?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Mentha longifolia
. is a perennial herb of Mentha in Labiatae. Considering the biological activities of Mentha longifolia essential oils, this work was planned to study the yields, relative percentage content and semi-quantitative change of chemical constituents as well as the biological activities of essential oils extracted by different extraction methods. Steam-distillation (SD), lipophilic solvents (n-hexane) extraction (LSE) and supercritical CO2 fluid extraction (SC-CO2) were employed to produce essential oils from Mentha longifolia. The essential oil obtained from LSE (1.21 ± 0.06%, w/w) showed the highest yield. A total of 39 compounds were identified by gas chromatography/flame ionization detector (GC-FID) and gas chromatography/mass spectrometry (GC-MS). The major compounds in SD and LSE were carvone, limonene, trans-caryophyllene and α-Terpineol, the major compounds in SC-CO2 were carvone, trans-caryophyllene, trans-β-Farnesene and Germacrene D. All essential oils showed varying degrees of antioxidant and anti-COX-2 activity. The IC50 ranging from (0.69 ± 0.01 ~ 15.61 ± 0.16 mg/mL) for DPPH and (0.16 ± 0.001 ~ 2.19 ± 0.11 mg/mL) for ABTS, SC-CO2 produced essential oil showed the highest scavenging activity both on DPPH and ABTS. Meanwhile, all the EOs showed the strong inhibition activity on COX-2, the EO obtained by SC-CO2 also showed the highest capacity to inhibit COX-2.
Introduction
Mentha longifolia is a perennial herb of Mentha in Labiatae. The tender leaves are not only common dishes, but also are an important flavoring. Mentha longifolia also is a commonly medicinal plant source. Mentha longifolia has been used in traditional medicine practices for the treatment of bronchitis, headache, cough, nausea, asthma, liver diseases, digestive disorders, stomach, abdominal disorders, etc.[1,Citation1, Citation2] Essential oil (EO) of Longifolia possesses a plethora of biological activities such as antispasmodic, anticancerous, antimicrobial, antioxidative, insect repellent, and neuroprotective effects.[Citation3,Citation4]
EO can be obtained from plants by many methods, including steam-distillation (SD), lipophilic solvents extraction (LSE) and supercritical CO2 fluid extraction (SC-CO2).[Citation5] Extraction methods have obvious influence on quality and exact chemical compositions of EO. Inflammation is one of the most common autoimmune diseases in our life. It is a complex natural defense mechanism of organism to infection or tissue damage.[Citation6] Free radicals and COX-2 are two important factors in the process of inflammation. Free radicals are the most common inflammatory mediators.[Citation7] When inflammation occurs, COX-2 is highly expressed in the body, which plays a key role in the induction and maintenance of inflammatory pain.[Citation8] It is well known that nonsteroidal anti-inflammatory drugs (NSAIDs) have good effects on treating inflammatory, but it also induces serious gastrointestinal reactions. Therefore, natural anti-inflammatory products are attracting more attention. EO is naturally synthesized in aromatic plants as secondary metabolites, having a wide array of bioactivities with minimal or zero toxicity to the mammals.[Citation5,Citation9] To the best of our knowledge, application of SC-CO2 to obtain essential oil from M. longifolia has not been previously reported. There is also negligible report available to compare the chemical constituents by semi-quantitative and biological activities, including the anti-COX-2 and antioxidant properties of essential oils which are obtained from different extraction methods. In this work, we investigated the yields, chemical constituents, and biological activities of EO extracted from M. longifolia by different methods, including, SD, LSE and SC-CO2. The EOs were analyzed by gas chromatography/flame ionization detector (GC-FID, equipped with polar and apolar capillary column) and gas chromatography/mass spectrometry (GC-MS). In order to identify the most effective extraction method, we investigated 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH), 2,2ʹ-Azinobis-(3-ethylbenzthiazoline-6-sulfonate) (ABTS) assays for antioxidant activities and inhibition effect of EO on COX-2. Moreover, the results of the present study might be of help to finding high-quality essential oil with anti-inflammatory activity.
