 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study compares the antioxidant capacity of non-sterilized lemon product (NLFP) and sterilized lemon fermented product (LFP). After gastrointestinal simulation reaction, the antioxidant capacity decreased by 50% in lemon juice (LJ), 30%–40% in NLFP, and 40% in LFP respectively. For the bioactive ingredients, sterilized LFP had a polyphenol of 25.8 mg GAE/mL, ascorbic acid of 55.2 mg/mL, and flavonoids of 75.2 mg/L, which were higher than LJ. NLFP and LFP effectively increased the activity of Glutathione Peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT) and mitochondrial membrane potential, reduced the reactive oxygen species (ROS) content. The results in this study indicate that LJ with lactic acid fermentation can increase biologically active ingredients and antioxidant capacity which is important for future development.
Introduction
Lemons have a special flavor and aroma and are widely consumed globally. Many countries have high lemon production. As of 2016, India had the highest production, at 298 million tons, while Mexico’s output was 243 million tons, China’s was 229 million tons, and that of the United States was 82 million tons. In Taiwan, lemons are the main fruit produce, with 1,699 hectares planted in 2010 and a production of 18,105 hectares (www. Atlasbig.com); lemons are mostly cultivated in the south of Taiwan, with 70% of the plantation in Pingtung. The main variety cultivated is Citrus limon (L.) Burm (Eureka lemon).[Citation1] The significant lemon cultivation indicates their commercial value and consumption circulation.
Eureka lemon [Citrus limon (L.) Burm] is the second most planted citrus fruit.[Citation2] Lemons are rich in bioactive compounds including hesperidin and naringin, while the main flavonoids in lemons are ferulic acid. Furthermore, lemons contain polyphenolic compounds such as catechin, which are antioxidant and free radical scavenging, and have the ability to prevent cardiovascular diseases.[Citation3] The total polyphenol content of lemons is higher than that of pomelos, with higher antioxidant potential.[Citation4] The antioxidant capacity of lemons is beneficial to humans.[Citation5]
The numerous benefits of lemons and their high processability have been recorded in many studies such as simple juice processing with high value effects.[Citation6] lemon slices.[Citation7] bread.[Citation8] lemon flavor fruit liquor.[Citation9] lemon oil essences on food antibacterial and quality improvement[Citation10–12] and the production of fermented lemon products.[Citation13] However, excluding studies on the use of probiotics in lemon fermentation and the improvement of the biological activity of food caused by lemons[Citation14] and how lactobacillus strains are also commonly used in food flavor and functional improvement.[Citation15] there is a lack of research on related products, especially in the application of lemon in lactic acid fermentation.
Therefore, in this study, we used Eureka lemons as an ingredient and performed 90 days of lactic acid fermentation to produce lemon-fermented products (LFP) and compared them to those of commercial lemon juice (LJ). This study examines changes in the components after the lactic acid fermentation process, changes in the components’ antioxidant capacity, and biological active components before and after the gastrointestinal tract simulation to detect the activity of antioxidant enzymes. Moreover, we investigated how lemon fermentation extracts can improve rat liver cells through anti-oxidation properties of the strain (Clone-9) cells, and finally, we performed sensory evaluation to understand the differences and product value before and after fermentation.
Materials and methods
Chemicals and reagents
In terms of the chemicals, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 1,2-Phthalic dicarb, and hydrogen peroxide (H2O2) were purchased from Alfa Aesar (Haverhill, MA, USA). Methanol, potassium persulfate, and hydrochloric acid were purchased from Aencore Chemical Co. (Melbourne, VIC, AU). Potassium ferricyanide was purchased from ThermoFisher Scientific (Waltham, MA, USA). Hydrochloric acid, ascorbic acid, R&D Systems (Minneapolis, MN, USA), Pepsin, Gallic acid, 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) (ABTS), Hydroperoxidases, 2,6-dichlorophenol Indophenol, Trypsin, Folin-Ciocalten were produced by Sigma-Aldrich (Saint Louis, USA).
