ABSTRACT
The aim of this study was to prepare physically stable nanoemulsions containing three different essential oils (Rosemary, Cuminum, and Zataria Multiflora Boiss) by using two different nanoemulsification methods. Then microbial growth rate, antioxidant, and oxidant content of fresh fish fillet were assessed at 4°C and 10°C temperatures. Essential oils (EOs) were purified from Rosmarinus officinalis L., Zataria multiflora Boiss, and Cuminum cyminum L. and emulsified by two different methods: Ultrasonic Homogenization (USH) and Emulsion Phase Inversion (EPI). Samples of Acipenser stellatus (weight of 10 ± 1 g) were immersed in Nanoemulsified essential oils. Then antibacterial and antioxidant potential of these essential oils was analyzed by culturing in a tube and measuring PV and TBA, respectively, during 1, 3, 7, and 14 days after treatment. Cuminum cyminum L., at 4°C in the USH method reduced the growth of bacteria in the fish sample on all studied bacteria at 5% concentration compared to 3%. Also the results of Cuminum cyminum L. EO made by both methods used to emulsify the oil were better than the other two extracts. As well these results were observed for PV and TBA contents. This study showed that nanoemulsified Cuminum cyminum L. prepared by the USH method has good antioxidant potential and antibacterial effect among other essential oils and it can be used to produce novel natural antioxidants as well as flavoring agents that can be applied in different food products.
Introduction
The most important goals in the food industry in all countries are healthy food production.[Citation1] One of the key issues related to the low shelf life of some foods is the growth of spoilage microorganisms. In addition, the formation of foodborne pathogens on them is the first cause of foodborne illness. During the production process, a large number of contaminants or toxins have been reported in raw foods.[Citation2] These contaminants are likely to remain in fresh or frozen foods and to cause disease. Food microbial contaminants can be reduced by using some preservatives, such as inorganic or organic acids, as well as some physical and chemical preservative methods.[Citation3] But most of these preservatives have adverse effects on humans. So the use of the natural plant, animals, or microbial preservatives that can improve the human immune system, is needed.[Citation4,Citation5]
Among natural antimicrobials, essential oils (EOs) (also called volatile or ethyl oily oils) are very interesting[Citation6] because of their antimicrobial (antifungal, antibacterial, antiviral), antioxidants, antimutagenic and anticarcinogenic properties.[Citation7,Citation8] EOs are aromatic, concentrated hydrophobic liquid containing volatile chemical compounds derived from plant materials (buds, flowers, leaves, barks, fruits, and roots).[Citation9] Rosmarinus officinalis L., Zataria multiflora Boiss and Cuminum cyminum L are popularly necessary oil. Rosmarinus officinalis, L., which originated in the Mediterranean is an aromatic plant belonging to the Lamiaceae family. In many areas, rosemary is a commercial spice that has attracted attention due to its antioxidant and therapeutic properties.[Citation10,Citation11] On the other hand, Rosmarinus is applied as a food preservator because have antioxidation and antimicrobial activity.[Citation12] Zataria multiflora Boiss is a member of the Lamiaceae family that grows geographically in Iran, Pakistan and Afghanistan .[Citation13] This plant, with its ethnic name Avicenna Shirazi (in Iran), is used as a spice (Cumin cyminum L.) and because of its anesthetic, antiseptic and spasmolytic properties is of interest to traditional herbal medicine.[Citation14] Cumin (Cumin cyminum) could be a spice traditionally used as a disinfectant and also encompasses a strong antimicrobial effect against various bacteria, pathogenic and nonpathogenic fungi against humans.[Citation15] The most constituent of cumin oil is aldehyde cumin.[Citation16]
Antimicrobial packaging has been developed as a way to extend food survival, reduce product waste and ensure consumer safety. The most prominent recently used device for preparing food packaging is the use of nanoemulsions.[Citation17,Citation18] One of the best carriers to deliver lipophilic materials are nanoemulsions, as they prepare easily, and small size of them leads to high optical clarity, good physical stability, and high bioavailability, accessibility and have biological efficacy and kinetic stability.[Citation19,Citation20] Nanoemulsions are known to be strong antimicrobials that protect themselves due to the low tide content in their structure, and thus there’s insufficient water for microorganisms during this structure.[Citation21,Citation22] Nanoemulsions can be produced using some methods commonly classified into low-energy and high-energy methods. The low-energy methods include spontaneous emulsification, phase inversion methods. These methods are more popular for production because they require cheaper equipment.[Citation23] It is stated that high energy including Ultrasonic Homogenization (USH). This method is more common in preparing food-grade nanoemulsion, cosmetics and pharmaceutical applications.[Citation24] The principles for an emulsion are taken into account to be a rather different nanoemulsion: droplets with diameters between 20 and 200 nm, 50 to 200 nm, and <500 nm intended for the assembly of nanoemulsions.[Citation25]
Fish is a special perishable food compared to other fresh produce. And it is one of the foods whose packaging has been considered. Increasing consumer demand for fresh and safe products with longer shelf life causes food science researchers to try to improve the process of preserving fresh fish.[Citation26,Citation27] Starry sturgeon (Acipenser stellatus) is a species of sturgeon which belongs to Acipenseridae family. This fish is one of the best fish found in the Caspian Sea which is most valuable caviar fish. As well as demand for fresh and safe fish, their meat can be contaminated by human pathogenic microorganisms.[Citation28–30] Therefore, a lot of interest has been shown in its packaging.