Materials and methods
Plant material and reagents
The aerial part of fresh plant materials were collected at the countryside of Kuche, in the province of Xinjiang, China in June 2019. The voucher of the species used in this study was deposited in the Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, with specimen number No. WY02663. The picked fresh M. longifolia was spread in the room without direct sunlight (25 ± 6°C) and natural ventilation. Drying process was continued for 14 days until constant weight and mechanically ground to a homogenous powder on a laboratory grinder.
2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH, purity≥98%), 2,2ʹ-Azinobis-(3-ethylbenzthiazoline-6-sulfonate) (ABTS, purity≥98%), tocopherol (purity≥98%), methyl octanoate (purity≥98%) and PGE2 (Prostaglandin E2) ELISA Kit (48 T). All other chemicals and solvents (n-hexane, anhydrous alcohol) used in the present study were analytical grade.
Extraction of essential oil
Steam-distillation
The 100 g of plant samples were extracted in a Clevenger apparatus. Fresh distilled water was used as extraction solvent and the ratio of material to liquid was 1:10. After four hours, the EOs were collected and dried by sodium sulfate anhydrous and then stored at 4°C until analysis.[Citation10] The extraction was performed three times.
Supercritical fluid extraction
SC-CO2 extraction was performed on a 1 L-SFE221-50-06 Supercritical CO2 Extraction System. The 50 g of plant materials were added to high present extraction kettle. The liquefied CO2 was introduced into the sample cartridge through a pump with a cooling jacket. The flow rate of CO2 was 5 L/min; the extraction pressure was 20 MPa, the static extraction time were 30 minutes and the dynamic extraction time were 120 minutes, respectively.[Citation11] Extracts were finally separated from the CO2 phase and collected in collection bottle at ambient temperature and atmospheric pressure. The extraction was performed three times.
Lipophilic solvent extract
The 50 g of plant materials were soaked in 250 mL of n-hexane for 24 hours at room temperature (RT, 25 ± 6°C), the operation repeated for 5 times. Then, the supernatants were mixed, filtered, and evaporated n-hexane under reduced pressure.[Citation12] The extraction was performed three times and the yield of EO was calculated according to formula 1[Citation13] as follows:
The yield of essential oil (%)
where M1 is the weight of collected essential oil (g) and M2 is the total weight of the plant material (g).
Chemical compositions analysis of EO by GC-FID
Analysis was carried out with an Agilent Technologies 7890B GC system equipped with flame ionization detectors. EO was analyzed by HP-5 (5%-phenyl)-methylpolysiloxane) apolar capillary column and HP-INNOWax (polyethylene glycol) polar capillary column. The oven temperature was started at 60°C for 5 min and raised to 240°C at rate of 4°C/min. The temperature of the injector and detector was set at 250°C. The carrier gas was nitrogen with a flow of 1.5 mL min−1. A sample of 0.3 μL was injected (split ratio 30:1). The polarity retention index (RIp) and apolar retention index (RIa) of each compound were calculated by using a series of n-alkanes (C7–C40). Methyl octanoate was used as an internal reference; the correction factor (CF) of each compound was calculated according to the calculation method described in literature.[Citation13] The result is shown in .
Table 1. Volatile compounds identified in extracts of Mentha longifolia prepared by different extraction methods
Chemical compositions analysis of EO by GC-Q-TOF-MS
The essential oil was analyzed with an Agilent Technologies 7890B GC system equipped with Quadrupole-Time of Flight Mass Spectrometer (Q-TOF-MS) and equipped with fused-silica capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm), HP-5 MS (5%-phenyl)-methylpolysiloxane). Injector temperature was set at 250°C. The carrier gas was nitrogen with a flow of 1 mL min−1. A sample of 0.3 μL was injected (split ratio 30:1). The oven temperature was started at 60°C for 5 min and then increased to 240°C at rate of 4°C/min. For GC-Q-TOF-MS detection, an electron impact ionization mode, with ionization energy of 70 eV was used. Mass range was 50 ~ 500 m/z while the injector and MS transfer line temperatures were set at 250 and 150°C, respectively.[Citation14] The components in essential oil were identified by comparing their retention index (RI) and mass spectra with NIST 14 libraries.
