during Intense Military Training. Mil. Med. 2007, 172(9), 1006–1011. DOI: 10.7205/milmed.172.9.1006.
- Mazani, M.; Nemati, A.; Baghi, A. N.; Amani, M.; Haedari, K.; Alipanah-Mogadam, R. The Effect of Probiotic Yoghurt Consumption on Oxidative Stress and Inflammatory Factors in Young Females after Exhaustive Exercise. J. Pak. Med. Assoc. 2018, 68(12), 1748–1754.
- Qamar, M.; Read, A. Effects of Exercise on Mesenteric Blood Flow in Man. Gut. 1987, 28(5), 583–587.
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J. F.; Probiotic Supplementation Affects Markers of Intestinal Barrier, Oxidation, and Inflammation in Trained Men; a Randomized, Double-blinded, Placebo-controlled Trial. J. Int. Soc. Sports. Nutr. 2012, 9(1), 45. DOI:10.1186/1550-2783-9-45.
- Salminen, S.; Bouley, C.; Boutron, M.-C.; Cummings, J.; Franck, A.; Gibson, G.; Isolauri, E.; Moreau, M.-C.; Roberfroid, M.; Rowland, I. Functional Food Science and Gastrointestinal Physiology and Function. Br. J. Nutr. 1998, 80(S1), S147–S171.
- Heshmati, J.; Farsi, F.; Shokri, F.; Rezaeinejad, M.; Almasi-Hashiani, A.; Vesali, S.; Sepidarkish, M. A Systematic Review and Meta-analysis of the Probiotics and Synbiotics Effects on Oxidative Stress. J. Funct. Foods. 2018, 46, 66–84.
- Malkov, S. V.; Markelov, V. V.; Polozov, G. Y.; Sobchuk, L. I.; Zakharova, N. G.; Barabanschikov, B. I.; Kozhevnikov, A. Y.; Vaphin, R. A.; Trushin, M. V. Antitumor Features of Bacillus Oligonitrophilus KU-1 Strain. J. Microbiol. Immunol. Infect. 2005, 38(2), 96–104.
- Lei, W.-T.; Shih, P.-C.; Liu, S.-J.; Lin, C.-Y.; Yeh, T.-L. Effect of Probiotics and Prebiotics on Immune Response to Influenza Vaccination in Adults: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Nutrients. 2017, 9(11), 1175.
- Ritchie, M. L.; Romanuk, T. N. A Meta-analysis of Probiotic Efficacy for Gastrointestinal Diseases. PloS One. 2012, 7(4), 1–11.
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L. A. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4(1), 1.
- Higgins, J. P.; Altman, D. G.; Gøtzsche, P. C.; Jüni, P.; Moher, D.; Oxman, A. D.; Savović, J.; Schulz, K. F.; Weeks, L.; Sterne, J. A. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. Bmj. 2011, 343, d5928.
- Borenstein M, Hedges LV, Higgins J, Rothstein H. Introduction to Meta-Analysis.Hoboken, NJ: John Wiley & Sons Inc; 2009.
- Follmann, D.; Elliott, P.; Suh, I.; Cutler, J. Variance Imputation for Overviews of Clinical Trials with Continuous Response. J. Clin. Epidemiol. 1992, 45(7), 769–773.
- Green, S.; Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions; Version, 2005.
- Komano, Y.; Shimada, K.; Naito, H.; Fukao, K.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H.; Efficacy of Heat-killed Lactococcus Lactis JCM 5805 on Immunity and Fatigue during Consecutive High Intensity Exercise in Male Athletes: A Randomized, Placebo-controlled, Double-blinded Trial. J. Int. Soc. Sports Nutr.. 2018, 15(1), 39. DOI:10.1186/s12970-018-0244-9.
- Michalickova, D.; Kotur-Stevuljevic, J.; Miljkovic, M.; Dikic, N.; Kostic-Vucicevic, M.; Andjelkovic, M.; Koricanac, V.; Djordjevic, B. Effects of Probiotic Supplementation on Selected Parameters of Blood Prooxidant-antioxidant Balance in Elite Athletes: A Double-blind Randomized Placebo-controlled Study. J. Human Kinetics. 2018, 64, 111–122. DOI: 10.1515/hukin-2017-0203.
- Dönmez, N.; Kisadere, I.; Balaban, C.; Kadiralieva, N.; Effects of Traditional Homemade Koumiss on Some Hematological and Biochemical Characteristics in Sedentary Men Exposed to Exercise. Biotech. Histochem. 2014, 89(8), 558–563. DOI:10.3109/10520295.2014.915428.
- Abbasi, M. M.; Moradi, N.; Narimani-Rad, M.; Lotfi, A. Effects of Probiotic Supplementation on Glycemic and Lipidemic Status in Trained Body Builders. Der Pharmacia Lettre. 2015, 7(3), 29–32.
- Brennan, C. J.; Axelrod, C. L.; Paul, D.; Hull, M.; Kirwan, J. P. Effects of a Novel Probiotic on Exercise-induced Gut Permeability and Microbiota in Endurance Athletes. Med. Sci. Sports Exercise. 2018, 50(5), 840.
- Cox, A. J.; West, N. P.; Horn, P. L.; Lehtinen, M. J.; Koerbin, G.; Pyne, D. B.; Lahtinen, S. J.; Fricker, P. A.; Cripps, A. W. Effects of Probiotic Supplementation over 5 Months on Routine Haematology and Clinical Chemistry Measures in Healthy Active Adults. Eur. J. Clin. Nutr. 2014, 68(11), 1255–1257. DOI: 10.1038/ejcn.2014.137.
- Gölünük, S. B.; Öztaşan, N.; Sözen, H.; Koca, H. B. Effects of Traditional Fermented Beverages on Some Blood Parameters in Aerobic Exercises. Biomed. Res. 2017, 28(21), 9475–9480.
- Cox, A. J.; Pyne, D. B.; Saunders, P. U.; Fricker, P. A.; Oral Administration of the Probiotic Lactobacillus Fermentum VRI-003 and Mucosal Immunity in Endurance Athletes. Br. J. Sports Med. 2010, 44(4), 222–226. DOI:10.1136/bjsm.2007.044628.
- O’Sullivan, O.; Cronin, O.; Clarke, S. F.; Murphy, E. F.; Molloy, M. G.; Shanahan, F.; Cotter, P. D. Exercise and the Microbiota. Gut Microbes. 2015, 6(2), 131–136.
- Allen, J. M.; Mailing, L. J.; Niemiro, G. M.; Moore, R.; Cook, M. D.; White, B. A.; Holscher, H. D.; Woods, J. A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports. Exerc. 2018, 50(4), 747–757.
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M. G.; Maté-Muñoz, J. L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in Gut Microbiota Profile between Women with Active Lifestyle and Sedentary Women. PLoS One. 2017, 12(2), e0171352.
- Cook, M. D.; Allen, J. M.; Pence, B. D.; Wallig, M. A.; Gaskins, H. R.; White, B. A.; Woods, J. A. Exercise and Gut Immune Function: Evidence of Alterations in Colon Immune Cell Homeostasis and Microbiome Characteristics with Exercise Training. Immunol. Cell. Biol. 2016, 94(2), 158–163.
- Sallam, Nada, and Ismail Laher. “Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases.” Oxidative medicine and cellular longevity vol. 2016 (2016): 7239639. doi:10.1155/2016/7239639.
- Lim, C. L.; Mackinnon, L. T. The Roles of Exercise-induced Immune System Disturbances in the Pathology of Heat Stroke. Sports Med. 2006, 36(1), 39–64.
- Lee, E. C.; Fragala, M. S.; Kavouras, S. A.; Queen, R. M.; Pryor, J. L.; Casa, D. J.; Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength. Condit. Res./Nat. Strength Condit. Assoc.. 2017, 31(10), 2920–2937. DOI:10.1519/jsc.0000000000002122.
- Hayes, L. D.; Grace, F. M.; Baker, J. S.; Sculthorpe, N. Exercise-induced Responses in Salivary Testosterone, Cortisol, and Their Ratios in Men: A Meta-analysis. Sports Med. 2015, 45(5), 713–726. DOI: 10.1007/s40279-015-0306-y.
- Lalitsuradej, E.; Sivamaruthi, B. S.; Sirilun, S.; Sittiprapaporn, P.; Peerajan, S.; Chaiyasut, C. The Effect of Supplementation of Lactobacillus Paracasei HII01 on Salivary Cortisol, and Dehydroepiandrosterone Sulfate (DHEA-S) Levels. Asian J. Med. Sci. 2020, 11(1), 12–15.
- Messaoudi, M.; Violle, N.; Bisson, J.-F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial Psychological Effects of a Probiotic Formulation (Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175) in Healthy Human Volunteers. Gut Microbes. 2011, 2(4), 256–261.
- Nishimura, M.; Ohkawara, T.; Tetsuka, K.; Kawasaki, Y.; Nakagawa, R.; Satoh, H.; Sato, Y.; Nishihira, J. Effects of Yogurt Containing Lactobacillus Plantarum HOKKAIDO on Immune Function and Stress Markers. J. Traditional Complementary Med. 2016, 6(3), 275–280.
- Fasano, A.;. Intestinal Permeability and Its Regulation by Zonulin: Diagnostic and Therapeutic Implications. Clin. Gastroenterol. Hepatol. 2012, 10(10), 1096–1100. DOI: 10.1016/j.cgh.2012.08.012.
- Moreno-Navarrete, J. M.; Sabater, M.; Ortega, F.; Ricart, W.; Fernandez-Real, J. M. Circulating Zonulin, a Marker of Intestinal Permeability, Is Increased in Association with Obesity-associated Insulin Resistance. PLoS One. 2012, 7(5), e37160. DOI: 10.1371/journal.pone.0037160.
- De Angelis, M.; Rizzello, C. G.; Fasano, A.; Clemente, M. G.; De Simone, C.; Silano, M.; De Vincenzi, M.; Losito, I.; Gobbetti, M. VSL# 3 Probiotic Preparation Has the Capacity to Hydrolyze Gliadin Polypeptides Responsible for Celiac Sprue Probiotics and Gluten Intolerance. Biochim. Biophys. Acta-Mol. Basis Dis. 2006, 1762(1), 80–93.
- Liu, Z.-H.; Huang, M.-J.; Zhang, X.-W.; Wang, L.; Huang, N.-Q.; Peng, H.; Lan, P.; Peng, J.-S.; Yang, Z.; Xia, Y. The Effects of Perioperative Probiotic Treatment on Serum Zonulin Concentration and Subsequent Postoperative Infectious Complications after Colorectal Cancer Surgery: A Double-center and Double-blind Randomized Clinical Trial. Am. J. Clin. Nutr. 2013, 97(1), 117–126.
- Lamprecht, M.; Bogner, S.; Steinbauer, K.; Schuetz, B.; Greilberger, J. F.; Leber, B.; Wagner, B.; Zinser, E.; Petek, T.; Wallner-Liebmann, S. Effects of Zeolite Supplementation on Parameters of Intestinal Barrier Integrity, Inflammation, Redoxbiology and Performance in Aerobically Trained Subjects. J. Int. Soc. Sports Nutr. 2015, 12(1), 40.
- Holland, A. M.; Hyatt, H. W.; Smuder, A. J.; Sollanek, K. J.; Morton, A. B.; Roberts, M. D.; Kavazis, A. N. Influence of Endurance Exercise Training on Antioxidant Enzymes, Tight Junction Proteins, and Inflammatory Markers in the Rat Ileum. BMC Res. Notes. 2015, 8(1), 514.
- Konstantinov, Sergey R et al. “S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions.” Proceedings of the National Academy of Sciences of the United States of America vol. 105,49 (2008): 19474–19479. doi:10.1073/pnas.0810305105
- Ouwehand, A. C.; Tiihonen, K.; Saarinen, M.; Putaala, H.; Rautonen, N. Influence of a Combination of Lactobacillus Acidophilus NCFM and Lactitol on Healthy Elderly: Intestinal and Immune Parameters. Br. J. Nutr. 2008, 101(3), 367–375.
- Brubaker, S. W.; Bonham, K. S.; Zanoni, I.; Kagan, J. C. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annu. Rev. Immunol. 2015, 33, 257–290. DOI: 10.1146/annurev-immunol-032414-112240.
- Dasu, M. R.; Devaraj, S.; Park, S.; Jialal, I. Increased Toll-like Receptor (TLR) Activation and TLR Ligands in Recently Diagnosed Type 2 Diabetic Subjects. Diabet. Care 2010, 33(4), 861–868.
- Kimoto, H.; Ohmomo, S.; Okamoto, T. Cholesterol Removal from Media by Lactococci. J. Dairy Sci. 2002, 85(12), 3182–3188.
- Lye, H.-S.; Rusul, G.; Liong, M.-T. Removal of Cholesterol by Lactobacilli via Incorporation and Conversion to Coprostanol. J. Dairy Sci. 2010, 93(4), 1383–1392.
- Begley, M.; Hill, C.; Gahan, C. G. Bile Salt Hydrolase Activity in Probiotics. Appl. Environ. Microbiol. 2006, 72(3), 1729–1738.
- Patel, A. K.; Singhania, R. R.; Pandey, A.; Chincholkar, S. B. Probiotic Bile Salt Hydrolase: Current Developments and Perspectives. Appl. Biochem. Biotechnol. 2010, 162(1), 166–180.
- Leung, C.; Rivera, L.; Furness, J. B.; Angus, P. W. The Role of the Gut Microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13(7), 412.
- Lee, J.; Hong, S.-W.; Rhee, E.-J.; Lee, W.-Y. GLP-1 Receptor Agonist and Non-alcoholic Fatty Liver Disease. Diabet. Metabol. J. 2012, 36(4), 262–267.
- Campbell, J. E.; Drucker, D. J. Pharmacology, Physiology, and Mechanisms of Incretin Hormone Action. Cell Metab. 2013, 17(6), 819–837.