Preparation of the lemon-fermented products
Organic lemons were obtained from Jiuru, Pingtung, Taiwan. The lemons were harvested, picked, washed, and dried, and Lactobacillus OPC1 were provided by Openmind Co. Ltd. The whole lemon was then squeezed into juice by citrus juicer machine, and the seed and pulp were removed. LJ, which has a Brix value of 7–10°Brix and pH value 2.5–2.8 was placed in a fermentation tank with Lactobacillus OPC1 (0.1%, powder and calculated by the weight of the lemon juice) and inoculated at 25°C. The strain was activated for 30 mins and agitated to prevent lumps.
After inoculation into the fermentation tank, the tank was opened and stirred for 15 mins. After standing for 24hrs, the tank was stirred for 30 mins, followed by stirring for 30 days every 48hrs. After 30 days of fermentation, then the mature process continued for 60 days at a frequency of stirring for 30 min every 72hrs. The sugar content was determined to be 3–6°Brix, and the pH value was 2.3–2.7. This is the index of the fermentation endpoint when the upper and lower layers of the finished product are obviously separated, and the upper layer is light yellow and the lower layer is dark yellow.
Non-sterilized lemon-fermented products (NLFP) were obtained from unsterilized samples; sterilized lemon-fermented products (LFP) were obtained after the sample was sterilized at 80°C for 1 min, and LJ was obtained after pressing and removing the seeds and pulp. Three samples were stored at 18°C prior to use. The NLFP and LFP were adjusted to concentrations of 1%, 10%, 25%, 50%, and 100% with distilled water for antioxidant analysis.
Antioxidant analysis
DPPH antioxidant analysis
The free radical scavenging activity was determined using the DPPH method.[Citation16] Then, 20 microliters of the sample were added to 180 μL DPPH reagent and reacted in the dark for 30 mins, and the absorbance was measured at 517 nm. Vitamin C (1000 ppm) was used as a control. The free radical scavenging activity was expressed as the percentage inhibition of DPPH and calculated using the following formula:
Reducing power analysis
Next, 1 mL of 200 mM phosphate buffer solution (pH 6.6) was added to 1 mL of the sample, and 1% potassium ferricyanide was added, mixed well, and incubated for 20 min in a 50°C water bath, rapidly cooled, with 1 mL of 10% trichloroacetic acid (TCA). Subsequently, the sample was centrifuged at 3000rpm for 10 mins. Moreover, 100 microliters of supernatant was drawn and 100 μL of distilled water was added, 100 μL of 0.1% ferric chloride (FeCl3‧6H2O) solution (prepared with 3.5% HCl solution) was mixed well and incubated for 10 min, and then the absorbance was measured at 700 nm. The higher the absorbance, the stronger the reducing power.[Citation16]
Trolox equivalent antioxidant activity
Potassium persulfate was added to 7 mM ABTS (2, 2ʹ-azinobis[3-ethylbenzothiazoline-6-sulfonic acid]) to prepare a final concentration of 2.45 mM solution, mixed well, and reacted in the dark for 12–16hrs at room temperature to stabilize the blue ABTS‧+ cation free radical aqueous solution. The solution was diluted using PBS to an absorbance of 0.70 (±0.02) at 734 nm for later use. Then, 1 mL of the diluted solution was added to 10 μL of different concentrations and allowed to stand for a minute, and the absorbance was measured at 734 nm. We then mixed in peroxidase, ABTS, and H2O2, and reacted it in the dark for an hour, then added 0.1 µL of sample solution and tested the absorbance at 734 nm. The total antioxidant capacity was calculated as follows:
Bioactive compound analysis
Analysis of ascorbic acid
The sample was centrifuged at 8000rpm for 15 mins, and 0.1 mL of supernatant was drawn, added with 0.9 mL of 1% of metaphosphoric acid and 9 mL of 2,6 – dichloroindophenol (50 μmol/L), mixed well, and tested under 515 nm.
