ABSTRACT
In this study, the banana flower was divided into bract and male flowers and extracted with water and ethanol, at different temperatures, and then the functionality of the obtained extract was evaluated and the bioactive compounds were analyzed. The findings showed that the banana flower with an ethanol extract of 50% and a temperature of 50°C (FE-50%-50°C) had the best DPPH radical scavenging ability and hydroxyl radical scavenging ability of 97.44% and 38.86%, respectively. Moreover, the FE-50%-50°Calso had the best reducing power. In terms of its ferrous ion chelating ability, the banana flower ethanol 50% extract at room temperature (FE-50%-RT) had the best ferrous ion chelating ability of 96.82%. The 95% ethanol extract of the banana flower was effective in inhibiting α-amylase, α-glucosidase, and lipase activity was 31.75%, 84.17%, and 70.59%, respectively. FE-50%-50°C had the highest alcohol dehydrogenase activity of 74.92%. In addition, the FE-95%-50°C had the highest content of lupeol and umbelliferone, which were 40.10 and 90.83 μg/mg, respectively.
Introduction
Diabetes mellitus is a chronic metabolic disease and is the primary cause of ill health: when the metabolic regulation of carbohydrates and lipids is damaged, it induces an improper insulin function, and the blood sugar increases. Postprandial hyperglycemia can initiate multiple secondary complications, such as nervous lesions, retinopathy, and kidney disease.[Citation1] Bananas, which are a staple food in many countries, are one of the most popular fruits globally, and forma very important agricultural food crop in global trade. The banana flower is one of the secondary products of the banana plant, and is considered to be a vegetable in many countries.[Citation2] Ivan[Citation3] indicated that the banana flower could be part of traditional medicine for treating diseases, such as renal calculus and ulcers, and its anti-hyperglycemic effect has been used in formulae for treating diabetes.[Citation2,Citation4] Some studies have indicated that the blood sugar activity in rats with alloxan diabetes could be reduced by using chloroform, water, and ethanol to extract the banana flower.[Citation5–7] Traditionally, the banana flower has been used for relieving cardiac pain, asthma, diabetes mellitus, hypermenorrhea, dysmenorrhea, diarrhea, and gastrospasms.[Citation8] Moreover, the banana flower extract has medicinal properties for diabetes mellitus, oxidative stress,[Citation9] and malaria.[Citation10] Some studies have indicated that the crude extract of the banana flower has a biological activity, e.g. it regulates or changesthe oxidation resistance and lysosomal enzyme activity (Bhaskar & Salimath),[Citation11] and it has the ability to heal wounds.[Citation12] The findings of Ramith et al.[Citation1] indicated that the ethanol extract of the banana flower, which contains high amounts of umbelliferone and lupeol, could have an antihyperglycemic activity by inhibiting α-glucosidase, and it could generate an antidiabetic effect by inhibiting the polyol pathway and protein glycosylation. Lupeol has been used as an in vitro and in vivo anti-inflammatory agent.[Citation13] It has an anticancer effect, which means that it can prevent different kinds of tumors from growing in vitro and in vivo,[Citation14] and it has an important therapeutic action on diabetes mellitus, cardiovascular disease, and arthritis.[Citation15] Umbelliferone also has extensive pharmacological activities, such as antioxidation[Citation16] and inhibitory activity for late glycosylation,[Citation1] and it also inhibits angiotensin-converting enzyme activity.[Citation17] In addition, some studies also indicated that umbelliferone can be used for antidiabetic activity,[Citation18] abirritation,[Citation19] antibacterial action,[Citation20] nerve protection,[Citation21] liver protection,[Citation22] as well as the anti-inflammatory and apoptosis-promoting effects of colon cancer.[Citation23]
The banana flower bract contains anthocyanins, such as delphinidin, pelargonidin, paeonidin, and malvidin.[Citation24,Citation25] Gunavathy et al.[Citation26] used dpetroleum ether to extract the bract and found alkaloids, as well as glucoside and flavonoid compounds, after its extraction with ethyl acetate. It also found alkaloid, saponin, flavonoid, terpenoid, coumarin, glucoside, phenolic compounds, and steroids, after its extraction with methanol, and it found coumarin and phenolic compounds after its extraction with water. The banana flower bract is used for treating hypertension in South Africa[Citation27] and for treating coughs, asthma, and bronchitis in Brazil.[Citation28]
As the banana flower consumes nutrients that are necessary for the growth of bananas, it is usually extirpated in the growing process, and large quantities of the banana flowers that are produced annually are discarded as agricultural waste. Although many studies have shown that banana flowers resist inflammation, are antidiabetic, and protect the liver, the banana bracts are seldom studied. Therefore, this paper studies the banana flower and the bract extract, in terms of their bioactive compounds and bioactivity, to obtain novel materials as a theoretical basis for new functional food or lead compounds for new drugs. This study may be used as a reference for the research and development of functional food and drugs.
Materials and method
Chemical
All reagents, including ferrozine [3-(2-pyridyl)-5,6-bis(4-sulfophenyl)-1,2,4-triazine], DPPH (2,2-diphenyl-1-picrylhydrazyl), 2-deoxy-D-Ribose cell culture tested, Folin & Ciocalteu’s phenol reagent, 3,5-dinitrosalicylic acid, P-nitrophenyl α-D-glucopyranoside, P-nitrophenyl palmitate, Triton X-100and solvents of highest purity available, were purchased from the Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The standards of lupeol, 7-hydroxycoumarin (umbelliferone), quercetin (C15H10O7.2H2O), gallic acid (C7H6O5) were purchased from the Sigma-Aldrich Chemical Co. (Milan, Italy). All enzymes in this study, includingα-Glucosidase (from Saccharomyces cerevisiae), α-Amylase (from Aspergillus oryzae spray-dried (powder)), lipase type II crude (from porcine pancreas), alcohol dehydrogenase (ADH, from yeast), pancreatin (from porcine pancreas), and pepsin (from porcine gastric mucosa powder), were also purchased from Sigma-Aldrich Chemical Co. (Milan, Italy).
