?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Antimicrobial packaging is used for improving shelf life of food. Propolis is a natural substance with antimicrobial effects that can be applied in packaging. The current study investigated the mechanical and antimicrobial properties of active packaging films of ethylene vinyl alcohol including propolis. Crude propolis was added to the polymer matrix at different ratios (20%, 15%, 10%, 5%, and 0%; w/w) and the films were primed by extrusion method. The mechanical attributes of the films showed that the incorporation of propolis to the EVOH film reduced the elongation to tear of the films and decreased the tensile strength. Scanning electron microscopy images also demonstrated that the films had a more heterogeneous morphology than pure ethylene vinyl alcohol and propolis particles had a good distribution within the polymer matrixes. Agglomeration did not observe. In addition, water permeability (WVP) and oxygen permeability (OTR) decreased with raising propolis percentage in comparison with the control film significantly (p < .05). Differential scanning calorimetry test showed that an increase propolis in the polymer matrix reduced the crystallization temperature and increased the crystallinity percentage. Antimicrobial effect on Aspergillus Niger and Saccharomyces cerevisiae, Staphylococcus aureus, and Escherichia coli was increased with increased percentage of propolis in the blended films. However, its effect on Gram-positive Staphylococcus aureus was much more than the other microorganisms studied.
Introduction
Active packaging is an innovative concept in which the packaging, product, and environment are linked to increase longevity and improve safety and the appearance of the product, which maintains the original quality of the product during its shelf life. Active packaging can play multiple roles that are not present in common packaging, depending on the used material into the active packaging film, the application aims of active packaging in food industry can vary based on antimicrobial activity, oxygen uptake, moisture, or ethylene release.[Citation1–3]
Since, microbial contamination is an important factor preventing for shelf life of food, antimicrobial packaging is used to destroy or inhibit the growth of them.[Citation4–6] In recent years, using and developing antimicrobial packaging was desired because of the improving shelf life of food products as well as its healthy aspects. Both natural and synthetic antimicrobial agents were applied to the packaging.[Citation7–9] Adding natural antimicrobial compounds to polymers to produce antimicrobial packaging eliminates some of the consumers and food industry producers concerns about the quality of foods.[Citation9,Citation10]
One of the most widely used polymers in this industry is ethylene vinyl alcohol (EVOH), a random copolymer of ethylene and vinyl alcohol.[Citation11–13] More than 35 years ago, Kuraray and his assistant made plastics from EVOH copolymer. Its high processability (forming and extruding) properties and a unique high barrier-to-permeability (pass-through) especially for gases and in particular oxygen characterize this resin.[Citation11,Citation14] This polymer is suitable for food, pharmaceutical, medical, cosmetic, and hygienic packaging applications as well as industrial packaging applications.[Citation9] Lopez de Dicastillo and his colleagues conducted a research on using ethylene vinyl alcohol copolymer and green tea extract in a new antioxidant active packaging films.[Citation15] They found positive results in this case. Jen et al. investigated the resistance to oxygen penetration in polyethylene and ethylene vinyl alcohol mixture bottles, polyethylene, modified polyethylene and ethylene vinyl alcohol, modified polyamide, and polyethylene as well as ethylene vinyl alcohol.[Citation16] Oxygen penetration significantly increased after mixing EVOH resin into PE matrix during melting process. Calatayad et al. expanded a copolymer of EVOH containing a flavonoid-rich cocoa extract and investigated the antimicrobial and antioxidant properties of the films on the cell line.[Citation17] Cocoa extraction showed antioxidant activity against cell line stimulated with hydrogen peroxide.
Propolis is one of the antimicrobial compounds that has gained a lot of drug and nutritional uses in recent years.[Citation1,Citation18–20] Propolis is a honeybee product that produces by worker bees to protect the hive. Many properties of propolis have been reported for example: antifungal, antibacterial, antiviral antioxidant and cytostatic properties.[Citation21–24] A significant portion of propolis consists of wax and resin, flavonoids and phenolic compounds. It also contains a high percentage of antioxidant, antibacterial, antiviral, anti-fungal, anti-cancer and anti-inflammatory properties that are analyzed in the previous studies.[Citation1,Citation25–27] Due to antibacterial and antioxidant properties of propolis, it has been studied for the use in food packaging. There are many studies on incorporation of propolis extract into polymeric or biopolymeric films.[Citation28,Citation29] Adomavičiūtė, et al. (2018) developed and characterized pure multi-filament yarns and yarns saturated with propolis. They evaluated the effect of preparation and formulation parameters on the characteristics of yarns and they determined the release of propolis extract components from yarns and their possible cytotoxicity on cell formation.[Citation30] On the other hand, incorporating raw propolis with in low-density polyethylene extruded film was indicated the antimicrobial activity of the films against Aspergillus niger, and Saccharomyces cerevisiae at the concentrations above of 15% w/w propolis in the film matrix.[Citation31]
Erica and his colleagues studied the development of antimicrobial films by releasing propolis compounds into poly lactic acid polymer. Propolis was detected in the poly lactic acid film as an antimicrobial and antioxidant release system in the packaging.[Citation32] Siripatrawan and Vitchayakitti studied the enhancement of useful attribute of chitosan films as active food packaging with using Propolis functional properties in a research study. These films were able to inhibit Escherichia coli, Staphylococcus aureus, Pseudomonas aerogens, and Salmonella enteritidis.[Citation33]
In the previous article, the characteristics of the extruded polyethylene-based active films containing different propolis percentages were investigated.[Citation31] In the literatures, limited researches have been reported on the usage of this copolymer as an antibacterial film. Therefore, in the present study, we focused on the incorporation of propolis with different percentages in the EVOH polymer film to investigate physicochemical as well as antimicrobial properties of the film.