Determination of polyphenols
Moreover, 100 microliters of the sample were added to 100 μL, 1 N of the Folin-Ciocalteu reagent, mixed well, and incubated for 5 mins. Then, 200 μL of 20% sodium carbonate was added, and the mixture stood for 20 mins. Then, the sample was centrifuged at 10000rpm for 30 mins and the supernatant was withdrawn for absorbance determination at 750 nm with a spectrophotometer.[Citation17]
Determination of flavanols
In all, 0.5 mL of the sample was added to 1.5 mL of 95% ethanol, followed by 0.1 mL of 10% AlCl3, 1 mL of (1 M) CH3COOK, and 2.8 mL of deionized water and tested at 415 nm. Quercetin was used to plot the standard curve and concentration calculation.[Citation18]
In vitro gastrointestinal digestion
Gastric phase
Then, 20 milligrams of the freeze-dried of LFP and NLFP was added to phosphate buffer (pH 7.0), and 1 M of HCl was used to adjust the pH to 2.0. Pepsin (enzyme/substrate 1:50 w/w) was then added and heated at 37°C for 2hrs before use.
Intestinal phase
Moreover, 0.9 M of NaHCO3 was used to adjust the pH to 5.3, and 1 M of NaOH was used to adjust it to 7.5. Then, pancreatin was added and heated at 37°C for 2hrs, centrifuged, and stored for later use.[Citation19]
Sensory evaluation
The sensory evaluation was carried out with four different samples and commercial LJ by 30 consumers. The questionnaire was designed to investigate flavor acceptability, acidity taste, sweetness taste, flavor distinctness, taste acceptability, and overall acceptability. The sensory evaluation was conducted using a 9-hedonic scale (dislike = 1, extremely like = 9)
Clone-9 cells oxidative activity
Determination of superoxide dismutase activity
The test was conducted using the superoxide dismutase activity assay kit (SD125, RANDOX). The activity is determined by understanding the superoxide dismutase (SOD) inhibition of INT (2-(4-indophenyl)-3-(4-nitrophenol-5-phenylterazolium chloride) where xanthine and xanthine oxidase (XOD) produced superoxide anion (O2-), which produced a red color when it reacted with INT and Formazan dye (red Formazan dye). Then, 10 microliters of cell solution were drawn to a 96-microwell plate, in addition to 200 μL of mixed substrate and 50 μL of XOD under dark conditions, and the absorbance (A1) was tested at 505 nm after a 30 sec reaction. The solution was tested again after 3 mins (A2). The SOD activity was calculated as follows:
Determination of Glutathione peroxidase activity
The test was carried out with a Glutathione Peroxidase (GPx) assay kit (RS505, RANDOX). Next, 10 μL of cell solution was drawn to a 96 microwell plate, and 100 μL of R1 buffer (containing 4 mM GSH, > 0.5 U/L GR, 0.034 mM NADPH, 0.05 M phosphate buffer, and 4.3 mM EDTA) and 4 μL R2 reagent (0.18 mM Cumene hydroperoxide), and the absorbance was measured at 340 nm after it stood for a minute. After 3 mins, the absorbance was measured again at 340 nm. The GPx activity was calculated as follows:
Determination of catalase activity
The test was performed using a catalase (CAT) assay kit (50283, Cayman). In all, 20 microliters of cell solution were drawn to 96 microwell plate, and 100 μL assay buffer (100 mM potassium phosphate), 30 μL of methanol, and 20 μL of H2O2 were vibrated in the dark for 20 mins. Then, adding 30 μL of potassium hydroxide and purpald, it was vibrated in the dark for 10 mins following by the addition of 10 μL of Potassium periodate, and vibrating in the dark for 5 mins. The absorbance was measured at 540 nm, and CAT activity was calculated by substituting the absorbance to the standard curve of CAT activity.
Endogenous levels of intracellular reactive oxygen production
When DCFH-DA enters the cell, it is deacetylated into DCHF where DCHF cannot enter the cell membrane freely but be left in the cell; then, it is oxidized by the reactive oxygen species (ROS) generated from the cell to a fluorescent DCF. The more fluorescent the DCF, the higher the ROS content in the cell. The experiment was carried out by removing 24hrs of cultured cells from the cultured solution, washed twice with PBS, and incubated at a constant temperature of 37°C and 5% CO2 for 3 mins after adding trypsin. Then, 1 milliliter of collected cell DMEM medium was added to a 1.5 mL microcentrifuge tube to centrifuge at 201 × g for 3 min at 25°C, and then, the supernatant and pellet was mixed well with 4°C PBS. The cell count of 1 × 106 cells per mL) was dissolved in 100 μL PBS, followed by the addition of 2 μL of Hoechst (500 μg/mL) at 37°C for 5 mins. Subsequently, the sample was centrifuged at 201 × g for 5 min at 25°C, the supernatant was removed, and the cells were washed three times with 100 μL of PBS. Then, 50 μL of PBS was added, and 30 μL of the solution was drawn to Slide-A2. The NC-3000 was set to ROS-DCF mode for determination of cell fluorescence intensity, where higher intensity represented higher production of ROS.