Sample preparation
Banana flowers (including the male flowers and bracts) of Musa acuminate (Banana) were obtained from farms in Nantou City, Taiwan. The banana flowers were extirpated 1 year after planting the banana trees, and the fresh samples were classified within 1 week, dried at 40°C, and pulverized by using a homogenizer for future use. In this study, 50 g of oven-dried bract and male flowers were dipped into and extracted with water and 500 mL ethanol at different concentrations (50%, 75%, and 95%) and temperatures (at room temperature and 50°C), where the extraction condition at room temperature was 72 h, and the extraction condition at 50°C was 24 h, and they were then concentrated by a reduced pressure concentrator and freeze-dried, and the obtained samples were stored at −20°C for future analysis.
Antioxidation activities
DPPH free radical scavenging activity: The DPPH free radical scavenging activity was measured by Wang et al.[Citation29] The absorbance rates of the resulting solution and the positive control (α-tocopherol methanolic solutions) were measured at 517 nm. The percentage of the DPPH scavenging activity was expressed as [1 − (Abs sample/Abs blank)]/100%.
Hydroxyl radical scavenging activity: The scavenging effect on a hydroxyl radical was measured following the method described by Nagai and Inoue,[Citation30] with some modifications. The absorbance of the solution was measured at 520 nm. The percentage inhibition of hydroxyl radical generation was expressed as [1 − (Abs sample/Abs blank)] × 100%.
Ferrous ion chelating ability: The ferrous ion chelating ability was measured and modified, based on Liao et al.[Citation31] The absorbance at 562 nm of the resulting EDTA (positive control) solutions was recorded. The percentage of the ferrous ion chelating ability was expressed as [1 − (Abs sample/Abs blank)]/100%.
Reducing power: The reducing power was measured and modified, based on Wang et al.[Citation29] The absorbance at 700 nm was then detected with a spectrophotometer for 10 min after the reaction; a higher absorbance (A700) represents a stronger reducing power.
Inhibition of the α-amylase
Referring to the method of Worthington et al.,[Citation32] the α-amylase (2 U/mL) was dissolved in a 0.02 M phosphate buffer saline, with a pH of 6.9. The various concentrations of the extract (0.5 mL) were added to a solution containing starch (0.5%) and a phosphate buffer (0.5 mL). The reaction was initiated by adding an enzyme solution (0.5 mL) to the incubation medium. After a 10 min incubation, the reaction was stopped by adding a 1.0 ml dinitrosalicylic (DNS) reagent (1% 3,5-dinitrosalicylic acid, 0.2% phenol, 0.05% Na2SO3 and 1% NaOH in aqueous solution) to the reaction mixture. The mixtures were heated at 100°C for 5 min in order to stop the reaction. Thereafter, 0.5 mL of a 40% potassium sodium tartrate solution was added to the mixtures to stabilize the color. After cooling it to room temperature in a cold water bath, the absorbance was recorded at 540 nm by a spectrophotometer, and the percentage of α-amylase inhibition was expressed as [1 − (Abs sample/Abs blank)]/100%.
Inhibition of the α-glucosidase
The modification was performed by referring to the method of Worawalai et al.,[Citation33]where 0.3 mL of extract (0.3 mL) at various concentrations was mixed with 2.1 mL of potassium phosphate buffer (0.05 M, pH 6.8) prior to the addition of 0.3 mL of yeast α-glucosidase (0.4 U/mL). The mixture was pre-incubated at 37°C for 10 min before the addition of 0.3 mL of pNPG (10 mM) to start the enzyme reaction, with a further incubation for 20 min at 37°C. The reaction was terminated by the addition of 0.75 mL of Na2CO3 (1 M), the absorbance of the liberated p-nitrophenol was measured at 405 nm, using a spectrophotometer, and the percentage of α-glucosidase inhibition was expressed as [1 − (Abs sample/Abs blank)]/100%.
Inhibition of the lipase
The determination was performed after the modification by referring to the method of McDougall et al.[Citation34] Solution I was prepared by dissolving 50 mg p-nitrophenylpalmitate in 30 mL Isopropanol. Solution II was prepared by quantifying 2 g Triton X-100 and 0.5 g Gum Arabic to 450 mL. The aforesaid two materials were configured in a ratio of 1:9, and called Solution III. An amount of 0.15 mL enzyme liquid (0.5 U/mL) and 0.15 mL sample solution were added in 2.7 mL of Solution III, mixed thoroughly before reacting in a 30°C aqueous thermostat for 5 mins, and the absorbance value was determined at the wavelength of 410 nm. The percentage of lipase inhibition was expressed as [1 − (Abs sample/Abs blank)]/100%.
In vitro alcohol dehydrogenase activity
The activity of alcohol dehydrogenase (ADH) was determined, after the method of Shim et al.[Citation35] was modified. An amount of a 1.5 mL sodium pyrophosphate buffer solution (pH8.8) was put in the test tube, mixed with oxidized NAD (1.0 mL, 0.027 mol/L NAD+) and 0.5 mL (11.5%) ethanol solution, and the samples at different concentrations (0.5 mL) were thoroughly mixed and placed in a 25°C water bath for 5 min, for a reaction. Afterward, the mixture was mixed with 0.1 mL ADH (0.25 U/mL), shaken up, the absorbance value at 340 nm was determined by a spectrophotometer, and the absorbance values were recorded at intervals of 5 s, for a period of 5 min. The percentage of ADH activation was expressed as [1 − (Abs sample/Abs blank)]/100%.
Determination of bioactive compounds
Total phenol content: The method of Julkunen et al.[Citation36] was modified, and the sample and standard (gallic acid) were determined at a wavelength of 760 nm by a spectrophotometer.
Flavonoid content: The method of Jia et al.[Citation37] was modified, and the sample and standard (quercetin) were determined at a wavelength of 415 nm by a spectrophotometer.
Lupeol: The lupeol was determined by using the HPLC (UV-VIS Spectrotometric detector, Shimadzu RID-6A, Japan), and the method was based on that of Xu et al.[Citation38]The mobile phase was methanol:acetonitrile = 1:1 (v: v). An elution was performed at a solvent flow rate of 1.0 mL/min, at a column temperature of25°C, and chromatograms were recorded at 280 nm
Umbelliferone: The umbelliferone was determined by using the HPLC (UV-VIS Spectrotometric detector, Shimadzu RID-6A, Japan), and the method was based on that of Xie et al.[Citation39]The mobile phase was 0.1% aqueous phosphoric acid/acetonitrile (75:25). An elution was performed at a solvent flow rate of 0.8 mL/min, at column temperature of25°C and chromatograms were recorded at 318 nm.
Gastrointestinal tract simulation test
Referring to the method of Wu et al.,[Citation40] the sample was dissolved in DI water. The pH was adjusted by 0.1 N HCl to 2, the sample was decomposed and digested by 1% (w/w) pepsin at 37°C for 4 h, and immediately heated in a boiling water bath for 15 min; then, after cooling, the pH was adjusted by 0.1 N NaOH to 7, and part of it was freeze-dried. The remaining sample was digested by 2% (w/w) pancreatin at 37°C for 4 h, immediately heated in a boiling water bath for 15 min to stop enzyme reaction, centrifuged (600 × g, 4°C) for 5 min, and the supernatant was freeze-dried. All of the freeze-dried samples were used to determine the antioxidation activities and to evaluate the metabolism ability and bioactive compounds.
Statistical analysis
The results are reported as the mean ± standard deviation (SD) of three separate experiments. The data were analyzed by using the one-way Analysis of Variance (ANOVA) and Duncan’s multiple range tests, which were performed by using statistical software (SAS Institute 2001). Values of p < .05 were regarded as statistically significant.
Results
Effect of the antioxidant activity
According to the results of this study in , when the concentration of banana flower extract is 1 mg/mL, there is no significant difference in the scavenging activity of the samples extracted with 50% and 75% ethanol at different temperatures, while the sample extracted with 50% ethanol at 50°C (FE-50%-50°C) has the maximum scavenging activity of 97.44%, and its IC50 is 0.46 mg/mL (). As shown in , there is no significant difference in the DPPH scavenging activity when water is used as the extraction solvent, and the DPPH scavenging activity of banana flower extract, using ethanol as the extraction solvent, is two or three times that of the bract extract.
Table 1. The antioxidant activities of different banana flower and bract extracts
The ferrous ion chelating abilities of the banana flower and bract extracted with 50% ethanol as the solvent are higher than 90% (), while the chelating ability of the other samples decrease, as the ethanol concentration increases, and the chelating ability of banana flower extract is higher than that of the bract extract.
The results of the hydroxyl radical scavenging ability are shown in . The hydroxyl radical scavenging abilities of the banana flower and bract extracted with 50% ethanol as the solvent are about 40%, while the scavenging activities of the other samples decline as the ethanol concentration increases, and the sample heated to 50°C has a higher scavenging activity than the sample extracted at a normal temperature. In addition, the result of reducing the power shows that the bract samples, as extracted with ethanol above 75%, have larger absorbance values than the banana flower samples.
Effect of the metabolic functions
It can be seen in that the samples extracted with 95% ethanol as the solvent have the ability to inhibit α-amylase. When the concentration is 1 mg/mL, the sample of the bract heated to 50°C has a higher inhibition ratio, which is 46.03%, and 1.3 times that of acarbose. In addition, the inhibition of the bract extracts extracted with 75% ethanol at room temperature, and at 50°C, are 12.70% and 14.29%, respectively, and 0.4 times that of acarbose. The result of the α-glucosidase activity inhibiting capacity shows that, when the concentration is 1 mg/mL, the banana flower extracted with 95% ethanol has a higher inhibition ratio, which is 84.17%, and 3.5 times that of acarbose. In addition, according to the results in , the inhibitory effect declines gradually as the ethanol concentration decreases, and the banana flower extracts have a better inhibitory effect than the bract extracts. There are no significant differences in the inhibition ratios of the water-extracted samples. The banana flower extracted with 95% ethanol at different temperatures has the best activity to inhibit lipase, which is70.59%. In addition, as shown in , among the samples extracted with ethanol, the banana flower extract has a better lipase inhibiting activity, while the bract has a better lipase inhibiting activity among the water-extracted samples.
Table 2. Evaluation of the metabolic functions of different banana flower and bract extracts
Effect of alcohol dehydrogenase activity
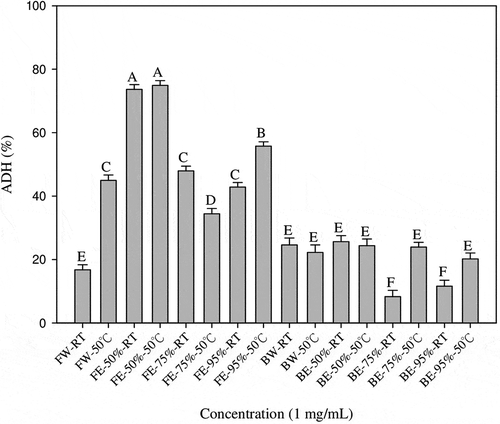
The results are shown in . There is no significant difference in the alcohol dehydrogenase activity of the samples of banana flowers extracted with 50% ethanol at different temperatures, and the banana flower extracts have a higher alcohol dehydrogenase activity than banana bract extracts. The banana flower extracted at 50°C has the best alcohol dehydrogenase activity, which is 74.92%.
Figure 1. Effects of different banana flowers and bract extracts on alcohol dehydrogenase activity. Results from three separate experiments are expressed as mean ±SD. Values with different letters are significantly different (P < 0.05).FW-RT: Banana flower water extract at room temperature; FW-50°C: Banana flower water extract at 50°C; FE-50%-RT: Banana flower 50% ethanol extract at room temperature, FE-50%-50°C: Banana flower 50% ethanol extract at 50°C; FE-75%-RT: Banana flower 75% ethanol extract at room temperature, FE-75%-50°C: Banana flower 75% ethanol extract at 50°C; FE-95%-RT: Banana flower 95% ethanol extract at room temperature, FE-95%-50°C: Banana flower ethanol 95% extract at 50°; FW-RT: Banana flower water extract at room temperature; BW-50°C: Bract water extract at 50°C; BE-50%-RT: Bract 50% ethanol extract at room temperature, BE-50%-50°C: Bract 50% ethanol extract at 50°C; BE-75%-RT: Bract 75% ethanol extract at room temperature, BE-75%-50°C: Bract 75% ethanol extract at 50°C; BE-95%-RT: Bract 95% ethanol extract at room temperature, BE-95%-50°C: Bract 95% ethanol extract at 50°C
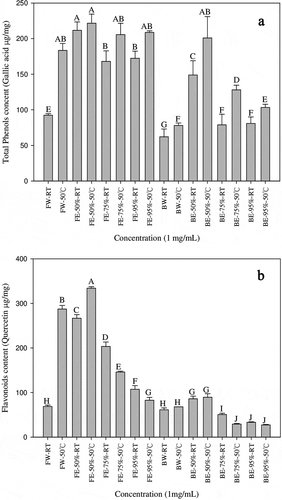
Total phenol and flavonoid content
As shown in (A), the banana flower extracted with 50% ethanol at 50°C (FE-50%-50°C) has the highest content, which is 221.67 μg/mg, the samples heated to 50°C have a higher total phenol content than the samples at a normal temperature, and the banana flower extract has a higher total phenol content than that of the bract. (B) shows that the maximum content in the banana flower extracted with 50% ethanol at 50°C (FE-50%-50°C) is 334 μg/mg. Moreover, the flavonoid content in the samples extracted with 50% ethanol and water, and heated to 50°C, is higher than that in the samples at a normal temperature, while the samples extracted with 75% and 95% ethanol at a normal temperature have a higher content, and the banana flower extract has higher flavonoid content than the bract.
Figure 2. The content of total phenol (A) and flavonoids (B) of different banana flower and bract extracts. Results from three separate experiments are expressed as mean ±SD. Values with different letters are significantly different (P < 0.05).FW-RT: Banana flower water extract at room temperature; FW-50°C: Banana flower water extract at 50°C FE-50%-RT: Banana flower 50% ethanol extract at room temperature, FE-50%-50°C: Banana flower 50% ethanol extract at 50°C; FE-75%-RT: Banana flower 75% ethanol extract at room temperature, FE-75%-50°C: Banana flower 75% ethanol extract at 50°C; FE-95%-RT: Banana flower 95% ethanol extract at room temperature, FE-95%-50°C: Banana flower ethanol 95% extract at 50°C; FW-RT: Banana flower water extract at room temperature; BW-50°C: Bract water extract at 50°C; BE-50%-RT: Bract 50% ethanol extract at room temperature, BE-50%-50°C: Bract 50% ethanol extract at 50°C; BE-75%-RT: Bract 75% ethanol extract at room temperature, BE-75%-50°C: Bract 75% ethanol extract at 50°C; BE-95%-RT: Bract 95% ethanol extract at room temperature, BE-95%-50°C: Bract 95% ethanol extract at 50°C
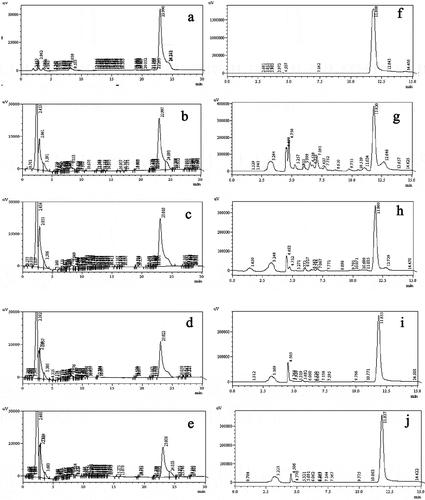
Lupeol and umbelliferone
The HPLC chromatographic profile of lupeol and umbelliferone of different banana flower and bract extracts are shown in , and the content of lupeol and umbelliferone in the different banana flower and bract extracts are shown in . The sample of the banana flower extracted with 95% ethanol at 50°C (FE-95%-50°C) has the highest lupeol and umbelliferone content of 40.10 and 90.83 μg/mg, respectively. The lupeol and umbelliferone content determined in the banana flower extract is higher than that in the bract extract, and the heated sample has a higher content than the sample at a normal temperature. In addition, shows that the water-extracted samples are free of umbelliferone.
Table 3. The lupeol and umbelliferone content of different banana flower and bract extracts
Figure 3. HPLC chromatographic profile of Lupeol and Umbelliferone of different banana flower and bract extracts. (A)Standard for Luepol; (B) FE-95%-RT for Luepol (C) FE-95%-50°C for Luepol; (D) BE-95%-RT for Luepol; (E) BE-95%-50° for Luepol; (F) Standard for Umbelliferone; (G) FE-95%-RT for Umbelliferone; (H) FE-95%-50° for Umbelliferone; (I) BE-95%-RT for Umbelliferone; (E)BE-95%-50°for UmbelliferoneFE-95%-RT: Banana flower 95% ethanol extract at room temperature, FE-95%-50°: Banana flower 95% ethanol extract at 50°, BE-95%-RT: Bract 95% ethanol extract at room temperature, BE-95%-50°: Bract 95% ethanol extract at 50°
Antioxidant activity and the total phenol and flavonoid
The results of the in vitro-simulated gastrointestinal digestion test are shown in . The DPPH radical scavenging ability and ferrous ion chelating ability have declined. The hydroxyl radical scavenging ability and the reducing power have increased in the extract of banana flower extracted with 50% ethanol (FE-50%), and the 50% ethanol 50°C banana flower extract (FE-50%-50°C) has the best effect, namely, 39.00% and 1.84, respectively (). The total phenol and flavonoid content decreases significantly after in vitro-simulated gastrointestinal digestion testing, and the possible reason is that the degradation and activity decrease of polyphenols during the digestion process is mainly caused by changes in the pH, where the 95%-50°C ethanol extracted banana flower extract (FE-95%-50°C) has the highest total phenol content, which is 164.39 μg/mg. The 50%-50°C ethanol extracted banana flower extract (FE-50%-50°C) has the highest flavonoid content, which is220.00 μg/mg ().
Table 4. The antioxidant activity of different banana flower and bract extracts afterin vitro-simulated gastrointestinal digestion
Table 5. The total phenols and flavonoids content of different banana flower and bract extracts after in vitro-simulated gastrointestinal digestion
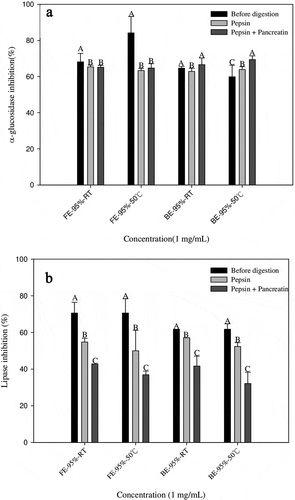
Inhibition of the α-glucosidase and lipase
As shown in (A), the α-glucosidase inhibition of the 95% ethanol banana flower extract declines after in vitro-simulated gastrointestinal digestion testing, while that of the 95% ethanol banana bract extract increases to 66.59% and 69.42%, respectively. In addition, (B) shows that the lipase enzyme inhibition of the 95% ethanol banana flower and bract extracts has decreased.
Figure 4. The α-glucosidase (A) and lipase inhibition (B) of different banana flower and bract extracts after simulated in vitro gastrointestinal digestion. Results from three separate experiments are expressed as mean ±SD. A-E: Different letters in the same column indicate significantly different (P < 0.05).FW-RT: Banana flower water extract at room temperature; FW-50°: Banana flower water extract at 50°; FE-50%-RT: Banana flower 50% ethanol extract at room temperature, FE-50%-50°C: Banana flower 50% ethanol extract at 50°; FE-75%-RT: Banana flower 75% ethanol extract at room temperature, FE-75%-50°: Banana flower 75% ethanol extract at 50°; FE-95%-RT: Banana flower 95% ethanol extract at room temperature, FE-95%-50°: Banana flower ethanol 95% extract at 50°; FW-RT: Banana flower water extract at room temperature; BW-50°: Bract water extract at 50°; BE-50%-RT: Bract 50% ethanol extract at room temperature, BE-50%-50°: Bract 50% ethanol extract at 50°; BE-75%-RT: Bract 75% ethanol extract at room temperature, BE-75%-50°: Bract 75% ethanol extract at 50°; BE-95%-RT: Bract 95% ethanol extract at room temperature, BE-95%-50°: Bract 95% ethanol extract at 50°
Analysis of the correlation of total phenol and flavonoid with antioxidant and metabolic activities
As shown in , the total phenol and flavonoid have greater positive correlations with antioxidant activity, and the flavonoid has a greater effect on antioxidant activity than the total phenol, especially in reducing power. The analysis result of the correlation of total phenol and flavonoid with metabolic activity is shown in , the total phenol and flavonoid have no positive correlation with the α-amylase, α-glucosidase and lipase inhibiting capacity; however, they have a greater positive correlation with the alcohol dehydrogenase activity. In addition, the total phenol has a greater effect on alcohol dehydrogenase activity than the flavonoid. To sum up the aforesaid results, the total phenol and flavonoid have a positive effect on the antioxidant activity and alcohol dehydrogenase activity, and only a slight effect on inhibiting α-amylase, α-glucosidase, and lipase activity.
Table 6. The correlation analysis of chemical components from different banana flower and bract extracts between the antioxidant and metabolic activity
Analysis of the correlation of lupeol and umbelliferone with antioxidant and metabolic activities
As shown in , the lupeol and umbelliferone are not only significantly correlated with antioxidant activity but they are also greatly positively correlated with α-amylase, α-glucosidase and lipase inhibition. also shows that the umbelliferone and the lupeol are not significantly correlated with alcohol dehydrogenase activity.
Discussion
This study reports that extracts from the banana flower and bract show a significant content of total phenol and flavonoids (), and the results also show that banana flower and bract extracts have significant antioxidant activities (). The banana flower extracts have higher concentrations of total phenolic and flavonoids than the bract extracts (), and they also show stronger antioxidant activities (). According to the correlation analysis of the total phenol and flavonoids with antioxidation (), the antioxidant activity increases with the total phenol and flavonoid content. These results are reliable.
The DPPH radical scavenging ability and ferrous ion chelating ability decline after in vitro-simulated gastrointestinal digestion testing, and the hydroxyl radical scavenging ability and reducing power are enhanced in the extract of the banana flower that is extracted with 50% ethanol (). In addition, the total phenol and flavonoid contents decrease significantly after in vitro-simulated gastrointestinal digestion testing (). Spinola et al.[Citation41] indicated that the phenol contents in different parts (the leaf, flower, and stem) of Oxalis, as extracted with methanol after in vitro-simulated gastrointestinal digestion, decreased significantly, which means that the antioxidant activity was reduced by 38.09–52.62%. Burgos-Edwards et al.[Citation42] indicated that the Ribesmagellanicum and R. punctatumin Chilean raisins were extracted with methanol, and the flavonoid content was reduced by 11.6% after in vitro-simulated peptic digestion; however, after intestinal digestion, the flavonoid content in Ribesmagellanicum was reduced by 46.2% and the flavonoid content in R. punctatum was reduced by 34.1%. Moreover, there is no significant difference in the total phenol content in Ribesmagellanicum after in vitro-simulated peptic digestion, while that in R. punctatum was reduced by 13.7%; however, the total phenol content in the two substances was reduced by about 50% after intestinal digestion. The digested extract still has antioxidant activity, which means that there is potential protection against diseases, in relation to oxidative stress.
Metabolic activity can influence human health, and an abnormal metabolism can even cause multiple diseases, in relation to metabolism, such as diabetes mellitus, obesity, and cardiovascular disease. The banana flower and bract extracts can inhibit α-amylase, α-glucosidase, and lipase activity (), especially the banana flower and bract extracted with 95% ethanol, which has a significant inhibiting capacity. The results in show that the extracts obtained by using 95% ethanol for extraction have a higher concentration of lupeol and umbelliferone. Ramith[Citation43] indicated that the separate identification of the banana flower extracted with ethanol showed that two effective chemical constituents, umbelliferone (0.38%) and lupeol (0.36%), were related to antidiabetic properties. Sim et al.[Citation44] indicated that umbelliferone, as a supplement, could effectively improve hypertriglyceridemia and hyperglycemia, but would not affect the energy intake or body weight. In addition, shows that lupeol and umbelliferone have a significantly positive correlation with inhibiting the α-amylase, α-glucosidase, and lipase activities.
ADH is mainly distributed in the liver, stomach, and intestine, and is the most critical enzyme for catalyzing ethanol into acetaldehyde.[Citation45] ADH is ubiquitous in higher organisms, takes part in metabolizing multiple alcohols and aldehydes, and is an important detoxification mechanism.[Citation46] The activity of ADH directly influences the concentration of ethanol and acetaldehyde, the in vivo enzyme activity of different organisms has different gene expressions, and the alcohol dehydrogenase activities are different; thus, individuals will have a very different tolerance to alcohol. If the activity of ADH in vivo can be enhanced, it will be favorable for the decomposition of ethanol, which will neutralize the effects of alcohol. shows that the banana flower and bract extracts have the ability to activate ADH. According to the results in and , the ADH activity increases with the total phenol and flavonoid content, which means that the total phenol and flavonoid are in a significantly positive correlation with ADH.
Conclusion
The banana flower and bract are bioactive, and their pharmacological action has been proved. The extract of FE-50%-50°C has the best antioxidant activity and ADH activity. In terms of metabolism, the extract of FE-95%-50°C has the best inhibition for the α-amylase, α-glucosidase, and lipase enzymes. Finally, in vitro-simulated gastrointestinal digestion testing was performed, and the results showed that, while a part of the antioxidant and metabolic activities declined after gastrointestinal digestion, there was some effect. Different dosage forms, or different coating materials, can be discussed in the future, in order to reduce the degradation of their valid function after gastrointestinal digestion. As health consciousness increases, improving the ingredients of food is much more important than reducing their hazards. Therefore, food research will continue to discuss the addition of materials with different biological activities, which can enhance the health-care function in functional foods, and be used as drugs. Thus, adding raw materials with biological activities to healthy food can enhance human health.
References
- Ramith, R.; Prithvi, S. S.; Farhan, Z.; Nagendra, M. N. P. Investigation of Antihyperglycaemic Activity of Banana (Musa sp. Var. Nanjangud Rasa Bale) Pseudostem in Normal and Diabetic Rats. J. Sci. Food Agric. 2014, 95, 165–173. DOI: https://doi.org/10.1016/j.sajb.2014.08.001.
- Joshi, S.;. Eugenia Jambolana L, Musa paradisiaca L. In Medicinal Plants; Joshi, S., Ed.; Oxford and IBH Publishing Co. Pvt. Ltd: New Delhi, 2000; pp 286–294.
- Ivan, A. R.; (2003). Medicinal Plants of the World: Chemical Constituents, Traditional Modern and Medicinal Uses. pp. 155-163. New Delhi, Humana Press.
- Bhaskar, J. J.; Kumar, A. P.; Salimath, P. V. Effect of Banana (Musa sp. Var. Elakki Bale) Flower and Pseudostem on Antioxidant and Lysosomal Enzyme Activities in Streptozotocin-induced Diabetic Rats. J. Pharm. Res. 2011, 4, 1087–1091. 0974-6943
- Dhanabal, S. P.; Sureshkumar, M.; Ramanathan, M.; Suresh, B. Hypoglycemic Effect of Ethanolic Extract of Musa sapientum on Alloxan Induced Diabetes Mellitus in Rats and Its Relation with Antioxidant Potential. J. Herbal Pharmacother. 2005, 5, 7–19. DOI: https://doi.org/10.1080/J157v05n02_02.
- Grover, J. K.; Yadav, S.; Vats, V. Medicinal Plants of India with Anti-diabetic Potential. Int. Soc. Ethnopharmacol. 2002, 81, 81–100. DOI: https://doi.org/10.1016/S0378-8741(02)00059-4.
- Pari, L.; Umamaheswari, J. Antihyperglycaemic Activity of Musa sapientum Flowers: Effect on Lipid Peroxidation in Alloxan Diabetic Rats. Phytotherapy Res. 2000, 14, 136–138. DOI: https://doi.org/10.1002/(SICI)1099-1573(200003)14:2<136::AID-PTR607>3.0.CO;2-K.
- Sumathy, V.; Lachumy, S. J.; Zakaria, Z.; Sasidharan, S. In Vitro Bioactivity and Phytochemical Screening of Musa acuminate Flower. Pharmacologyonline. 2011, 2, 118–127. Corpus ID: 85659963
- Vinaykumar, T.; Sumanth, M. H.; Suman, L.; Vijayan, V.; Srinivasarao, D.; Sharmila, A.; Naveen, M.; Ramana, B. Reno Protective and Testicular Protective Effect of Musa paradisiaca Flower Extract in Streptozotocin Induced Diabetic Rats. J. Innovative Trends Pharm. Sci., 2010, 1, 106–114. 0975-8593.
- Bagavan, A.; Rahuman, A. A.; Kaushik, N. K.; Sahal, D. In Vitro Antimalarial Activity of Medicinal Plant Extracts against Plasmodium falciparum. Parasitol. Res. 2011, 108, 15–22. DOI: https://doi.org/10.1007/s00436-010-2034-4.
- Bhaskar, J. J.; Salimath, P. V.; Nandini, C. D. Stimulation of Glucose Uptake by Musa sp. (Cv. Elakki Bale) Flower and Pseudostem Extracts in Ehrlich Ascites Tumor Cells. Asian J. Agric. Food Sci. 2011, 91, 1482–1487. DOI: https://doi.org/10.1002/jsfa.4337.
- Calixtro, R. S.; Malalay, A. P.; Epino, P. B.; Avelino, L. E. 2014. Wound Healing Potential to the Ethanolic Extract of Banana Flower (Musa sapientum, BBB ’Saba’, Family Musaceae). Int. J. Pharmacy, 4:33–37. 2249-1848.
- Geetha, T.; Varalakshmi, P. Effect of Lupeol and Lupeol Linoleate on Lysosomal Enzymes and Collagen in Adjuvant-induced Arthritis in Rats. Mol. Cell. Biochem. 1999, 201, 83–87. DOI: https://doi.org/10.1023/a:1007056300503.
- Sunitha, S.; Nagaraj, M.; Varalakshmi, P. Hepatoprotective Effect of Lupeol and Lupeol Linoleate on Tissue Antioxidant Defence System in Cadmium-induced Hepatotoxicity in Rats. Fitoterapia. 2001, 7, 516–523. DOI: https://doi.org/10.1016/s0367-326x(01)00259-3.
- Siddique, H. R.; Saleem, M. Beneficial Health Effects of Lupeol Triterpene: A Review of Preclinical Studies. Life Sci. 2011, 88, 285–293. DOI: https://doi.org/10.1016/j.lfs.2010.11.020.
- Singh, R.; Singh, B.; Singh, S.; Kumar, N.; Kumar, S.; Arora, S. Umbelliferone – An Antioxidant Isolated from Acacia Nilotica (L.) Willd. Ex. Del. Food Chem. 2010, 120, 825–830. DOI: https://doi.org/10.1016/j.foodchem.2009.11.022.
- Hyun, S. K.; Oh, Y. N.; Kwon, H. J.; Kim, B. W. Angiotensin Converting Enzyme Inhibitory Benzopyranoids from Angelica Gigas. Food Sci. Biotechnol. 2013, 22, 1741–1745. DOI: https://doi.org/10.1007/s10068-013-0275-6.
- Ramesh, B.; Pugalendi, K. V. Antihyperlipidemic and Antidiabetic Effects Ofumbelliferone in Streptozotocin Diabetic Rats. Yale J. Biol. Med. 2005, PMCID: PMC2259150 78, 189–196.
- Rauf, A.; Khan, R.; Khan, H.; Pervez, S.; Pirzada, A. S. In Vivo Antinociceptive and Anti-inflammatory Activities of Umbelliferone Isolated from Potentilla evestita. Nat. Prod. Res. 2014, 28, 1371–1374. DOI: https://doi.org/10.1080/14786419.2014.901317.
- Jurd, L.; King, A. D.; Mihara, K. Antimicrobial Properties of Umbelliferone Derivatives. Phytochemistry. 1971, 10, 2965–2970.. DOI: https://doi.org/10.1016/S0031-9422(00)97333-3.
- Subramaniam, S. R.; Ellis, E. M. Neuroprotective Effects of Umbelliferone and Esculetin in a Mouse Model of Parkinson’s Disease. J. Neurosci. Res. 2013, 91, 453–461. DOI: https://doi.org/10.1002/jnr.23164.
- Ramesh, B.; Pugalendi, K. V. Effect of Umbelliferone on Tail Tendon Collagen Andhaemostatic Function in streptozotocin-Diabetic Rats. Basic Clin. Pharmacol. Toxicol. 2007, 101, 73–77. DOI: https://doi.org/10.1111/j.1742-7843.2007.00090.x.
- Muthu, R.; Selvaraj, N.; Vaiyapuri, M. Anti-inflammatory and Proapoptotic Effects of Umbelliferone in Colon Carcinogenesis. Hum. Exp. Toxicol. 2016, 35, 1041–1054. DOI: https://doi.org/10.1177/0960327115618245.
- Pazmino-Duran, E. A.; Giusti, M. M.; Wrolstad, R. E.; Gloria, M. B. A. Anthocyanins from Banana Bracts (Musa X Paradisiaca) as Potential Food Colorants. Food Chem. 2001, 73, 327–332. DOI: https://doi.org/10.1016/S0308-8146(00)00305-8.
- Pothavorn, P.; Kitdamrongsont, K.; Swangpol, S.; Wongniam, S.; Atawongsa, K.; Svasti, J.; Somana, J. Sap Phenolic Compositions in Some Bananas in Thailand. J. Agric. Food Chem. 2010, 58, 8782–8787. DOI: https://doi.org/10.1021/jf101220k.
- Gunavathy, N.; Padmavathy, S.; Murugavel, S. C. Phytochemical Evaluation of Musa acuminate Bract Using Screening of FTIR and UV-VIS Spectroscopic Analysis. J. Int. Acad. Res. Multidiscip., 2014, 2, 212–221. 2320–5083.
- De Wet, H.; Ramulondi, M.; Ngcobo, Z. N. The Use of Indigenous Medicine for the Treatment of Hypertension by a Rural Community in Northern Maputaland, South Africa. S. Afr. J. Bot. 2016, 103, 78–88. DOI: https://doi.org/10.1016/j.sajb.2015.08.011.
- Di Stasia, L. C.; Oliveiraa, G. P.; Carvalhaesa, M. A.; Queiroz-Juniora, M.; Tiena, O. S.; Kakinamia, S. H.; Reis, M. S. Medicinal Plants Popularly Used in the Brazilian Tropical Atlantic Forest. Fitoterapia. 2002, 73, 69–91. doi: https://doi.org/10.1016/s0367-326x(01)00362-8.
- Wang, S. Y.; Chang, C. Y.; Chen, C. W. Effect of Vinegar-egg on Growth Inhibition, Differentiation Human Leukemic U937 Cells and Its Immunomodulatory Activity. J. Food Drug Anal. 2018, 26, 731–740. doi: https://doi.org/10.1016/j.jfda.2017.10.007.
- Nagai, T.; Inoue, R. Preparation and the Functional Properties of Water Extract and Alkaline Extract of Royal Jelly. Food Chem. 2004, 84, 181–186. DOI:https://doi.org/10.1089/jmf.2006.9.363.
- Liao, D. Y.; Chai, Y. C.; Wang, S. H.; Chen, C. W.; Tsai, M. S. Antioxidant Activities and Contents of Flavonoids and Phenolic Acids of Talinum Triangulareextracts and Their Immunomodulatory Effects. J. Food Drug Anal. 2015, 23, 294–302. DOI: https://doi.org/10.1016/j.jfda.2014.07.010.
- Worthington, V.;. Alpha Amylase. In Worthington Enzyme Manual; Worthington, V., Ed.; Worthington Biochemical Corp.: Freehold, NJ, 1993; pp 36–41.
- Worawalai, W.; Wacharasindhu, S.; Phuwapraisirisan, P. Synthesis of New N-Substituted Aminoquercitols from Naturally Available (+)-Proto-Quercitol and Their α-Glucosidase Inhibitory Activity. Med. Chem. Commun. 2012, 3, 1466–1470. DOI: https://doi.org/10.1039/C2MD20227A.
- McDougall, G. J.; Kulkarni, N. N.; Stewart, D. Berry Polyphenols Inhibit Pancreatic Lipase Activity in Vitro. Food Chem. 2009, 115, 193–199. DOI: https://doi.org/10.1016/j.foodchem.2008.11.093.
- Shim, K. B.; Lee, H. J.; Lee, S. J.; Cho, H. A.; Yoon, N. Y.; Lim, C. W. Changes in Alcohol Dehydrogenase (ADH) and Acetaldehyde Dehydrogenase (ALDH) Activity during the Processing of Salt-dried Rockfish Sebastes schlegel. Korean J. Fish. Aquat. Sci. 2012, 45, 594–599. doi: https://doi.org/10.5657/KFAS.2012.0594.
- Julkunen, T. R.;. Phenolic Constituents in the Leaves of Northern Willows: Methods for the Analysis of Certain Phenolics. J. Agric. Food Chem. 1985, 33, 213–217. DOI: https://doi.org/10.1021/jf00062a013.
- Jia, Z.; Tang, M.; Wu, J. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. DOI: https://doi.org/10.1016/S0308-8146(98)00102-2.
- Xu, F.; Wu, H. M.; Wang, X. P.; Yang, Y.; Wang, Y. M.; Qian, H. B.; Zhang, Y. Y. RP-HPLC Characterization of Lupenone and β-Sitosterol in Rhizoma musae and Evaluation of the Anti-Diabetic Activity of Lupenone in Diabetic Sprague-Dawley Rats. Molecules. 2014, 19, 14114–14127. DOI: https://doi.org/10.3390/molecules190914114.
- Xie, L. S.; Gong, Z. Q.; Huang, Z. Y.; Meng, T. X. Determination of Umbelliferone in Rhinacanthus nasutus (L.) Lindau by HPLC. J. Nat. Sci. Hunan Normal Univ. 36, 52–55. 1000–2537. 2013.
- Wu, J. P.; Ding, X. L. Characterization of Inhibition and Stability of Soy-protein-derived Angiotensin I-converting Enzyme Inhibitory Peptides. Food Res. Int. 2002, 57, 619–625. doi:https://doi.org/10.1016/S0963-9969(01)00131-4.
- Spínola, V.; Llorent-Martínez, E. J.; Castilho, P. C. Antioxidant Polyphenols of Madeira Sorrel (Rumexmaderensis): How Do They Survive to in Vitro Simulated Gastrointestinal Digestion? Food Chem. 2018, 259, 105–112. DOI: https://doi.org/10.1016/j.foodchem.2018.03.112.
- Burgos-Edwards, A.; Jiménez-Aspee, F.; Thomas-Valdés, S.; Schmeda-Hirschmann, G.; Theoduloz, C. Qualitative and Quantitative Changes in Polyphenol Composition and Bioactivity of Ribesmagellanicum and R. Punctatum after in Vitro Gastrointestinal Digestion. Food Chem. 2017, 237, 1073–1082. DOI: https://doi.org/10.1016/j.foodchem.2017.06.060.
- Ramith, R.; Prithvi, S. S.; Nanjunda, S. S.; Farhan, Z.; Bhadrapura, L. D.; Nagendra, M. N. P.; Essop, M. F. Assessment of in Vivo Antidiabetic Properties of Umbelliferone and Lupeolconstituents of Banana (Musa sp. Var. Nanjangud Rasa Bale) Flower in Hyperglycaemic Rodent Model. PLoS One. 2016, 11, e0151135. DOI: https://doi.org/10.1371/journal.pone.0151135.
- Sim, M. O.; Ham, J. R.; Lee, H. I.; Seo, K. I.; Lee, M. K. Long-term Supplementation of Umbelliferone and 4-methylumbelliferone Alleviates High-fat Diet Induced Hypertriglyceridemia and Hyperglycemia in Mice. Chem.-Biol. Interact. 2014, 216, 9–16. DOI: https://doi.org/10.1016/j.cbi.2014.03.003.
- Lee, S. L.; Chau, G. Y.; Yao, C. T.; Wu, C. W.; Yin, S. J. Functional Assessment of Human Alcohol Dehydrogenase Family in Ethanol Metabolism: Significance of First-Pass Metabolism. Alcohol. Clin. Exp. Res. 2006, 30(7), 1132–1142. DOI: https://doi.org/10.1111/j.1530-0277.2006.00139.x.
- Jörnvall, H.; Hedlund, J.; Bergman, T.; Kallberg, Y.; Cederlund, E.; Persson, B. Origin and Evolution of Medium Chain Alcohol Dehydrogenases. Chem. Biol. Interact. 2013, 202, 91–96. doi: https://doi.org/10.1016/j.cbi.2012.11.008.