Materials and methods
Materials
Raw propolis was purchased from western Iran (Hamadan province), the ethylene vinyl alcohol copolymer (F171B grade; 32% by weight of ethylene) was purchased from Kuraray industry. Nutrient Agar (NA), Muller Hinton Agar (MHA), Muller Hinton Broth (MHB), Nutrient Broth (NB), Potato Dextrose Broth (PDB), Potato Dextrose Agar (PDA), Yeast Mold Agar (YMA) and Yeast Mold Broth (YMB) were purchased from Merck. Banks of bacteria include Escherichia cola (PTCC 1330), Staphylococcus aureus (PTCC 1764), and fungi including Saccharomyces cerevisiae yeast (PTCC 2601) and Aspergillus niger (PTCC 5011) were obtained from Fungi and Bacteria Collection Center of Iran (Scientific and Industrial Research Organization).
Methods
Preparation of propolis sample
Raw propolis first was frozen in liquid nitrogen then was milled. The gained powder was screened (No. 40 mesh) then stored in a special dish at −19°C until the tests were done.
Film preparation
The EVOH polymer was first incubated in an oven (Memert, UF55 model and made in Germany) for 1 h at 80°C to remove moisture. In order to produce active film, a propolis master batch with EVOH polymer was prepared. Propolis powder was blended with ethylene vinyl alcohol polymer with a portion of 60:40 (polymer to propolis) in an internal mixer (Brabender, W50 model, Germany). The temperatures of the three zones were set at 195°C and time was set for 8 min. Initially raised the speed to 120 rpm until the polymer was completely melted, then lowered the speed to 20 rpm and added the propolis and raised the speed to 100 rpm for 8 min. Mixtures obtained by semi-industrial hammer mill (Weiser, Germany) were transformed into the granules. Subsequently, master batch granules with a ratio of 60:40 (EVOH to propolis) were added to ethylene vinyl alcohol in different proportions to achieve the propolis to ethylene vinyl alcohol ratios of 20%, 15%, 10%, 5%, and 0% (w/w) in the final film. This granulation was accomplished by using a double-coil screw extruder (ZSK Mc18, Germany) with a screw diameter of 25 mm (D), screw length (L) of 100 cm and speed of 80 rpm. Temperature profiles from feed to die were 175–185- 190- 200- 205- 210°C. After preparing the granules, they were placed in the oven at 80°C for 2–3 h to remove moisture of ethylene vinyl alcohol. Subsequently, the films were produced with single screw extruder (Brabender, PL 2200, Germany) at 40 rpm and 50 m/min speed and temperature profile of 160–170- 180- 200-190°C.
Specification of the prepared films
Thermal analysis
The thermal stabilities of crude propolis, EVOH copolymer granule, and EVOH/propolis blended films were measured with a thermogravimetric analyzer (TGA apparatus (Mettler Toledo, Model TGA1, Switzerland)); heating was applied from 25°C to 600°C at a rate of 10°C/min with a constant nitrogen flow (20 mL/min).
To understand the thermal behaviour of the samples, a differential scanning calorimeter (DSC) device (METTLER TOLEDO, Model DSCI, Switzerland) was used. Heating was applied from five to 200°C at a rate of 10°C/min with a constant nitrogen flow (50 mL/min). The melting enthalpy (ΔHm) and temperature (Tm) of the EVOH/propolis films were measured. To calculate the crystallinities (Xc) related to these films, EquationEq. (1)(1)
(1) was employed.
In the above equation, ΔHf and ΔH0f (157.8 J g−1) denote the temperature for melting of the polymer samples and perfect crystalline EVOH, respectively.[Citation34]
Water Vapor Permeability (WVP)
The water vapour permeability (WVP) values related to the EVOH/propolis films were determined in accordance with the ASTM E96 the diameter and height of the cups utilized were 65 and 52 mm, respectively.[Citation35] The inlet of the cups (63 mm in diameter) was sealed by the films after the cups had been filled with CaCl2 (10 g). After weighing the vials with their contents, they were placed within a desiccator that contained a saturated solution of NaCl. After placing the desiccator in an oven set at 25°C and 30% relative humidity (RH), the vials were weighed every 24 h for 10 days. The entire process was repeated thrice. The change in weight was analyzed with linear regression in terms of time (water vapour transmission rate; WVTR); with the slope being used to obtain the WVP value after reaching steady state, as shown in EquationEq. (2)(2)
(2) :
where X denotes the thickness of the film and P denotes the vapour pressure of pure water at 25°C.[Citation7,Citation36]
Oxygen Transmission Rate (OTR)
The Oxygen Transmission Rate evaluation of the EVOH/propolis films was performed using an oxygen permeability tester (Variable – Pressure Constant – Volume). Evaluation was done at 30% RH 25°C with three replicates.
Tensile properties
The tensile properties measurement of EVOH/propolis blended films was done using the Universal testing machine (SMT-50, IRAN), according to ASTM-D882-01, in three replicates. For experiments, cut strips of the films (dimensions: 50 mm×20 mm×0.15 mm) were placed between two jaws with a distance 50 mm and tensioned at a rate of 500 mm/min.[Citation37]
Scanning Electron Microscopy (SEM)
Seeing of the scattering of the propolis in the EVOH/propolis blended films were obtained by a scanning electron microscope (SEM) (INCA, Oxford instruments Co). The samples were first immersed in liquid nitrogen for 2 minutes, to obtain SEM image of their cross-sectional area. After coating the sample with a thin layer of gold, the samples were placed on the base of the machine and images were prepared.[Citation38]
Fourier Transform Infrared Spectroscopy (FTIR)
The chemical structures of the EVOH and EVOH/propolis blended films and raw propolis were evaluated by a Fourier transform infrared spectroscopy (FTIR) in the region of 400–4,000 cm−1 using a Spectrum 65 FTIR spectrometer (Avater, 370 FTIR, Canada) as 16 scans with a resolution of 2 cm−1.[Citation39]
Antimicrobial efficacy
The antibacterial properties of the films were evaluated according to Japanese Industrial Standard Method (JIS Z 2801:2000).[Citation40] Briefly, an aqueous solution of antimicrobial substances was prepared and mixed in NB medium containing 105 colony-forming unit (CFU)/mL of E. coli and S. aureus. After the incubation period (24 h at 35 ± 1°C and 90% RH), colony counting was done immediately.
The antimicrobial efficiency of the films against Saccharomyces cerevisiae and Aspergillus niger was evaluated according to the National Standard of Iran method (2008).[Citation41] Briefly, the slices of the film (diameter of 20 mm) were inserted into tubes containing 10 mL of liquid culture medium (YMB for Saccharomyces and PDB for Aspergillus) containing 1.5 × 108 activated spores per mL. After incubation (72 h at 25°C for Aspergillus and 24 h at 30°C for Saccharomyces), survival of microorganism was determined by conventional plating. Pure film and Ampicillin were used as a negative and positive controls, respectively. The number of colonies grown was counted and was reported in CFU/mL.[Citation42]
Statistical analysis
Data were analyzed by statistical software in a completely randomized design. ANOVA and Duncan test with 95% significance level and Minitab version 4.2.16 were used to examine the possibility of significant differences between groups. Charts were drawn using Excel 2010 software. All tests were performed in three replications.
Results and discussions
Thermal analysis measurements
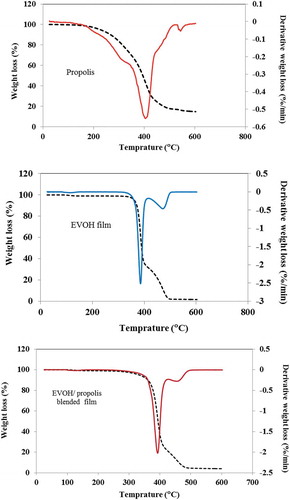
Materials made of polymers must maintain adequate thermal stability in order to withstand treatment at high temperatures during various packaging processes including melt-extrusion, pasteurization, and/or sterilization. The thermal characteristics of such polymers may be significantly affected by greater dispersion of materials within their matrices and more powerful interfacial interactions.[Citation9] To determine how the thermal stabilities of the EVOH/propolis films were impacted by the propolis content and the method of mixing used, DSC and TGA analyses were employed, with the results being depicted in . In order to determine the appropriate temperature for film production, the degradation temperature of the propolis sample and the ethylene vinyl alcohol film were studied by thermal gravimetric method (). Including a steep decrease in the range of 230 − 450°C and then a slow decrease to 500°C. The degradation temperature of the ethylene vinyl alcohol polymer is in the range of 500°C, whereas the degradation temperature of the propolis is 225°C. The TGA results of the ethylene vinyl alcohol film containing 20% propolis. indicates that the thermal degradation of the polymer blended with propolis was carried out after 200°C and about 500°C is almost complete destruction. The thermal behaviour of the blend film indicates that the blend of propolis and ethylene vinyl alcohol lacked strong interactions between them, which would have caused the destruction of the blend film at higher temperatures. From the results obtained, it was concluded that a temperature of less than 230°C should be used to prepare ethylene vinyl alcohol and propolis films. Moreover, as shown in , T 10% of the mix film is between the range of propolis T 10% and pure film T 10%. Apparently, propolis was effective in decreasing the thermal stability of EVOH film.[Citation9] DSC test was performed to evaluate the effect of propolis on thermal behaviour and crystallization of EVOH. The crystallinity and melting behaviour of EVOH in the samples tested are illustrated in . [Citation43]
Table 1. Characteristics of crystallization and melting behaviour of EVOH pure sample and EVOH films containing 0%, 5%, 10%, 15%, and 20% (w/w) propolis