Mitochondrial membrane potential assay
JC-1 (5, 5ʹ, 6, 6ʹ-tetrachloro-1, 1ʹ, 3, 3ʹ-tetraethylbenzimidazolyl-carbocyanine iodide) is a fluorescent dye that reveals red fluorescence after reacting with the dye. Apoptotic cells show green fluorescence after reaction with JC-1. The stronger the red fluorescence, the more complete the mitochondria. The test was conducted by drawing 24hrs cultured cells and washed twice with PBS after removing the medium, then incubated at a constant temperature of 37°C and 5% CO2 for 3 mins after adding trypsin. Next, 1 milliliter of collected cell DMEM medium was added to a 1.5 mL microcentrifuge tube to centrifuge at 201 × g for 3 mins at 25°C; the supernatant was then removed, and the pellet was mixed well with 4°C of PBS, followed by the addition of 12.5 μL, 200 μg/mL JC-1 dye, and allowed to stand for 30 min at 37°C in a dry bath. The cells were then centrifuged for 5 mins at 100 × g and 25°C, and the pellet was mixed well with 4°C PBS; the action was repeated twice, followed by reconstitution with 1 mL of 4°C PBS. The solution was analyzed with a flow cytometer (BD Accuri C6). The cell distribution diagram shows the ratio of healthy cells to apoptotic cells. The higher the ratio of healthy cells, the more complete the mitochondrial membrane.
Statistical analysis
The results were analyzed within group with (one-way ANOVA) of SPSS 12.0 (SPSS, USA), and significant differences between groups were analyzed with Duncan’s multiple range test and Tukey’s test (P < .05).
Results and discussion
Antioxidant capacity of non-sterilized lemon fermented product (NLFP), lemon fermented product (LFP), and lemon juice (LJ)
The lemon fermented product were classified as NLFP and LFP to understand the differences in component and efficacy, and later compared to commercial LJ. shows the antioxidant capacity determination under different conditions and concentrations. The sample concentrations were 1%, 10%, 25%, 50%, 100%, and vitamin C was used as the control to determine the functionality of the diluted samples. Under different conditions but with the same concentration of lemon products, LHP had the highest DPPH scavenging capacity, while commercial LJ had the lowest. The LFP undergoes lactic acid fermentation, where lactic acid bacteria produce enzymes to decompose part of lemons to form free active ingredients. This explains why NLFP and LFP had a higher antioxidant capacity than LJ. Sterilized products showed a lower scavenging activity than NLFP, but did not show a significant difference in the statistical analysis. Research has shown that the antioxidant capacity of citrus is related to the content of vitamin C and phenols. In addition, the pulp and flesh are good bioactive ingredients.[Citation17]
Figure 1. Antioxidant capacity analysis of LFP before and after in vitro gastrointestinal digestion. DPPH radical scavenging activity (%). (a) DPPH analysis before in-vitro gastrointestinal digestion. (b) DPPH analysis after in-vitro gastrointestinal digestion. (c) Reducing power before in-vitro gastrointestinal digestion. (d) Reducing power after in-vitro gastrointestinal digestion. (e) TEAC analysis before in-vitro gastrointestinal digestion. (f) TEAC analysis after in-vitro gastrointestinal digestion. Vit C concentration:1000ppm
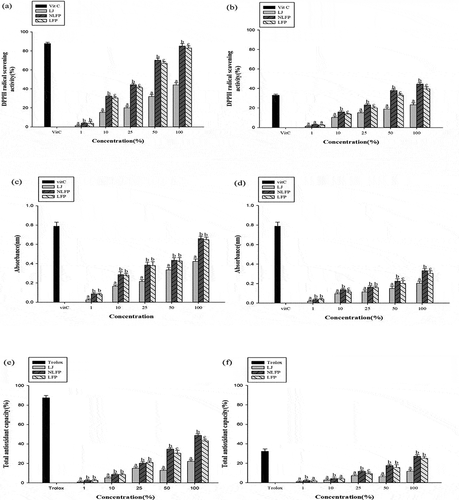
As seen in , the scavenging activity ranged between 82%–85% at 100% concentration. The scavenging activity results are equivalent to 800ppm of the standard. Research has also reported that the DPPH free radical scavenging test is a rapid method that can be used to evaluate the antioxidant activity of a sample by changing the absorbance of the solution in the DPPH free radical.[Citation20] In addition, the species of lemon used is a factor that influences the antioxidant activity, while maturity of lemon and type of antioxidant activity are related, such as ABTS and DPPH.[Citation21]
The LFP is used to determine the bioactive component and antioxidant capacity affected by acid and gastrointestinal systems after simulation of gastric acid and bile salt. In DPPH scavenging activity, vitamin C standard decreased 60% of the total scavenging activity, while LFP and NLFP decreased by 40%, LJ decreased from 62.7% to 23.2% (), which differs by 20% from the LFP. Therefore, the fermentation process improves the tolerance of LJ in the gastrointestinal phase, which can resist the bile salt environment. Furthermore, the in vitro gastrointestinal digestion system simulates changes in ingredients and can be used as basic data for further utilization of food functionality.[Citation19,Citation22]
During the lactic fermentation of lemons, microorganisms breakdown the lemon fiber for sugar metabolism, producing various reducing substances such as polyphenols and free the bioactive substance from the lemon peel, as shown in . reveals that LFP has a significant difference from other samples, taking 100% concentration as an example. The absorbance of commercial LJ was 0.41, while the samples ranged between 0.62–0.71, showing higher absorbance than the commercial LJ. These results demonstrate that fermentation could improve the bioactivity component.
By comparing samples with different concentrations, NLFP showed the strongest reducing power and commercial LJ showed the weakest. However, based on statistical analysis, NLFP and LFP did not show significant differences. These results were similar to , and many reducing substances are present in lemon essential oil and released from fiber, which shows that the reducing substances of commercial LJ are able to increase the content of reducing substances through fermentation such as Vitamin C. Therefore, 2 groups of LFPs were added to compare the reducing power of commercial LJ.
The reducing power of commercial LJ after gastrointestinal simulation decreased more than that of LFPs, as shown in . At 100% concentration, the reducing power decreases from 0.41 to 0.21, while NLFP decreases from 0.69 to 0.39. The reducing power of the control decreased by approximately 60%, that of LJ decreased by about 50%, and NLFP decreased by 30%–40%, as seen in . This is because the fermentation product of microorganisms was protected in the gastrointestinal system, and the results showed that fermentation helps in producing reducing substances and maintaining the reducing power.
The results of total antioxidant capacity were similar to . As seen in , samples from each concentration (except 1%) showed significant differences from the control. Previous studies have revealed that lactic acid fermentation increases the antioxidant capacity.[Citation23,Citation24] Studies have also found that metabolites from different strains are related to antioxidant capacity.[Citation25]
Bioactivity compounds of non-sterilized lemon fermented product (NLFP), lemon fermented product (LFP), and lemon juice (LJ)
In this study, the bioactive components of the samples before and after gastrointestinal simulation were analyzed. The results are reported in . From the analysis of the original sample, we understand the differences between the control group and the four groups of samples, such as total polyphenols – the total polyphenol content of the LJ was approximately 4.14 mg GAE. Studies have stated that lemon peel has a large number of biologically active ingredients.[Citation26] however, variations in crushing and pressing methods lead to different active ingredient contents.[Citation27] Thus, in the present study, the polyphenol-rich peel might not have been crushed properly, causing the polyphenols in the peel to be release. Polyphenols in NLFP were higher than LJ, since the whole lemon was used to undergo fermentation after crushing, which means the fiber on the peel decomposed and freed the bioactive substances. The polyphenol, ascorbic acid, and flavonoids of NLFP had a higher value than other samples, which might be due to the non-sterilization of samples. Studies have also stated that the antioxidant capacity, total polyphenol, and total flavonoids are correlated, and phenolic acid and flavonoids are natural antioxidants of citrus fruits [Citation28]
Table 1. Bioactive compounds before and after in vitro gastrointestinal digestion
The bioactive substances in gastrointestinal simulated samples were reduced because of the environment of bile salt and gastric acid. The analyses of the control group observed a 50% decrease in each analysis items, while in NLFP groups the results only showed a 30%–40% decrease. This might be the bioactivity of NLFP groups protected by essential oil which produce from peel. Although every sample showed a decline, when it reached the gastrointestinal system, it had a higher value than the control group, for example, except for the non-fermented LJ, it retained a higher value of ascorbic acid. Thus, we concluded that the fermented samples retained bioactive compounds after gastrointestinal simulation, which is good for nutrition retention and product development.
Cell oxidative stress of non-sterilized lemon fermented product (NLFP), lemon fermented product (LFP), and lemon juice (LJ)
Oxidative stress is considered a dangerous factor of diseases, which is mainly caused by excessive ROS generated. ROS are mainly produced by mitochondria, and excessive generation will cause damage to protein and DNA and lead to cell damage.[Citation29] Generation of oxidative stress will cause inflammation, cancer, neurological disease, cardiovascular disease, diabetes, and kidney disease.[Citation13] Our body balances oxidative stress by regulating antioxidant enzyme activity, active oxygen content, and mitochondrial membrane potential.
Superoxide is one of the main ROS in cells. Our body will increase antioxidant enzyme activity for protection to avoid being stimulated by oxidative stress. SOD is an enzyme that decomposes superoxide and metabolizes superoxide into H2O2, then decomposes H2O2 with GPx or CAT into H2O, reducing oxidative stress stimulation in the body.
shows the effect of sterilized and unsterilized LFP on the activity of the antioxidant enzyme SOD in Clone-9 cells. After 24hrs of combined induction Clone-9 cells with different concentrations (0.1, 0.5, 0.75, and 1 mg/mL) of LFP and NLFP, the SOD activity increased significantly compared to the induction group. The SOD activity of the 0.1 mg/mL and 0.75 mg/mL NLFP combined induction group was significantly higher than that of the 0.1 mg/mL and 0.75 mg/mL LFP combined induction group. These results indicate that LFP or NLFP can increase the activity of the antioxidant enzyme SOD in Clone-9 cells and reduce oxidative stress in the cell.
Figure 2. Effect of sterilized lemon-fermented products (LFP) or non-sterilized lemon-fermented products (NLFP) on antioxidant enzyme activity in Clone-9 cells. (a) SOD: Superoxide dismutase; (b) GPx: Glutathione peroxidase; (c) CAT: Catalase; (d) ROS: Reactive oxygen species. Significance of difference in activities of different dose was evaluated by Tukey’s multiple range test statistical analysis
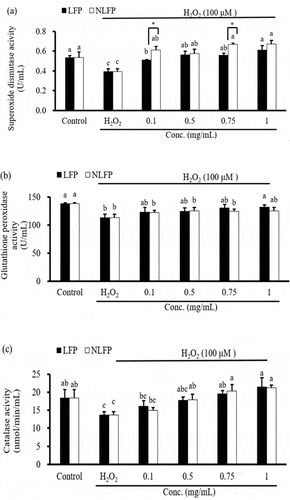
shows the activity of GPx. After 24hrs of combined induction Clone-9 cells with 1 mg/mL LFP. GPx activity increased significantly compared to the induction group. By using different concentrations (0.1, 0.5, 0.75, and 1 mg/mL) of NLFP, the GPx activity did not show a significant effect. The results indicated that LFP increased GPx activity in Clone-9 cells and reduced oxidative stress in the cell.
shows the activity of catalase. After 24hrs of combined induction Clone-9 cells with 0.75, 1 mg/mL LFP, or 0.5, 0.75, and 1 mg/mL NLFP, the CAT activity increased significantly compared to the induction group. The causes of these increases may include the increased activity of antioxidant enzyme from citrus bioactive ingredients. Recent studies have indicated that flavonoids can increase the activity of SOD, CAT, and GPx, such as citrusin, hesperidin.[Citation30,Citation31] and the bioactive ingredients of LFP can increase the activity of catalase.
shows the effect of sterilized and unsterilized LFP on the content of reactive oxygen in Clone-9 cells. After 24hrs of combined induction Clone-9 cells with different concentrations (0.1, 0.5, 0.75, and 1 mg/mL) of LFP or NLFP, the intracellular ROS content decreased significantly compared to the induction group. The ROS content of the 0.1 mg/mL 0.75 mg/mL NLFP combined induction group was significantly lower than that of the 0.1 mg/mL and 0.75 mg/mL LFP combined induction group.
Figure 3. Effect of sterilized lemon-fermented products (LFP) or non-sterilized lemon-fermented products (NLFP) on ROS content and MMP in Clone-9 cells. Cells were treated simultaneously with both different dose LFP or NLFP and 100 μM H2O2 for 24hrs. (a) ROS: Reactive oxygen species; (b) MMPA: Mitochondrial membrane potential. Significance of difference in activities of different dose was evaluated by Tukey’s multiple range test statistical analysis
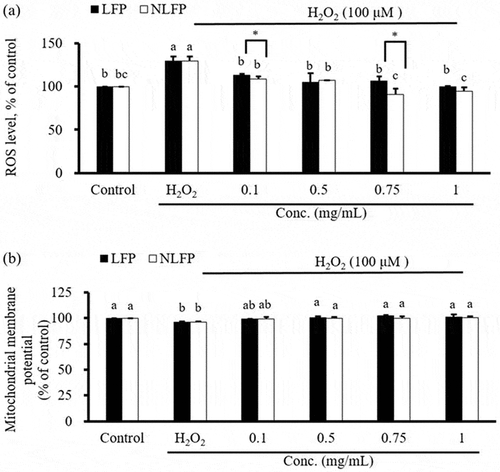
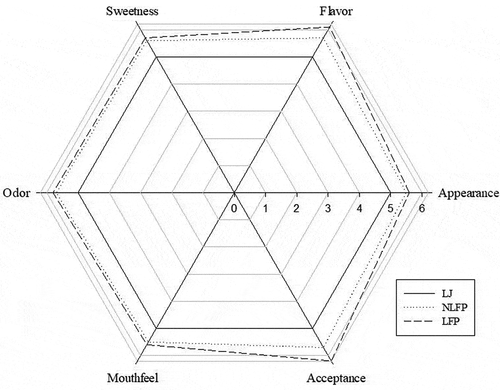
Figure 4. Sensory evaluation of LJ, NLFP, and LFP
shows the effect of sterilized and unsterilized LFP on the mitochondrial membrane potential in Clone-9 cells. ROS are mainly generated in mitochondria, where mitochondrial dynamics represent the continuous fission and fusion of mitochondria. The citric acid cycle is the main pathway of ATP production in humans. MMP is generated by a redox reaction related to the citric acid cycle and then acts as ATP synthase for ATP storage. Sterilized and unsterilized LFP significantly increases cellular antioxidant enzyme activity, while the increase in MMAP reduces the generation of oxidative stress.
Sensory evaluation of non-sterilized lemon fermented product (NLFP), lemon fermented product (LFP), and lemon juice (LJ)
Essential oil is found in the peel of a plant and has a unique and representative flavor. However, a high concentration of essential oil leads to an unpleasant odor and flavor; therefore, it is rarely used in the food industry and its application needs to be developed. This study used four samples for sensory evaluation analysis, and the results are shown in . Regarding sweetness, flavor, odor, and overall acceptance, LFP had the highest acceptance, while NLFP had the second highest acceptance. The resulting LFP sample with sterilization caused flavor changes due to the high temperature of sterilization. For example, the degradation of polysaccharides and oligosaccharides caused the presentation of sweetness in the sample increase and loss of volatile compounds during heating. The NLFP groups had a lower value than LFP, which might be due to the irritating flavor and the lemon flavor caused by the unpleasant odor of the sample in addition to the bitter flavor. The LFP samples had higher scores, which may be caused by the decomposition of the fiber and the peel, which produced a granular texture and a smooth taste. The sweetness, flavor, odor, and overall acceptance of fermented samples was higher than those of commercial LJ. This stated that the flavor of LJ can be improved by lactic acid fermentation.
Conclusion
Lemon juice is often used as a beverage and condiment. Fermented lemon products were produced by crushing and fermenting the whole lemon, including the peel and fiber. The LFP produced had a higher nutritious value, such as antioxidant capacity, reducing power, and bioactive substances. After gastrointestinal simulation, the retained active ingredients were higher than those from the control group, which means that the fermented samples could retain higher bioactive components to facilitate human absorption. Through cell experiments, LFP and NLFP could increase the activity of antioxidant enzymes GPx, SOD, and CAT, and reduce the ROS content in Clone-9 cells. Moreover, by increasing the mitochondrial membrane potential, it could maintain mitochondrial integrity and reduce oxidative stress damage. Furthermore, the sensory evaluation results showed that fermentation had a positive effect, where sweetness and overall acceptance had the highest score in fermentation samples. The results of this study have various applications in the fermentation of lemons and can be used as an important basis for future experimental planning, product development, and application.
Acknowledgments
The author would like to thank everyone who volunteered to assist with the study. The authors declare no conflicts of interest.
Additional information
Funding
References
- www.coa.gov.tw/ws.php?id=23876 “ Regulation of production and sales of lemons”Council of Agriculture, Executive Yuen R.O.C. ; 2011.
- García-Salas, P.; Gómez-Caravaca, A. M.; Arráez-Román, D.; Segura-Carretero, A.; Guerra-Hernández, E.; García-Villanova, B.; Fernández-Gutiérrez, A. Influence of Technological Processes on Phenolic Compounds, Organic Acids, Furanic Derivatives, and Antioxidant Activity of Whole-Lemon Powder. Food Chem. 2013, 141(2), 869–878. DOI: https://doi.org/10.1016/j.foodchem.2013.02.124.
- Binello, A.; Cravotto, G.; Boffa, L.; Stevanato, L.; Bellumori, M.; Innocenti, M.; Mulinacci, N. Efficient and Selective Green Extraction of Polyphenols from Lemon Balm. C. R. Chim. 2017, 20(9–10), 921–926. DOI: https://doi.org/10.1016/j.crci.2017.06.003.
- Gorinstein, S.; Martin-Belloso, O.; Park, Y.-S.; Haruenkit, R.; Lojek, A.; ĈIž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of Some Biochemical Characteristics of Different Citrus Fruits. Food Chem. 2001, 74(3), 309–315. DOI: https://doi.org/10.1016/S0308-8146(01)00157-1.
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid Profile and Radical-Scavenging Activity of Mediterranean Sweet Lemon (Citrus Limetta Risso) Juice. Food Chem. 2011, 129(2), 417–422. DOI: https://doi.org/10.1016/j.foodchem.2011.04.093.
- Lorente, J.; Vegara, S.; Martí, N.; Ibarz, A.; Coll, L.; Hernández, J.; Valero, M.; Saura, D. Chemical Guide Parameters for Spanish Lemon (Citrus Limon (L.) Burm.) Juices. Food Chem. 2014, 162, 186–191. DOI: https://doi.org/10.1016/j.foodchem.2014.04.042.
- Guo, T.; Yin, T.; Ma, Z.; Wang, Z.; Sun, X.; Yuan, W. Characterization of Different Processes Lemon Slice Using Electronic Tongue. IFAC-PapersOnLine. 2018, 51(17), 683–688. DOI: https://doi.org/10.1016/j.ifacol.2018.08.117.
- Fu, J. T.; Ho Chang, Y.; Shiau, S. Y. Rheological, Antioxidative and Sensory Properties of Dough and Mantou (Steamed Bread) Enriched with Lemon Fiber. LWT - Food Sci. Technol. 2015, 61(1), 56–62. DOI: https://doi.org/10.1016/j.lwt.2014.11.034.
- Altınel, T.; Cherlin, G. Limoncello. J. Algebra 2005, 291(2), 373–415. DOI: https://doi.org/10.1016/j.jalgebra.2005.02.033.
- Alfonzo, A.; Martorana, A.; Guarrasi, V.; Barbera, M.; Gaglio, R.; Santulli, A.; Settanni, L.; