?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to determine the effects of different drying methods on the physicochemical properties of mango powders (Amropali cultivar) and its reconstituted products. Six types of drying methods were employed, which were spray drying (SD), cabinet drying (CD), vacuum drying (VD), tunnel drying (TD), rotary oven drying (RoD) and gas oven drying (GoD). For drying temperature was maintained around 60 to 70°C for all dryers except for SD for which temperature was kept at 150°C. It was found that drying methods affected the physicochemical properties of mango powders (Amropali cultivar) and its reconstituted products. SD and CD powders showed better physicochemical properties (water activity, rehydration, solubility, and porosity) than other mango powder samples. In addition, mango powder produced through SD and CD methods showed lighter color, whereas mango powder prepared using other drying methods showed yellowish, dull, and dark colors. However, reconstituted products of SD and CD powders gave almost similar physicochemical properties compared to other dried samples. The present study concludes that SD powder and CD powder are more preferable in terms of producing high-quality products compared to other drying techniques.
Introduction
The season of mango (Mangifera indica L.) in Bangladesh is summer (April-July). There are several varieties of mangoes available in Bangladesh. The most widely cultivated mango cultivars in Bangladesh are BARI AAM- 3 (Amropali), Fazlee, Lengra, Gopalbogh, Himsagar, Khirsapat, Ashhwina, Khisanbogh, Kuapahadi, Lata Bombai, Foria, Bombai, Kohitoor, Laksmanbhog, Mohanbhog and Misribhog. Fazlee, Lengra, Gopalbhog, and Khisrapat are considered as premier varieties.[Citation1] Rashid et al.[Citation2] reviewed extensively about the physicochemical properties of five mango varieties, viz. Fazli, Amropali, Langra, Gopalbogh and Misribhog, which are available in Northern Bangladesh. Among those five varieties, Fazli variety contained high amount of moisture. Protein and non-reducing sugar were high in Langra variety, whereas the Gopalbogh variety had high reducing sugar. The Misribhog variety was rich in high amount of total sugar and fiber. However, the Amrapali variety comprised high fat, ash and carbohydrate which provides ultimately more energy. It holds high total soluble solids. Ali et al.[Citation3] also highlighted the physical and chemical changes of various cultivars, such as, Guti aam, Amropali, Harivanga, Khirsapat, Fazli, Langra, and Ashwina over various experimental analysis. The authors also stated that Amropali and Khirsapat variety were not big in size. However, in terms of taste, Amropali leads the most preferred fruit for consumption and processing in food industries. Amropali is very popular all over the world due to its sweetness and non-fibrous flesh. Therefore, in this study, BARI AAM-3 variety was used, which is also known as “Amropali”.
The global production volume of mangoes reached 50.65 million metric tons in 2017.[Citation4] Bangladesh is among the major producer of mangoes alongside India, Pakistan, Mexico, Brazil, and the Philippines.[Citation5] The total cultivation area of mango was 102,939 acres with a total production of 128,833,15 metric tons in Bangladesh in 2017.[Citation6] However, the post-harvest losses have been affecting the profitability of mango cultivation. Although the global production of mango is increasing, a significant proportion of mango is spoiled every year due to its perishable nature, inappropriate handling, and lack of conservation and storage facilities. In Bangladesh, the average post-harvest loss is about 34%.[Citation7]
Mangoes are not fully utilized in the producing countries due to their perishable nature and short harvest season. Roughly 20% of mangoes are used to prepare processed items such as puree, nectar, pickles, leather, chutney and canned slices.[Citation7] Moreover, a large number of people prefer mango juice which can be found in either liquid form or powder form. Mango powder can be used for preparing drinks, desserts, baby foods, and confectionaries. In addition, dried green mango powder can be added to curries, chutneys, and soups to impart fruity flavor.
Dehydration of mango juice produces powder form, which gives a significant decrease in volume and is a good strategy to preserve mangoes.[Citation8] The dehydrated powdered mango puree increases the handling properties and shelf-life of the product. There are several ways to convert fruit juice into powder. However, the most effective ways are spray drying, freeze-drying, foam mat drying, drum drying, and vacuum drying.[Citation9]
Spray drying is generally used in order to produce milk, fruits and vegetable powders.[Citation10] In this process, the feed is pumped into a drying chamber through an atomizer, which breaks the liquid stream into droplets and dehydration occurs by the contact of hot air. The fluid droplets dry up in seconds due to profoundly effective heat and mass exchanges.[Citation11] However, it is difficult to splash dry sugar-rich materials, for example, mangoes easily, since they tend to adhere to the walls of the dryer.[Citation12] To make the drying easy, carrier material, such as maltodextrin is broadly added to the feed to build the glass transition temperature of the dried item which eventually helps to solve the issue of stickiness during spray drying.[Citation13]
In vacuum drying, moisture is removed from food products under low pressure. Moisture is removed from the food products by the production of latent heat required for evaporation, after spreading a thin layer of food on a heated plate in the vacuum dryer.[Citation14] Another method of drying is a cabinet drying in which, the air is flowed into the warming wire and warmed up to the necessary temperature. The material which is being dried is scattered on the levels of the plate.[Citation15] The plate, which is utilized in this procedure probably punctured, strong or wirework bottoms.[Citation16] For the dissemination of the air over the drying materials, the screen plate is fixed with paper. A constrained measure of warmth is given to each withdraw when the breeze ignores it to give the inert warmth of vaporization. This sort of dryer gives legitimate control of dampness and temperature.[Citation17]
Tunnel dryer is a direct and consistent sort of dryer which is used as a large-scale dryer. The materials to be dried are transferred to the air warmed passage for drying purposes in this drying method.[Citation15] The material is entered through one side, and the dried material is gathered at the opposite ends of the passage. The active material meets the approaching air to guarantee the most extreme drying and the active air contacts the wettest material with the goal that the air is as almost soaked as could reasonably be expected.[Citation18] A rotary dryer is also known as a tumbling dryer. Heat is added to or expelled from the solids by direct exchange between the gas and solids.[Citation19]
All gas broilers have the primary burner on the base of the stove compartment, generally protected by a metal sheet.[Citation15] This fundamental burner gives extraordinarily brilliant warmth upwards into the principle stove compartment. Gas is extremely effective as gas broilers will generally warmth the product rapidly.[Citation20] Although the slices of dried mangoes dripped in sugar are sold as snack item, dried mango powder is not still widely available commercially. Dried mango powder can not only be added diversely to food products, but it also increases the value addition with desired functionality to health benefits with minimum transportation cost. However, drying can affect the nutritional quality and antioxidant properties of fresh fruits.[Citation21] Thus, the development of mango powder with minimum losses of nutritional and other physicochemical properties is a major challenge. Therefore, it is very important to investigate the effect of different drying methods on the physicochemical properties of dried mango product. To the best of our knowledge, no research has been carried out on the physicochemical properties of Amropali mango cultivar and its reconstituted product. Thus, this study aimed to investigate the physicochemical properties of dried powders prepared by different drying techniques and the reconstituted products.