Antioxidant activity
DPPH radical-scavenging assay
The antioxidant ability of EO against DPPH radical scavenging was based on Reis et al.[Citation15] with slightly modified. Briefly, the EO sample (100 μL) at different concentrations was mixed with 100 μL of 0.2 mmol/L DPPH solution. The absorbance was measured at 517 nm by microplate reader (bio-red 550) after the reaction was incubated in the dark at room temperature for 30 minutes. The blank control was the EO solution of corresponding concentration.
ABTS radical-scavenging assay
The ABTS radical scavenging assay was measured based on R. Re et al.[Citation16] The ABTS stock solution was produced by mixed 7 mM ABTS solution with 2.45 mM potassium persulfate solution, and kept in the dark at room temperature for 16 h before used. Then, the ABTS stock solution was diluted with absolute alcohol to an absorbance of approximately 0.70 at 734 nm. The EOs (100 μL) at different concentrations was mixed with ABTS (100 µL) solution. The absorbance was measured at 734 nm by microplate reader after the reaction was incubated in the dark at room temperature for 5 minutes. The blank control was the EO solution of corresponding concentration. The scavenging ability of EO to radical was calculated according to formula 2[Citation17]:
Scavenging rate (%)
Ad is the absorbance of free radical solution does not contain sample, Ae is the absorbance of reaction solution and Abg is the background color of EO.
COX-2 inhibition assay
Enzyme immunoassay (EIA) was used to determine the inhibitory activity of sample to COX-2.[Citation18] The determination process can be divided into the following steps: First, prepare reaction solutions with various concentrations according to the instructions of the kit (Multi Sciences (Lianke) Biotechnology Corporate Limited), prepare positive control solutions (celecoxib:1 μmoL/mL) and EO sample solutions (100 μg/mL). Secondly, sample solutions (EO and celecoxib) react with COX-2. Then, the substrate arachidonic acid (AA) was added, and AA was catalyzed to prostaglandin (PG) by COX-2. Then, the enzyme-labeled pore was stained with3,3ʹ,5,5ʹ-tetramethylbenzidine (TMB). The optical density (OD) was measured at 450 nm (absorption wavelength) and 570 nm (reference wavelength) by microplate reader. The inhibition ability of EO to COX-2 was calculated according to formula 3.[Citation19]
Inhibition rate (%)
Ae is the absorbance of the EOs, Aec is the absorbance of the EOs control group, Ac is the absorbance of the enzyme activity group, Acc is the absorbance of the enzyme activity control group.
Statistical analysis
All assay was performed with three repetitions in independent experiments. The statistical analysis was performed by the one-way analysis of variance (ANOVA), and p < .05 or p < .01 of the difference means was considered to be significant.