The crystallization temperature (Tc) of the pure sample (EVOH) is 164.1°C. After adding propolis the temperature decreased about 7 degrees, indicating that the propolis was not a nucleating agent but aiding the growth of the nucleus.[Citation44] Changes in Tm also indicate that the size of the crystals has changed in the ethylene vinyl alcohol phase. The addition of propolis increased the crystallinity percentage of the ethylene vinyl alcohol phase from 46% to about 55% due to the acting of propolis as a lubricating agent and probably as a result, the mobility of polymer chains increases and the chains can be more easily placed in regular crystalline formats. In addition, increases in the percentage of crystallinity have a great effect on the mechanical properties of the films and it enhances the antimicrobial properties of the films. This is because crystallization and higher conductivity reduce the access of microorganisms to moisture and oxygen, which reduces microbial growth.[Citation45,Citation46]
Scanning Electron Microscope (SEM)
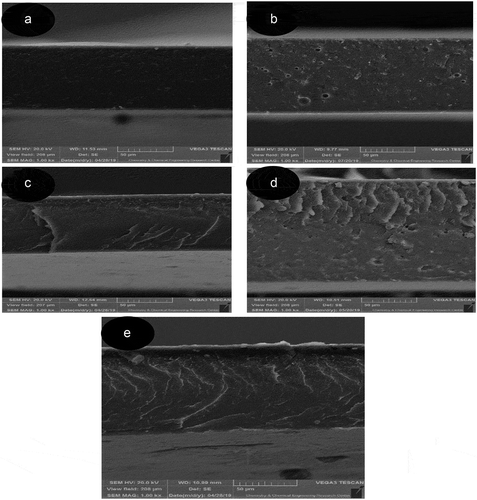
Scanning electron microscopy was used to investigate the fracture surface morphology of the film samples. The microstructural images of the analyzed samples are shown in the images show that the structure of the ethylene vinyl alcohol polymer containing propolis was physically affected. The particle distribution is almost uniform and aggregation and agglomeration are not observed at different fracture areas. It is also noticeable that with the increase in the percentage of propolis in the film composition, the gaps have widened and their number has increased in the film context. These gaps and bumps in the film structure can disrupt the polymer structure and affect the physical and mechanical properties of the films. The results of other researchers also confirm the decrease in surface uniformity and the increase in pores resulting from the addition of an additive to the polymer matrix.[Citation38,Citation47]
Fourier Transform Red Infrared Spectroscopy (FTIR)
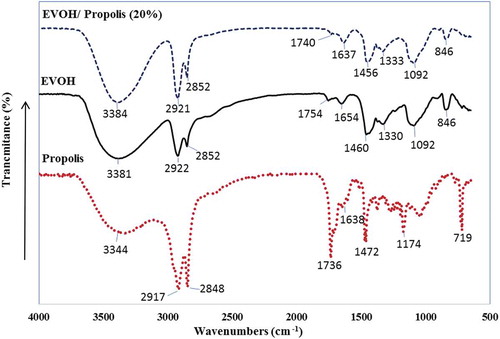
FTIR test was performed to investigate the new functional groups created in the mixture of propolis and ethylene vinyl alcohol in the range of 400 to 4000 cm−1. The corresponding spectra are shown in . As depicted in , four distinct absorption peak areas are seen for propolis in wave numbers 720/730, 1376, 2917, and 3344 cm−1. These peaks, respectively, can be related to the C-H group with vibration movement in aromatic rings, the C-H group with rotational movement, the C-H group with asymmetric tensile vibration, and the O-H groups in the phenolic compounds of propolis or moisture. Four distinct absorption peak areas were found in wave numbers 722, 1754, 2922, and 3381 cm−1 of ethylene vinyl alcohol, which can be classified into CH group with vibrational movement, C = O functional group, CH group with asymmetric tensile vibration and stretching of OH groups. As shown in new peaks appeared due to the presence of the propolis and some displacements appeared because of chemical interactions. In addition, the blue shift (elongation at lower wavelengths) was observed.[Citation48,Citation49]
Mechanical properties
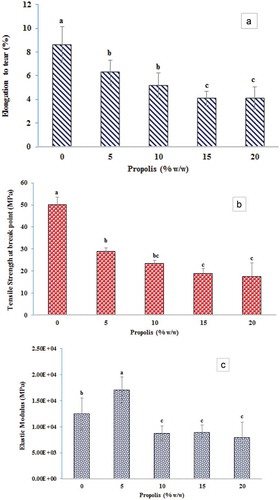
Depending on the amount of interfacial interactions between the filler and the matrix, how the particles diffuse into the matrix, and the number of voids filled, varying amounts of tensile strength can be observed in the films. To study the effect of propolis particles on the mechanical properties of the film, tensile strength, relative elongation to tear and elastic modulus were measured. The results for the ethylene vinyl alcohol films containing propolis compared to the control film (ethylene vinyl alcohol without propolis) are illustrated in , which shows the active films having a lower relative elongation and tensile strength than the control film, indicating that the propolis particles are embedded within the polymer matrix and across the chains, causing disturbances in the uniform matrix. In addition, since the propolis modulus is lower than EVOH, the mixed film has less modulus than the control film. However, the modulus of the active film containing 5% propolis increased more than the control film. This may be due to better diffusion of propolis, which acted as a nucleating agent and thus increase the crystallinity and consequently modulus.[Citation48,Citation50]
Water sorption and barrier properties
The exchange of moisture between the nutrient and the environment can cause some problems such as moisture absorption by hygroscope materials and loss of nutrient quality. Water loss happens in foods where the water vapour pressure is higher than the ambient water pressure and causes loss of freshness of the food. Absorption of water by certain nutrients and increased aqueous activity in them cause microbial, chemical, and enzymatic degradation. Therefore, packaging materials must have a minimum permeability to water vapour and oxygen to maintain the quality of the nutrient.
As it is shown in , active ethylene vinyl alcohol films containing propolis had significant lower moisture permeability than pure ethylene vinyl alcohol films (P < .05). However, there was no significant difference between different concentrations of propolis. On the other hand, oxygen permeability of EVOH active films containing different concentrations of propolis compared to pure EVOH film were similar to moisture permeability, which means blending of propolis in EVOH film matrix decreased the permeability to oxygen. By increasing propolis, permeability decreased but this difference was not significant. In fact, propolis had an adaptive role that increased the crystallinity of the substrate phase, as shown in the DSC test, which increased the crystallinity percentage, and thereby increased the barrier properties (moisture and oxygen permeability) of the film.[Citation3,Citation51]
Table 2. Results of permeability to moisture and oxygen for EVOH films containing different concentrations of propolis
Study of antimicrobial effects
According to the studies, there was a significant difference between the antimicrobial activity of the pure EVOH film and the blended films of EVOH with different amounts of propolis (5, 10, 15, and 20 w/w%) in testicular microorganisms with 95% coefficient of determination (P < .05). With increasing propolis, the antimicrobial activity of the film increased, although the difference between 15% and 20% is not significant as shown in .
Figure 5. Antimicrobial results of EVOH films containing different concentrations of propolis
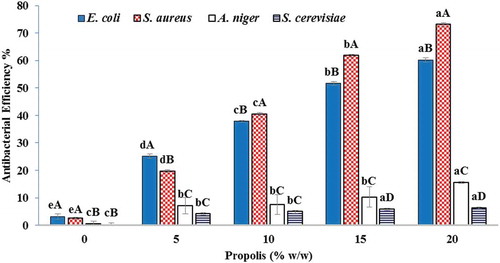
Phenols and flavonoids are found in many natural compounds including propolis. These compounds not only have high antioxidant effects, but can also affect microorganisms and destroy or inhibit their growth.[Citation20] In a previous research, it was determined that the propolis with the content of 44.82 mg Gallic acid/g flavonoid and 32.17 mg Gallic acid/g phenolic had antimicrobial effect. The expected antimicrobial effect of propolis on some microorganisms, including Gram-positive bacteria, has been pronounced more. Moreover, the microbial tests confirm this and its antimicrobial effect on Staphylococcus aureus is significantly greater than other microorganisms. These findings are in line with the results of the Ghisalberti and Papova experiments. They studied the antimicrobial activity of propolis and investigated its chemical composition using GC-MS and spectrophotometry. The results showed a high percentage of phenolic and flavonoid compounds that showed a good antimicrobial activity against Gram-positive bacteria.[Citation52–54]
Conclusion
In this study for the first time, the antimicrobial EVOH film carrying propolis extract was produced. The produced film had good homogeneity as well as an improved barrier property to oxygen while being more permeable to moisture. In addition, the mechanical strength of the obtained active films was reduced, it was acceptable. The active films obtained showed good antimicrobial properties, especially against E.coli and S. aureus. However, more research is needed on the release of effective compounds of propolis from films and its efficiency in food packaging and extending the shelf life.
References
- Król, W.; Bankova, V.; Sforcin, J. M.; Szliszka, E.; Czuba, Z.; Kuropatnicki, A. K. Propolis: Properties, Application, and Its Potential. Evid. Based Complement. Altern. Med. 2013, 2013, 807578. DOI: 10.1155/2013/807578.
- Rambabu, K.; Bharath, G.; Fawzi, B.; Show, P. L.; Cocoletzi, H. H. Mango Leaf Extract Incorporated Chitosan Antioxidant Film for Active Food Packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. DOI: 10.1016/j.ijbiomac.2018.12.196.
- López de Dicastillo, C.; Nerín, C.; Alfaro, P.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Development of New Antioxidant Active Packaging Films Based on Ethylene Vinyl Alcohol Copolymer (EVOH) and Green Tea Extract. J. Agric. Food Chem. 2011, 59, 7832–7840. DOI: 10.1021/jf201246g.
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S. W. Active Packaging Technologies with an Emphasis on Antimicrobial Packaging and Its Applications. J. Food Sci. 2003, 68(2), 408–420. DOI: 10.1111/j.1365-2621.2003.tb05687.x.
- Wang H, Wang W, Jiang S,Jiang S, Zhai L, and JiangQ. Poly (vinyl alcohol)/oxidized starch fibres via electrospinning technique: fabrication and characterization. Iran polym J 2011; 20:551–558.
- Quintavalla, S.; Vicini, L. Antimicrobial Food Packaging in Meat Industry. Meat Sci. 2002, 62(3), 373–380. DOI: 10.1016/S0309-1740(02)00121-3.
- Muramatsu, M.; Okura, M.; Kuboyama, K.; Ougizawa, T.; Yamamoto, T.; Nishihara, Y.; Saito, Y.; Ito, K.; Hirata, K.; Kobayashi, Y.;, et al. Oxygen Permeability and Free Volume Hole Size in Ethylene–vinyl Alcohol Copolymer Film: Temperature and Humidity Dependence. Radiat. Phys. Chem. 2003, 68(3–4), 561–564. DOI: 10.1016/S0969-806X(03)00231-7.
- Sadeghi, K.; Shahedi, M. Physical, Mechanical, and Antimicrobial Properties of Ethylene Vinyl Alcohol copolymer/chitosan/nano-ZnO (Ecnzn) Nanocomposite Films Incorporating Glycerol Plasticizer. J. Food Meas. Charact. 2016, 10(1), 137–147. DOI: 10.1007/s11694-015-9287-7.
- Kwon, H.; Kim, D.; Seo, J. Thermal and Barrier Properties of EVOH/EFG Nanocomposite Films for Packaging Applications: Effect of the Mixing Method. Polym. Compos. 2016, 37(6), 1744–1753. DOI: 10.1002/pc.23347.
- Muriel-Galet, V.; Cran, M. J.; Bigger, S. W.; Hernández-Muñoz, P.; Gavara, R. Antioxidant and Antimicrobial Properties of Ethylene Vinyl Alcohol Copolymer Films Based on the Release of Oregano Essential Oil and Green Tea Extract Components. J. Food Eng. 2015, 149, 9–16. DOI: 10.1016/j.jfoodeng.2014.10.007.
- Mokwena, K.; Tang, J. Ethylene Vinyl Alcohol: A Review of Barrier Properties for Packaging Shelf Stable Foods. Crit. Rev. Food Sci. Nutr. 2012, 52(7), 640–650. DOI: 10.1080/10408398.2010.504903.
- Yeh, J.-T.; Yao, W.-H.; Du, Q.; Chen, -C.-C. Blending and Barrier Properties of Blends of Modified Polyamide and Ethylene Vinyl Alcohol Copolymer. J. Polym. Sci. B Polym. Phys. 2005, 43, 511–521. DOI: 10.1002/polb.20344.
- Anjum, S. I.; Ullah, A.; Khan, K. A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M. A.; Tahir, M.; Ansari, M. J.; Ghramh, H. A.;, et al. Composition and Functional Properties of Propolis (Bee Glue): A Review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. DOI: 0.1016/j.sjbs.2018.08.013.
- Muriel-Galet, V.; López-Carballo, G.; Hernández-Muñoz, P.; Gavara, R. Characterization of Ethylene-vinyl Alcohol Copolymer Containing Lauril Arginate (LAE) as Material for Active Antimicrobial Food Packaging. Food Pack. Shelf Life. 2014, 1(1), 10–18. DOI: 10.1016/j.fpsl.2013.09.002.
- Martı́nez-Abad, A.; Lagaron, J. M.; Ocio, M. J. Development and Characterization of Silver-Based Antimicrobial Ethylene–Vinyl Alcohol Copolymer (EVOH) Films for Food-Packaging Applications. J. Agric. Food Chem. 2012, 60(21), 5350–5359. DOI: 10.1021/jf300334z.
- Matsuda, N.; Shirasaka, H.; Takayama, K.; Ishikawa, T.; Takeda, K. Thermal Degradation and Flame Retardancy of Ethylene-vinyl Alcohol Copolymer Blended with Ammonium Polyphosphate. Polym. Degrad. Stab. 2003, 79(1), 13–20. DOI: 10.1016/S0141-3910(02)00229-X.
- Calatayud, M.; López-de-Dicastillo, C.; López-Carballo, G.; Vélez, D.; Hernández Muñoz, P.; Gavara, R. Active Films Based on Cocoa Extract with Antioxidant, Antimicrobial and Biological Applications. Food Chem. 2013, 139(1–4), 51–58. DOI: 10.1016/j.foodchem.2013.01.097.
- Yildirim S, Röcker B, Pettersen MK, Nilsen-Nygaard J, Ayhan Z, Rutkaite R, Radusin T, Suminska P, Marcos B, and Coma V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018; 17:165-199. DOI: 10.1111/1541-4337.12322.
- Asawahame, C.; Sutjarittangtham, K.; Eitssayeam, S.; Tragoolpua, Y.; Sirithunyalug, B.; Sirithunyalug, J. Antibacterial Activity and Inhibition of Adherence of Streptococcus Mutans by Propolis Electrospun Fibers. AAPS PharmSciTech. 2015, 16(1), 182–191. DOI: 10.1208/s12249-014-0209-5.
- Siheri W, Alenezi S, Tusiimire J, Watson DG. The Chemical and Biological Properties of Propolis. In: Alvarez-Suarez J. (eds): Bee Products - Chemical and Biological Properties. Cham: Springer; 2017; 137-178. DOI: 10.1007/978-3-319-59689-1_7.
- Gutiérrez, L.; Escudero, A.; Batlle, R.; Nerín, C. Effect of Mixed Antimicrobial Agents and Flavors in Active Packaging Films. J. Agric. Food Chem. 2009, 57, 8564–8571. DOI: 10.1021/jf901459e.
- Wang, H.; Chen, M.; Jin, C.; Niu, B.; Jiang, S.; Li, X.; Jiang, S. Antibacterial [2-(Methacryloyloxy) ethyl] Trimethylammonium Chloride Functionalized Reduced Graphene Oxide/Poly(ethylene-co-vinyl alcohol) Multilayer Barrier Film for Food Packaging. J. Agric. Food Chem. 2018, 66, 732–739. DOI: 10.1021/acs.jafc.7b04784.
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant Activity of Propolis of Various Geographic Origins. Food Chem. 2004, 84(3), 329–339. DOI: 10.1016/S0308-8146(03)00216-4.
- Kubiliene, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Barcauskaite, K.; Kubilius, R.; Kasparaviciene, G.; Savickas, A. Alternative Preparation of Propolis Extracts: Comparison of Their Composition and Biological Activities. BMC Complementary Altern. Med. 2015, 15(1), 156. DOI: 10.1186/s12906-015-0677-5.
- Mello, B. C. B. S.; Petrus, J. C. C.; Hubinger, M. D. Concentration of Flavonoids and Phenolic Compounds in Aqueous and Ethanolic Propolis Extracts through Nanofiltration. J. Food Eng. 2010, 96, 533–539. DOI: 10.1016/j.jfoodeng.2009.08.040.
- Pellati, F.; Prencipe, F. P.; Bertelli, D.; Benvenuti, S. An Efficient Chemical Analysis of Phenolic Acids and Flavonoids in Raw Propolis by Microwave-assisted Extraction Combined with High-performance Liquid Chromatography Using the Fused-core Technology. J. Pharm. Biomed. Anal. 2013, 81-82, 126–132. DOI: 10.1016/j.jpba.2013.04.003.
- de la Cruz-Cervantes JA, Benavides-González F, Sánchez-Martínez JG, de la Luz Vázquez-Sauceda M, and Ruiz-Uribe AJ. Propolis in Aquaculture: A Review of Its Potential. Rev. Fish. Sci. Aquacult. 2018; 26:1-13.DOI: 10.1080/23308249.2018.1424798.
- Ismail MI, Roslan A, Saari NS, Hashim KH, Kalamullah MR. Ethanolic extract of propolis for biodegradable films packaging enhanced with chitosan. 3rd Electronicand Green Materials International Conference 2017 (EGM 2017), Thailand. AIP Conference Proceedings 2017; 1885(1):020231. DOI: 10.1063/1.5002425
- Jaganathan SK, Mani MP, Ismail AF, Prabhakaran P, and Nageswaran G. Tailor-made multicomponent electrospun polyurethane nanofibrous composite scaffold comprising olive oil, honey, and propolis for bone tissue engineering: Electrospun polyurethane incorporated olive oil/honey/propolis. Polym. Compos. 2018; 40.DOI: 10.1002/pc.24985.
- Adomavičiūtė E, Baltušnikaitė-Guzaitienė J, Juškaitė V, Žilius M, Briedis V, and Stanys S. Formation and characterization of melt-spun polypropylene fibers with propolis for medical applications. The J. Text. Inst. 2018; 109:278-284.DOI: 10.1080/00405000.2017.1341295.
- Hajinezhad, S.; Razavizadeh, B. M.; Niazmand, R. Study of Antimicrobial and Physicochemical Properties of LDPE/Propolis Extruded Films. Polym. Bull. 2020, 77, 4335–4355. DOI: 10.1007/s00289-019-02965-y.
- Mascheroni, E.; Guillard, V.; Nalin, F.; Mora, L.; Piergiovanni, L. Diffusivity of Propolis Compounds in Polylactic Acid Polymer for the Development of Anti-microbial Packaging Films. J. Food Eng. 2010, 98(3), 294–301. DOI: 10.1016/j.jfoodeng.2009.12.028.
- Siripatrawan, U.; Vitchayakitti, W. Improving Functional Properties of Chitosan Films as Active Food Packaging by Incorporating with Propolis. Food Hydrocolloids. 2016, 61, 695–702. DOI: 10.1016/j.foodhyd.2016.06.001.
- Lagaron, J. M.; Powell, A. K.; Bonner, G. Permeation of Water, Methanol, Fuel and Alcohol-containing Fuels in High-barrier Ethylene–vinyl Alcohol Copolymer. Polym. Test. 2001, 20(5), 569–577. DOI: 10.1016/S0142-9418(00)00077-5.
- ASTM. Standard Test Methods for Water Vapor Transmission of Material; American Society for Testing and Materials: Annual book of ASTM: Philadelphia, PA, 1995.
- Kołodziejska, I.; Piotrowska, B. The Water Vapour Permeability, Mechanical Properties and Solubility of Fish Gelatin–chitosan Films Modified with Transglutaminase or 1-ethyl-3-(3-dimethylaminopropyl) Carbodiimide (EDC) and Plasticized with Glycerol. Food Chem. 2007, 103(2), 295–300. DOI: 10.1016/j.foodchem.2006.07.049.
- Byun, Y.; Kim, Y. T.; Whiteside, S. Characterization of an Antioxidant Polylactic Acid (PLA) Film Prepared with α-tocopherol, BHT and Polyethylene Glycol Using Film Cast Extruder. J. Food Eng. 2010, 100, 239–244. DOI: 10.1016/j.jfoodeng.2010.04.005.
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M. C. Characterization and Antimicrobial Activity Studies of Polypropylene Films with Carvacrol and Thymol for Active Packaging. J. Food Eng. 2012, 109(3), 513–519. DOI: 10.1016/j.jfoodeng.2011.10.031.
- Oliveira RN, Mancini MC, de Oliveira FCS, Passos TM, Quilty B, da Silva Moreira Thiré RM, McGuinness GB. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. 62;Matéria (Rio de Janeiro)62; 2016; 21:767-779.DOI: 10.1590/S1517-707620160003.0072.
- JIS, Z.;. 2801. Antimicrobial Products Test for Antimicrobial Activity and Efficacy; Japanese Standards Association: Japan, 2000.
- Isiri-10899-2. Microbiology of food and animal feeding stuffs - Horizontal method for the enumeration of yeasts and moulds In Part 2 : Colony count technique in products with water activity less thanor equal to 0.95. Islamic Republic of Iran: Institute of Standards and Industrial Research of Iran; 2008; 8-20.
- Meshkani, M.; Mortazavi, A.; Pourfallah, Z. Antimicrobial and Physical Properties of a Chickpea Protein Isolate-based Film Containing Essential Oil of Thyme Using Response Surface Methodology. Iran. J. Nutr. Sci. Food Technol. 2013, 8, 93–104.
- Cabedo, L.; Giménez, E.; Lagaron, J. M.; Gavara, R.; Saura, J. J. Development of EVOH-kaolinite Nanocomposites. Polymer. 2004, 45(15), 5233–5238. DOI: 10.1016/j.polymer.2004.05.018.
- Matsuda, A. H.; Machado, L. B.; Del Mastro, N. L. Thermal Analysis Applied to Irradiated Propolis. Radiat. Phys. Chem. 2002, 63(3–6), 353–355. DOI: 10.1016/S0969-806X(01)00524-2.
- Rosa, M. F.; Chiou, B.-S.; Medeiros, E. S.; Wood, D. F.; Williams, T. G.; Mattoso, L. H. C.; Orts, W. J.; Imam, S. H. Effect of Fiber Treatments on Tensile and Thermal Properties of Starch/ethylene Vinyl Alcohol Copolymers/coir Biocomposites. Bioresour. Technol. 2009, 100(21), 5196–5202. DOI: 10.1016/j.biortech.2009.03.085.
- Durmuş, A.; Woo, M.; Kaşgöz, A.; Macosko, C. W.; Tsapatsis, M. Intercalated Linear Low Density Polyethylene (Lldpe)/clay Nanocomposites Prepared with Oxidized Polyethylene as a New Type Compatibilizer: Structural, Mechanical and Barrier Properties. Eur. Polym. J. 2007, 43(9), 3737–3749. DOI: 10.1016/j.eurpolymj.2007.06.019.
- Cui, L.; Xu, L.-P.; Tsai, F.-C.; Zhu, P.; Jiang, T.; Yeh, J.-T. Oxygen Depletion Properties of Glucose-grafted Polyethylene Resins Filled with Sodium Ascorbate/modified Iron Compounds. J. Polym. Res. 2011, 18(6), 1301–1313. DOI: 10.1007/s10965-010-9533-y.
- López-Rubio, A.; Lagarón, J. M.; Hernández-Muñoz, P.; Almenar, E.; Catalá, R.; Gavara, R.; Pascall, M. A. Effect of High Pressure Treatments on the Properties of EVOH-based Food Packaging Materials. Innovative Food Sci. Emerg. Technol. 2005, 6(1), 51–58. DOI: 10.1016/j.ifset.2004.09.002.
- Oliveira RN, Mancini MC, de Oliveira FCS, Passos TM, Quilty B, da Silva Moreira Thiré RM, McGuinness GB. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria (Rio de Janeiro), 2016; 21(3):767-779. DOI: 10.1590/S1517-707620160003.0072.
- López-Rubio, A.; Hernández-Muñoz, P.; Gimenez, E.; Yamamoto, T.; Gavara, R.; Lagarón, J. M. Gas Barrier Changes and Morphological Alterations Induced by Retorting in Ethylene Vinyl Alcohol–based Food Packaging Structures. J. Appl. Polym. Sci. 2005, 96, 2192–2202. DOI: 10.1002/app.21690.
- Shin, Y.; Shin, J.; Lee, Y. S. Preparation and Characterization of Multilayer Film Incorporating Oxygen Scavenger. Macromol. Res. 2011, 19(9), 869. DOI: 10.1007/s13233-011-0912-y.
- Popova, M.; Silici, S.; Kaftanoglu, O.; Bankova, V. Antibacterial Activity of Turkish Propolis and Its Qualitative and Quantitative Chemical Composition. Phytomedicine. 2005, 12(3), 221–228. DOI: 10.1016/j.phymed.2003.09.007.
- Dias, L. G.; Pereira, A. P.; Estevinho, L. M. Comparative Study of Different Portuguese Samples of Propolis: Pollinic, Sensorial, Physicochemical, Microbiological Characterization and Antibacterial Activity. Food Chem. Toxicol. 2012, 50(12), 4246–4253. DOI: 10.1016/j.fct.2012.08.056.
- Razavizadeh, B. M.; Niazmand, R.; Hajinezhad, S.; Akbari, E. Physicochemical and Antimicrobial Properties and Determination of Phenols and Flavonoids Content of Propolis from Bee Hives in Khorasan Razavi Province. J. res. Innovation Food Sci. Technol. 2020, 9, 27–40. DOI: 10.22101/JRIFST.2019.09.17.e1031.