Materials and methods
Materials
Whole, ripe fresh mangoes (Amropali cultivar) were purchased from Chapainawabganj, Rajshahi, Bangladesh. The average weight of each mango was 241 ± 2.5 g. The homogeneity in size and peel color was visually maintained. All chemicals used in this study were of analytical grades.
Sample preparation
The ripe fruits were washed thoroughly and trimmed to remove the stem and blossom ends. The fruits were peeled, cut into quarters and blended using a blender (Philips HR 7761, China) to prepare concentrated mango juice/pulp. The yield of mango pulp was 70%, the total soluble solids (TSS) was 17 ± 0.45° Brix and the pH was 4.92 ± 0.04.
Spray drying
A mini spray dryer B-290 (Buchi, Switzerland) with a dehumidifier (B-296, Switzerland) was used for the production of mango powder. A carrier agent, maltodextrin (MD) (DE = 12) (Sigma, USA) with a moisture content of 4.15 ± 0.02% was used. Spray dryer feed was prepared by mixing 1 kg mango pulp, 60 g maltodextrin and 187 mL water. A peristaltic pump (Dingo-QI, China) was used to feed the sample in the dryer. The parameters used were as follows: feed rate, 15 mL/min; compressed air flow rate, 35 m3/h; and inlet temperature, 150°C. The outlet temperature was monitored at around 103°C.
Cabinet drying
Two types of cabinet dryer were used to prepare dried powder, laboratory scale (Genlab 1000-L, UK) and large scale (Sinmag, rack oven-F3, Taiwan). Mango pulp was dried at 70°C for three days.
Vacuum drying
Mango pulp was dried in a vacuum oven (JP Selecta S.A, 4001490-Vaciotem-TV, Spain) operated at 70°C at 1 bar for 24 h.
Tunnel drying
The tunnel dryer used was a direct, continuous type, large scale dryer (NRO-1624, Naogaon Engineering Workshop, Bangladesh). The mango pulp was fed at one end at 70°C and the dried material was collected at the other end of the tunnel after two days.
Rotary oven drying
Rotary rack oven (Arun Rega Three phase Rack Oven, Tamil Nadu, India) was used to dry mango pulp. The drying was continued at 70°C for two days with a rotation of 700 rpm.
Gas oven drying
Mango pulp was dried by a gas oven or deck oven (Sinmag, SM-803A, Taiwan) at 60°C for two days. The principle of gas oven was to generate heat for drying using gas as the fuel.
Preparation of mango powder
The dried mango prepared using different types of dryers was ground using a grinder (Panasonic MX-AC300, India) to prepare fine powder (except spray dried powder). All the dried mango powder samples were stored in plastic airtight containers at 4°C in a refrigerator for further analysis. The sampling codes of mango powders prepared by different drying methods are shown in . Different types of dryers are shown in .
Table 1. Sampling codes of mango powders
Analysis of proximate composition
The proximate composition (moisture, crude protein, crude fat and ash contents) of mango powder was measured according to the method of the Association of Official Analytical Chemists (AOAC).[Citation22] Moisture content was measured by drying the powder samples at 105°C for 24 hrs in an air oven (Eco cell, LSIS-B2V/EC55, Germany). Crude protein content was measured by the Kjeldahl procedure with subsequent steps of digestion (Raypa, MB-12/N), distillation (DNP-1500 MP) and titration. Crude fat content was determined by the Soxhlet extraction method using the Soxhlet apparatus (Gerhardt, EV14, Germany), while anhydrous ether was used as an extracting solvent. Ash content was measured gravimetrically by taking oven-dried sample in a muffle furnace (Witeg FH-12, Korea) at 600°C for four h after charging over an electric heater (Prestige TC-45, China). All the analyses were carried out in triplicates.