Results and discussion
Yield of essential oil extracted by different methods
An ANOVA statistical analysis was used to analyze the significant difference among the yield of EO extracted by different methods (p < .05). The highest yield of EO was obtained by LSE (1.21 ± 0.06%, w/w) method, followed by SC-CO2 (1.09 ± 0.08%, w/w) method and the lowest yield was SD (0.82 ± 0.16%, w/w). The result was shown in . This is consistent with a previous report that LSE was the optimal process for obtaining a high yield of EOs from lavender.[Citation20]
Figure 1. Yield of essential oil extract by different methods, SD was the essential oil obtained by steam-distillation, SC-CO2 was supercritical CO2 fluid extraction and LSE was organic solvent extract
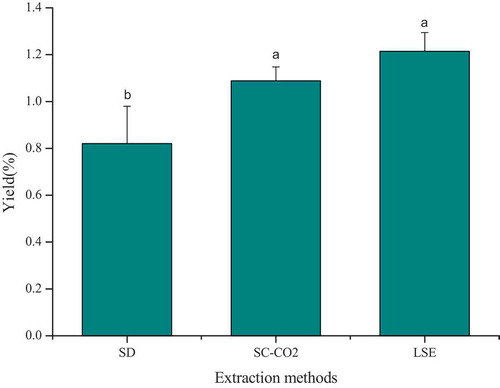
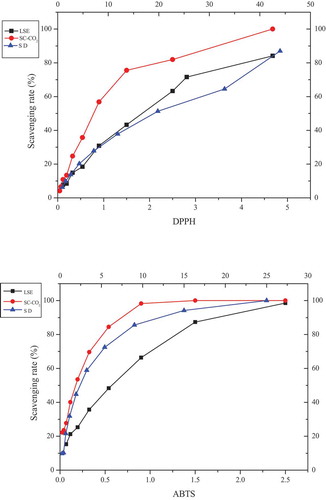
Figure 2. Scavenging ability of EOs against DPPH (upper panel) and ABTS (lower panel) free radicals, SD corresponds to the upper X-axis, LSE and SC-CO2 corresponds to the lower X-axis, the value of each point is represented in terms of mean (n = 3) ± SD (Standard deviation)
Supercritical fluids generally display physicochemical properties between of a liquid and gas, typically characterized by low viscosity, high density, and diffusivity, its viscosity is smaller than the liquid and diffusion speed faster than the liquid, therefore, supercritical fluids have better fluidity and transmission performance,[Citation21] Theoretically, based on the characteristics of supercritical fluid, the yield of EO produced by SC-CO2 might was higher than LSE, but the result showed that the yield of LSE was slightly higher than SC-CO2, it may due to LSE extract contain more plant pigments.
Identification of the chemical compounds from M. longifolia essential oil
Peak area normalizing method and internal standard method were used to determine the relative contents and semi-qualification of the compounds in the EO ( and ). A total of 39 compounds were identified by GC/MS and GC/FID, including eight aldehydes and ketones, 18 monoterpenes and sesquiterpenes, nine alcohols, two esters and two others compounds. Peak area normalizing method showed that terpenes (38.22 ± 1.53%-13.25 ± 0.87%) compounds and aldehydes and ketones (54.44 ± 1.62%-36.73 ± 1.44%) compounds made the most important contribution for essential oil of M. longifolia. An ANOVA statistical analysis showed that the contents of major compounds in three EO had significant difference (p < .01). The main components in SD and LSE were carvone (52.81 ± 0.46% and 47.52 ± 1.37%), limonene (30.10 ± 0.37% and 14.66 ± 0.81%), respectively, trans-caryophyllene (2.59 ± 0.11% and 2.70 ± 0.69%) and α-terpineol (1.34 ± 0.09%-1.47 ± 0.13%), the major compounds in the EO produced by SC-CO2 were carvone (33.07 ± 1.54%), trans-caryophyllene (4.87 ± 0.29%), trans-β-farnesene (2.26 ± 0.15%) and germacrene D (2.09 ± 0.16%) respectively.
Table 2. Numbers of the classes of compounds in extracts obtained by different extraction methods
Table 3. Antioxidant activities of essential oils extracted by different methods
The identified 39 compounds in the EO accounted for 91.54 ± 0.72 g/100 g by SD, 21.41 ± 1.35 g/100 g by LSE and only 7.15 ± 0.61 g/100 g by SC-CO2. The compositions of EO extracted by SD were mainly made up of volatile components, and the compositions of EO extracted by LSE and SC-CO2 were mainly formed by high boiling point and nonvolatile components. The content of limonene in the EO was 23.14 ± 0.19 (g/100 g) extracted by SD, 4.23 ± 0.34 (g/100 g) in LSE and only 0.03 ± 0.004 (g/100 g) in SC-CO2. In addition, α-pinene, β-thujene, sabinene, β-myrcene, eucalyptol, and (Z)-ocimene were not be detected in the essential oil by SC-CO2. It might be due to the lower boiling point of those compounds, and those compounds volatilized during the continuous purging of the collection bottle by carbon dioxide fluid.
Antioxidant activity
A large number of free radicals lead to disease deterioration, inflammation, radiation damage and ischemia in the body.[Citation22] Other studies had confirmed that free radicals can promote the progression and persistence of inflammatory reaction, which may be related to the lipid peroxidation induced by free radicals in vivo.[Citation23] Therefore, it is necessary to evaluate the ability of EO to scavenge the free radicals.
Two groups of experiments (DPPH and ABTS) were conducted to evaluate the antioxidant activity of EO ( and ), with tocopherol as a positive control. IC50 values (mg/mL) calculated from log dose inhibition curves were expressed as means ± standard deviation (SD) of triplicate experiments. Higher DPPH radical scavenging activities were associated with lower IC50 values. An ANOVA was used to analyze the significant (p < .01) variation in the radical scavenging activity of essential oil and different extracts. Extracting methods had an obvious effect on the antioxidant activity of essential oil. The essential oil extracted by SC-CO2 presented the highest radical scavenging activity both on DPPH (0.69 ± 0.014 mg/mL) and ABTS (0.156 ± 0.001 mg/mL), and the IC50 value of SC-CO2 on DPPH was better than positive control, but the IC50 value on ABTS was weaker than positive control. Followed by the EO extracted by n-hexane (DPPH: 1.58 ± 0.03 mg/mL, ABTS: 0.42 ± 0.006 mg/mL), and the lowest was SD (DPPH: 15.61 mg/mL, ABTS: 2.19 ± 0.11).
More variety of compounds were contained in the EO extracted by SC-CO2 method, because of the enhanced transport properties, low viscosity and moderately high diffusivity[Citation21] the supercritical CO2 fluids possessed. According to reports, the antioxidant activities of essential oils may act synergistically and the activity may be higher than a single compound.[Citation24]
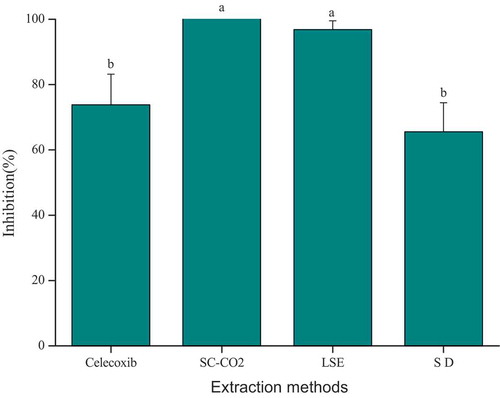
COX-2 inhibitory activity
The essential oil extracted by different methods were also evaluated for inhibitory effect against COX-2 activity with celecoxib (1 μM/mL) was used as positive control. An ANOVA was used to analyze the significant (p < .05) variation in the COX-2 inhibitory activity of essential oil (). SC-CO2 (100%) showed the highest inhibition rate on COX-2, followed by LSE (96.85 ± 2.68%) and the lowest was SD (65.55 ± 8.87%). The inhibitory effects of EO obtained by SC-CO2 and LSE on COX-2 had a significant difference from positive control and EO obtained by SD (p < .05), the result showed that extraction methods has a great effect on the properties of essential oil.
Conclusion
In this study, various extraction methods were used to analyze the essential oil obtained from M. longifolia. The highest yield of the EO was obtained by LSE method. The SD produced EO possessed the most principal component (carvone and limonene). The most bioactive essential oil was extracted by SC-CO2 method. These results indicated that the extraction methods had significant impact on the yield, chemical composition and bioactivities of EOs. Therefore, the type of extraction method could be chosen according to the purpose of the use of essential oil.
Compliance with ethical standards
The authors declare that there is no conflict of interest in this study.
Additional information
Funding
References
- Mikaili, P.; Mojaverrostami, S.; Moloudizargari, M.; Aghajanshakeri, S. Pharmacological and Therapeutic Effects of Mentha Longifolia L. And Its Main Constituent, Menthol. Ancient Sci. Life. 2013, 33(2), 131–138. DOI: 10.4103/0257-7941.139059.
- Brahmi, F.; Khodir, M.; Mohamed, C.; Pierre, D. Chemical Composition and Biological Activities of Mentha Species. In Aromatic and Medicinal Plants Back to Nature, 3rd ed, London: In Tech. 2017; pp 48–50.
- Hussain, A. I.; Anwar, F.; Nigam, P. S.; Ashraf, M.; Gilani, A. H. Seasonal Variation in Content, Chemical Composition and Antimicrobial and Cytotoxic Activities of Essential Oils from Four Mentha Species. J. Sci. Food Agric. 2010, 90(11), 1827–1836. DOI: 10.1002/jsfa.4021.
- Zouari-Bouassida, K.; Trigui, M.; Makni, S.; Jlaiel, L.; Tounsi, S. Seasonal Variation in Essential Oils Composition and the Biological and Pharmaceutical Protective Effects of Mentha Longifolia Leaves Grown in Tunisia. Biomed Res. Int. 2018, 7856517. DOI: 10.1155/2018/7856517.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils-a Review. Food Chem. Toxicol. 2008, 46(2), 446–475. DOI: 10.1016/j.fct.2007.09.106.
- Seth, K.; Garg, S. K.; Kumar, R.; Purohit, P.; Meena, V. S.; Goyal, R.; Banerjee, U. C.; Chakraborti, A. K. 2-(2-Arylphenyl) Benzoxazole as a Novel Anti-inflammatory Scaffold: Synthesis and Biological Evaluation. ACS Med. Chem. Lett. 2014, 5(5), 512–516. DOI: 10.1021/ml400500e.
- Barnes, P. J.; Chung, K. F.; Page, C. P. Inflammatory Mediators of Asthma: An Update. Pharmacol. Rev. 1998, 50(4), 515–596.
- Kundu, N.; Fulton, A. M. Selective Cyclooxygenase (COX)-1 or COX-2 Inhibitors Control Metastatic Disease in a Murine Model of Breast Cancer. Cancer Res. 2002, 62(8), 2343–2346.
- ElAsbahani, A.; Miladi, K.; Badri, W.; Sala, M.; AïtAddi, E. H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F. N.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483(1–2), 220–243. DOI: 10.1016/j.ijpharm.2014.12.069.
- Garcia, G. P.; Sutour, S.; Rabehaja, D.; Tissandié, L.; Filippi, J. J.; Tomi, F. Essential Oil of the Malagasy Grass Elionurus Tristis Hack. Contains Several Undescribed Sesquiterpenoids. Phytochemistry. 2019, 6(162), 29–38. DOI: 10.1016/j.phytochem.2019.02.012.
- Jing, C. L.; Huang, R. H.; Su, Y.; Li, Y. Q.; Zhang, C. S. Variation in Chemical Composition and Biological Activities of Flos Chrysanthemiindici Essential Oil under Different Extraction Methods. Biomolecules. 2019, 9(10), 518. DOI: 10.3390/biom9100518.
- Abdullah, I. H.; Shahzad, A. S. C.; Ghulam, M. K.; Muhammad, A. A.; Muhammad, A. H.; Muhammad, I. L. Chemical Composition and Biological Activities of Essential Oil and Extracts from Ocimum Sanctum. Int. J. Food Prop. 2017, 20(7), 1569–1581. DOI: 10.1080/10942912.2016.1214145.
