ABSTRACT
The effects of steam explosion (SE) treatment, acid hydrolysis (AH), and the combination of steam explosion and acid hydrolysis treatment (SE+AH) on structural and rheological properties of sago starch were investigated. Steam explosion (SE) is a hydrothermal pre-treatment in high-pressure which caused the expansion of water vapor and hydrolyzing the material into low molecular weight. It is a potential method for changing the structural and functional properties by hydrolyzing the starch with low cost and environmentally friendly. Sago starch and distilled water (1:12) were added into a cylinder of SE reactor at 140°C. The slurry was exploded when the temperature reached. And then sago starch was hydrolyzed in hydrochloric acid at 50°C, 60°C, 70°C for 1, 1.5, 2 h. The solubility, dextrose equivalent, hygroscopicity, viscosity, crystallinity, and rheological properties of acid-hydrolyzed sago starches (AH) were analyzed. Steam explosion (SE) treatment increased the solubility up to 40.1%; meanwhile, the combination of SE+AH increased the solubility up to 70.8%. While the viscosity decreased with increasing degree of acid hydrolysis (i.e. from 2155 cP to 27 cP). The results showed that combination SE+AH could be an effective method to produce maltodextrin. The combination of SE+AH at 70°C for 2 h has dextrose-equivalent value was increased up to 11.97 almost similar to the dextrose equivalent of maltodextrin. It changed the morphology and decreased the crystallinity up to 9.6%. Thus, SE+AH methods could be used to modify properties of starch for a specific product such as maltodextrin.
Introduction
Maltodextrin is one kind of polysaccharide that is produced by enzymatic or acid hydrolysis treatments which can hydrolyze the glycosidic bond of starch. Maltodextrin is characterized by dextrose equivalent (DE) value of less than 20, highly soluble in water, low molecular weight, and low viscosity.[,Citation1,Citation2] Sago starch (Metroxylon sago) is a source of starch with high productivity which is higher than rice and wheat and over 10 times higher than potato and cassava. The sago starch yield can reach up to 25 MT/ha and each sago palm tree contains up to 400 kg of dry starch.[Citation3] Sago starch has some similar physicochemical properties with common starches like potato and tapioca.[,Citation3,Citation4] Native sago starch still has some weaknesses, such as a low water absorption index, low solubility, semi-crystalline granules, high hygroscopicity properties, tendency for retrogradation, an opacity of cooked pasta, and high viscosity.[Citation5] Many attempts have been made to develop industrial-scale applications. Therefore, starch modification is needed to expand the functional properties of starch.[Citation6]
Starch modification can be done by chemical, physical, or enzymatic methods. The chemical method is the most common modification because it can be readily and easily controlled in comparison to enzymatic hydrolysis. Enzymatic hydrolysis has high yield but the reaction rate of enzyme hydrolysis is still low compared to acid hydrolysis.[Citation7]Chemical reagents are typical chemical modifications that have a wide range of applications in the starch industry.[Citation8] Acid hydrolysis is an inexpensive and effective method for increasing sago starch solubility.[Citation9] Acid hydrolysis of sago starch decreases the molecular weight of starch, especially amylopectin, increases water solubility, and decreases the viscosity of starch.[,Citation10,Citation11] Acid attacks of the hydronium ion on glycosidic oxygen atoms thus hydrolyze the glycosidic linkage. However, acid hydrolysis has weaknesses such as requiring a long time and high temperature of hydrolysis so it requires high energy and costs. Acid hydrolysis in sago starch needs 6 h for solubility 58,90%. While sorghum starch hydrolysis needs 1.5 h for solubility 31.8% and at 8 h has solubility 77%.[Citation4] Then, maize starch has a solubility of 40.7% at 75°C for 1 h acid hydrolysis, the cassava starch has solubility 58,9% at 1 h hydrolysis.[Citation12] Thus, starch modification using acid hydrolysis needs combination with other methods to increase solubility in water up to 99% and a short time. The acid hydrolyzed could be combined using physical methods.
One of the alternatives to physical modifications of starch could be done by a steam explosion.[Citation13] Hydrothermal steam explosion is a modification option that offers a potential method for changing the functional properties and hydrolyzing the glycosidic of the starch with low cost and environmental-friendly method.[Citation14] Steam explosion (SE) technology is a hydrothermal pretreatment in which the material exposed with high-pressure saturated steam, followed formation of organic acids during the process, and shearing forces resulting in the expansion of the moisture. Two stages compose the SE process: vapocracking and explosive decompression which include modification of the material components: hydrolysis of hemicellulosic structure to low molecular weight.[Citation15] The energy in the form of steam causes the expansion of water vapor and hydrolyzing of the glycosidic bonds.[Citation13] The occurrence of hydrolyzed glycosidic bonds conducts an increase in the solubility of starches. Application of SE in cellulose is a pre-treatment to make the cellulose disrupt the crystalline structure to be more accessible for enzymatic hydrolysis processes in bioethanol production.[Citation16] Modification of potato starch with a steam explosion decreased the molecular weight and crystallinity because the amylopectin degraded the 1,6-α-glycosidic bond thereby reducing the number of large molecules (amylopectin) and breaking the 1,4-α-glycosidic linkages in the long amylose chain to the shorter amylose chain in potato starches.[Citation13] The decrease in the molecular weight can increase the solubility of the starch. A pretreatment SE decreased the crystallinity of cellulose from 52.1% to 25.9%.[Citation17] The other previous study shows that dually modified sago starch using hydrolyzed-hydroxypropylated add with carrageenan can increase the solubility up to 100%.[Citation18] A specific study on the effect of acid hydrolysis and using steam explosion treatment on sago starch was very scarce. This study aimed to characterize steam explosion, acid-hydrolyzed, and the combination of steam explosion and acid hydrolysis to provide and find the effect of hydrolysis on the structural and rheological properties of sago starch to produce maltodextrin.
Material and methods
Materials
Sago starch (10 g water content per 100 g starch) was purchased from Selat Panjang (Riau, Indonesia). It was used without any further treatment. All chemicals were analytical of grade.
Treatment steam explosion sago starches
The native sago starch (NA) was treated with steam explosion (SE) treatment following the methods with modification.[Citation13,Citation18]Native sago starches and distilled water with ratio (1:12) were added into a cylinder of SE reactor. The slurry in SE reactor was treated at 140°C then the materials extruded from the cylindrical were collected and dried in a cabinet dryer. The starch was milled until it becomes a powder.
Acid hydrolysis of sago starch
AH of the sago starch sample was prepared based on methods described previously with modifications.[Citation19] The NA and SE treated sago starch was hydrolyzed using 0,1 M HCl solution with the ratio 30:100. The suspension was continuously stirred at different temperatures 50°C, 60°C, 70°C and different times at 1 h, 1.5 h, and 2 h. After the reaction, the suspension was neutralized, used 0.1 M NaOH, and washed three times. The final products were dried then saved and became AH, and SE+AH. Each sample was compared with maltodextrin (MA) as standard.
Solubility of starch granules
The solubility of starch analysis conducted by 0.5 g modified sago starch samples was dissolved into 50 ml aquadest and stirred at 4000 rpm for 2 min using a homogenizer (Ultraturax Basic IKA, Werke, Germany). The starch suspension was placed on centrifuge tubes, then centrifuged at 4000 rpm for 15 min. 25 ml supernatants were taken then placed in a weighing bottle and dried in the oven with a temperature of 105⁰C for 48 h and obtained dry weight.[Citation20]
Dextrose equivalent (DE) of starch
Dextrose-equivalent value was analyzed using Nelson Somogyi and Lane Eynon methods with modification.[Citation21] DE was calculated by reducing sugar content per dry weight of the sample in units of percent. Analysis of reducing sugar levels using the Nelson-Somogyi methods was carried out to make standard solutions. The solution was made using 10 mg glucose/100 ml diluted with concentration variation of 2; 4;6; 8; 10 mg/100 ml. Then 1 ml of solution was transferred to a reaction jar and each Nelson A: B tube (1:25) was added and warmed in boiling water for 20 min. After that, cooled and added 1 ml of arsenomolybdate then followed by shaking. 7 ml of distilled water was added and measured with a spectrophotometer at 540 nm. The determination of reducing sugar was carried out by 1 g sample diluted with distilled water and taking 1 ml of solution in a tube and carried out stages such as a standard solution.[Citation22]
Hygroscopicity
Hygroscopicity analysis using 1 g of sample that was put in an aluminum cup then dried with the oven over the past 24 h (as dry weight). Then the sample was conditioned at RH 96% saturated K2SO4 solution and weighed in every 1 h for 9 times. The samples were covered with gauze and stood in an ambient moist environment. Weight was performed daily until equilibrium reached; percent moisture reabsorption of the sample was calculated concerning its initial dried weight.[Citation23,Citation24]
Morphology and size of granule
The sago starch particles were attached to SEM stubs or cradle samples with a diameter of 10 mm using double-sided adhesive tape. Then the sample coated with gold and observed at magnification 1000 up to 10,000 times and voltage 20 kV.[Citation25]
FTIR spectroscopy
FTIR spectra of native and modified sago starch were recorded in KBr tablets on a Nicolet 6700 FTIR spectrometer (ThermoScientific, USA) within the spectral range of 4000–400 cm−1, the resolution 2 cm−1 and the number of scans being 64. Spectra were smoothed and the baselines were corrected using Omnic 8.0 software (ThermoScientific, USA) and exported as CSW files to Origin 6.0 software (OriginLab, USA) for grouping the graphic.[Citation17]
X-ray diffraction
The crystallinity of sago starch samples was studied using an X-ray diffractometer (Rigaku Miniflex 600, Kyoto, Japan) with a copper anode X-ray tube at 40 kV and 15 mA. The monochromatic radiation wavelength was 0.154 nm. The starch sample was packed into a 25 mm (diameter) × 1 mm (d) aluminum sample holder for analysis. The scanning region of the diffraction angle (2) was adjusted from 3º to 35º. The other operating conditions were as follows: scanning rate at of 2º/min, step interval 0.02º, divergence, and scatter slit 1º.[Citation26] Relative crystallinity (%) is defined as the peak area ratio of the crystalline component and was calculated using Rietveld analysis software developed by Shimadzu.
Pasting and thermal properties
The pasting and thermal properties of sago starch were determined by Rapid Visco Analyzer (Model RVA-4, Newport Scientific Pvt.Ltd., Warriewood NSW, Australia). In an RVA sample cup, 2.5 g of native starch or 3.5 g of hydrolyzed sago starch was added to 25 g distilled water. The slurry starch was analyzed using the procedure described by Huijbrechts et al.[Citation27]
Statistical analysis
The physicochemical analysis was conducted and used as a completely randomized design factorial. The comprehensive study of the effects and their interactions was carried out with the aid of statistical software SPSS 2023. Each experiment was performed in triplicate and data corresponds to mean values ± standard deviation of the series. An analysis of variances and the Tukey test was applied to a confidence level of 95% to determine statistically significant differences (p < .05) between samples.
Result and discussion
Solubility of sago starch
AH treatments, and SE+AH at 50°C; 60°C; 70°C for 1; 1.5; 2 h on sago starches are shown in . Modification using acid slightly increased the solubility of starch with the increasing temperature and duration of hydrolysis. The results were consistent with the previous study reported by Omojola, that the solubility increased with the increase of acid hydrolysis durations in cola starches.[Citation28] The solubility (0.5 g starch in 50 g water) increased from 1.8% for NA starches to 28.65% AH starches at 70°C for 2 h. The solubility increased slightly up to 70.80% and was shown in SE+AH treatment at 70°C for 2 h. The acid solution can attack the amorphous and crystalline regions of the starch granules to produce starch with low molecular weight and cause dissolve easily in water. The study from Aminian has the same results that using a higher temperature of modified sago starch with propylene oxide can increase the solubility of starch.[Citation29] Besides acid hydrolysis caused a shortening of the starch polymer chain as a result of weakening hydrogen bonds, therefore, it improved solubilization. The same results showed increasing of solubility because of increasing acid hydrolysis duration on waxy corn starch.[Citation30] The sample from SE+AH at 70°C for 2 h has solubility 70.80% which approaches the solubility of maltodextrin at 99.59%. The combination of SE+AH has a higher solubility than a single treatment of AH at 70°C for 2 h (28.65%) or single treatment SE (40.14%). The steam explosion process makes the stretching of granules at high temperature and pressure. It can easily break the glycosidic bonds of starches.[Citation31] The combination with acid hydrolysis completely reduces the molecular weight of starch and increased the percentage of solubility. The other previous study shows that dually modified sago starch using hydrolyzed-hydroxypropylated add with carrageenan can increase the solubility because of the reduction of the molecular weight of starch.[Citation32]
Table 1. Solubility of sago starch with acid hydrolysis, combination steam explosion and acid hydrolysis, native, steam explosion, and maltodextrin.
Dextrose equivalent (DE)
Degradation of starch glucoside bonds was characterized by a dextrose equivalent (DE) that there is calculated by the amount of starch which is hydrolyzed become as reducing sugar.[Citation24] Dextrose equivalent (DE) value analysis is shown in . Based on these results, hydrolysis time and temperature give effect to the DE value of starch modification. The length of time (1; 1.5; 2 h) and higher of temperature (50°C; 60°C; 70°C) were used for hydrolysis. In other words, the amount of reducing sugar was formed from hydrolyzed starch and can increase DE value. However, the duration of AH affected a longer contact acid with the suspension of starch. It can promote the acid breakdown of the polysaccharide bond into reducing sugars and increased the DE values. The acid can attack the C-O-C bond which causes increasing low molecular weight of sugar. The first corresponding degradation is amorphous regions of granules, and the second corresponding degradation is crystalline regions.[Citation33] The difference of acid hydrolysis rates of starch granules depends on the source of the starch such as potato and sweet potato starches. Both potatoes are the most resistant to the acid and have similar hydrolysis percentages during the treatment.[Citation34] Single modification uses steam explosion treatment which has DE value 6.17, while DE value of maltodextrin 12.02. The combination of SE+AH at 70°C for 2 h has DE value 11.97 higher than a single modification. Stretching of granules by treatment of SE at high temperature and pressure facilitated the process of acid hydrolysis to be more easily accessible to attack the glycosidic of starches.[Citation31] Thus, based on these results, DE values of SE+AH treatment are in accordance with the requirements of maltodextrin. The DE values of maltodextrin are less than 20 average degrees of polymerization (DP).[Citation35]
Table 2. Dextrose Equivalent of sago starch with acid hydrolysis, combination steam explosion and acid hydrolysis, native, steam explosion, and maltodextrin.