This study was designed to introduce new emulsion formulations with essential oils for preserving fresh food. Thus, Rosmarinus officinalis, L., Zataria multiflora Boiss and Cuminum cyminum L essential oil nanoemulsion were fabricated by two emulsification methods and their effects on microbial growth rate, antioxidant and oxidant content of fresh fish fillet were assessed at room and 4°C temperatures. This study was performed on days 1, 3, 7 and 14. The finding of the current research could result in better development of food in industry as well as enhancement of the food quality mainly the protein products.
Materials and methodS
Preparation of fish fillet
Acipenser stellatus (starry sturgeon) with an average weight of 2000 ± 100 g was purchased from a farm in Ahmad-Abad Mostophi, Tehran, Iran. Fishes in a cool condition transported to the Food Science Department at Azad University of Tehran within 25 min after catching. They were eviscerated, beheaded and filleted (approximately every fillet of uniform 25 cm × 10 cm and weight 100 ± 5gr fillet) by hand in the lab.
Preparation of essential oil
The vegetal material used was Cuminum cyminum L., Zataria multiflora Boiss and Rosmarinus officinalis provided by a local herbal store in Tehran, Iran, and fresh leaves of Wild mint were collected (June 2019) from the botanical garden of the research center of medicinal plants in Islamic Azad University, Tehran, Iran. We applied the steam distillation method commonly used for commercial purposes.[Citation31] Briefly, leaves of each plant were dried by an oven (Shimi Az, Iran) at 55°C to a constant weight. Then all the samples were well powdered with a blender. (Pars khazar, Iran). The plant materials that passed through an 80-emesh sieve were retained for use. Then the plants were immersed in water, where the system was heated up to water’s boiling point. In the second part, the plants were inserted in contact with water and fatty acid ethyl esters. The system was heated up to boiling point. The homogeneous mixture is composed of the essential oil. The protocol essential oil extraction used in cohdrydistillation depended on the vegetal structure used, based on reporting by Hernández-Ochoa (2005). Extraction was done by using 200 g of vegetal material, 4 L of water and 20 mL of ethyl.
Nanoemulsion preparation
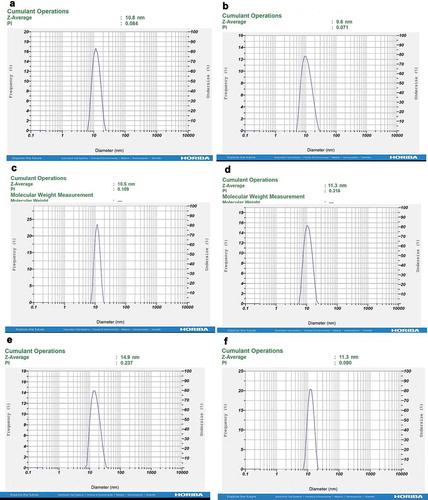
In the synthesis of 3% Nanoemulsion of the essential oil by ultrasonic homogenization, first, at 50°C, the aqueous phase consisting of 10 g surfactant tween 80 (Sigma_Aldrich, Germany), 4 g surfactant tween 20 (Sigma_Aldrich, Germany), 2 g surfactant span 80 (Sigma_Aldrich,Germany) and 3 g of essential oil were mixed completely. Then at room temperature 81 ml deionized distilled water was added to mixture to uniform solution. The solution was sonicated for 30 minutes (with a probe of 7 ml diameter and 85% power) to form a homogeneous and transparent mixture. The Nano emulsified droplet structure for measuring of the size distribution of droplets was analyzed using dynamic light scattering (DLS) (Mason, Wilking, Meleson, Chang, & Graves, 2006). In this study, the size distribution in the Nanoemulsion was measured by dynamic light scattering (DLS) technique using a Malvern Zeta seizer Nano-series instrument (HORIBA SZ-100 for Windows [Z Type] Ver2.20)
Low-energy method (Emulsion phase inversion: catastrophic phase inversion)[Citation32] was used as a second Nanoemulsion method. In this study, we focused on the formation of nanoemulsions using the emulsion phase inversion (EPI) method, which is based on a catastrophic phase inversion that occurs when water is titrated into a system containing a mixture of oil and a hydrophilic surfactant.[Citation33,Citation34]
Treatment of fish samples
Fish samples were divided into three groups, each one consisted of eight sub-groups. The weights of the samples were considered to be 10 ± 1 g for microbial tests and 12 ± 1 g for chemical tests. Group 1 consisting of eight sub-groups of nanoemulsified Cuminum cyminum L. (Cuminum group), group 2 consisting of eight sub-groups of nanoemulsified Zataria multiflora Boiss (Zataria group) and group 3 consisting eight sub-groups of nanoemulsified Rosmarinus officinalis (Rosemary groups) oils. Samples of each sub-group were prepared by floating fish fillet into 3.0% and 5.0% of USH and EPI nanoemulsified EOs for 1, 3, 7 and 14 days. Two close EOs concentration were considered for each treatment to evaluate the importance of conservator value. After draining, all samples separately put in Low-density polyethylene (LDPE) bags were stored at 4 ± 1°C or 10 ± 1°C for short and long time. In each group, two sub-groups were considered as control which their samples dipped in distilled water. Chemical, microbiological and sensory characteristics were investigated during storage at 1, 3, 7 for up to 14 days.