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC‐FID Quantification Technique without Authentic Samples Using Predicted Response Factors. Flavour Fragance J. 2012, 274, 290–296. DOI:10.1002/ffj.3098.
- Li, Z. H.; Bai, X.; Ma, Q. L.; Aisa, H. A.; Maiwulanjiang, M. Detection of Antibacterial and Antioxidant Compounds in the Essential Oil of Schizonepeta Annua (Pall.) Schischk. Using High-performance Thin-layer Chromatography-direct Bioautography and Gas Chromatography-quadrupole Time-of-flight Mass Spectrometry. JPC-J. Planar Chromatogr. 2019, 32(5), 359–364. DOI:10.1556/1006.2019.32.5.2.
- Reis, F. S.; Martins, A.; Barros, L.; Ferreira, I. C. Antioxidant Properties and Phenolic Profile of the Most Widely Appreciated Cultivated Mushrooms: A Comparative Study between in Vivo and in Vitro Samples. Food Chem. Toxicol. 2012, 50(5), 1201–1207. DOI: 10.1016/j.fct.2012.02.013.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26(9–10), 1231–1237. DOI: 10.1016/s0891-5849(98)00315-3.
- Nabet, N.; Boudries, H.; Chougui, N.; Loupassaki, S.; Souagui, S.; Burló, F.; Hernández, F.; Carbonell-Barrachina, Á. A.; Madani, K.; Larbat, R. Biological Activities and Secondary Compound Composition from Crithmummaritimum Aerial Parts. Int. J. Food Prop. 2017, 20(8), 1843–1855. DOI: 10.1080/10942912.2016.1222541.
- Pradelles, P.; Grassi, J.; Maclouf, J. Enzyme Immunoassays of Eicosanoids Using Acetylcholine Esterase as Label: An Alternative to Radioimmunoassay. Anal. Chem. 1985, 57(7), 1170–1173. DOI: 10.1021/ac00284a003.
- Jones, D. A.; Carlton, D. P.; McIntyre, T. M.; Zimmerman, G. A.; Prescott, S. M. Molecular Cloning of Human Prostaglandin Endoperoxide Synthase Type II and Demonstration of Expression in Response to Cytokines. J. Biol. Chem. 1993, 268(12), 9049–9054.
- Danh, L. T.; Han, L. N.; Triet, N. D. A.; Zhao, J. Comparison of Chemical Composition, Antioxidant and Antimicrobial Activity of Lavender (Lavandula Angustifolia L.) Essential Oils Extracted by Supercritical CO2, Hexane and Hydrodistillation. Food Bioprocess. Tech. 2013. DOI: 10.1007/s11947-012-1026–z.
- Allawzi, M.; Allaboun, H.; Almasri, A. CO2 Supercritical Extraction of Essential Oil of Jordanian Rosemary. J. AOAC Int. 2019, 102(2), 662–665. DOI: 10.5740/jaoacint.18-0221.
- Kourounakis, A. P.; Assimopoulou, A. N.; Papageorgiou, V. P.; Gavalas, A.; Kourounakis, P. N. Alkannin and Shikonin: Effect on Free Radical Processes and on Inflammation - a Preliminary Pharmacochemical Investigation. Arch. Pharm. (Weinheim).2002, 335(6), 262–266. DOI: 10.1002/1521-4184(200208)335:6<262::AID-ARDP262>3.0.CO;2-Y.
- Kataki, M. S.; Kakoti, B. B.; Bhuyan, B.; Rajkumari, A.; Rajak, P. Garden Rue Inhibits the Arachidonic Acid Pathway, Scavenges Free Radicals, and Elevates FRAP: Role in Inflammation. Chin. J. Nat. Med. 2014, 12(3), 172–179. DOI: 10.1016/S1875-5364(14)60029-7.
- Miguel, M. G. Antioxidant and Anti-inflammatory Activities of Essential Oils: A Short Review. Molecules. 2010, 15(12), 9252–9287. DOI: 10.3390/molecules15129252.