Hygroscopicity of sago starch
Hygroscopicity shows the ability of starch granules to absorb moisture from the environment. Water absorption is an important factor in a material that causes damage to powdered food products.[Citation24] shows the hygroscopicity of starch modification used single treatments of AH, SE+AH, NA, SE, and MA. The research showed that the hygroscopicity of AH decreased with higher temperature (50°C; 60°C; 70°C) and longer hydrolysis time (1; 1.5; 2 h). Decreasing of hygroscopicity up to 12.95% was due to single treatments of AH at 70°C for 2 h, while the SE+AH has hygroscopicity 12.38%. It indicates the decreasing ability of water absorption which caused by a reduction in free hydroxyl groups in amylose due to the reaction of hydroxyl groups with carboxyl. The reaction of both can form strong bonds and it has hydrophobic properties.[Citation36] The result of SE+AH at 70°C at 2 h is the same with the hygroscopicity of maltodextrin. It forms a strong cross-link bond between hydroxyl and carboxyl groups which makes the free hydroxyl group losing the affinity to bind the free water.[Citation36] The hygroscopicity of modified sago starch produced by SE+AH methods at 70ºC at 2 h had 12.38% and the closest value with the maltodextrin hygroscopicity value 12.43%.
Table 3. Hygroscopicity of sago starch with acid hydrolysis, combination steam explosion and acid hydrolysis, native, steam explosion, and maltodextrin.
Table 4. Pasting and thermal properties of sago starch with acid hydrolysis and a combination steam explosion and acid hydrolysis.
Morphology of starch granule
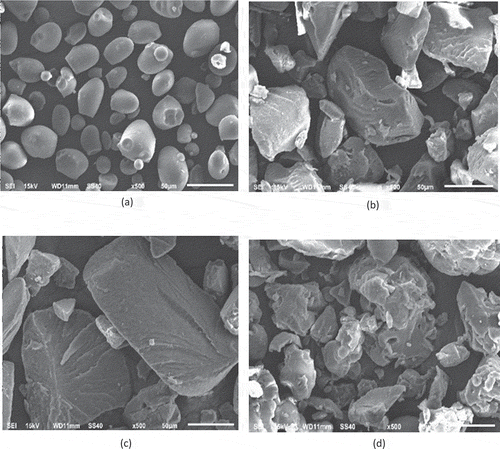
NA starch, AH starch, SE+AH were observed by SEM (.) The native starch (NA) granules were mainly oval and spherical in shape with a smooth surface and no cracks or cavities. The granules of single treatment SE starch have an uneven surface with some cracking and increased porosity. Whereas the surface of AH granules becomes more porous, rougher, and mostly eroded than native granules. However, the granule’s surfaces of single treatments of AH at 70°C for 2 h present irregularities such as cavities that were not found in the native starch (NA) and increased the porosity. Acid hydrolysis causes pitting or cracking, which indicates the formation of pores or channels.[Citation37] While increasing the rupture of granules is seen in starches with SE+AH at 70°C for 2 h. The steam explosion process at high temperature and pressure causes stretching and disintegrating the granules. Besides the presence of hydrolysis with acids causes starch granules to be more eroded, breaking the glycosidic bonds of starch. The complete destruction of the starch granule promoted the generating fragments of amylose and amylopectin.[Citation34] The average granule size of native sago starch is 1.233 µm, in which after acid hydrolysis reduced to 0.313 µm.
FTIR spectroscopy
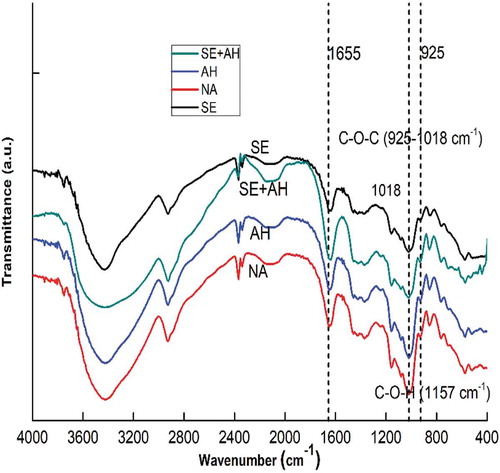
illustrates the spectrogram infrared for all samples of the sago starch. The detected peaks were the same for all samples; however, several slight differences in intensity were detected between the NA starch and the other sample modifications, as evidenced by the lower line in . Hydrolyzed samples showed sharper bands indicate that there is a possibility of alterations on amorphous and crystalline regions of the starch.[Citation38]
Starch natives have a major chemical group at the main cluster of C-H; C-O-C; C = C; O-H; C-O; C-O-C. The absorbance at 3300–2500 cm−1 indicates the O-H bond stretching, 2932; 927 and 859 cm−1 were assigned as C-H stretching and C-H bending, respectively, C = C showed at 1643 cm−1 and C-O stretching appeared at 1077 cm−1. The absorbance at 1635–1655 and 925–1018 cm−1 was assigned as C-O-C.[,39Citation40] Based on this result, the shifting vibration band happened in all sample treatments C-O-C bond from 995.27to 1018.41 in native. C = C bond in combination SE+AH has shifting vibration band of 2090,84 to 2098,55; C-H bond in all sample treatments has shifting vibration band 2931,8 to 2924,09. Treatment of single modification such as acid hydrolysis, and the combination of SE+AH causing loss of one peak in wavenumber band 995–1018 as C-O-C. The glycosidic linkages of starch related with C-O-C and C-O-H bond, it indicates that acid breakdown the glycosidic linkages on starch polymers and causes losses some of the C-O-C bond.
X-ray diffraction
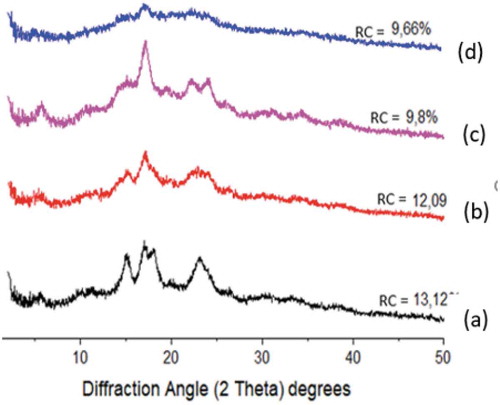
The degree of crystallinity of native starch granules ranges from 20%–35% and the average of the amorphous region is 70% classified into three types, namely, A; B; and C.[Citation26] The X-ray diffraction patterns of NA and differently treated (AH; SE; SE+AH) sago starch samples are shown in . Based on these results, NA starch granules have the highest Relative Crystallinity (RC) value of 13.12% compared to AH, SE, and the combination of SE+AH at 70°C for 2 h. Relative crystallinity was directly related to the amylopectin content and inversely proportional to the amylose content.[Citation41] Modification of starches using AH and SE decreased the relative crystallinity value from NA 13.12% to 12.09 and 9.8%. While the combination of SE+AH at 70°C for 2 h decreased up to 9.66%. Decreasing RC value of modified sago starch related to depolymerization of the molecular chain especially the amylopectin. It can cleavage of a-(1.4)-glycosidic linkages and hydrogen bonds. These results were the same as earlier observation by Chung.[Citation42] The decrease in relative crystallinity of HMT (Heatmoisture Treatment) starch samples indicated that the hydrogen bonds were destroyed as long temperature of HMT.
Pasting and thermal properties
The pasting properties of NA, AH, SE, and combination of SE+AH are shown in The results indicated a significant effect of the SE treatment, AH at 70°C for 2 h, and a combination of SE+AH on sago starch pasting properties. There was a significant decrease in pasting properties upon the increased duration of hydrolysis and in combination SE and AH. Pasting properties of starches depend on amylose leaching, starch crystallinity, amylose, and amylopectin chain lengths.[Citation41] As shown in , the peak viscosity of NA 3869 cP decreased drastically to 285 cP after the SE treatment, and there was a significantly decreased in acid hydrolysis and the combination of SE+AH at 70°C for 2 h.
This reduction of peak viscosity during SE treatment, AH, and combination SE+AH was likely due to extensive disruption of amorphous regions of starch granule and the conversion of amylose to low molecular weight chains.[Citation9] Acid hydrolysis greatly alters the pasting profile of starch granules, resulting in decreased peak, trough, final viscosities, breakdown, and setback.[Citation43] The decrease in the peak viscosity was indicated to the hydrolysis of amorphous regions and the production of low molecular weight dextrins.[Citation4,Citation44] Low molecular weight dextrins tend to dissolve rather than swell during heating in water, resulting in the low viscosity profiles of acid-hydrolyzed starches.[Citation37]
Based on this research, the peak viscosity of combination SE+AH at 70°C for 2 h was lower than other modifications and has almost similar to the viscosity of maltodextrin. Breakdown viscosity represents the vulnerability of cooked granules to disintegration.[Citation45] Final viscosity represents the ability of starch to form a gel after heating and cooling process or the resistance of the paste and how easily the structure granules break due to heating. Breakdown and final viscosity were drastically reduced by steam explosion treatment, acid hydrolysis process, and a combination of SE and AH at 70°C for 2 h. These results were the same as other research on corn, potato, and other hydrolyzed starches.[Citation30,Citation31] The pasting temperature of native starches was higher than other modifications likely pretreatment steam explosion, acid hydrolysis, and combination SE and AH. Those modifications are no peak observed and indicate that they do not form a paste or gel during heating. Decreasing of viscosity, breakdown viscosity, final viscosity, and the pasting temperature was not detected the same with the previous study on potato starch acid hydrolysis with HCL 5%.[Citation46]
Conclusion
Modification of starch using a single SE treatment or AH, and a combination of SE+AH at 70°C for 2 h which can improve the characteristics of sago starch significantly. The combination method using SE+AH at 70°C for 2 h affected most of the functional, structural, and rheological properties of modified sago starches compared to single treatments. It has higher solubility (70.80%), dextrose equivalent (11.97%), and lower viscosity than native starch. The results showed that the combination SE+AH can be an effective method to produce maltodextrin. The reason is that combination SE+AH at 70°C for 2 h can approach the characteristic of maltodextrins such as dextrose-equivalent value, solubility, and hygroscopicity. The combination of SE+AH treatment can decrease the crystallinity starch and degrade the starch granules. In the future research need to optimize the SE treatment in starch that can improve the characteristics results such as dextrose-equivalent value, solubility, and hygroscopicity.
Acknowledgments
The authors would like to thank the Directorate of Research of Universitas Gadjah Mada for supporting the grant through Final Research Project 2019.
References
- Sadeghi, A.; Shahidi, F.; Mortazavi, S.; Nassiri Mahallati, M. Evaluation of Different Parameters Effect on Maltodextrin Production By- Amylase Termamyl2-x. World Appl. Sci. J. 2008, 3(1), 34–39.
- Takeiti, C. Y.; Kieckbusch, T. G.; Collares-Queiroz, F. P. Morphological and Physicochemical Characterization of Commercial Maltodextrins with Different Degrees of Dextrose-Equivalent. Int. J. Food Prop. 2010, 13(2), 411–425. DOI: 10.1080/10942910802181024.
- Ehara, H.; Toyoda, Y.; Johnson, D. V. Sago Palm: Sago Palm: Multiple Contributions to Food Security and Sustainable Livelihoods; Springer Singapore, 2018.
- Singh, H.; Sodhi, N. S.; Singh, N. Structure and Functional Properties of Acid Thinned Sorghum Starch. Int. J. Food Prop. 2009, 12(4), 713–725. DOI: 10.1080/10942910801995614.
- Zainal Abiddin, N. F.; Yusoff, A.; Ahmad, N. Effect of Octenylsuccinylation on Physicochemical, Thermal, Morphological and Stability of Octenyl Succinic Anhydride (OSA) Modified Sago Starch. Food Hydrocoll. 2018, 75, 138–146. DOI: 10.1016/j.foodhyd.2017.09.003.
- Bai, Y.; Shi, Y. C. Structure and Preparation of Octenyl Succinic Esters of Granular Starch, Microporous Starch and Soluble Maltodextrin. Carbohydr. Polym. 2011, 83(2), 520–527. DOI: 10.1016/j.carbpol.2010.08.012.
- Azmi, A. S.; Malek, M. I. A.; Puad, N. I. M. A Review on Acid and Enzymatic Hydrolyses of Sago Starch. Int. Food Res. J. 2017, 24(December), 265–273.
- Nishinari, K. Polysaccharide Rheology and In-mouth Perception. In Food Polysaccharides and Their Applications, 2nd ed.; Stephen, A. M., Phillips, G.O., Williams, P. A. Eds. CRC Press: Boca Raton, 2016.
- Mohammadi Nafchi, A.; Robal, M.; Cheng, L. H.; Tajul, A. Y.; Karim, A. A. Physicochemical, Thermal, and Rheological Properties of Acid-Hydrolyzed Sago (Metroxylon Sagu) Starch. LWT - Food Sci. Technol. 2012, 46(1), 135–141. DOI: 10.1016/j.lwt.2011.10.015.
- Fouladi, E.; Mohammadi, N. A. Effects of Acid-hydrolysis and Hydroxypropylation on Functional Properties of Sago Starch. Int. J. Biol. Macromol. 2014, 68, 251–257. DOI: 10.1016/j.ijbiomac.2014.05.013.
- Luchese, C. L.; Frick, J. M.; Patzer, V. L.; Spada, J. C.; Tessaro, I. C. Synthesis and Characterization of Biofilms Using Native and Modified Pinhão Starch. Food Hydrocoll. 2015, 45, 203–210. DOI: 10.1016/j.foodhyd.2014.11.015.
- Ferrini, L. M. K.; Rocha, T. S.; Demiate, I. M.; Franco, C. M. L. Effect of Acid-Methanol Treatment on the Physicochemical and Structural Characteristics of Cassava and Maize Starches. Starch/Staerke. 2008, 60(8), 417–425. DOI: 10.1002/star.200700712.
- Li, G.; Chen, M.; Li, F.; Zeng, J.; Sun, J. Effect of Steam Explosion Pre-treatment on Molecular Structure of Sweet Potato Starch. Trop. J. Pharm. Res. 2017, 16(5), 1113–1119. DOI: 10.4314/tjpr.v16i5.20.
- Kurosumi, A.; Sasaki, C.; Kumada, K.; Kobayashi, F.; Mtui, G.; Nakamura, Y. Novel Extraction Method of Antioxidant Compounds from Sasa Palmata (Bean) Nakai Using Steam Explosion. Process Biochem. 2007, 42(10), 1449–1453. DOI: 10.1016/j.procbio.2007.06.007.
- Jiang, S. T.; Guo, N. The Steam Explosion Pretreatment and Enzymatic Hydrolysis of Wheat Bran. Energy Sour Part A Recov. Util. Environ. Eff. 2016, 38(2), 295–299. DOI: 10.1080/15567036.2012.744118.
- Xu, W.; Ke, G.; Huang, J.; Wu, J. Xungai 2006-09, Modification of Wool Fiber Using Steam Explosion, European Polymer Journal. 2006, 42(9), 2168–2173.
- Xiao, Z.; Xu, Z. A.; Zhu, G. Production and Characterization of Nanocapsules Encapsulated Linalool by Ionic Gelation Method Using Chitosan as Wall Material. Food Sci. Technol. 2017, 37(4), 613–619. DOI: 10.1590/1678-457x.27616.
- Suharjono, I. K. Cassava Starch Modification with Steam Explosion and Oxidation of H2O2 and Its Application as Encapsulant for Nanoencapsulation Cacao Leaves Crude Extract; M.Sc. Thesis of Faculty of Agricultural Technology, Universitas Gadjah Mada, 2018.
- Falade, K. O.; Ayetigbo, O. E. Effects of Annealing, Acid Hydrolysis and Citric Acid Modifications on Physical and Functional Properties of Starches from Four Yam (Dioscorea Spp.) Cultivars. Food Hydrocoll. 2015, 43, 529–539. DOI: 10.1016/j.foodhyd.2014.07.008.
- Cano-Chauca, M.; Stringheta, P. C.; Ramos, A. M.; Cal-Vidal, J. Effect of the Carriers on the Microstructure of Mango Powder Obtained by Spray Drying and Its Functional Characterization. Innov. Food Sci. Emerg. Technol. 2005, 6(4), 420–428. DOI: 10.1016/j.ifset.2005.05.003.
- Lane, L.; Eynon, J. H. Determination of Reducing Sugars by Means of Fehling’s Solution with Methylene Blue as Internal Indicator. J. Soc. Chem. Ind. Trans. 1923, 42, 32–36.
- Loksuwan, J. Characteristics of Microencapsulated β-carotene Formed by Spray Drying with Modified Tapioca Starch, Native Tapioca Starch and Maltodextrin. Food Hydrocoll. 2007, 21(5–6), 928–935. DOI: 10.1016/j.foodhyd.2006.10.011.
- Chandumpai, A.; Singhpibulporn, N.; Faroongsarng, D.; Sornprasit, P. Preparation and Physico-chemical Characterization of Chitin and Chitosan from the Pens of the Squid Species, Loligo Lessoniana and Loligo Formosana. Carbohydr. Polym. 2004, 58,(4), 467–474. DOI: 10.1016/j.carbpol.2004.08.015.
- Zheng, M.; Jin, Z.; Zhang, Y. Effect of Cross-linking and Esterification on Hygroscopicity and Surface Activity of Cassava Maltodextrins. Food Chem. 2007, 103(4), 1375–1379. DOI: 10.1016/j.foodchem.2006.10.053.
- Caliskan, G.; Dirim, S. N. The Effect of Different Drying Processes and the Amounts of Maltodextrin Addition on the Powder Properties of Sumac Extract Powders. Powder Technol. 2016, 287, 308–314. DOI: 10.1016/j.powtec.2015.10.019.
- Cheetham, N. W. H.; Tao, L. Variation in Crystalline Type with Amylose Content in Maize Starch Granules: An X-ray Powder Diffraction Study. Carbohydr. Polym. 1998, 36(4), 277–284. DOI: 10.1016/S0144-8617(98)00007-1.
- Huijbrechts, A. M. L.; Desse, M.; Budtova, T.; Franssen, M. C. R.; Visser, G. M.; Boeriu, C. G.; Sudhölter, E. J. R. Physicochemical Properties of Etherified Maize Starches. Carbohydr. Polym. 2008, 74(2), 170–184.
- Omojola, A.; Olufunsho, E. Isolation and Physico-chemical Characterization of Cola Starch. Afr. J. Food Agric. Nutrition Dev. 2010, 53(9), 1689–1699.
- Aminian, M.; Nafchi, A. M.; Bolandi, M.; Alias, A. K. Preparation and Characterization of High Degree Substituted Sago (Metroxylon Sagu) Starch with Propylene Oxide. Starch. 2013, 65(7–8), 686–693. DOI: 10.1002/star.201200137.
- Singh Sandhu, K.; Singh, N.; Lim, S. T. A Comparison of Native and Acid Thinned Normal and Waxy Corn Starches: Physicochemical, Thermal, Morphological and Pasting Properties. LWT - Food Sci. Technol. 2007, 40(9), 1527–1536. DOI: 10.1016/j.lwt.2006.12.012.
- Xing, J.; Liu, Y.; Li, D.; Wang, L.; Adhikari, B. Heat-Moisture Treatment and Acid Hydrolysis of Corn Starch in Different Sequences. LWT - Food Sci. Technol. 2017, 79, 11–20. DOI: 10.1016/j.lwt.2016.12.055.
- Oladzadabbasabadi, N.; Ebadi, S.; Mohammadi Nafchi, A.; Karim, A. A.; Kiahosseini, S. R. Functional Properties of Dually Modified Sago starch/κ-carrageenan Films: An Alternative to Gelatin in Pharmaceutical Capsules. Carbohydr. Polym. 2017, 160, 43–51. DOI: 10.1016/j.carbpol.2016.12.042.
- Srichuwong, S.; Isono, N.; Mishima, T.; Hisamatsu, M. Structure of Lintnerized Starch Is Related to X-ray Diffraction Pattern and Susceptibility to Acid and Enzyme Hydrolysis of Starch Granules. Int. J. Biol. Macromol. 2005, 37(3), 115–121. DOI: 10.1016/j.ijbiomac.2005.09.006.
- Campanha, R. B.; Franco, C. M. L. Gelatinization Properties of Native Starches and Their Näegeli Dextrins. J. Therm. Anal. Calorim. 2011, 106(3), 799–804. DOI: 10.1007/s10973-011-1682-7.
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; McClements, D. J. Stability and Rheology of Corn Oil-in-Water Emulsions Containing Maltodextrin. Food Res. Int. 2004, 37(9), 851–859. DOI: 10.1016/j.foodres.2004.05.001.
- Thiebaud, S.; Aburto, J.; Alric, I.; Borredon, E.; Bikiaris, D.; Prinos, J.; Panayiotou, C. Properties of Fatty‐Acid Esters of Starch and Their Blends with LDPE. J. Appl. Polym. Sci. 1997, 65(4), 705–721. DOI: 10.1002/(SICI)1097-4628(19970725)65:4<705::AID-APP9>3.0.CO;2-O.
- Wang, S.; Copeland,; Copeland, L. New Insights into Loss of Swelling Power and Pasting Profiles of Acid Hydrolyzed Starch Granules. Starch/Staerke. 2012, 64(7), 538–544. DOI: 10.1002/star.201100186.
- Das, R.; Kayastha, A. M. Enzymatic Hydrolysis of Native Granular Starches by A New β-amylase from Peanut (Arachis Hypogaea). Food Chem. 2019, 276, 583–590. DOI: 10.1016/j.foodchem.2018.10.058.
- Colussi, R.; Pinto, V. Z.; El Halal, S. L. M.; Vanier, N. L.; Villanova, F. A.; Marques E Silva, R.; da Rosa Zavareze, E.; Dias, A. R. G. Structural, Morphological, and Physicochemical Properties of Acetylated High-, Medium-, and Low-Amylose Rice Starches. Carbohydr. Polym. 2014, 103(1), 405–413. DOI: 10.1016/j.carbpol.2013.12.070.
- Yang, X.; Wang, -Y.-Y.; Li, -K.-K.; Li, J.; Li, C.-R.; Shi, X.-G.; Ko, C.-H.; Leung, P.-C.; Ye, C.-X.; Song, X.-H. Cocoa Tea (Camellia Ptilophylla Chang), a Natural Decaffeinated Species of Tea – Recommendations on the Proper Way of Preparation for Consumption. Journal of Functional Foods. 2011, 3(4), 305–312. DOI: 10.1016/j.jff.2011.06.001.
- Gunaratne, A. Effect of Heat–moisture Treatment on the Structure and Physicochemical Properties of Tuber and Root Starches. Carbohydr. Polym. 2002, 49(4), 425–437. DOI: 10.1016/S0144-8617(01)00354-X.
- Chung, H.-J.; Liu, Q.; Hoover, R. Effect of Single and Dual Hydrothermal Treatments on the Crystalline Structure, Thermal Properties, and Nutritional Fractions of Pea, Lentil, and Navy Bean Starches. Food Res. Int. 2010, 43(2), 501–508. DOI: 10.1016/j.foodres.2009.07.030.
- Amaya-Llano, S. L.; Martínez-Bustos, F.; Alegría, A. L. M.; de Jesús Zazueta-morales, J. Comparative Studies on Some Physico-chemical, Thermal, Morphological, and Pasting Properties of Acid-thinned Jicama and Maize Starches. Food Bioprocess Technol. 2011, 4(1), 48–60. DOI: 10.1007/s11947-008-0153-z.
- Polesi, L. F.; Sarmento, S. B. Structural and Physicochemical Characterization of RS Prepared Using Hydrolysis and Heat Treatments of Chickpea Starch. Starch/Staerke. 2011, 63(4), 226–235. DOI: 10.1002/star.201000114.
- Mohd Hanim, A. B.; Chin, N. L.; Yusof, Y. A. Physico-chemical and Flowability Characteristics of a New Variety of Malaysian Sweet Potato, VitA to Flour. Int. Food Res. J. 2014, 21(5), 2099–2107.
- Babu, A. S.; Parimalavalli, R.; Jagannadham, K.; Rao, J. S. Chemical and Structural Properties of Sweet Potato Starch Treated with Organic and Inorganic Acid. J. Food Sci. Technol. 2015, 52(9), 5745–5753. DOI: 10.1007/s13197-014-1650-x.