Microbiological analysis of nanoemulsified herbal oils
The Microbiological analysis was done according to Cava et al. (2007) research. The samples were cultured with sterile trypticase soy broth (BDDIFCO) and each tube was inoculated with 1.5 × 108 cell/mL of the different bacterial strains. Sufficient concentration of bacteria got by diluting bacteria in a phosphate buffer solution until the turbidity comparable to the McFarland 0.5 nephelometer tube was achieved. The minimum inhibitory concentration (MIC) was used to observe the growth of bacteria in the different tubes up to 24 h incubation at 37°C.
Antioxidant activity assay
Peroxidase value (PV) and Malondialdehyde levels (MDA) were taken as the parameters for the assessment of lipid peroxidation and antioxidative activity of nanoemulsion EOs in all groups during 14 days of storage at 4°C and 10°C. Thiobarbituric acid assay (TBA) is the most commonly used method to determine the MDA levels. PV and TBA were analyzed according to the previously reported researches (AOCS, 1989; Bhanger et al., 2008; AOCS, 2006). These assessments were carried out three times and all data were represented as mean ± STD.
Statistical analysis
To analyze the effect of the addition of oils and extracts of meat, a one-way ANOVA was used. Tukey was used for analysis of media, using a 0.95% confidence level. Statistical analysis was done using the SPSS software (version 16.0). All analysis was done by triplicate.
Results
Particle size characteristics
Droplet size was analyzed by dynamic light scattering (DLS) technique. Mean droplet sizes were found in the range of 10.5–10.8 nm for Cuminum cyminum L. 5% (EPI; 10.4 ± 2.8 & USH; 10.7 ± 1.7) ()) and 9.4–9.9 nm for Cuminum cyminum L. 3% (EPI; 9.5 ± 2.1 & USH; 9.9 ± 2.5) ()) and 9.3–10.5 nm for Rosmarinus officinalis 5% (EPI; 9.3 ± 4.5 & USH; 10.5 ± 1.6) ()) and 11.2–11.8 for Rosmarinus officinalis 3% (EPI; 11.8 ± 1.6 & USH; 11.2 ± 4.3) ()). Moreover, the results showed that the mean droplet sizes for Zataria multiflora Boiss oil 5% ()) was 14.5–14.9 and for Zataria multiflora Boiss oil 3% was 10.9–11.5 in both methods ()). After one month, DLS is taken again and their durability was confirmed (Cuminum cyminum L. have the best response) (supplementary material).
Antimicrobial activity of nanoemulsion EOs
The results of the Rosemary groups fabricated by USH method did not show significant bacterial growth in both 3% and 5% concentration and at both temperatures of 4°C and 10°C in comparison to control group (p > .05). But bacterial growth reduced by those was made by EPI method in 3% concentration 24 h after incubation (p < .05), however this reduction was not observed at days 3, 7 and 14. The results showed that Rosmarinus officinalis did not inhibit bacteria growth at different EO concentrations, temperatures and emulsification methods () compared to control groups (P > .05).
Table 1. Antibacterial properties of Rosmarinus officinalis evaluation after applying two different emulsification methods in different concentrations, temperatures and treatment duration with herbal oil
The results of Cuminum group made by USH method maintained at 4°C presented biological activity on all studied bacteria and inhibited their growth at 5% compared to 3% concentration significantly (p < .05). These results were not seen in those made by EPI methods at both concentrations and both temperatures (). Based on the Zataria group results, the number of bacteria in fish sample treated with 3% concentration of emulsified Zataria multiflora Boiss was less in sample prepared by USH method maintain at 4°C than that treated in other situation (p < .05). In the Zataria sub-groups the bacterial count increased over time. In addition, at 5% concentration at 4°C, the rate of bacterial growth was acceptable until day 7 after storage. At 10°C, the results at 3% concentration were better than those of 5% concentration, although this was significantly lower at 4°C. The other results are presented in in days, concentrations, and in two different ways of emulsifying the EOs (). Together, the results showed that the USH method were better than EPI to develop antibacterial properties. Also three nanoemulsifications had better antibacterial activity at 4°C. Concentration of extract had different effects on antibacterial properties ().
Table 2. Antibacterial properties of Cuminum cyminum L. evaluation after applying two different emulsification methods in different concentrations, temperatures and treatment duration with essential oil
Table 3. Antibacterial properties of Zataria multiflora Boiss. Evaluation after applying two different emulsification methods in different concentrations, temperatures and treatment duration with herbal oil
Table 4. Antibacterial properties of Zatariamultiflora Boiss., Cuminumcyminum L., and Rosmarinusofficinalis evaluation after applying two different emulsification methods in different concentrations and treatment duration with herbal oil at 4°C temperature
Table 5. Antibacterial properties of Zatariamultiflora Boiss., Cuminumcyminum L., and Rosmarinusofficinalis evaluation after applying two different emulsification methods in different concentrations and treatment duration with herbal oil at 10°C temperature
Peroxide value in the fish samples during storage period
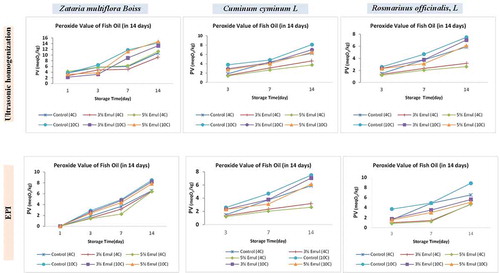
The assessment of peroxide value (PV) as an indicator of oxidative stress was used to detect antioxidative activity of the prepared Cuminum, Zataria and Rosmarinus nanoemulsions. The results showed that the PV values of fish samples treated with all three nanoemulsions prepared by either USH or EPI method were less than that of untreated (control) groups (p < .05). On the other hand, it was observed that the PV significantly increased in all control and treated samples during storage period (14 days) (). The results obtained from the impact of each nanoemulsion of PV are described as follows: the PV showed no significant changes in samples treated with 3% Zataria prepared by USH during storage period at 4°C, comparing to 10°C temperature in which the PV increased in 3% Zataria group. Moreover, it has been demonstrated that an increase of USH-prepared Zataria concentration to 5% had no effect on PV reduction and the elevated temperature led to a significant increase of PV in both 3% and 5% Zataria-treated groups. In both 3% and 5% Rosmarinus groups, no significant change in the PV was observed at 4°C condition, however, an increasing rate of PV was recorded at 10°C temperature during storage period. The Cuminum-treated groups exhibited the same results as Rosmarinus groups. Among three nanoemulsions prepared by USH method, Cuminum and Rosmarinus presented more effective impacts on the reduction of PV. Furthermore, comparing to USH, the nanoemulsions prepared by EPI method demonstrated more proper effects on PV reduction at 4°C condition. The highest efficiency in reduction of PV was attributed to Rosmarinus group. Consequently, the most significant antioxidative activity in order for reduction of PV was observed in both 3% and 5% nanoemulsions prepared by EPI method and at 4°C condition.
Figure 2. Evaluation of peroxidase value (PV) in control and treated samples at 4 and 10°C temperatures during 14 days as the storage period. The treatments were performed using three Cuminum, Rosmarinus, and Zataria nanoemulsions developed by two USH and EPI emulsification methods and prepared in 3% and 5% concentrations
Alterations in MDA levels in the fish samples during storage period
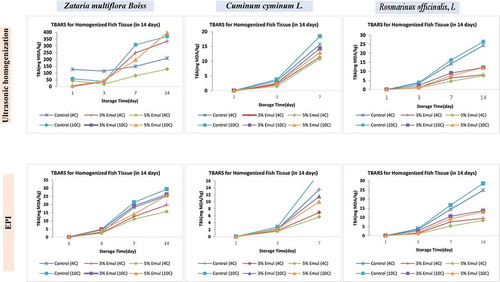
Evaluation of alterations in MDA values was used to describe the degree of lipid oxidation as the second stage of auto-oxidation process. The results showed that MDA levels in control sub-groups were significantly higher than that of the nanoemulsion-treated sub-groups (p < .05) (). In case of Zataria nanoemulsion, the resulting data showed at none of 3% and 5% concentrations of USH-prepared Zataria were effective on reduction of MDA production. Comparing to Zataria, it has been demonstrated that USH-prepared Cuminum in both concentrations of 3% and 5% and at both 4°C and 10°C exhibited more effective antioxidative activity. Nevertheless, the most impressive antioxidative effects belonged to USH-prepared Rosmarinus nanoemulsion with both 3% and 5% concentration, and at 4°C temperature. In case of nanoemulsions prepared through EPI method, 3% and 5% Cuminum treatment notably led to a decrease in lipid oxidation at 4°C, however, the reducing effect of 3% and 5% Cuminum was less at 10°C condition. Similarly, 3% and 5% Rosmarinus nanoemulsions were able to significantly reduce the MDA levels at 4°C, whereas this reduction rate of MDA was considerably low at 10°C. As a result, the obtained results elucidated that the reduction of MDA level occurred more efficiently in EPI-prepared nanoemulsions than USH-prepared ones. Furthermore, among three nanoemulsions, Cuminum manifested a higher antioxidative activity in comparison to Zataria and Rosmarinus.
Figure 3. Evaluation of MDA levels (lipid oxidation) in control and treated samples at two 4 and 10°C temperatures during 14 days as the storage period. The treatments were performed by three Cuminum, Rosmarinus, and Zataria nanoemulsions developed via two USH and EPI emulsification methods and prepared in 3% and 5% concentrations
Discussion and conclusion
One of the current applications of nanotechnology in food industry is to increase the quality and durability of food packaging via nanoscale additives and developing the delivery systems of biologically active compounds.[Citation35,Citation36] Nanoemulsions are the most new device for preparing food packaging. In this study, we fabricate three herbal EO nanoemulsions via two available mechanisms. Our results showed that those made through Ultrasonic Homogenization method compared to EPI have better antibacterial effect on fish samples. This seems to be due to the production procedure. For this, in the first step, unstable interfacial waves were produced by an acoustic field, so oil phase erupts into the water medium forming droplets.[Citation37] Another reason is that acoustic cavitation occurs by using low-frequency ultrasound. It causes the formation and consequent collapse of microbubbles by the pressure fluctuations due to a simple sound wave applying. These microscopic implosions resulted in huge level of highly localized turbulence. The turbulent micro-implosions as a very effective method cause to break up initial droplets of dispersed oil into droplets of sub-micron scope.[Citation38] It seems that the better results in applyingUltrasonic Homogenization in the present study are related to the physical properties of the ultrasound compared to the EPI emulsification method.
The results also exhibited that Cuminum has the best anti-bacterial results among the three examined essential oils. Parallel to our research, other scientific teams revealed that the essential oils, such as cumin’s applied to fillet samples to prolong their shelf life, are effectiveness via inhibiting pathogen growth and raising the antioxidant activity.[Citation39–41]
Iacobellis and coworkers were reported the antibacterial activity of Cuminum L. .[Citation42] It has lethal effects on either gram-negative or gram-positive bacteria. Besides, our findings were in line with the results of Singh, Kapoor, Pandey, Singh, & Singh (2002), who reported that the essential oil of cyminum L. oil is more effective compared with conventional antibiotics, even at low doses.[Citation43] In the case of Cuminum L., the results of the disc diffusion method and minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) assays demonstrated that E. coli is the most sensitive microorganism that possesses the lowest MBC value.[Citation44] The polyphenol profile of Cuminum has been widely described in the scientific literature .[Citation45–48] According to the results of GC and GC–MS analyses Cyminum L. contains the active compounds including α-pinene (29.1%), 1,8-cineole (17.9%) and linalool (10.4%) (Reisi et al.). Cumin is a potent aromatic compound derived from the dried ripe fruit (seed) of Cuminum L. belongs to the Apiaceous family (parsley family).[Citation49]
Also nanoemulsified Zataria multiflora Boiss decreased growth of bacteria especially those prepared by Ultrasonic Homogenization method compared to EPI. This data is in accordance with results of Moosavy et al. study. They evaluated the impact of Zataria multiflora Boiss and reported that the essential oil and nisin had a great effect on Salmonella typhymurium and Staphylococcus aurous in a food model system.[Citation50] Also Basti-Akhondzadeh et al. reported that Zataria multiflora Boiss had inhibitory properties against Salmonella typhimurium and Staphylococcus aurous in brain heart infusion broth.[Citation51] Carvacrol (26.08%), p-cymene (20.34%), thymol (17.23%), and linalool (10.09%) are the most plentiful components constituting 73.74% of the oil.[Citation52] They observed that thymol (37.59%), carvacrol (33.6%), ρ-cymene (7.72%), γ-terpinene (3.88%), and β-caryophyllene (2.06%) were the major ingredient of Zataria multiflora Boiss.[Citation53] The active components of Zataria multiflora Boiss are phenolic compounds such as thymol and caracole. Carvacrol (61.29%) and thymol (25.18%) were the major components of ZMEO in the Yazd Province area of Iran. Carvacrol enhances the inhibitory properties of several antibiotics against zoonotic pathogens and food spoilage bacteria such as Salmonella typhimurium and Streptococcus pyogeneserm B.[Citation53]
In this research the essential oil of Rosemary oil exhibited less potent activity than C. cyminum. Sienkiewicz et al. reported that the essential oil of rosemary mainly has 1,8-cineole (46.4%), camphor (11.4%) and α-pinene (11.0%).[Citation54] The elements of the rosemary essential oil applied by Jiang et al. chiefly composed of 1, 8-cineole (26.54%) and α-pinene (20.14%).[Citation55] Bendeddouche et al. reported that the tested essential oil contains mainly camphor (37.6%), 1, 8-cineole (10.0%), p-cymene-7-ol (7.8%) and borneol (5.4%).[Citation56] The anti-inflammatory, anti-diabetes mellitus and analgesic properties and of camphor are reported more than its other properties such as antibacterial effect [Citation57; Citation58] which are in parallel by our results.
As well, nanoemulfication of Cuminum cyminum L, Zataria multiflora Boiss and Rosmarinus officinalis has antioxidant activity. This essential oil is useful in decreasing the peroxide agents and lipid peroxidation evaluated by Peroxide value (PV) assay. Other studies have revealed similar effects that the usefulness of essential oils such as cumin in increasing the life of fillet samples was through increasing the antioxidant activity.[Citation39–41] In rosemary extraction, the other compounds found are rosmarinic acid and hydroxy hydrocaffeic acid, showing some complementary antioxidant activity. The extraction of rosemary also has other caffeic acid derivatives. These compounds are capable of reacting with metal ions, thus forming chelates. These substances can react with peroxide radicals’s thus stabilizing these free radical agents. The essential oil of Rosemary is used as a food seasoning, [Citation59] as a result of its chemical compound possessing the antifungal, antibacterial, and antioxidant activity.
Also, the rate of TBARS formation in control samples was markedly higher than treated samples during the storage time. Such a difference in TBARS formation between the treated and untreated samples confirmed the antioxidant properties of the plant compounds used in this study. Essential oils applied in this study had acceptable antioxidant potential, the best of them was C. cyminum particularly those that were made using the Ultrasonic Homogenization method. These compounds in its nonvolatile extracts have high antioxidant activity.
The antioxidant activity of Cuminum L. oil showed the optimal results when used at the concentrations of 3% and 5% at 4°C according to the methods of Ultrasonic Homogenization and EPI. Besides, the results obtained from Rosmarinus officinalis were as the same as Cuminum L. in both emulsifying methods. The findings of our study revealed that the use of emulsified EOs at suitable temperature can lead to decrease in the growth of bacteria during fish fillet storage. This observation displayed that the temperature had a significant effect on food preservation also in the case of emulsification forms of all three types of vegetable oils. We could not obligate the temperature of storage also by this fabrication formula.
It can be concluded that using Ultrasonic Homogenization method to produce nanoemolusifaction has better consequence at anti-bacterial and antioxidant activity. These properties can be employed in the food industry to achieve suitable alternatives to synthetic chemicals (namely BHT, phenolic compounds). In this context, the essential oils of R. Officinal are and C. cyminum, showed the optimal results in terms of antimicrobial activity, the ability to neutralize free radicals, and preventing the oxidation of unsaturated fatty acids. They can be utilized for the production of new natural antioxidants as well as flavoring agents in different food products. The results may help to understand how these essential oils have antioxidative and antimicrobial activity.
