ABSTRACT
Chopped pepper, a traditionally fermented condiment product in China, is widely consumed because of its good flavor. However, large amounts of by-product, namely, chopped pepper seeds (CPS) are produced annually during the manufacture of chopped pepper product, and this material is generally classified as solid waste, which causes waste of resource. In this work, the total phenolics (TPC) and capsaicinoids content, antioxidant property, and α-glucosidase inhibitory activity (GIA) of three varieties CPS (i.e., WPS, LPS, and BRPS) were assessed. The effects of solvents with different polarities, including 80% methanol, 80% acetone, and 80% ethanol, in extracting the bioactive compounds were also investigated. The results indicated that the studied CPS were rich in total phenolics and capsaicinoids with contents of 3,934–6,208 μg GAE/g dw and 1,052–3,692 μg/g dw, respectively. Three capsaicinoid compounds, namely, capsaicin, dihydrocapsaicin, and nordihydrocapsaicin, in the CPS were identified and quantified by HPLC. All CPS samples exhibited potent antioxidant property and GIA. Moreover, the different extraction solvents exhibited considerable influence on TPC, capsaicinoid content, antioxidant capacity, and GIA. Regardless of different polarities extraction solvents, BRPS had the highest level of phenolics, capsaicin, dihydrocapsaicin and nordihydrocapsaicin and exhibited the strongest antioxidant activity and GIA among the three varieties CPS studied. In practical applications, the use of 80% ethanol is recommended for the extraction of bioactive compounds from BRPS, because it is environment friendly. Positive correlations between phenolics, capsaicinoids, antioxidant activity, and GIA were observed by PCA and linear correlation analysis. Thus, CPS, especially BRPS, is abundant and can be a cheap source of natural antioxidants and anti-diabetic compounds, making it an interesting ingredient in the potential future development of nutraceuticals or functional food products and bringing great advantages for food and pharmaceutical industries, consumers, and producers.
Abbreviations
CPS: chopped pepper seeds; WPS: wild pepper seeds; LPS: linear pepper seeds; BRPS: bright red pepper seeds; TPC: total phenolics content; HPLC: high-performance liquid chromatography; ABTS: 2, 2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt; FRAP: ferric reducing antioxidant power; DPPH: 2,2-diphenyl-1-picrylhydrazyl; vitamin C: ascorbic acid; RP: reducing power; GIA: α-glucosidase inhibitory activity; PCA: principal component analysis.
Introduction
In the human body, reactive oxygen species (ROS), oxidants or free radicals could accumulate not only from normal intracellular metabolisms, but also from surrounding environment factors (e.g., cigarette smoke, pollution, medication, and radiation).[1] Excessive production of ROS, oxidants, or free radicals would result in oxidative damage in the human body,[Citation2] which leads to the occurrence of many chronic illnesses (e.g., cancer, arthritis, neurodegenerative, etc.), and specially, increased oxidative stress in human have been proved to cause diabetes mellitus (DM).[Citation3] It was reported by World Health Organization[Citation4] that 422 million adults aged over 18 years in the worldwide were living with diabetes in 2014 and International Diabetes Federation[Citation5] predicted that there will be 578 million adults with diabetes by 2030, and 700 million by 2045. One of the effective targets for regulating and managing DM is to suppress the activity of α-glucosidase responsible for carbohydrates hydrolysis.[Citation6,Citation7] At present, in order to reduce the damage caused by oxidation and meet people’s requirements, a large amounts of commercial synthetic antioxidants/drugs or α-glucosidase inhibitors have been developed and widely used in the food and pharmaceutical industries.[Citation8,Citation9] Nonetheless, many of these synthetic compounds have been noted to associate with adverse side impacts on human health,[Citation10–Citation12] which necessitates the search for alternatives. By now, natural antioxidants from plants have attracted a lot of interest because of their capacity to prevent the human body from oxidative stress and thus the progressions of induced chronic diseases were retarded.[Citation7,Citation13,Citation14] Plant materials are greatly potential sources of antioxidants/drugs that are of great importance to combat oxidative stress-associated diseases, and a lot of the presently commercial drugs were obtained from the natural plants.[Citation2,Citation7,Citation13] Thus, the importance of utilization of antioxidants or α-glucosidase inhibitors derived from natural sources has globally increased recently for their low cost, no adverse side effects as well as availability in large amounts as raw material.
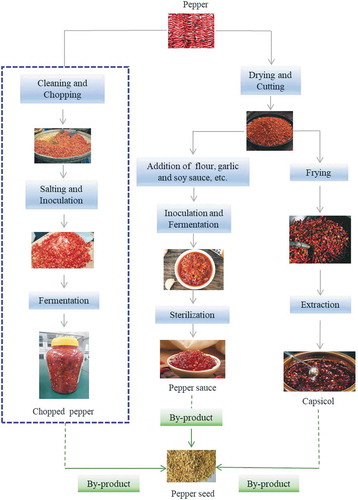
Pepper (Capsicum annuum L.) is widely cultivated in the worldwide such as Africa, Asia, and Mediterranean countries.[Citation15] According to the Statistics of FAO,[Citation15] Asia is the largest pepper producer region, which accounting for 70% of global production. Pepper fruits have considerably utilizations in culinary preparations that make this is one of the most important vegetables in the worldwide. Pepper fruits are mostly consumed as fresh; however, they are also widely used as pickled, dried, or processed items, such as power, pickle, pepper paste, and chopped pepper (). Fresh peppers are rich in many of health benefit compounds such as vitamins E and C, phenolic compounds and capsaicinoids, which could be effectively in preventing oxidative damage-induced chronic diseases.[Citation16–Citation20] Among these compounds, capsaicinoids are a group of molecules unique to pepper fruits, which are accounting for the fruit’s pungent sensation and exert greatly promising valuable biological effects (e.g., anti-obesity and anti-diabetes).[Citation19,Citation20] It has been reported in previous studies that more than 10 structures of capsaicinoids existed in the pepper.[Citation21] However, the most predominantly forms are dihydrocapsaicin, capsaicin, and nordihydrocapsaicin (Figure S1), especially dihydrocapsaicin and capsaicin that accounting for almost 90% of total capsaicinoids content.[Citation17,Citation21] Pepper seed is a by-product from the processing of pepper products such as pepper power, pepper paste, chopper pepper, and chili pepper, among others.[Citation22–Citation26] Substantial quantities of pepper seeds are generated annually, and these by-products are usually disposed and regarded as solid waste,[Citation22,Citation24,Citation27] which created a major problem and aggravated the industry’s burden on the treatment of waste concurrently. For instance, it has been reported that around 17,600 tons of pepper seeds are produced annually during producing red pepper powder (a traditional Korean cooking ingredient) in the Republic of Korea.[Citation28] Besides, Marion and Dempsey[Citation29] reported that 35174 tons of pimento pepper fruit were harvested in the southeastern US in 1964, and those fruit produced approximately 1456 tons of seed. It is expensive to handle, transport and dispose this waste,[Citation30] to date, these solid wastes pepper seed resources have not been fully utilized. Nevertheless, similar to pepper fruits, pepper seeds are also a promising source of nutritional constitutes and bioactive compounds such as phenolics and capsaicinoids, which can be used for its nutritional properties and biological potential.[Citation24,Citation27] Chopped pepper, a traditional fermented condiment in China and produced in large quantities annually, is widely received great interest for its good flavor. Traditionally produced chopped pepper was achieved by natural fermentation for 7–14 days, and the microorganisms used in the fermentation usually derived from the pepper fruit itself as well as from the surrounding environment.[Citation31] However, similar to the other pepper products processing, large amounts of pepper seeds were also produced during the manufacture of chopped pepper product and generally recognized as solid waste.
Recently, great efforts are being made in the worldwide to utilize food processing by-products with the requirement of sustainable development, and thus could also increase profits for local and global economies.[Citation32,Citation33] Besides, the utilization of food by-products would help maximize the use of available resources as well as for the development of a variety of new foods.[Citation33] To achieve these purposes, it is essential to investigate the valorization of waste and recovery of agricultural by-products.[Citation34] Since chopped pepper seeds (CPS) might contain sufficient valuable compounds, use of CPS wastes as bioactive compounds sources may have great economic benefits for food producers as well as crucial importance to the benefits in human health. To the best of our knowledge, there has been no information regards the investigation of CPS. Hence, the objective of this research was to assess the phenolics, capsaicinoids, antioxidant activity, and α-glucosidase inhibitory activity (GIA) of three varieties of CPS from different chopped pepper producers. A variety of solvent systems with different polarities were used to extract bioactive compounds from CPS and assaying their subsequent influences on antioxidant activity and GIA. HPLC was performed to evaluate the main individual capsaicinoids of the various extracts of different CPS. The bioactivities of the extracts were assessed, being, to our knowledge, the first research concerning the antioxidant activity and GIA of these CPS byproducts. Accordingly, the current results investigated would provide new theoretical basis about CPS with added-value and to apply it into potentially functional ingredients for use in food products, thus was considered of great application in the food industry.
Materials and methods
Materials and chemicals
Acetonitrile was HPLC grade and bought from Tedia (Fairfield, OH, USA). 2, 2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2, 4, 6-tris (2-pyridyl)-S-triazine (TPTZ), gallic acid, Folin-Ciocalteuf’s reagent, 2, 2-diphenyl-1-picrylhydrazyl (DPPH), p-nitrophenyl glucopyranoside (pNPG), α-glucosidase from Saccharomyces cerevisiae (EC 3.2.1.20), p-nitro phenol, capsaicin, dihydrocapsaicin, and nordihydrocapsaicin were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All other employed chemicals and reagents in the current work were of analytical grade.