Water activity, rehydration, hygroscopicity
Water Activity of mango powder was measured using a water activity meter (Novasina AG, Switzerland) at temperature 25.5 ± 1°C. Rehydration of mango powder was determined according to the method described by Goula et al.[Citation23] The hygroscopicity of mango powder was determined according to the method described by Cai and Corke.[Citation24]
Solubility, bulk density, tapped density
The solubility of mango powder was determined using the procedure developed by Eastman and Moore.[Citation25] as adopted by Cano-Chauca et al.[Citation26] Two grams of mango powder was poured to an empty 10 mL graduated cylinder and the cylinder was kept in a vibrator for 1 min.[Citation23] The volume was then recorded and used to calculate the bulk density () in g/mL. The tapped density (
) was measured by tapping the cylinder for 2.5 min (60 taps/min) using powder characteristics measuring instruments (A.B.D-72, Tsutsui Physics and Chemistry Instrument Co., Ltd. Japan). Then the final volume was recorded and tapped density was expressed as g/mL.[Citation27]
Particle density and porosity
Particle density was measured according to the method described by Jinapong et al.[Citation28] Briefly, 1 g of powder was poured into a 10 mL measuring cylinder with a glass stopper. Then, 5 mL of petroleum ether was gently added, and the mixture was shaken for a few mins until all the powder particles were suspended. Finally, the wall of the cylinder was rinsed with 1 mL petroleum ether and the total volume of petroleum ether and suspended particles were recorded. Particle density was calculated as follows:
Porosity () was calculated using powders particle density (ρP) and tapped density (ρT) as follows Jinapong et al.[Citation28]
Hausner ratio and Carr index
The cohesiveness and the flowability of powders are expressed in terms of the Hausner ratio (HR) and Carr index (CI), respectively. HR and CI were determined using the method described by Jinapong et al.[Citation28]
Water absorption indices and oil absorption indices
The water absorption indices (WAI) and oil absorption indices (OAI) were determined using a method as described by Beuchat.[Citation29] One gram of sample was mixed with 10 mL distilled water or oil for 30 s. The mixture was kept standing for 30 min and centrifugation was performed at 5000 rpm for 30 min. Finally, water or oil was drained up and reweighed the sample. The differences between the final weight and the initial weight was expressed as WAI or OAI.
Color characteristics
The color parameters of the mango powder were determined by a Colorimeter (CR-400, Minolta Co., Japan) with D65 illuminant and 0° viewing angle. The results were expressed by the Hunter color values, L*, a*, and b*, where large and small L* denotes lightness and darkness, +a* and – a* denotes redness and greenness, and +b* and – b* denote yellowness and blueness.
Morphology of powder particle
A small quantity of dried mango powder was coated with a fine layer of platinum, using a fine auto coater (Jeol JEC-3000FC, Japan). The morphology of the samples was observed by field emission scanning electron microscope (Jeol JSM-7610 F, Japan), operated at an accelerating voltage of 5.0 kV.
Preparation of reconstituted product from mango powder
The mango powder samples prepared from different drying methods were rehydrated to the same TSS as of the fresh mango pulp. The quantity of distilled water/g of powder was calculated to obtain the TSS exactly 17 ± 0.45° Brix. The physicochemical properties were determined by the following methods.
Physicochemical and thermo-physical properties of reconstituted product
Titratable acidity
The total titratable acidity was determined by titration with standard sodium hydroxide (0.1 N) solution and values were expressed as citric acid/100 mL of juice.[Citation30]
Total soluble solids (°Brix)
The total soluble solids content of mango juice as °Brix content was determined by a refractometer (Atago PAL-1, Japan). The temperature for the measurement of ○Brix was 25°C.
pH of reconstituted product
The pH of the reconstituted product from mango powder was measured using a pH meter (Hanna, Romania).
Density
The density of the mango juice was determined by the pycnometer.[Citation31] Briefly, a pre-weighed pycnometer (50 mL) was filled with distilled water at 20°C and stoppered. The mass of water was calculated by subtracting the pycnometer weight with water to the empty weight of the pycnometer. The mass of the reconstituted mango juice was calculated following the similar procedure. The density was determined by using the following formula:
Viscosity
The viscosity of the reconstituted product was determined by a viscometer (DV-E Brookfield LV viscometer, USA) with spindle No. 62 at 25°C and velocity of 12 rpm.
Specific heat capacity, thermal conductivity, and thermal diffusivity
Specific heat capacity by the cooling method was determined according to Ahmad and Shahabuddin.[Citation32] Thermal conductivity (k) was determined by oven drying method and it was calculated by the following formula described by Bon et al.[Citation33]
Where, w is the moisture content of the sample. T is the room temp (25°C). The thermal diffusivity was determined by the method described by Gundurao et al.[Citation34]
Where, α is thermal diffusivity (×10−1m2/s), k is the thermal conductivity (Wm−1k−1), ρ is density (g/cm3) and is specific heat (kJ/kg°C).
Statistical analysis
The obtained data in this study were analyzed by one-way ANOVA (Tukey’s Multiple Comparison Test) using SPSS (Statistical Package for the Social Sciences) version 16.0. A significant difference was considered at the level of p < 0.05.
Results and discussion
Proximate composition
(a and b) shows the proximate composition of fresh mango pulp and powder samples prepared by different drying methods. Moisture content is the most important parameter for handling and keeping the long shelf-life of dried ingredients. The initial moisture content of fresh mango pulp was 79.9%. After drying, the moisture content of mango powders prepared using different dryers ranged from 5.80 to 12% after dehydration. For spray drying sample, maltodextrin has been added as anticaking. The lowest moisture content was found in the SD powder (5.80%) and the highest was found in the GoD powder (12%). The moisture content of mango powders in decreasing order are as follows: GoD > TD > VD >RoD > CD(L) > CD(I) > SD. The variation was significantly different, which may be due to the temperature differences in different drying methods.
Table 2. (a). Proximate composition of Fresh Mango. (b). Analysis of proximate composition of mango powders
Crude protein, crude fiber, crude fat and ash contents increased in mango powders due to the elimination of moisture content i.e. increasing concentration of nutrients. Different drying methods produced the powders with significantly different crude protein content. The values varied from 3.3 to 4.4%, where SD powders had the lowest amount of crude protein content. The high temperature (150°C) of spray drying could initiate the Maillard reaction and affect the protein content. The Maillard reaction could occur by the reaction of reducing sugar and amino acid of mango pulp, which typically starts above 120°C. Furthermore, the quality and digestibility of protein also could be lost due to Maillard reaction.[Citation35] Haque and Adhikari[Citation36] reviewed extensively about the protein denaturation during spray drying. The authors summarized that due to thermal or air interface related stresses a significant amount of protein could be inactivated or denatured. On the other hand, VD powder gave a higher value of crude protein. The convective heat transfer of vacuum drying reduces the boiling point of the products to be dried and thus reduces the oxidation, discoloration, other chemical changes[Citation37] and retain much higher nutritional components.[Citation38] However, there was no significant difference between GoD and VD; RoD and TD, which indicates no effect of these drying on the protein content of the powders.
Similar to crude protein, crude fiber, ash content and crude fat were comparatively low in SD powders than other dried powders. The reason might be the higher temperature applied in SD. Islam et al.[Citation39] too highlight the high temperature as the drawback of SD which reduces the quality of heat-sensitive ingredients. Mango powder produced by GoD had the highest fiber content (3.75%) and ash content (1.58%) than other powder samples. In terms of fat content, high value (1.8%) was found in CD(L) and second-highest fat content (1.7%) was found in CD(I). Cabinet drying method showed the most efficient method for retaining fat content, when the temperature was maintained at 70°C.
Physicochemical properties of mango powder
The physicochemical properties of mango powders prepared by different drying methods are shown in .
Table 3. Physicochemical properties of mango powders
Water activity
Water activity is liable for microbiological and biochemical reactions in foods. Water activity of mango powders ranged between 0.27 and 0.42. The water activity of mango powders in decreasing order was as follows: CD(L) > TD, RoD > SD, CD(L), VD, GoD. Lower water activity gives better stability of powder. The current result is in similar range with orange juice powder (0.3–0.4) as reported by Shrestha et al. .[Citation40] Similar range of water activity values has been reported for spray dried date powder (around 0.25) and pumpkin powder prepared by cabinet hot-air drying (0.42 at 60°C and reduced to 0.3 at 70°C) .[Citation41] Water activity might be affected by different processing type (different drying methods), processing time and temperature, carrier agents etc.[Citation42]
Rehydration, solubility and hygroscopicity
The rehydration index for mango powders prepared using different drying methods ranged from 66 to156 s. The rehydration of mango powders in decreasing order was as follows: TD, GoD > VD > RoD, CD(I) > SD. While CD(L) was not significantly different from SD, RoD and GoD. From , there is a positive trend between the moisture content of dried powder and its rehydration index. The agglomerated form of powder due to higher moisture content might have been the reason to obtain higher rehydration value. Indeed, there is a relation between the rehydration and solubility. SD powder (96.4%) had a higher solubility as like CD powder (around 98%). During operation of cabinet dryer, processed air flows to heat wire and is heated up to the desired temperature, then flows through a manifold with evenly scattered holes. This kind of dryers provides proper control of humidity and temperature. However, the solubility of other dried powders showed an almost reverse situation with the rehydration index, which is rational. The low rehydration or high solubility of SD powder might be due to the cell rupture of the mango puree during the processing stage. Caparino et al.[Citation9] also found the highest level of solubility (95%) of SD mango powder compared with other drying techniques. The author mentioned that high atomization of spray drying could break the mango fiber into tiny pieces and increase the solubility. Additionally, the addition of maltodextrin as a carrier agent might also be contributed to the solubility of SD powder. Many researchers observed that maltodextrin has superior solubility, and the solubility of powder increases with an increased amount of maltodextrin.[Citation26,Citation39] On the other hand, GoD powder had the highest rehydration time, which happened to give the highest moisture content. Gas oven has the tendency to heat very quickly. Thus, the outer surface (crust) of the powder may become harder than the internal surface and may increase the overall moisture content of the powder. Additionally, gas ovens also tend to be more humid than either electric or convection ovens because the combustion of the gas releases some moisture into the air of the oven, which leads to issues with browning and crisping of dried sample.
The hygroscopicity of mango powders varied from 0.170 to 0.192 gH2O/g with different drying techniques. The hygroscopicity of mango powders in decreasing order was as follows: TD > GoD > CD(I), SD, CD(L). However, VD was not significantly different than TD and RoD was not significantly different than GoD. Mango powders with high moisture content showed highly hygroscopic behavior. Powders with low moisture content i.e., the concentrated acid and sugar amount in the mango puree may influence to impart high hygroscopicity and stickiness to the powder. Similar results were found in some previous studies.[Citation24,Citation39,Citation43] Although, SD powder had the lower moisture content, the presence of maltodextrin could lower the hygroscopicity. Caparino et al.[Citation9] also mentioned that maltodextrin has very high efficiency to lower the hygroscopicity of dried material.
Density and porosity
Bulk density represents the ratio of the mass of several particles of the material to the total volume they occupy, whereas particle density is the true density of the particles that make up the powder. The result of bulk density, tapped density and particle density significantly varied from 0.48 to 0.58, 0.61 to 0.72, and 1.42 to 1.76 g/mL, respectively. SD powder yielded higher bulk density, tapped density and particle density, which might be due to excessive shrinkage during drying and the addition of maltodextrin. Although, Islam et al.[Citation44] stated that a positive correlation is present between moisture content and bulk density, as the presence of water could provide the denser material than dry solid. However, this study could not find a direct relationship between moisture content and density. This might be due to the heating pattern and drying time applied to dry the mango puree. Lower bulk densities can be obtained due to the direct heating process, as direct heating results the compact particles (drying shrinking) due to the collapse of the solid structure.[Citation1] The porosity of dried powders was ranged from 56 to 63% (). The RoD powder had the highest porosity. The higher porosity indicates the higher void space in the particle, which has a tendency to become degraded or oxidized. [Citation1,Citation44]
Hausner ratio and Carr index
Hausner ratio (HR) and Carr index (CI) of powder were ranged from 1.21 to 1.37; 17.35 to 27.01, respectively (). These values represent the free-flowing properties i.e. the cohesiveness of the powder. The present study indicated that all the powders had the good to fair flowability and intermediate level of cohesiveness, as HR and CI were in the range of 1.2–1.4 and 15–35, respectively.[Citation28] Similar results were reported by Ong et al.[Citation27] Statistically pairwise comparisons were indifferent from each other except TD and GoD regarding HR. There was no significant difference between TD and GoD. It was observed that all pairwise comparisons were significantly different from one another regarding CI.
Water and oil absorption indices
The water absorption indices (WAI) of dried mango powders ranged from 1.41 to 2.15%, which is attributed to the superior water absorption properties of the powder. Highest WAI was observed in vacuum dried mango powder, which might be due to the maintenance of very low pressure of an enclosed area. Vacuum dryers are made up of either cast iron or stainless steel. Thus, it can allow the high vacuum pressure without any kind of deformation. Statistical analysis revealed that WAI of dried powders differed significantly with the drying methods used except CD (L) and CD (I). OAI of dried mango powders (0.9–1.5%) too differed significantly with the drying methods used. SD powder had the highest OAI due to controlled drying conditions and specifications.
Color characteristics of fresh mango puree and dried powders
The color properties of fresh mango puree and dried powders are shown in . Visual examination of the color of fresh mango puree and dried mango powders showed variations in luminosity (L*). Using CIE (Commission International deI’Eclairage), L* indicates the lightness of the powder samples, whereas a* represents red/green coordinate. All the dried powders showed darker except SD powder (with added maltodextrin). The redness of all the powders increased as a* values increased. b* values are the approximate measurement of blue/yellow color. The yellowish and dull colors of all dried powders were observed except SD powder (lower b* and c* (chroma) values). Lower ∆C* values lead to the lower color intensity of samples and dull color perceives by humans. The probable reason of having lighter color and distinct superiority of SD powder over other dried powders is the addition of maltodextrin with mango pulp, which gave lower intensity. Islam et al.[Citation44] also found the great influences of color with the addition of maltodextrin. Hue angle (H*), measured the qualitative attribute of color which is related to the differences in absorbance at different wavelengths. shows that the H* values and b*/a* values decreased in all dried mango powder. Finally, among all samples, total color differences increased in the following order: TD > GoD > RoD > VD > CD (L) > CD (I) > SD. The direct heating system of GoD and the drying pattern might be the reason for obtaining the darker color of the powder. Direct heating may accelerate the browning reaction of sugar in mango puree and thus could give the dark color. Statistically, it was observed that all pairwise comparisons were significantly different from one another regarding L*, b*, C*, H* and b*/a*. Regarding a*, it was observed that all pairwise comparisons were significantly different from one another except RoD and VD. There was significant difference from one another regarding ∆E except GoD and TD, which indicates no significant difference between GoD and TD.
Table 4. Color characteristics of fresh mango puree and dried powders
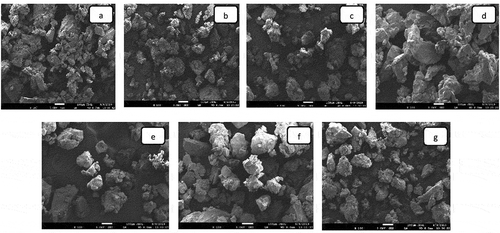
Morphology of dried powder
The morphological images (SEM images) of mango powders are shown in . All the dried powders looked aggregated and irregular in shapes. From the figure, it was not clear how drying affected the outer surface of the powders. However, the powders looked crushed with wrinkled surface, which might be due to the shrinkage of the surface owing to moisture evaporation. The sugar content present in the mango puree could give the sticky agglomerated form of the powders.
Physicochemical and thermo-physical properties of reconstituted product
Reconstituted fruit juice or reconstituted fruit product (puree/pulp) is usually termed as the juice/product produced from the concentrated juice or powdered fruit puree/pulp. Briefly, a reconstituted product (RP) is the diluted form of concentrated/powdered fruit juice. Typically, RP may differ in the texture and taste compared with real fruit juice. However, the nearest physicochemical or other properties of RP with fresh fruit juice/pulp are more desirable to the consumers’, as consumers prefer to take the taste of real juice. Additionally, to maintain the taste, texture and other nutritional properties, it is very important to obtain the closest relationship between RP and fresh fruit juice/pulp.
shows the physicochemical and thermo-physical properties of fresh mango puree and reconstituted product. Although, moisture content of dehydrated mango powder decreased, after reconstitution, it increased for all the RP compared with fresh mango puree. The probable reason for being low moisture content (high total solids) of fresh puree is the presence of pulp compared with RP. However, RP of SD powder had the highest moisture content, which might be due to the addition of maltodextrin as it has higher water absorption capacities. The moisture absorption of RP depends on powder size, porosity, hygroscopicity and solubility of mango powder. All pairwise comparisons were significantly different from one another regarding moisture except GoD and RoD. There was no significant difference between GoD and RoD.
Table 5. Physicochemical and thermo-physical properties of fresh mango puree and its reconstituted products resulted from various drying methods
Titratable acidity and pH have the reverse correlation and this study also showed the same correlation among all the samples. Titratable acidity decreased for all RP, which might be due to the occurrence of Maillard reaction during heat treatment by a different dryer. RP of SD and CD powder had the lowest titratable acidity and highest pH. The highest moisture content of those products could reduce the acidity level.
Although, the values of density of the RP and fresh puree varied a little, but there was no significant difference observed among those products. The viscosity of RP from different dried powders significantly decreased compared with the fresh mango puree. Viscosity can be correlated to the moisture content or the total solid of the product. Typically, the viscosity of liquid indicates the tendency of the resistance of the free flow of the liquid. Manjunatha et al.[Citation45] stated that viscosity had an inverse relationship with moisture content. According to the authors, the increasing magnitude of viscosity might be due to the increasing number of hydrated molecules and intermolecular bonding with solute molecules. The present study showed the similarity with the above statement and thus RP of SD powder had the lowest viscosity (highest moisture content).
The specific heat capacity of RP of SD powder was significantly different from all other RP and fresh puree. However, it was affected by the moisture content of the products. Manjunatha et al.[Citation45] also found a positive correlation of specific heat and moisture content when they studied about the lime juice. The higher specific heat capacity of water than fruit juice showed that kind of behavior. No significant differences observed in terms of thermal conductivity and thermal diffusivity between the RP and fresh mango puree.
Drying effects or thermal damage occur on a product during drying, which depend on time, temperature, thickness and dryer atmospheric conditions. In this study, time, temperature and thickness were almost similar except the dryer’s atmospheric conditions. The atmospheric conditions varied with dryer to dryer for their different drying techniques involved. In cabinet drying, more uniform, attractive and hygienic product can be formed rapidly using hot air as a heating medium, but it needs uniformity of hot air flow and heat supply. In spray dryer, any suspension, solution, emulsion or dispersion feed can be dried as powders, agglomerates or granules form depending upon the feed properties and the dryer configuration. Apart from that, under low pressure, vacuum dryer allows effective moisture elimination by enhancing the mass transfer due to the increased vapor pressure gradient between inside and outside of the product. However, this drying technique offers low-temperature drying with limited capacity and mainly suitable for thermolabile materials and puff-dried foods. Tunnel drying resembles a continuous type and suitable for large scale production. The produce to be dried are placed through a long-insulated tunnel or chamber, where the hot and dry air meets the wetted produce. Due to high initial drying rates, case hardening and too wet granules are the consequences of this type of drying method. In rotary oven dryers, tube rotates by tumbling effects, which enhance the drying rates due to continuous agitation, hence internal moisture is greatly driven out. Careful maintenance is necessary to avoid explosion. In gas oven drying, natural gas is used as a fuel and by gas combustion, the gas burner heats directly, leads to instant heats. Hence, the method is not suitable for large scale production, due to the possibility of non-uniform heat transfer. Finally, products quantity, sensitivity, availability of heat source, and cost involved are the main factors for choosing an effective drying method.
Conclusion
This study shows that the physicochemical properties of mango powders were significantly affected by different drying methods applied. Rehydration value of SD powder was only 66 s, whereas it was more than 140 s for other dried powders. Water activity of SD powder was 0.27, which showed better stability of the powder. The solubility of dried powders ranged from 91–98%, where CD (98%) and SD (96%) powder had the highest value. Lower bulk density, tapped density and particle density was found in laboratory cabinet drying methods which indicates more solubility. Lower hygroscopicity and lower color degradation were found in SD mango powder due to addition of maltodextrin. The Carr index and Hausner ratio of all the powders indicated good to fair flowability and intermediate cohesiveness. In terms of color characteristics, SD powder showed lighter color followed by CD powder, whereas other powders showed yellowish and dark color. The physicochemical properties of reconstituted products were quite similar to fresh mango puree. Overall, this study concludes that spray drying method produces superior quality mango powder product compared to other drying techniques, especially TD, RoD and GoD, the direct heating methods. On the other hand, cabinet drying method can play an effective way in terms of powder production due to higher production cost of spray dryer. Further investigations should be carried out to compare the nutritional, bioactive and antioxidant properties of dried powders by different drying methods and its reconstituted products compared to fresh mango puree.
Acknowledgments
The authors are deeply grateful to Bangabandhu Science and Technology Fellowship for providing fund to continue PhD program and University Grant Commission of Bangladesh for funding to carry out this research. Authors would like to express gratefulness to Department of Food Processing and Engineering, Poultry Research and Training Center (PRTC) of Chattogram Veterinary and Animal Sciences University (CVASU) for providing facilities to conduct this research. Authors would like to give a special thanks to Mr. Abdul Rahman for his cordial help to check English. There is no conflict of interest. Shireen Akther has generated the idea and carried out the research. Shireen Akther, Afroza Sultana and Amiza Mat Amin have written the manuscript. Md. Rahim Badsha has conducted the research work and helped to write a portion of the manuscript. Md. Mokhlesur Rahman and Md. Abdul Alim supervised the research.
Additional information
Funding
References
- Zotarelli, M. F.; da Silva, V. M.; Durigon, A.; Hubinger, M. D.; Laurindo, J. B. Production of Mango Powder by Spray Drying and Cast-tape Drying. Powder Technol. 2017, 305, 447–454. DOI: 10.1016/j.powtec.2016.10.027.
- Rashid, M.; Khatun, H.; Rayhan, M.; Plabon, M.; Hossain, M.; Mozid, M.; Kamal, M.; Hasan, M.; El Sabagh, A.; Islam, M. Comparative Study on Physicochemical Properties of Selected Mango (Mangifera Indica L.) Varieties in Northern Bangladesh. Cercetari Agronomice in Moldova (Agronomic Research in Moldavia). 2019, 52(1), 54–65.
- Ali, S. Y.; Hossain, M.; Zakaria, M.; Haque, M.; Ahiduzzaman, M. Physio-chemical Characteristics of Seven Cultivars Mango (Mangifera Indica L.)” In Bangladesh. Int. J. Bus. Soc. Sci. Res. 2019, 7(4), 01–08.
- Akin-Idowu, P. E.; Adebo, G. U.; Egbekunle, K. O.; Olagunju, Y. O.; Aderonmu, O. I.; Aduloju, A. O. Diversity of Mango (Mangifera Indica L.) Cultivars Based on Physicochemical, Nutritional, Antioxidant, and Phytochemical Traits in South West Nigeria. Int. J. Fruit Sci. 2020, 1–25. https://doi.org/10.1080/15538362.2020.1735601.
- Alam, M.; Islam, M.; Uddin, M.; Barman, J.; Quamruzzaman, A. Effect of Age of Seedling and Variety of Scion in Stone Grafting of Mango. Int. J. Sustain. Crop Prod. 2006, 1(2), 27–32.
- BBS. Yearbook of Agricultural Statistics of Bangladesh; Bangladesh Bureau of Statistics, Ministry of Planning: Bangladesh, 2017; pp 203–204.
- Sarkar, K.; Alam, M.; Rahman, A.; Bhuiyan, M. Post-harvest Losses in Mango Value Chain. Int J. Biores. 2011, 10(5), 25–31.
- Mahendran, T.;. Physico-chemical Properties and Sensory Characteristics of Dehydrated Guava Concentrate: Effect of Drying Method and Maltodextrin Concentration. Trop. Agric. Res. Ext. 2011, 13(2), 48–54.
- Caparino, O.; Tang, J.; Nindo, C.; Sablani, S.; Powers, J.; Fellman, J. Effect of Drying Methods on the Physical Properties and Microstructures of Mango (Philippine ‘Carabao’var.) Powder. J. Food Eng. 2012, 111(1), 135–148. DOI: 10.1016/j.jfoodeng.2012.01.010.
- Kim, E. H. J.; Chen, X. D.; Pearce, D. Surface Composition of Industrial Spray-dried Milk Powders, 2, Effects of Spray Drying Conditions on the Surface Composition. J. Food Eng. 2009, 94(2), 169–181. DOI: 10.1016/j.jfoodeng.2008.10.020.
- Muzaffar, K.; Dar, B.; Kumar, P. Assessment of Nutritional, Physicochemical, Antioxidant, Structural and Rheological Properties of Spray Dried Tamarind Pulp Powder. J. Food Measurem. Charact. 2017, 11(2), 746–757.
- Bhandari, B. R.; Datta, N.; Howes, T. Problems Associated with Spray Drying of Sugar-rich Foods. Dry. Technol. 1997, 15(2), 671–684. DOI: 10.1080/07373939708917253.
- Krokida, M.; Maroulis, Z. Effect of Drying Method on Shrinkage and Porosity. Dry. Technol. 1997, 15(10), 2441–2458. DOI: 10.1080/07373939708917369.
- Pap, L.;. Production of Pure Vegetable Juice Powders of Full Biological Value. Fruit Process. 1995, 3, 55–60.
- Mujumdar, A. S.;. Handbook of Industrial Drying, 3rd ed.; CRC Press, Boca Raton, USA, 2006.
- Hutchinson, J. E.; Drying Cabinet, United States Patent: US1752797A, 1930.
- Chua, K.; Hawlader, M.; Chou, S.; Ho, J. On the Study of Time-varying Temperature drying—Effect on Drying Kinetics and Product Quality. Dry. Technol. 2002, 20(8), 1559–1577. DOI: 10.1081/DRT-120014052.
- Fuller, R.; Charters, W. Performance of a Solar Tunnel Dryer with Microcomputer Control. Solar Energy. 1997, 59(4–6), 151–154. DOI: 10.1016/S0038-092X(96)00143-0.
- Mujumdar, A. S.; Menon, A. S. Drying of Solids: Principles, Classification, and Selection of Dryers, Handbook of Industrial Drying;3rd; Taylor and Francis Group: Boca Raton, USA, 1995; pp 1–39.
- Kruse, O. O.;.Drying Oven Control, United States Patent: US2412990A, 1946.
- Dorta, E.; Lobo, M. G.; González, M. Using Drying Treatments to Stabilise Mango Peel and Seed: Effect on Antioxidant Activity. LWT Food Sci. Technol. 2012, 45(2), 261–268. DOI: 10.1016/j.lwt.2011.08.016.
- Official Methods of Analysis, 20th ed.; AOAC International, Rockville, Maryland, USA, 2016.
- Goula, A. M.; Adamopoulos, K. G.; Kazakis, N. A. Influence of Spray Drying Conditions on Tomato Powder Properties. Dry. Technol. 2004, 22(5), 1129–1151. DOI: 10.1081/DRT-120038584.
- Cai, Y.; Corke, H. Production and Properties of Spray‐dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65(7), 1248–1252. DOI: 10.1111/j.1365-2621.2000.tb10273.x.
- Eastman, J. E.; Moore, C. O. Cold-water-soluble Granular Starch for Gelled Food Compositions, United States Patent: US4465702A, 1984.
- Cano-Chauca, M.; Stringheta, P.; Ramos, A.; Cal-Vidal, J. Effect of the Carriers on the Microstructure of Mango Powder Obtained by Spray Drying and Its Functional Characterization. Innov. Food Sci. Emerg. Technol. 2005, 6(4), 420–428. DOI: 10.1016/j.ifset.2005.05.003.
- Ong, M.; Yusof, Y.; Aziz, M.; Chin, N.; Amin, N. M. Characterisation of Fast Dispersible Fruit Tablets Made from Green and Ripe Mango Fruit Powders. J. Food Eng. 2014, 125, 17–23. DOI: 10.1016/j.jfoodeng.2013.10.014.
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of Instant Soymilk Powders by Ultrafiltration, Spray Drying and Fluidized Bed Agglomeration. J. Food Eng. 2008, 84(2), 194–205. DOI: 10.1016/j.jfoodeng.2007.04.032.
- Beuchat, L. R.;. Functional and Electrophoretic Characteristics of Succinylated Peanut Flour Protein. J. Agric. Food Chem. 1977, 25(2), 258–261. DOI: 10.1021/jf60210a044.
- Sadler, G. D.; Murphy, P. A. pH and Titratable Acidity, In: Food Analysis. Food Analysis; Springer: Boston, 2010; pp 219–238.
- Chang, C.;. Measuring Density and Porosity of Grain Kernels Using a Gas Pycnometer. Cereal Chem. 1988, 65(1), 13–15.
- Ahmad, G.; Shahabuddin, M. To Determine the Specific Heat of a Liquid by the Method, Practical Physics for Degree Students, 1st ed.; Hafiz Book Centre: Dhaka, 1969.
- Bon, J.; Vaquiro, H.; Benedito, J.; Telis-Romero, J. Thermophysical Properties of Mango Pulp (Mangifera Indica L. Cv. Tommy Atkins). J. Food Eng. 2010, 97(4), 563–568. DOI: 10.1016/j.jfoodeng.2009.12.001.
- Gundurao, A.; Ramaswamy, H. S.; Ahmed, J. Effect of Soluble Solids Concentration and Temperature on Thermo-physical and Rheological Properties of Mango Puree. Int. J. Food Prop. 2011, 14(5), 1018–1036. DOI: 10.1080/10942910903580876.
- Tanaka, M.; Kimiagar, M.; Lee, T. C.; Chichester, C. O. Effect of Maillard Browning Reaction on Nutritional Quality of Protein. In Protein Crosslinking, Advances in Experimental Medicine and Biology; Friedman, M., Ed.; Springer: Boston, MA, 1977; pp 321–341.
- Haque, M. A.; Adhikari, B. Drying and Denaturation of Proteins in Spray Drying Process. In Handbook of Industrial Drying; Mujumdar, A. S., Ed.; Taylor and Francis Group: Boca Raton, USA, 2006; pp 971–981.
- Afolabi, I. S.;. Moisture Migration and Bulk Nutrients Interaction in a Drying Food Systems: A Review. Food Nutri. Sci. 2014, 58(8), 692–714.
- Burova, N.; Kislitsina, N.; Gryazina, F.; Pashkova, G.; Kuzminykh, A. A Review of Techniques for Drying Food Products in Vacuum Drying Plants and Methods for Quality Control of Dried Samples (Technical Note). Revista Espacios. 2017, 38, 35.
- Islam, M.; Kitamura, Y.; Yamano, Y.; Kitamura, M. Effect of Vacuum Spray Drying on the Physicochemical Properties, Water Sorption and Glass Transition Phenomenon of Orange Juice Powder. J. Food Eng. 2016, 169, 131–140. DOI: 10.1016/j.jfoodeng.2015.08.024.
- Shrestha, A. K.; Ua-Arak, T.; Adhikari, B. P.; Howes, T.; Bhandari, B. R. Glass Transition Behavior of Spray Dried Orange Juice Powder Measured by Differential Scanning Calorimetry (DSC) and Thermal Mechanical Compression Test (TMCT). Int. J. Food Prop. 2007, 10(3), 661–673. DOI: 10.1080/10942910601109218.
- Roongruangsri, W.; Bronlund, J. Effect of Air-drying Temperature on Physico-chemical, Powder Properties and Sorption Characteristics of Pumpkin Powders. Int. Food Res. J. 2016, 23(3), 962–972.
- Manickavasagan, A.; Thangavel, K.; Dev, S. R.; Delfiya, D. A.; Nambi, E.; Orsat, V.; Raghavan, G. Physicochemical Characteristics of Date Powder Produced in a Pilot-scale Spray Dryer. Dry. Technol. 2015, 33(9), 1114–1123. DOI: 10.1080/07373937.2015.1014045.
- Rodriguez-Hernandez, G.; Gonzalez-Garcia, R.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.; Abud-Archila, M. Spray-drying of Cactus Pear Juice (Opuntia Streptacantha): Effect on the Physicochemical Properties of Powder and Reconstituted Product. Dry. Technol. 2005, 23(4), 955–973. DOI: 10.1080/DRT-200054251.
- Islam, M.; Kitamura, Y.; Kokawa, M.; Monalisa, K.; Tsai, F.-H.; Miyamura, S. Effects of Micro Wet Milling and Vacuum Spray Drying on the Physicochemical and Antioxidant Properties of Orange (Citrus Unshiu) Juice with Pulp Powder. Food Bioprod. Process. 2017, 101, 132–144. DOI: 10.1016/j.fbp.2016.11.002.
- Manjunatha, S. S.; Raju, P. S.; Bawa, A. S. Modelling the Rheological Behaviour of Enzyme Clarified Lime (Citrus Aurantifolia L.) Juice Concentrate. Czech. J. Food Sci. 2012, 30(5), 456–466.

![Figure 1. Illustration of different dryes [(A) spray drying, (B) cabinet drying (laboratary scale) (C) cabinet drying (large scale), (D) vaccum drying, (E) Tunnel drying (F) Rotary oven drying (G) Gas oven drying]](/cms/asset/a1a91aaf-d742-4354-b7cd-d044d4e9dec6/ljfp_a_1849278_f0001_oc.jpg)