Supplemental Material
Download MS Word (1.7 MB)Disclosure statement
The authors have declared no conflicts of interest.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
References
- Organization W. H. Healthy Diet; World Health Organization. Regional Office for the Eastern Mediterranean, 2019.
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I. P.; Speijers, G.; Chiodini, A.; Recker T. Impact of Food Processing and Detoxification Treatments on Mycotoxin Contamination. Mycotoxin Res. 2016, 32(4), 179–205.
- Chatterjee, A.; Abraham, J. Microbial Contamination, Prevention, and Early Detection in Food Industry. In Microbial Contamination and Food Degradation; Elsevier, 2018; pp 21–47.
- Saeed, F.; Afzaal, M.; Tufail, T.; Ahmad, A. Use of Natural Antimicrobial Agents: A Safe Preservation Approach. Active Antimicrobial Food Packag. 2019, 0–18.
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial Peptides as Natural Bio-preservative to Enhance the Shelf-life of Food. J. Food Sci. Technol. 2016, 53(9), 3381–3394.
- Hassoun, A.; Çoban, Ö. E. Essential Oils for Antimicrobial and Antioxidant Applications in Fish and Other Seafood Products. Trends Food Sci. Technol. 2017, 68, 26–36. DOI: 10.1016/j.tifs.2017.07.016.
- Al-Mansoori, D. H. Efficacy of Essential Oil Nanoemulsion Delivery System for strong Antimicrobial Action against Pathogen Listeria Monocytogenes: Rutgers the State University of New Jersey; School of Graduate Studies, 2018.
- Alarcón-Moyano, J.; Matiacevich, S. Active Emulsions Based on Alginate and Lemongrass/citral Essential Oils: Effect of Encapsulating Agents on Physical and Antimicrobial Properties. Int. J. Food Prop. 2019, 22(1), 1952–1965. DOI: 10.1080/10942912.2019.1698605.
- Eslahi, H.; Fahimi, N.; Sardarian, A. R. Chemical Composition of Essential Oils. Essent. Oils Food Process. 2017, 119–171.
- Senanayake, S. N. Rosemary Extract as a Natural Source of Bioactive Compounds. J. Food Bioactives. 2018, 2, 51–57. DOI: 10.31665/JFB.2018.2140.
- Tyagi, A. Rosemary: An Overview of Potential Health Benefits, 2017.
- Georgantelis, D.; Ambrosiadis, I.; Katikou, P.; Blekas, G.; Georgakis, S. A. Effect of Rosemary Extract, Chitosan and α-tocopherol on Microbiological Parameters and Lipid Oxidation of Fresh Pork Sausages Stored at 4 C. Meat Sci. 2007, 76(1), 172–181.
- Hadian, J.; Ebrahimi, S. N.; Mirjalili, M. H.; Azizi, A.; Ranjbar, H.; Friedt, W. Chemical and Genetic Diversity of Zataria Multiflora Boiss. Accessions Growing Wild in Iran. Chem. Biodiversity. 2011, 8(1), 176–188.
- Sajed, H.; Sahebkar, A.; Iranshahi, M. Zataria Multiflora Boiss. (Shirazi Thyme)—an Ancient Condiment with Modern Pharmaceutical Uses. J. Ethnopharmacol. 2013, 145(3), 686–698. DOI: 10.1016/j.jep.2012.12.018.
- Gajera, H.; Savaliya, D. D.; Hirapara, D. G.; Patel, S.; Golakiya, B. Biocontrol Mechanism of Bacillus for Fusarium Wilt Management in Cumin (Cuminum Cyminum L.). In Current Trends in Plant Disease Diagnostics and Management Practices; Springer, 2016; pp. 29–47.
- Wanner, J.; Bail, S.; Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Gochev, V.; Girova, T.; Atanasova, T.; Stoyanova, A. Chemical Composition and Antimicrobial Activity of Cumin Oil (Cuminum Cyminum, Apiaceae). Nat. Prod. Communicat. 2010, 5(9), 1934578 × 1000500904.
- Bhushani, A.; Anandharamakrishnan, C. Food-grade Nanoemulsions for Protection and Delivery of Nutrients. In Nanoscience in Food and Agriculture 4; Springer, 2017; pp 99–139.
- Putatunda, S.; Bhattacharya, S.; Sen, D.; Bhattacharjee, C. A Review on the Application of Different Treatment Processes for Emulsified Oily Wastewater. Int. J. Environ. Sci. Technol. 2019, 16(5), 2525–2536.
- Komaiko, J. S.; McClements, D. J. Formation of Food‐grade Nanoemulsions Using Low‐energy Preparation Methods: A Review of Available Methods. Compr. Rev. Food Sci. Food Saf. 2016, 15(2), 331–352. DOI: 10.1111/1541-4337.12189.
- McClements, D. J.; Xiao, H. Potential Biological Fate of Ingested Nanoemulsions: Influence of Particle Characteristics. Food Funct. 2012 Mar, 3(3), 202–220. DOI: 10.1039/c1fo10193e. PubMed PMID: 22105669; eng.
- Donsì, F.; Ferrari, G. Essential Oil Nanoemulsions as Antimicrobial Agents in Food. J. Biotechnol. 2016, 233, 106–120. DOI: 10.1016/j.jbiotec.2016.07.005.
- Dasgupta, N.; Ranjan, S.; Mundra, S.; Ramalingam, C.; Kumar, A. Fabrication of Food Grade Vitamin E Nanoemulsion by Low Energy Approach, Characterization and Its Application. Int. J. Food Prop. 2016, 19(3), 700–708. DOI: 10.1080/10942912.2015.1042587.
- Jasmina, H.; Džana, O.; Alisa, E.; Edina, V.; Ognjenka, R. Preparation of Nanoemulsions by High-energy and Lowenergy Emulsification Methods. In Cmbebih 2017; Springer, 2017; pp. 317–322.
- Solans, C.; Solé, I. Nano-emulsions: Formation by Low-energy Methods. Curr. Opin. Colloid Interface Sci. 2012, 17(5), 246–254. DOI: 10.1016/j.cocis.2012.07.003.
- Okamoto, T.; Tomomasa, S.; Kakoki, H.; Nishiyama, S.; Nakajima, H. Oil-in-water Type Emulsion Composition. Google Patents; 1997.
- Muela, E.; Alonso, V.; Morago, P.; Calanche, J. B.; Roncalés, P.; Beltrán, J. Effect of Gas Packaging Conditions on Thawed Thunnus Obesus Preservation. Food Control. 2014, 46, 217–224. DOI: 10.1016/j.foodcont.2014.05.022.
- Alfnes, F.; Rickertsen, K.; Shogren, J. F. Test‐Retesting in Experimental Valuation of Perishable Food Products: Unstable Individual Bids and Reliable Market Demand. J. Agricultural Econ. 2018, 69(2), 382–392. DOI: 10.1111/1477-9552.12248.
- Wang, C.; Shelef, L. A. Behavior of Listeria Monocytogenes and the Spoilage Microflora in Fresh Cod Fish Treated with Lysozyme and EDTA. Food Microbiol. 1992, 9(3), 207–213. DOI: 10.1016/0740-0020(92)80048-9.
- Prasad, M.; Seenayya, G. Effect of Spices on the Growth of Red Halophilic Cocci Isolated from Salt Cured Fish and Solar Salt. Food Res. Int. 2000, 33(9), 793–798. DOI: 10.1016/S0963-9969(00)00100-9.
- Barakat, S. Reconstructing War-torn Societies; Palgrave Macmillan: Afghanistan, 2004.
- Conde-Hernández, L. A.; Espinosa-Victoria, J. R.; Trejo, A.; Guerrero-Beltrán, J. Á. CO2-supercritical Extraction, Hydrodistillation and Steam Distillation of Essential Oil of Rosemary (Rosmarinus Officinalis). J. Food Eng. 2017, 200, 81–86. DOI: 10.1016/j.jfoodeng.2016.12.022.
- Ostertag, F.; Weiss, J.; McClements, D. J. Low-energy Formation of Edible Nanoemulsions: Factors Influencing Droplet Size Produced by Emulsion Phase Inversion. J. Colloid Interface Sci. 2012, 388(1), 95–102.
- Fernandez, P.; Andre¢, V.; Rieger, J.; Kuhnle, A. Nano-emulsion Formation by Emulsion Phase Inversion. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251(1–3), 53–58. DOI: 10.1016/j.colsurfa.2004.09.029.
- Thakur, R. K.; Villette, C.; Aubry, J.; Delaplace, G. Dynamic Emulsification and Catastrophic Phase Inversion of Lecithin-based Emulsions. Colloids Surf. A. 2008, 315(1–3), 285–293.
- Handford, C. E.; Dean, M.; Spence, M.; Henchion, M.; Elliott, C. T.; Campbell, K. Awareness and Attitudes Towards the Emerging Use of Nanotechnology in the Agri-food Sector. Food Control. 2015, 57, 24–34. DOI: 10.1016/j.foodcont.2015.03.033.
- Kishore, P.; Panda, S. K.; Minimol, V.; Mohan, C.; Ravishankar, C. Nanotechnology for Pathogen Detection and Food Safety. 2018; Research Methodology in Food Sciences: Apple Academic Press; p. 21–36.
- Li, M.; Fogler, H. Acoustic Emulsification. Part 1. The Instability of the Oil-water Interface to Form the Initial Droplets. J. Fluid Mechan. 1978, 88(3), 499–511. DOI: 10.1017/S0022112078002232.
- Li, M.; Fogler, H. Acoustic Emulsification. Part 2. Breakup of the Large Primary Oil Droplets in a Water Medium. J. Fluid Mechan. 1978, 88(3), 513–528. DOI: 10.1017/S0022112078002244.
- Jayathilakan, K.; Sharma, G.; Radhakrishna, K.; Bawa, A. Antioxidant Potential of Synthetic and Natural Antioxidants and Its Effect on Warmed-over-flavour in Different Species of Meat. Food Chem. 2007, 105(3), 908–916.
- Kalchayanand, N.; Arthur, T. M.; Bosilevac, J. M.; BRICHTA-HARHAY, D. M.; GUERINI, M. N.; WHEELER, T. L.; KOOHMARAIE, M. Evaluation of Various Antimicrobial Interventions for the Reduction of Escherichia Coli O157: H7 on Bovine Heads during Processing. J. Food Prot. 2008, 71(3), 621–624.
- Farias, P. K. S.; Silva, J. C. R. L.; Souza, C. N. d.; Fonseca, F.S.A.d.; Brandi, I.V.; Martins, E.R.; Azevedo, A.M.; Almeida, A.C.d. Antioxidant Activity of Essential Oils from Condiment Plants and Their Effect on Lactic Cultures and Pathogenic Bacteria. Ciência Rural 2019, 49(2). DOI: 10.1590/0103-8478cr20180140.
- Iacobellis, N. S.; Lo Cantore, P.; Capasso, F.; Senatore, F. Antibacterial Activity of Cuminum Cyminum L. And Carum Carvi L. Essential Oils. J. Agric. Food Chem. 2005, 53(1), 57–61.
- Petretto, G.; Fancello, F.; Bakhy, K.; Faiz, C. A.; Sibawayh, Z.; Chessa, M.; Zara, S.; Sanna, M. L.; Maldini, M.; Rourke, J. P.; et al. Chemical Composition and Antimicrobial Activity of Essential Oils from Cuminum Cyminum L. Collected in Different Areas of Morocco. Food Biosci. 2018, 22, 50–58. DOI: 10.1016/j.fbio.2018.01.004.
- Singh, G.; Kapoor, I.; Pandey, S.; Singh, U. K.; Singh, R. K. Studies on Essential Oils: Part 10; Antibacterial Activity of Volatile Oils of Some Spices. Phytother. Res. 2002, 16(7), 680–682.
- Žegura, B.; Dobnik, D.; Niderl, M. H.; Filipič, M. Antioxidant and Antigenotoxic Effects of Rosemary (Rosmarinus Officinalis L.) Extracts in Salmonella Typhimurium TA98 and HepG2 Cells. Environ. Toxicol. Pharmacol. 2011, 32(2), 296–305.
- Visentin, A.; Rodríguez-Rojo, S.; Navarrete, A.; Maestri, D.; Cocero, M. J. Precipitation and Encapsulation of Rosemary Antioxidants by Supercritical Antisolvent Process. J. Food Eng. 2012, 109(1), 9–15.
- Sasaki, K.; El Omri, A.; Kondo, S.; Han, J.; Isoda, H. Rosmarinus Officinalis Polyphenols Produce Anti-depressant like Effect through Monoaminergic and Cholinergic Functions Modulation. Behav. Brain Res. 2013, 238, 86–94.
- Kontogianni, V. G.; Tomic, G.; Nikolic, I.; Nerantzaki, A. A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I. P.; Tzakos, A. G. Phytochemical Profile of Rosmarinus Officinalis and Salvia Officinalis Extracts and Correlation to Their Antioxidant and Anti-proliferative Activity. Food Chem. 2013, 136(1), 120–129.
- Al-Snafi, A. E. The Pharmacological Activities of Cuminum cyminum-A Review. IOSR J. Pharm. 2016, 6(6), 46–65.
- Moosavy, M.-H.; Basti, A. A.; Misaghi, A.; Salehi, T. Z.; Abbasifar, R.; Mousavi, H. A. E.; Alipour, M.; Razavi, N. E.; Gandomi, H.; Noori, N.; et al. Effect of Zataria Multiflora Boiss. Essential Oil and Nisin on Salmonella Typhimurium and Staphylococcus Aureus in a Food Model System and on the Bacterial Cell Membranes. Food Res. Int. 2008, 41(10), 1050–1057.
- Basti, A., {published data only}; Akhondzadeh Basti, A.; Moshiri, E.; Noorbala, A. A.; Jamshidi, A. H.; Abbasi, S. H.; Akhondzadeh, S. Comparison of Petal of Crocus Sativus L. And Fluoxetine in the Treatment of Depressed Outpatients: A Pilot Double-blind Randomised Trial. Progress Neuropsychopharmacol. Biol. Psychiatry. 2007, 31(2), 439–442.
- Shahsavari, N.; Barzegar, M.; Sahari, M.; Naghdi Badi, H. An Investigation on the Antioxidant Activity of Essential Oil of Zataria Multiflora Boiss. In Soy Bean Oil. J. Med. Plants. 2008, 4(28), 56–68.
- Shafiee, A.; Javidnia, K. Composition of Essential Oil of Zataria Multiflora. Planta Med. 1997, 63(04), 371–372. DOI: 10.1055/s-2006-957707.
- Sienkiewicz, M.; Łysakowska, M.; Pastuszka, M.; Bienias, W.; Kowalczyk, E. The Potential of Use Basil and Rosemary Essential Oils as Effective Antibacterial Agents. Molecules. 2013, 18(8), 9334–9351.
- Jiang, Y.; Wu, N.; Fu, Y.-J.; Wang, W.; Luo, M.; Zhao, C. -J.; Zu, Y. -G.; Liu, X.-L. Chemical Composition and Antimicrobial Activity of the Essential Oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32(1), 63–68.
- Bendeddouche, M. S.; Benhassaini, H.; Hazem, Z.; Romane, A. Essential Oil Analysis and Antibacterial Activity of Rosmarinus Tournefortii from Algeria. Nat. Prod. Communicat. 2011, 6(10), 1934578 × 1100601026.
- Arranz, E.; Jaime, L.; García‐Risco, M. R.; Fornari, T.; Reglero, G.; Santoyo, S. Anti‐inflammatory Activity of Rosemary Extracts Obtained by Supercritical Carbon Dioxide Enriched in Carnosic Acid and Carnosol. Int. J. Food Sci. Technol. 2015, 50(3), 674–681.
- Kültür, Ş. Medicinal Plants Used in Kırklareli Province (Turkey). J. Ethnopharmacol. 2007, 111(2), 341–364. DOI: 10.1016/j.jep.2006.11.035.
- Lo Presti, M.; Ragusa, S.; Trozzi, A.; Dugo, P.; Visinoni, F.; Fazio, A.; Dugo, G.; Mondello, L. A Comparison between Different Techniques for the Isolation of Rosemary Essential Oil. J. Sep. Sci. 2005, 28(3), 273–280.