Three varieties of CPS, namely, wild pepper seed (WPS), liner pepper seed (LPS), and bright-red pepper seed (BRPS) were studied in this work for comparison. They are the by-products from chopped pepper processing, which were obtained from three different chopped pepper producers in Hunan province (China). The obtained CPS were firstly freeze-dried and grounded into powder using an electric mill (FW 100, Beijing Yongguangming Medical Instrument Co., Ltd., Beijing, China). The crushed pepper seeds flour were passed through a 60-mesh sieve and then deposited in sealed polyethylene bags at 4°C in the dark for further analysis.
Preparation of solvent extracts
Two gram of CPS powder was extracted with 20 vol. (v/w) of 80% ethanol, 80% methanol, or 80% acetone and maintained for 40 min in an ultrasonic bath (40 KHz) at 40°C. Then, the supernatants were obtained after the extracts were centrifuged at 10,000 × g (3–18 R refrigerated centrifuge, Hunan Hengnuo Instrument Equipment Co., Ltd., Hunan, China) for 15 min at 4°C. The residues were then re-extracted with two times under the same conditions. After centrifugation, the obtained supernatants concentrated at 40°C with a vacuum evaporator RE-52 (Shanghai Yarong Biochemical Instrument Factory, Shanghai, China) to remove the organic solvent, and then the extracts were reconstituted with the respective solvent system to a final volume of 50 mL in volumetric flask. The obtained resultant extracts stored in the refrigerator at −20°C until use.
Evaluation of total phenolic content (TPC)
Folin–Ciocalteu method described by Xiao et al.[Citation35] was employed to determine the TPC in all studied CPS samples. Briefly, 200 μL of sample extracts added into 2.3 mL of deionized water, 0.5 mL of Folin-Ciocalteu reagent was then added into the mixture and maintained for 1 min by a vortex mixer. Then, 2 mL of 75 g/L sodium carbonate solution was added and the reaction was allowed to proceed at 25°C for 60 min. Absorbance of the mixture was recorded at 760 nm against a blank. Gallic acid solution was employed for plotting the standard curve (y = 0.0021x + 0.0033, R2 = 0.9996). The TPC of each sample was presented as μg of gallic acid equivalent per g of dry weight CPS (μg GAE/g dw).
Capsaicinoids content by high-performance liquid chromatography (HPLC) analysis
Identification and quantification of capsaicinoid contents of the three varieties of CPS samples extracted with different polarities solvents were determined using HPLC. First, the obtained CPS extracts were filtered using a 0.45 µm PVDF membrane, then 20 µL of filtrate was injected to the Shimadzu LC-20AT Prominence liquid chromatograph system (Shimadzu, Kyoto, Japan) for analysis of capsaicinoid compositions. A ZORBAX SB-C18 reverse-phase column (Eclipse Plus, Agilent Technologies, USA) (5 μm particle size; 4.6 × 250 mm) was performed and kept at 30°C. Deionized water was used as mobile A and acetonitrile was used as mobile B. The gradient system was carried out as follows: starting with 45% B at 0 min, then increased to 100% B by linear gradient in 30 min, solvent B then decreased to 45% B over 5 min. Solvent flow rate was maintained at 0.8 mL/min. Capsaicinoids were monitored at 280 nm[Citation18] and measured with a Shimadzu SPD-20A Prominence UV-Visible detector (Shimadzu, Kyoto, Japan). The identification and quantification of capsaicin, dihydrocapsaicin, and nordihydrocapsaicin were conducted by comparing the UV spectra and retention times with their authentic standards, respectively. The contents were presented as micrograms per one-gram of dry weight CPS (µg/g dw). Total capsaicinoids content was calculated by the sum of capsaicin, dihydrocapsaicin, and nordihydrocapsaicin contents.
Antioxidant activity
Four commonly antioxidant activity assays with different reaction mechanisms, i.e., ferric reducing antioxidant power (FRAP), scavenging activities against DPPH radical and ABTS·+, and reducing power, was performed to estimate the antioxidant activity of test CPS samples.
FRAP evaluation
The FRAP evaluation was carried out based on the procedure of Xiao et al.[Citation36] The FRAP reagent was freshly prepared by mixing 20 mM ferric chloride (10 mL) with 10 mM TPTZ (10 mL, dissolved in 40 mM HCl) in 0.3 M acetate buffer (100 mL, pH 3.6). Then, 1 mL of test sample was added with 5 mL of working FRAP solution, and allowed to react at 37°C in the dark for 20 min. Absorbance at 593 nm was read against a blank. Ferrous sulfate solution with different concentrations (100–1600 µM) was performed as standard solutions to establish the calibration curve. The FRAP of CPS was calculated as µmol Fe (II) per g of dry weight CPS.
Assay of DPPH radical scavenging activity
Scavenging activity against DPPH radical was carried out referred to the method previously mentioned by Xiao et al.[Citation36] Briefly, 2 mL of CPS extract incubated with 2 mL of methanol solution containing DPPH radicals (0.4 mmol/L). Then, the mixture was vortexed thoroughly and left to stand for 30 min at 25°C in the dark place before the absorbance was recorded at 517 nm against a methanol blank. All samples were performed in triplicate. A calibration curve was constructed by using a variety of concentrations of ascorbic acid (vitamin C). Scavenging effects of DPPH radical were presented in micrograms vitamin C equivalents in one-gram of dry weight CPS (µg VCE/g dw).
ABTS·+ scavenging activity
The procedure of Wang et al.[Citation37] was applied to evaluate ABTS·+ scavenging activity. ABTS·+ stock solution was generated by reacting potassium persulfate solution (2.45 mM) with ABTS stock solution (7 mM), and allowing the mixture to stand for 12 h in dark at 25°C to reach a stable oxidative state. The resulting solution was diluted with ethanol to obtain the freshly prepared ABTS·+ working solution, which absorbance at 734 nm is 0.70 ± 0.02. One milliliter of eightfold diluted CPS extract was mixed thoroughly with 4 mL ABTS·+ working solution, then absorbance at 734 nm was taken after the mixture reacted at 25°C in dark for 6 min. The ABTS·+ scavenging activity of samples were also calculated as µg VCE/g dw.
Reducing power (RP)
RP of the CPS extract was assayed in light of the approach reported by our previous work.[Citation35] Briefly, 0.5 mL of the evaluated sample solution was mixed with 2.5 mL potassium ferricyanide solution (10 mg/mL) and 2.5 mL phosphate buffer (pH 6.6, 0.2 M), then left to stand at 50°C for 20 min, followed by adding 2.5 mL of 100 mg/mL trichloroacetic acid solution to terminated the reaction. After centrifuged for 10 min at 420 g, 2.5 mL of the collected supernatant was transferred to a centrifuge tube with 0.5 mL of 1 mg/mL ferric chloride solution and maintained for 10 min at 25°C in dark. Absorbance of the mixture was immediately taken at 700 nm. Vitamin C with a variety of concentrations was used to construct a standard calibration curve, and RP was presented as µg VCE/g dw.
α-Glucosidase inhibitory activity (GIA) assay
To assay the GIA of the test samples, the obtained extracts of CPS were firstly diluted ten-fold and then performed with the procedure described previously with Chen et al.[Citation6] Reaction mixture was performed as follows: Firstly, 100 μL of CPS extract incubated with 100 μL α-glucosidase solution (1.0 U/mL, dissolved in 0.1 M phosphate buffer, pH 6.9) in a 2.0 mL centrifuge tube. Then, the mixture was incubated at 37°C for 10 min in a water bath, and the reaction was initiated by the addition of 200 μL 5 mM p-nitrophenyl glucopyranoside (pNPG). The reaction mixtures further incubated for 20 min at 37°C, and then reaction terminated by the addition of 100 μL 1 M sodium carbonate solution. The absorbance of liberated product (i.e., p-nitro phenol) from pNPG was read at 405 nm. The GIA was calculated following the equation: GIA (%) = (1- A/A0) × 100% (where A is the absorbance of the test sample, and A0 is the absorbance of the control).
Statistical analysis
All the assays were independently carried out in triplicate. The experiment data were represented as the mean value ± standard deviation (SD) and analyzed with one-way analysis of variance (ANOVA). Duncan’s multiple range test which conducted with SPSS version 17.0 software package for Windows (SPSS Inc., Chicago, USA) was used to analyze the significant differences (p< .05) between the means. Principal component analysis (PCA) was performed to detect an insight overview of the relationships among total phenolic, capsaicin, dihydrocapsaicin, nordihydrocapsaicin, total capsaicinoids, antioxidant activity, and α-glucosidase inhibitory activity of CPS as well as clustering samples in the present study. OriginPro 8.1 statistical software (OriginLab Co., USA) was employed for Pearson correlation, regression analysis as well as preparation of the data graphs.
Results and discussion
Total phenolics content
Phenolic compounds are considered as natural antioxidants and important biological compounds in plant materials. These compounds protect the human body against free radicals and display a crucial role in preventing certain diseases, including cardiovascular disease, cancer, and neurodegenerative disorders.[Citation37] The phenolic compounds in plant materials could be extracted using different organic solvents (e.g., ethanol, methanol, or acetone), and no single solvent could extract all of the phenolics in the samples.[Citation38,Citation39] Therefore, three commonly extraction solvents were performed in this study, and their extraction capacities were compared with those of bioactive compounds from CPS. The TPC results of WPS, LPS, and BRPS extracted using 80% ethanol, 80% methanol, and 80% acetone are depicted in . Depending on the solvent used for extraction, the TPC ranged from 3,934 ± 77 μg GAE/g dw to 6,208 ± 32 μg GAE/g dw with the extracts of WPS, LPS, and BRPS. These results implied that the different extraction solvents had a remarkable effect on the extraction ability of phenolics depending on their polarities, solubilities, and chemical structures. The TPC values observed in all the three CPS in the present study were higher than the values (0.43–2.93 mg GAE/g dw) reported for the n-hexane and chloroform extracts from red chili seeds (Capsicum frutescens L.) grown in India[Citation40] and methanol extract from hot pepper seeds (1.54 mg GAE/g dw) cultivated in Korea.[Citation25] Moreover, the TPC values of the three CPS were significantly higher than those of the four varieties of hot pepper fruit (2.33–2.84 mg GAE/g dw) from Mexico.[Citation41] However, the TPC of the pepper seed byproduct samples in the present study was lower than that of 80% ethanol extract from raw and scalded Jalapeño pepper industrial byproduct (10.01–13.09 mg GAE/g dry defatted byproduct) from Chihuahua and Sinaloa in Mexico.[Citation42]
Table 1. Total phenolics content (TPC) of WPS, LPS, and BRPS extracted with various solvents.
In addition, shows that 80% of acetone extracts exhibited the highest TPC among the various solvent extracts of WPS and LPS examined. Furthermore, shows that regardless of the variant extraction solvents used in the current study, the TPC of three cultivated CPS displayed a large variability, and notably, the TPC values of the extracts of BRPS were significantly greater (p< .05) than those of the two other extracts of WPS and LPS. For instance, the TPC of the methanol extract of BRPS is 6,208 ± 32 μg GAE/g dw, which is almost 1.47- and 1.50- fold higher, than those of the extracts of WPS and LPS, respectively. Different cultivars that considerably influenced the TPC of pepper derived samples were stated in previous studies.[Citation18] Furthermore, a significant difference was not observed (p> .05) among the 80% methanol, 80% ethanol, and 80% acetone extracts of BRPS. Therefore, the use of 80% ethanol is recommended for the extraction of phenolic compounds from BRPS in practical applications, because this solvent is more environment friendly than 80% methanol and 80% acetone.
Capsaicinoid contents determined by HPLC analysis
Pepper is the only plant that can produce capsaicinoids, and it is characterized by its strong spicy taste and exhibit effective health-stimulating effects.[Citation19–Citation21] In the present investigation, three capsaicinoids in the various extracts of three varieties CPS samples, namely, capsaicin, dihydrocapsaicin, and nordihydrocapsaicin, were identified and quantified by HPLC analysis, and the results are shown in and Figure S2. The result showed that capsaicin and dihydrocapsaicin were the dominant capsaicinoids in the pepper samples, and this result is consistent with previous studies.[Citation18,Citation43] Furthermore, shows that the levels of capsaicinoids varied dramatically (p< .05) among the different CPS samples. Although solvents with different polarities were employed for extraction, for the three kinds of capsaicinoids, namely, capsaicin, dihydrocapsaicin, and nordihydrocapsaicin, the BRPS exhibited the highest value, followed LPS and then WPS. For the methanolic extracts, the order of the contents of capsaicin assayed is BRPS (1,950 μg/g) > LPS (968 μg/g) > WPS (723 μg/g), that of dihydrocapsaicin is BRPS (1,108 μg/g) > LPS (391 μg/g) > WPS (341 μg/g), that of nordihydrocapsaicin is BRPS (271 μg/g) > LPS (69 μg/g) > WPS (44 μg/g), and that of the total capsaicinoids is BRPS (3,329 μg/g) > LPS (1,428 μg/g) > WPS (1,109 μg/g). The capsaicin and dihydrocapsaicin contents of pepper seed byproduct samples in this study were significantly higher than those of raw and scalded Jalapeño pepper industrial byproduct from Chihuahua and Sinaloa in Mexico, with values of 0.14–0.29 and 0.10–0.12 mg/g dry-defatted byproduct, respectively.[Citation42] Loizzo et al.[Citation44] found that the capsaicin and dihydrocapsaicin contents of nine Capsicum annum cultivars were 344.3–2,932.1 μg/g dw and 27.8–1,291.2 μg/g dw, respectively. Our findings had a similar trend with their study, but the capsaicin and dihydrocapsaicin contents of BRPS were higher than most of the Capsicum samples. In a previous study, pepper seeds had low capsaicin (32.1 μg/g seeds) and dihydrocapsaicin contents (9.2 μg/g seeds) because of cultivar differences and extraction solvent.[Citation25] Alvarez-Parrilla et al.[Citation45] have determined the capsaicinoid content of seven different fresh and processed hot peppers, the total capsaicinoid contents of the four pepper samples, including Ascencion, chipotle, pickled Jalapeño, and pickled Serrano were 742.0, 525.7, 539.5, and 254.9 μg/g dw, respectively, which were significantly lower than those of the three CPS in the present study, and nordihydrocapsaicin was not detected in these four pepper samples. The Serrano sample had the highest total capsaicinoid content (3,330.9 μg/g dw), which is similar to the result of BRPS, the capsaicin, and nordihydrocapsaicin contents were lower than that of BRPS, whereas the dihydrocapsaicin content was higher than that of BRPS ().[Citation45] Blanco-Rios et al.[Citation46] found that the capsaicin and dihydrocapsaicin contents of the different dried red peppers were 17.91–76.71 and 9.73–25.36 μg/g dry weight, respectively, which were approximately 30–120 and 50–130-folds lower than those of BRPS. Ku et al.[Citation28] have determined the capsaicin and dihydrocapsaicin contents of 20 domestic varieties of red pepper seeds cultivated in Korea and four foreign varieties of samples cultivated in New Mexico, USA. The results showed that the capsaicin content of various red pepper seeds was 0.09–5.32 mg/100 g dw, while that of dihydrocapsaicin was 0.00–2.17 mg/100 g dw. Thus, the studied CPS, especially for BRPS, is rich in capsaicinoids.
Table 2. Capsaicin, dihydrocapsaicin, and nordihydrocapsaicin in terms of total capsaicinoids contents of WPS, LPS, and BRPS extracted with various solvents.
In addition, similar to TPC, reveals that the variant solvent extracts from WPS, LPS, or BRPS exhibited a significant difference (p< .05) in capsaicinoid concentrations. The extraction solvents used in the current investigation have different polarities and chemical characteristics, and a chosen discrepant solvent system might exhibit variant efficacy for extracting capsaicinoids. In general, based on the results in , regardless of the variety of the pepper seed samples used, 80% acetone extracts exhibited higher capsaicinoid contents than the two other solvents. However, no significant difference (p> .05) was observed upon using 80% ethanol and 80% acetone to extract capsaicinoids from LPS or BRPS. Thus, the use of 80% ethanol is recommended for the extraction of capsaicinoids from LPS or BRPS in practical applications, because this solvent possesses little or no toxicity to human health and the environment. A lot of researches have reported that capsaicinoids have potent antioxidant activity.[Citation47–Citation49] Moreover, the present study indicated that capsaicinoids showed a linear relationship with the total phenolics content (Fig. S3), and the R2 of capsaicinoids and TPC ranged from 0.9387 to 0.9590 (p< .01). Moreover, the antioxidant properties of capsaicinoids can be primarily ascribed to the presence of the phenolic moiety in the molecules (Fig. S1), whereas the amide group in capsaicin does not display a major effect on its antioxidant activity.[Citation43] However, Kogure et al.[Citation50] found that the C7-benzyl carbon in capsaicin is the crucial functional group with potent-free radical scavenging ability and antioxidant properties.
Antioxidant activity
By now, many assays were applied to evaluate the antioxidant capacity of complex food constituents because of the unique antioxidant mechanism of different antioxidant compounds. It is not adequate to determine the antioxidant capacity by only one single method as different antioxidant assays are based on discrepant reaction mechanisms.[Citation36] Many researchers have emphasized the necessary to carry out more than one type of antioxidant property assessment to estimate the antioxidant activity of studied samples. In this work, four commonly used antioxidant assays with different mechanisms were performed to estimate the change of antioxidant activity of CPS extracted with different polarities solvent and the results are showed in –d.
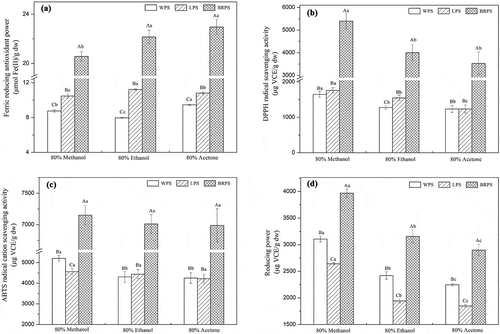
Figure 2. Ferric reducing antioxidant power (FRAP) (a), scavenging effect of DPPH radical (b), ABTS·+ scavenging ability (c) and reducing power (RP) (d) of the various extracts from WPS, LPS, and BRPS. Values were represented as mean ± standard deviation of triplicate assay. Values followed by different superscript capital letters (a–c) are remarkably different (p< .05) among WPS, LPS and BRPS from the same extracted solvent. Values followed by variant superscript small letters (a-c) suggest considerably different (p< .05) among 80% methanol, 80% ethanol, and 80% acetone extracts from the same CPS samples (WPS, LPS, or BRPS).
Ferric reducing antioxidant power (FRAP)
FRAP was conducted to evaluate the ability of antioxidants that in reducing TPTZ–Fe (III) complex compound to TPTZ–Fe (II) form. elucidates that depending on the solvent employed for extraction, FRAP ranged from 7.96 to 23.0 µmol Fe (II)/g dw with the various extracts of different CPS. Grozeva et al.[Citation51] reported the FRAP of pepper fruit ranged from 3.2 to 15.9 µmol Fe (II)/g fresh weight peppers. A similar trend of FRAP was observed in the present study. In the previous study, after taking the extraction yield into consideration, the FRAP of 20 varieties of fresh, boiled, and frozen processed chili peppers had significant lower values when compared with the various extracts of WPS, LPS, and BRPS in this study.[Citation52] However, Loizzo et al.[Citation44] have observed that the FRAP of nine Capsicum annum cultivars ranged from 21.0 to 43.9 µmol Fe (II)/g peppers, which was higher in comparison to the result of CPS in this study. Besides, it is clearly noted in that the FRAP ability of different CPS decreased in the following order: BRPS > LPS > WPS regardless of different polarities solvent used for extraction. FRAP of the methanol, ethanol, and acetone of BRPS were 20.6, 22.2 and 23.0 µmol Fe (II)/g dw, respectively, which were approximately 2.35-, 2.78-, and 2.43-fold higher, respectively, in comparison to the respective extracts of WPS, and 1.97-, 1.97-, and 2.12-fold higher, respectively, compared with the respective extracts of LPS. The BRPS showed the highest FRAP whereas WPS showed the lowest FRAP observed in , which was highly consistent with the data obtained for total phenolics and capsaicinoids contents ( and ). Herein, in order to precisely elucidate the relationship among TPC, capsaicinoids contents, and FRAP, liner correlation analysis was conducted and the obtained data are displayed in Table S1. It was indicated that there was a greatly positive association (p< .01) between TPC, capsaicinoids and FRAP (TPC, R2 = 0.9262; capsaicin, R2 = 0.9885; dihydrocapsaicin, R2 = 0.9853; nordihydrocapsaicin, R2 = 0.9879; total capsaicinoids, R2 = 0.9915). Our findings are consistent with those from previous studies with Capsicum.[Citation44,Citation53] For instance, high positive correlations among TPC and FRAP (R2 = 0.956) was reported by Bogusz et al.[Citation53] for Brazilian Capsicum peppers. Besides, Loizzo et al.[Citation44] reported that there is a positive Pearson’s correlation coefficients of 0.7 and 0.6 for capsaicin and dihydrocapsaicin with FRAP, respectively. Thus, it seems that the phenolic and capsaicinoid compounds in the CPS samples contributed to their FRAP ability, and the strongest FRAP of BRPS among three CPS are mainly attributed to its highest TPC and capsaicinoid concentrations.
In addition, it is noteworthy to mention that different organic solvent extracts of WPS and LPS revealed statistically significant in their FRAP (p< .05). However, we found that there is no statistical difference (p> .05) for FRAP values between 80% ethanol and 80% acetone extracts of BRPS (), but significant (p< .05) higher compared with 80% methanol extract of BRPS. Thus, 80% of ethanol might be a suitable solvent for extracting antioxidants from BRPS when FRAP was used as an evaluation index. It was stated by Dirar et al.[Citation38] that different polarity solvents have a considerable influence on the extraction efficiency of antioxidant compounds. Changes in the polarity of solvent will alter the extraction ability of the antioxidant compounds, and consequently affect the antioxidant activity of the extract.[Citation36] In the present study, as phenolics and capsaicinoids greatly correlated with FRAP (Table S1), the difference in FRAP of variant extracts might be caused by the different TPC and capsaicinoids content of various extracts of WPS, LPS, and BRPS ( and ). For instance, acetone extracts of BRPS showed the highest of TPC, capsaicin, dihydrocapsaicin, nordihydrocapsaicin, and total capsaicinoids among all the various extracts of studied CPS, which consequently contributing to its strongest FRAP observed in .
Scavenging activity of DPPH radical
DPPH is a stable organic-free radical which generally applied for the assessment of in vitro antioxidant property. The distinct purple color of DPPH radical solution disappeared as it reacts with antioxidants, which prevent the radical chain reaction by providing hydrogen atoms. Through the assay of radical scavenging against DPPH, it was possible to study the ability of the CPS to donate hydrogen and stabilize a radical molecule. In this study, the scavenging ability against DPPH radical of different solvent extracts from the three varieties of CPS is illustrated in . The result revealed that the scavenging activity of DPPH radical ranged from 1,235 to 5,398 µg VCE/g dw with the various extracts of CPS, depicting a 4.37-fold variation between test samples. Bae et al.[Citation47] have analyzed the DPPH radical scavenging activity of pepper cultivars at mature and immature stages in 2008 and 2009, which ranged from 880 to 4,400 μg VCE/g fresh weight. Wangcharoen et al.[Citation54] have also reported that DPPH radical scavenging activities of fresh green and red bird chili were 3.06 and 2.39 mg VCE/g dw, respectively. A similar trend of DPPH radical scavenging activity was observed in the present study. However, Zimmer et al.[Citation55] found that the DPPH radical scavenging activities of the seed and fruit of Capsicum baccatum was very low, which ranged from 0.006 to 0.020 and from 0.017 to 0.071 μg VCE/g dw sample, respectively. Škrovánková et al.[Citation56] reported higher DPPH values of 20 pepper spices (from Austrian, Czech, and Slovak producers) compared with the CPS, which ranged from 7.07 to 15.81 mg VCE/g sample for aqueous extracts and from 8.25 to 15.93 mg VCE/g sample for ethanolic extracts, respectively. Besides, similarly to FRAP, DPPH radical scavenging ability of the methanol, ethanol, and acetone extracts of BRPS were remarkably (p< .05) stronger in comparison to those of the respective extracts of WPS and LPS, respectively. The strongest scavenging effect against DPPH radical of BRPS observed might be due to its highest TPC and capsaicinoids content among the studied CPS. This explanation could be further verified by the linear correlation analysis (Table S1), which revealed that the correlation coefficients R2 ranged from 0.7828 to 0.8212 (p< .01) between TPC, capsaicinoids, and scavenging effect of DPPH radical. These findings are consistence with previous studies in which extracts enriched with higher phenolics or capsaicinoids concentrations exhibited stronger DPPH radical scavenging effect in comparison to the other extracts.[Citation42,Citation44,Citation57] Generally, phenolic compounds are ubiquitous in most plants and usually responsible for the scavenging activity of DPPH radical. Several studies have conclusively noted the closely positive relationship between antioxidant activity and total phenolic content in vegetables and fruits samples. For instance, Zhuang et al.[Citation18] stated that DPPH radical scavenging effect and TPC are highly correlated with R2 of the relation is 0.8504, which obtained from nine peppers studied. Sandoval-Castro et al.[Citation42] have reported that the Pearson correlation coefficient between TPC, capsaicin, dihydrocapsaicin, and DPPH radical scavenging activity were 0.857, 0.756 and 0.761, respectively, when the investigation of Jalapeño pepper industrial byproduct. Accordingly, these extracts of CPS can react with free radicals and transforming them into more stable substances, thus the radical chain reactions were terminated.
In addition, various solvent extracts from WPS, LPS, and BRPS depicted remarkable difference in their scavenging activity of DPPH radical, suggesting that this free radical scavenging activity evaluation was also greatly influenced by extraction solvent, this finding was in consonance with previous studies.[Citation55,Citation58] Besides, it was noted that 80% methanol extract showed the highest DPPH radical scavenging regardless of varieties of CPS, which demonstrated that 80% methanol was more efficient solvent than other solvent mixtures for extracting antioxidants in CPS with higher DPPH radical scavenging activity. However, it was noted that there is no statistical difference (p> .05) between 80% ethanol and 80% acetone extracts of BRPS or WPS for the DPPH radical scavenging activity. Although the discrepancies in scavenging activity of DPPH radical exhibited from different extracts, this antioxidant activity was likely to associate with the phenolics and capsaicinoids in the samples as shown in , and .
Scavenging activity of ABTS·+
ABTS·+ scavenging effect is applicable for both hydrophilic and hydrophilic antioxidants assay. The ABTS assay is an excellent approach to assess the antioxidant activity of hydrogen donating and chain-breaking antioxidants, which has already reported in earlier researches.[Citation13] Scavenging activity against ABTS·+ of WPS, LPS, and BRPS is displayed in . The result showed that ABTS·+ scavenging abilities of 80% methanol, 80% ethanol, and 80% acetone extracts of BRPS were 7,151, 7,012, and 6,988 µg VCE/g dw, respectively, which were considerably greater in comparison those of the respective extracts of WPS and LPS, regardless of the extraction solvents conducted. This result providing strong evidence that BRPS was very effectively in scavenging ABTS·+, which is mainly attributed to its highest phenolics and capsaicinoids content ( and ). Interestingly, Table S1 also revealed that significantly positive correlations were existed between the phenolics, capsaicinoids, and ABTS·+ scavenging effect (the correlation coefficient R2 ranged from 0.7940 to 0.8989, p< .01), the trend in the results was supported with previous studies.[Citation42,Citation45] However, for ethanol or acetone extracts, it was noted that there have no significant (p> .05) ABTS·+ scavenging effect between WPS and LPS even though LPS exhibited higher TPC when compared with the WPS (). Besides, it was also found that different solvent extracts of BRPS or LPS exhibited no significant (p> .05) ABTS·+ scavenging effect. Previous studies have reported that there existed synergistic actions among the antioxidants in the mixture. As a result, the antioxidant activity of the obtained mixture could not be solely explained on the basis of the concentration of antioxidants, the structure, and interaction among the antioxidants should also be taken into consideration.[Citation39,Citation59] For example, it has been well documented that phenolic compounds with a hydroxy and a methoxy group or para- and ortho- dihydroxylation are more effective in antioxidant activity than simple phenolics.[Citation60] The findings noted above was most likely owing to the point that phenolic compounds in the extracts of WPS and LPS exhibited discrepant contributions to the scavenging effect of ABTS·+. Furthermore, synergistic, additive, or antagonistical actions of the phenolics, capsaicinoids, and other bioactive compounds present in the extracts cannot be ruled out.[Citation47] Besides, as seen from , different solvent extracts from WPS, LPS, and BRPS displayed insignificant differences (p> .05) in their ABTS·+ scavenging activity except for the methanol and ethanol extract of WPS. In addition, it was found that the ABTS·+ scavenging activities of WPS were lower compared with previous studies. For instance, Wangcharoen et al.[Citation54] have reported that higher ABTS·+ scavenging activities for fresh green and red bird chili, which were 30.06 and 19.59 mg VCE/g dw, respectively. Besides, Dutta et al.[Citation61] found that the ABTS·+ scavenging activities of 72 Capsicum landraces in North-East India ranged from 20.5 to 44.3 mM VCE/g dw. []
Reducing power (RP)
shows the RP of different solvent extracts from WPS, LPS, and BRPS. RP ranged from 1,847 to 3,967 µg VCE/g dw among the various extracts of CPS. Roy et al.[Citation62] have reported that the RP of green, red, and yellow variants raw Capsicum were 451, 466, and 697 μg VCE/g sample, higher RP of CPS was observed in the present study. The RP of nine peppers have been investigated by Zhuang et al.[Citation18] with half-maximal effective concentration (EC50) ranged from 65.91 to 125.08 μg/mL. Significant differences (p< .05) in the RP were also noted with the solvent carried out for extraction, and the methanol extract of BRPS possessed the strongest RP in the variety of extracts of WPS, LPS, and BRPS. Regardless of the different varieties CPS studied, the RP of variant solvent extracts of CPS reduced followed the order: 80% methanol extract > 80% ethanol extract > 80% acetone extract, which is similar with the DPPH radical scavenging activity. However, these results obtained is inconsistence with TPC, capsaicinoids, FRAP, and ABTS·+ scavenging activity evaluation as illustrated in , and . For instance, shows that acetone extracts of WPS, LPS, and BRPS have the highest capsaicinoids, nevertheless, the RP of their respective extracts are the weakest. Additionally, the RP of ethanol extract of WPS is greatly stronger when compared with the respective extract of LPS, which is in contrary to their phenolics content (). Our results obtained are similar to earlier investigations. Previous studies emphasized that the antioxidant ability of complex mixture not only associate with the concentration of antioxidant compounds, but also closely relate with the structure and interaction among the antioxidants.[Citation36,Citation38] Thus, the phenomenon obtained probably owing to the variety of antioxidant compounds existed in the extracts of CPS as the RP of a compound is associated with its structure-function configuration. Additionally, since the studied extracts or fractions were not pure single compounds, a synergistic or antagonistic effect might have been occurred by a combination of some specific structures of antioxidant compounds,[Citation63] which also could at least partly explain the observed findings.
Furthermore, also reveals that different varieties of CPS extracts differed significantly (p< .05) in their RP. It was noted that the RP of WPS is remarkably greater (p< .05) in comparison those of the respective extracts of LPS, regardless of the different performed extraction solvents. However, the extracts of TPC, capsaicinoids, FRAP, and DPPH radical scavenging activity of LPS is greater when compared with the respective extracts of WPS. Despite of the inconsistence in the RP with TPC and capsaicinoids in terms of WPS and LPS, the extracts of BRPS also exhibited the highest RP regardless of the different extraction conducted. The correlation analysis revealed that this antioxidant assay was positively correlated (p< .01) with the content of phenolics and capsaicinoids compounds in the extracts ( and ), but the correlation coefficients were much lower when compared with those of correlation coefficients between phenolics, capsaicinoids and FRAP, DPPH and ABTS (Table S1). However, Zhuang et al.[Citation18] reported a higher correlation coefficient (R2 = 0.8832) between TPC and reducing the power of pepper fruits. The observed results implied that phenolics and capsaicinoids in the extracts of CPS contributed to its RP, and the presence of other antioxidant compounds in the extracts might be highly related to RP of the samples. In addition, the characteristics of phenolics, which can act additively, antagonistically, or synergistically should also be taken into consideration for the obtained RP. For instance, a synergism effect was noted among different polyphenols or between other phytochemical compounds and polyphenols in earlier studies,[Citation13] which should also be taken into consideration for RP. The findings suggested that the different RP observed not only from the concentrations of phenolics and capsaicinoids in the extract, but also attributed by the chemical structures characteristics of phenolic and capsaicinoids constituent. Herein, all of the above-mentioned reasons might interpret the observed variant RP of discrepant CPS extracts. By comparing antioxidant abilities carried out with a variety of methods, i.e., FRAP, scavenging effects of DPPH radical and ABTS·+, and RP, we conclusively reported that all of these assays were in coincided with each other that BRPS exhibited the strongest antioxidant activity regardless of different extraction solvents used.
Inhibitory activity of α-glucosidase (GIA)
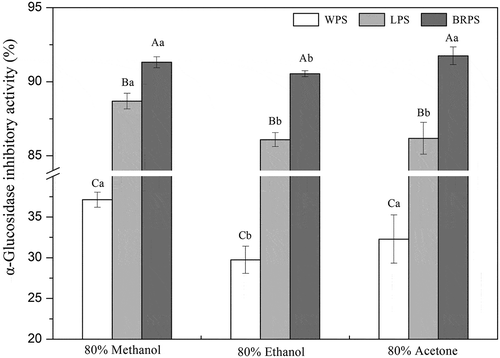
Alpha-glucosidase, a key enzyme responsible for the digestion of dietary polysaccharides, has been considered as an effective therapeutic target for regulation and treatment of elevations in postprandial blood glucose levels. According to a lot of current in vivo researches, inhibition of α-glucosidase is considered to be one of the most effective approaches for diabetes care.[Citation1,Citation7] Alpha-glucosidase inhibitors could effectively decrease the catalyzation of starch into glucose and contributed to the reduced absorption of glucose in the small intestine, and consequently prevent postprandial hyperglycemia. Thus, α-glucosidase inhibitors were widely used as a useful hypoglycemic agent in the prevention or treatment of diabetes.[Citation7,Citation64] Great efforts have been made recently to seek α-glucosidase inhibitors derived from nature materials, which are effectively and safety to utilize as physiological functional compounds for managing anti-diabetic.[Citation1] In this study, various extracts of WPS, LPS, and BRPS were assayed for their ability to inhibit α-glucosidase, and the data is shown in . The result demonstrated that all the extracts of CPS exhibited GIA, but their GIA varied mainly depend on the varieties of CPS, and also were significant influenced by the different extraction solvent used. According to the result depicted in , BRPS and LPS exhibited strong GIA since all of the variant extracts of BRPS and LPS are more than 90.0% and 85.0%, respectively. However, for WPS, all its extracts are less than 40.0% which exhibited the weakest effect when compared with LPS and BRPS. The GIA decreased in the order of BRPS > LPS > WPS (), which is consistence with the result trendy of TPC and capsaicinoids ( and ). Besides, it was noted that methanol and acetone extract of BRPS showed higher GIA when compared with the ethanol extract, however, ethanol extract of BRPS also exhibited strong GIA (> 90.0%). Therefore, it is recommended that use of 80% ethanol for the extraction of GIA compounds from BRPS in practical applications because it is solvent more friendly with the environment compared with 80% methanol and 80% acetone. Despite the variation in the GIA exerted by the varieties of CPS and diverse solvent extracts conducted, this inhibitory ability was noted to be connected with the concentrations of phenolic compounds and capsaicinoids in the extracts. Many earlier studies have verified that α-glucosidase activity could be effectively inhibited by phenolic compounds in the samples.[Citation1,Citation6] Oboh et al.[Citation65] have studied the antioxidant and α-glucosidase activities of tropical pepper varieties, they also attributed to the observed effects to the phenolics. In addition, the present study revealed that α-glucosidase inhibitory activity was moderately correlated with capsaicinoids in the test samples. For instance, the acetone extract of the BRPS possessed the greatest capsaicin, dihydrocapsaicin, nordihydrocapsaicin, total capsaicinoids, and exhibited the strongest GIA in the variously performed solvent extracts. In contrast, the lowest GIA was found in the ethanol extract of WPS that showed the lowest capsaicin, dihydrocapsaicin, nordihydrocapsaicin, and total capsaicinoids (). The correlation analysis result observed in Table S2 also supported that the interpretation as the correlation coefficients between GIA and capsaicinoids ranged from 0.5943 to 0.6833 (p< .01). Therefore, these results implied that CPS enriched with phenolics and capsaicinoids display the greatly promising to contribute to the regulation and treatment of diabetes. BRPS is the best potential source of future alternatives to medicinal anti-diabetics among the three varieties CPS because BRPS contains the highest active compounds that exhibit GIA. This result implies that BRPS shows the greatly promising effect to suppress the postprandial hyperglycemia in patients which influenced by diabetes type II, and BRPS consumption could possibly be used for the prevention of diabetic. A previous in vivo study performed by Tolan et al.[Citation66] demonstrated that capsaicin showed a strong hypoglycemic activity, which involved in the insulin pancreas secretion and also adrenal medulla and liver. More investigation in terms of the molecular level is crucially essential to elucidate the reaction mechanisms of GIA by CPS phenolics and capsaicinoids.
Figure 3. Inhibitory activity against α-glucosidase of the various extracts from WPS, LPS, and BRPS. Values are means ± standard deviation of triplicate assay. Values with different superscript uppercase letters (a–c) indicated significantly different (p< .05) among WPS, LPS, and BRPS from the same extracted solvent. Values with different superscript lowercase letters (a-c) demonstrated remarkably different (p< .05) among 80% methanol, 80% ethanol, and 80% acetone extracts from the same CPS samples (WPS, LPS, or BRPS).
Principal component analysis (PCA) of total phenolics, capsaicinoids, antioxidant activities, and α-glucosidase inhibitory activity
To get an insight overview of the relationships between total phenolics, capsaicinoids, antioxidant activities, and α-glucosidase inhibitory activity (GIA), PCA model was carried out in this work. depicts the PCA loading plot of each evaluation index. The original variables can be reduced to two validated principal components (PCs), which together accounted for 95.01% of the total variability information, suggesting that the two PCs could well reflect the information of the original variables. It can been seen from that the first PC (PC1) correlated well with TPC, capsaicinoids, all evaluated antioxidant assays and GIA due to their high loading on PC1 (>0.65). What’s more, TPC, capsaicinoids, all evaluated antioxidant assays and GIA were found on the right location of PC1, which implied that the antioxidant abilities and GIA of the studied CPS was predominately attributed to the TPC as well as capsaicinoids. Furthermore, depicts that FRAP, DPPH, ABTS, TPC, capsaicin, dihydrocapsaicin, nordihydrocapsaicin, and total capsaicinoids were closely correlated to each other, with the high PC1 loadings 0.986, 0.944, 0.963, 0.962, 0.989, 0.988, 0.992, and 0.991, respectively, which indicated considerably positive correlations between FRAP, DPPH, ABTS, phenolics and capsaicinoids. Results observed by PCA are consistent with phenomena obtained through correlation analysis (Table S1 and Table S2). What’s more, phenolics and capsaicinoids also have been reported to exhibit potent antioxidant capacity according to some earlier reports in terms of pepper fruits,[Citation18,Citation43,Citation48,Citation49] and this findings is also coincided to the current correlation analysis results. Thus, as discussed above, PCA result further proved that phenolics and capsaicinoids contributed to the antioxidant activity and GIA of the studied samples, and the highest content of phenolics and capsaicinoids in BRPS leading its strongest antioxidant activity and GIA among the three varieties of CPS samples.
Figure 4. (a) Loading plot of principal component analysis (PCA) of total phenolic content (TPC), capsaicinoids (capsaicin, dihydrocapsaicin, nordihydrocapsaicin, total capsaicinoids), antioxidant activities, and α-glucosidase inhibitory activity. (b) Score plot of principal component analysis (PCA) of discrepant solvent extracts of WPS, LPS, and BRPS. M-, E- and A- indicate CPS (i.e., WPS, LPS, and BRPS) were extracted with 80% methanol, 80% ethanol, and 80% acetone, respectively.
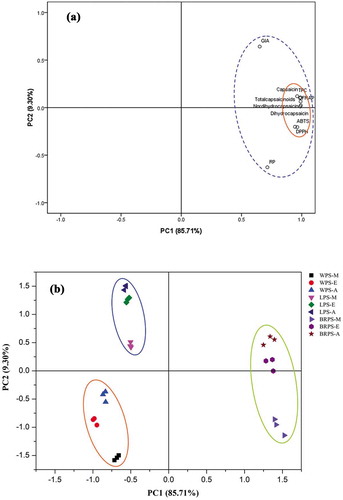
The PCA score plot was also carried out to study the similarities and differences of antioxidant activities and GIA among CPS samples extracted with different polarities solvent, and the results are illustrated in . It can be apparently discovered that all of the CPS samples could be completely separated into three sections (WPS, LPS, BRPS) along with the vector of PC1 from left to right. shows that WPS-M, WPS-E, WPS-A were similarly distributed, LPS-M, LPS-E, LPS-A were similarly distributed, whereas BRPS-M, BRPS-E, and BRPS-A were closely distributed in the PCA score plot, which can be interpreted that the bioactive compounds, antioxidant activity, and GIA of these similarly distributed samples were relatively similar. However, it should be noted that the similarly distributed samples also displayed different distributions to each other in the PCA score plot, implying that the total amount of bioactive compounds are different, which extracted from WFS, LPS, and BRPS with discrepant polarities solvents. Furthermore, it also should be noted that BRPS-M, BRPS-E, and BRPS-A were distributed on the right point of PCA score plot and having a high score on PC1, these test samples could be well characterized as possessing greater antioxidant activity, GIA, phenolics, and capsaicinoids content, these interpretations are further concurrently supported by the data illustrated in , , and . Nevertheless, it is clearly find that negative scores of PC1 are occupied by WPS-M, WPS-E, WPS-A, LPS-M, LPS-E, LPS-A, which implied that all of these test samples displayed lower GIA, antioxidant capacity, phenolics, and capsaicinoids content. Thus, this finding implied that PCA could be useful to provide crucial insight into the discrimination and classification of the investigated chopped pepper seed samples, along with the relationships between phenolics, capsaicinoids, antioxidant ability, and α-glucosidase inhibitory activity.
Conclusion
CPS is the byproduct of the processing of chopped pepper products, and it is generally regarded as a solid waste, which causes environmental pollution. However, valuable products could be obtained from the utilization of CPS by adopting appropriate methods, thus contributing to the human society. The findings of the current study revealed that three varieties of chopped pepper seeds (especially for BRPS) are rich in phenolics and capsaicinoids and exhibited potent antioxidant activity and α-glucosidase inhibitory activity (GIA). In addition, solvents with variant extraction polarities had different extraction abilities for phenolics and capsaicinoids from CPS, thus influencing the antioxidant activity and GIA. Among the three varieties of CPS studied, the highest level of phenolics, capsaicin, dihydrocapsaicin, and nordihydrocapsaicin was detected in BRPS, regardless of solvents with different polarities were employed. Furthermore, BRPS exhibited the strongest FRAP, scavenging effects of DPPH radical and ABTS·+, reducing power, and α-GIA. Moreover, the 80% ethanol and 80% acetone extracts of BRPS exhibited significantly (p< .05) high capsaicinoid content and strong FRAP and did not have significant difference (p> .05) with the TPC and ABTS·+ scavenging activity compared with 80% methanol extracts. In practical applications, the use of 80% ethanol is recommended for the extraction of phenolic and capsaicinoid compounds from BRPS, because this solvent possesses little or no toxicity to human health and is more environment friendly than 80% methanol and 80% acetone. Among the investigated CPS, BRPS is a naturally excellent source of anti-diabetic and antioxidant compounds, followed by LPS, and then WPS. Both PCA and linear correlation analysis verified the positive correlations (p< .01) between phenolic and capsaicinoid contents, antioxidant activity, and GIA. The findings obtained in this study are of great concern, because this work first reported the potent antioxidant activity and GIA of these chopped pepper seed byproducts. The overall results suggest that the chopped pepper seeds are potentially good and cheap sources of natural antioxidants and anti-diabetic compounds and can serve as a nutraceuticals or functional ingredients in new food formulations. The exploitation of these low-cost and high amounts of renewable resources could be used in the food and pharmaceutical industries. Further work is needed to estimate their potential human health-promoting effects of CPS by in vivo studies. Furthermore, this approach could ultimately improve the economy and the environment. The reutilization of these byproducts used as ingredients in functional foods or nutraceuticals provides a new opportunity to support sustainable development from an economic and environmental point of insight.
Supplemental Material
Download MS Word (3.7 MB)Acknowledgments
The authors thank the Hunan Agricultural University Undergraduate Students Innovation Training Project (No. xcx19103), Natural Science Foundation of Hunan Province (No. 2018JJ3224), Hainan Province Science and Technology Major Project (No. ZDKJ2016003) and Hunan Province Science and Technology Innovation Plan Project (No. 2017SK2430) for their financial support. The authors declare no conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Indrianingsih, A. W.; Tachibana, S.; Dewi, R. T.; Itoh, K. Antioxidant and α-glucosidase Inhibitor Activities of Natural Compounds Isolated from Quercus Gilva Blume Leaves. Asian Pac. J. Trop. Bio. 2015, 5, 748–755. DOI: 10.1016/j.apjtb.2015.07.004.
- Pham-Huy, L. A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96.
- Rahman, K.;. Studies on Free Radicals, Antioxidants, and Co-factors. Clin. Interv. Aging. 2007, 2, 219–236.
- World Health Organization (2017). Global Report on Diabetes. World Health Organization, 2016.
- IDF Diabetes Atlas. 2019. 9th ed. International Diabetes Federation: Brussels, Belgium. http://www.diabetesatlas.org
- Chen, Y.; Wang, E.; Wei, Z.; Zheng, Y.; Yan, R.; Ma, X. Phytochemical Analysis, Cellular Antioxidant, α-glucosidase Inhibitory Activities of Various Herb Plant Organs. Ind. Crop. Prod. 2019, 141, 111771. DOI: 10.1016/j.indcrop.2019.111771.
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase Inhibitors from Plants: A Natural Approach to Treat Diabetes. Pharmacogn. Rev. 2011, 5, 19–29. DOI: 10.4103/0973-7847.79096.
- Nieva-Echevarría, B.; Manzanos, M. J.; Goicoechea, E.; Guillén, M. D. 2, 6‐Di‐tert‐butyl‐hydroxytoluene and Its Metabolites in Foods. Compr. Rev. Food Sci. F. 2015, 14, 67–80. DOI: 10.1111/1541-4337.12121.
- Williams, G.; Iatropoulos, M.; Whysner, J. Safety Assessment of Butylated Hydroxyanisole and Butylated Hydroxytoluene as Antioxidant Food Additives. Food Chem. Toxicol. 1999, 37, 1027–1038. DOI: 10.1016/S0278-6915(99)00085-X.
- Santeusanio, F.; Compagnucci, P. A Risk-benefit Appraisal of Acarbose in the Management of Non-insulin-dependent Diabetes Mellitus. Drug Safety. 1994, 11, 432–444. DOI: 10.2165/00002018-199411060-00005.
- Husøy, T.; Andreassen, M.; Lillegaard, I. T. L.; Mathisen, G. H.; Rohloff, J.; Starrfelt, J.; Svendsen, C. Risk Assessment of Butylated Hydroxytoluene (BHT). Opinion of the Panel on Food Additives, Flavourings, Processing Aids, Materials in Contact with Food, and Cosmetics of the Norwegian Scientific Committee for Food and Environment. VKM Report. 2019, pp. 15.
- Gharavi, N.; Haggarty, S.; El-Kadi, S.; Ayman, O. Chemoprotective and Carcinogenic Effects of Tert-butylhydroquinone and Its Metabolites. Curr. Drug Metab. 2007, 8, 1–7. DOI: 10.2174/138920007779315035.
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Hayes, A. W.; Tsatsakis, A. M.; Kouretas, D. Assessment of Polyphenolic Content, Antioxidant Activity, Protection against Ros-induced DNA Damage and Anticancer Activity of Vitis Vinifera Stem Extracts. Food Chem. Toxicol. 2013, 61, 60–68. DOI: 10.1016/j.fct.2013.01.029.
- Ma, Y.; Wu, Y.; Yang, J.; Liang, H.; Shang, F. Flavonoids Content and Antioxidant Activity of Ethanol Extracts of Osmanthus Fragrans Flowers. Bangl. J. Bot. 2017, 46, 907–915.
- Food and Agriculture Organization of the United Nations. The Annual Production of Dry Chillies and Peppers Reports from the United Nations 2018, http://www.fao.org/faostat/en/#data/QC/visualize, Accessed date: 30 July 2018.
- Hamed, M.; Kalita, D.; Bartolo, M. E.; Jayanty, S. S. Capsaicinoids, Polyphenols and Antioxidant Activities of Capsicum Annuum: Comparative Study of the Effect of Ripening Stage and Cooking Methods. Antioxidants. 2019, 8, 364. DOI: 10.3390/antiox8090364.
- Meghvansi, M.; Siddiqui, S.; Khan, M. H.; Gupta, V.; Vairale, M.; Gogoi, H.; Singh, L. Naga Chilli: A Potential Source of Capsaicinoids with Broad-spectrum Ethnopharmacological Applications. J. Ethnopharmacol. 2010, 132, 1–14. DOI: 10.1016/j.jep.2010.08.034.
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive Characteristics and Antioxidant Activities of Nine Peppers. J. Funct. Foods. 2012, 4, 331–338. DOI: 10.1016/j.jff.2012.01.001.
- Rosa, A.; Deiana, M.; Casu, V.; Paccagnini, S.; Appendino, G.; Ballero, M.; Dessí, M. A. Antioxidant Activity of Capsinoids. J. Agr. Food Chem. 2002, 50, 7396–7401. DOI: 10.1021/jf020431w.
- Whiting, S.; Derbyshire, E.; Tiwari, B. Capsaicinoids and Capsinoids. A Potential Role for Weight Management? A Systematic Review of the Evidence. Appetite. 2012, 59, 341–348. DOI: 10.1016/j.appet.2012.05.015.
- Lu, M.; Ho, C.-T.; Huang, Q. Extraction, Bioavailability, and Bioefficacy of Capsaicinoids. J. Food Drug Anal. 2017, 25, 27–36. DOI: 10.1016/j.jfda.2016.10.023.
- Li, M.; Wen, X.; Peng, Y.; Wang, Y.; Wang, K.; Ni, Y. Functional Properties of Protein Isolates from Bell Pepper (Capsicum Annuum L. Var. Annuum) Seeds. LWT. 2018, 97, 802–810. DOI: 10.1016/j.lwt.2018.07.069.
- Firatligil-Durmus, E.; Evranuz, O. Response Surface Methodology for Protein Extraction Optimization of Red Pepper Seed (Capsicum Frutescens). LWT-Food Sci. Technol. 2010, 43, 226–231. DOI: 10.1016/j.lwt.2009.08.017.
- Sung, J.; Bang, M.-H.; Lee, J. Bioassay-guided Isolation of Anti-adipogenic Compounds from Defatted Pepper (Capsicum Annuum L) Seeds. J. Funct. Foods. 2015, 14, 670–675. DOI: 10.1016/j.jff.2015.02.043.
- Jeon, G.; Choi, Y.; Lee, S. M.; Kim, Y.; Oh, M.; Jeong, H. S.; Lee, J. Antioxidant and Antiproliferative Properties of Hot Pepper (Capsicum Annuum L.) Seeds. J. Food Biochem. 2012, 36, 595–603. DOI: 10.1111/j.1745-4514.2011.00571.x.
- Sandoval-Castro, C.; Valdez-Morales, M.; Perea-Domínguez, X.; Medina-Godoy, S.; Espinosa-Alonso, L. Antioxidant Activity of Processed and Raw Seed Byproduct from Jalapeño Pepper. J. Chem. Biol. Phys. Sci. Spec. 2014, 4, 26–34.
- Zou, Y.; Ma, K.; Tian, M. Chemical Composition and Nutritive Value of Hot Pepper Seed (Capsicum Annuum) Grown in Northeast Region of China. Food Sci. Tech. 2015, 35, 659–663. DOI: 10.1590/1678-457X.6803.
- Ku, K.-H.; Choi, E.-J.; Park, J.-B. Chemical Component Analysis of Red Pepper (Capsicum Annuum L.) Seeds with Various Cultivars. J. Korean Soc. Food Sci. Nutr. 2008, 37, 1084–1089. DOI: 10.3746/jkfn.2008.37.8.1084.
- Marion, J.; Dempsey, A. Fatty Acids of Pimiento Pepper Seed Oil. J. Am. Oil Chem. Soc. 1964, 41, 548–549. DOI: 10.1007/BF02898131.
- Silva, L. R.; Azevedo, J.; Pereira, M. J.; Valentão, P.; Andrade, P. B. Chemical Assessment and Antioxidant Capacity of Pepper (Capsicum Annuum L.). Seeds. Food Chem. Toxicol. 2013, 53, 240–248. DOI: 10.1016/j.fct.2012.11.036.
- Wang, J.; Wang, R.; Xiao, Q.; Liu, C.; Deng, F.; Zhou, H. SPME/GC-MS Characterization of Volatile Compounds of Chinese Traditional-chopped Pepper during Fermentation. Int. J. Food Prop. 2019, 22, 1863–1872. DOI: 10.1080/10942912.2019.1684320.
- Loizzo, M. R.; Sicari, V.; Pellicanò, T.; Xiao, J.; Poiana, M.; Tundis, R. Comparative Analysis of Chemical Composition, Antioxidant and Anti-proliferative Activities of Italian Vitis Vinifera By-products for a Sustainable Agro-industry. Food Chem. Toxicol. 2019, 127, 127–134. DOI: 10.1016/j.fct.2019.03.007.
- Lai, W. T.; Khong, N. M.; Lim, S. S.; Hee, Y. Y.; Sim, B. I.; Lau, K. Y.; Lai, O. M. A Review: Modified Agricultural By-products for the Development and Fortification of Food Products and Nutraceuticals. Trends Food Sci. Tech. 2017, 59, 148–160. DOI: 10.1016/j.tifs.2016.11.014.
- Costa, A. S.; Alves, R. C.; Vinha, A. F.; Costa, E.; Costa, C. S.; Nunes, M. A.; Almeida, A. A.; Santos-Silva, A.; Oliveira, M. B. P. Nutritional, Chemical and Antioxidant/pro-oxidant Profiles of Silverskin, a Coffee Roasting By-product. Food Chem. 2018, 267, 28–35. DOI: 10.1016/j.foodchem.2017.03.106.
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the Antioxidant Capacity of Chickpeas by Solid State Fermentation with Cordyceps Militaris SN-18. J. Funct. Foods. 2014, 10, 210–222. DOI: 10.1016/j.jff.2014.06.008.
- Xiao, Y.; Rui, X.; Xing, G.; Wu, H.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Solid State Fermentation with Cordyceps Militaris SN-18 Enhanced Antioxidant Capacity and DNA Damage Protective Effect of Oats (Avena Sativa L.). J. Funct. Foods. 2015, 16, 58–73. DOI: 10.1016/j.jff.2015.04.032.
- Wang, Q.; Rehman, M.; Peng, D.; Liu, L. Antioxidant Capacity and α-glucosidase Inhibitory Activity of Leaf Extracts from Ten Ramie Cultivars. Ind. Crop. Prod. 2018, 122, 430–437. DOI: 10.1016/j.indcrop.2018.06.020.
- Dirar, A.; Alsaadi, D.; Wada, M.; Mohamed, M.; Watanabe, T.; Devkota, H. Effects of Extraction Solvents on Total Phenolic and Flavonoid Contents and Biological Activities of Extracts from Sudanese Medicinal Plants. S. Afr. J. Bot. 2019, 120, 261–267. DOI: 10.1016/j.sajb.2018.07.003.
- Zhao, H.; Dong, J.; Lu, J.; Chen, J.; Li, Y.; Shan, L.; Lin, Y.; Fan, W.; Gu, G. Effects of Extraction Solvent Mixtures on Antioxidant Activity Evaluation and Their Extraction Capacity and Selectivity for Free Phenolic Compounds in Barley (Hordeum Vulgare L.). J. Agr. Food Chem. 2006, 54, 7277–7286. DOI: 10.1021/jf061087w.
- Gurnani, N.; Gupta, M.; Mehta, D.; Mehta, B. K. Chemical Composition, Total Phenolic and Flavonoid Contents, and in Vitro Antimicrobial and Antioxidant Activities of Crude Extracts from Red Chilli Seeds (Capsicum Frutescens L.). J. Taibah Univ. Sci. 2016, 10, 462–470. DOI: 10.1016/j.jtusci.2015.06.011.
- Hervert-Hernández, D.; Sáyago-Ayerdi, S. G.; GONi, I. Bioactive Compounds of Four Hot Pepper Varieties (Capsicum Annuum L.), Antioxidant Capacity, and Intestinal Bioaccessibility. J. Agr. Food Chem. 2010, 58, 3399–3406. DOI: 10.1021/jf904220w.
- Sandoval-Castro, C. J.; Valdez-Morales, M.; Oomah, B. D.; Gutiérrez-Dorado, R.; Medina-Godoy, S.; Espinosa-Alonso, L. G. Bioactive Compounds and Antioxidant Activity in Scalded Jalapeño Pepper Industrial Byproduct (Capsicum Annuum). J. Food Sci. Techn. 2017, 54, 1999–2010. DOI: 10.1007/s13197-017-2636-2.
- Tundis, R.; Menichini, F.; Bonesi, M.; Conforti, F.; Statti, G.; Menichini, F.; Loizzo, M. R. Antioxidant and Hypoglycaemic Activities and Their Relationship to Phytochemicals in Capsicum Annuum Cultivars during Fruit Development. LWT-Food Sci. Technol. 2013, 53, 370–377. DOI: 10.1016/j.lwt.2013.02.013.
- Loizzo, M. R.; Bonesi, M.; Serio, A.; Chaves-López, C.; Falco, T.; Paparella, A.; Menichini, F.; Tundis, R. Application of Nine Air-dried Capsicum Annum Cultivars as Food Preservative: Micronutrient Content, Antioxidant Activity, and Foodborne Pathogens Inhibitory Effects. Int. J. Food Prop. 2017, 20, 899–910. DOI: 10.1080/10942912.2016.1188310.
- Alvarez-Parrilla, E.; de la Rosa, L. A.; Amarowicz, R.; Shahidi, F. Antioxidant Activity of Fresh and Processed Jalapeno and Serrano Peppers. J. Agr. Food Chem. 2011, 59, 163–173. DOI: 10.1021/jf103434u.
- Blanco-Rios, A. K.; Medina-Juarez, L. A.; Gamez-Meza, N. Drying and Pickling on Phenols, Capsaicinoids, and Free Radical-scavenging Activity in Anaheim and Jalapeño Peppers. Ciência Rural. 2017, 47, e20160722. DOI: 10.1590/0103-8478cr20160722.
- Bae, H.; Jayaprakasha, G.; Crosby, K.; Yoo, K. S.; Leskovar, D. I.; Jifon, J.; Patil, B. S. Ascorbic Acid, Capsaicinoid, and Flavonoid Aglycone Concentrations as a Function of Fruit Maturity Stage in Greenhouse-grown Peppers. J. Food Compos. Anal. 2014, 33, 195–202. DOI: 10.1016/j.jfca.2013.11.009.
- Lavorgna, M.; Orlo, E.; Nugnes, R.; Piscitelli, C.; Russo, C.; Isidori, M. Capsaicin in Hot Chili Peppers: In Vitro Evaluation of Its Antiradical, Antiproliferative and Apoptotic Activities. Plant Food Hum. Nutr. 2019, 74, 164–170. DOI: 10.1007/s11130-019-00722-0.
- Chaudhary, A.; Gour, J. K.; Rizvi, S. I. Capsaicin Has Potent Anti-oxidative Effects in Vivo through a Mechanism Which Is Non-receptor Mediated. Arch. Physiol. Biochem. 2019, 1–7. doi:10.1080/13813455.2019.1669056.
- Kogure, K.; Goto, S.; Nishimura, M.; Yasumoto, M.; Abe, K.; Ohiwa, C.; Sassa, H.; Kusumi, T.; Terada, H. Mechanism of Potent Antiperoxidative Effect of Capsaicin. BBA-Gen. Subjects. 2002, 1573, 84–92. DOI: 10.1016/S0304-4165(02)00335-5.
- Grozeva, S.; Tringovska, I.; Nankar, A. N.; Todorova, V.; Kostova, D. Assessment of Fruit Quality and Fruit Morphology in Androgenic Pepper Lines (Capsicum Annuum L.). Crop Breeding, Genetics Genomics. 2020, 2, e200005.
- Loizzo, M. R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of Chemical Profile and Antioxidant Activity of Twenty Cultivars from Capsicum Annuum, Capsicum Baccatum, Capsicum Chacoense and Capsicum Chinense: A Comparison between Fresh and Processed Peppers. LWT-Food Sci. Technol. 2015, 64, 623–631. DOI: 10.1016/j.lwt.2015.06.042.
- Bogusz, S., Jr; Libardi, S. H.; Dias, F. F.; Coutinho, J. P.; Bochi, V. C.; Rodrigues, D.; Melo, A. M.; Godoy, H. T. Brazilian Capsicum Peppers: Capsaicinoid Content and Antioxidant Activity. J. Sci. Of Food Agr. 2018, 98, 217–224. DOI: 10.1002/jsfa.8459.
- Wangcharoen, W.; Morasuk, W. Antioxidant Capacity Changes of Bird Chili (Capsicum Frutescens Linn.) During Hot Air Drying. Kasetsart J. (Nat. Sci.). 2009, 43, 12–20.
- Zimmer, A. R.; Leonardi, B.; Miron, D.; Schapoval, E.; de Oliveira, J. R.; Gosmann, G. Antioxidant and Anti-inflammatory Properties of Capsicum Baccatum: From Traditional Use to Scientific Approach. J. Ethnopharmacol. 2012, 139, 228–233. DOI: 10.1016/j.jep.2011.11.005.
- Škrovánková, S.; Mlček, J.; Orsavová, J.; Juríková, T.; Dřímalová, P. Polyphenols Content and Antioxidant Activity of Paprika and Pepper Spices. Potravinarstvo Slovak J. Food Sci. 2017, 11, 52–57.
- Sun, T.; Xu, Z.; Wu, C. T.; Janes, M.; Prinyawiwatkul, W.; No, H. Antioxidant Activities of Different Colored Sweet Bell Peppers (Capsicum Annuum L.). J. Food Sci. 2007, 72, S98–S102. DOI: 10.1111/j.1750-3841.2006.00245.x.
- Bae, H.; Jayaprakasha, G.; Crosby, K.; Jifon, J. L.; Patil, B. S. Influence of Extraction Solvents on Antioxidant Activity and the Content of Bioactive Compounds in Non-pungent Peppers. Plant Food Hum. Nutr. 2012, 67, 120–128. DOI: 10.1007/s11130-012-0290-4.
- Jayaprakasha, G.; Murthy, K. C.; Etlinger, M.; Mantur, S. M.; Patil, B. S. Radical Scavenging Capacities and Inhibition of Human Prostate (Lncap) Cell Proliferation by Fortunella Margarita. Food Chem. 2012, 131, 184–191. DOI: 10.1016/j.foodchem.2011.08.058.
- Hossain, M. A.; Rahman, S. M. Total Phenolics, Flavonoids and Antioxidant Activity of Tropical Fruit Pineapple. Food Res. Int. 2011, 44, 672–676. DOI: 10.1016/j.foodres.2010.11.036.
- Dutta, S.; Singh, S.; Saha, S.; Akoijam, R.; Boopathi, T.; Banerjee, A.; Roy, S. Diversity in Bird’s Eye Chilli (Capsicum Frutescens L.) Landraces of North-east India in Terms of Antioxidant Activities. P. NatL. A. Sci. India B. 2017, 87, 1317–1326. DOI: 10.1007/s40011-016-0707-1.
- Roy, S. A.; Pal, T. K.; Bhattacharyya, S. Effect of Thermal Processing on in Vitro Antioxidant Potential of Capsicum (Capsicum Annuum) of Different Ripening Stages. J. Pharm. Res. 2014, 8, 1751–1756.
- Tan, Y.; Chang, S. K.; Zhang, Y. Comparison of α-amylase, α-glucosidase and Lipase Inhibitory Activity of the Phenolic Substances in Two Black Legumes of Different Genera. Food Chem. 2017, 214, 259–268. DOI: 10.1016/j.foodchem.2016.06.100.
- Sivasothy, Y.; Loo, K. Y.; Leong, K. H.; Litaudon, M.; Awang, K. A Potent Alpha-glucosidase Inhibitor from Myristica Cinnamomea King. Phytochemistry. 2016, 122, 265–269. DOI: 10.1016/j.phytochem.2015.12.007.
- Oboh, G.; Ademiluyi, A. O.; Faloye, Y. M. Effect of Combination on the Antioxidant and Inhibitory Properties of Tropical Pepper Varieties against α-amylase and α-glucosidase Activities in Vitro. J. Med. Food. 2011, 14, 1152–1158. DOI: 10.1089/jmf.2010.0194.
- Tolan, I.; Ragoobirsingh, D.; Morrison, E. Y. S. A. Isolation and Purification of the Hypoglycaemic Principle Present in Capsicum Frutescens. Phytother. Res. 2004, 18, 95–96. DOI: 10.1002/ptr.1328.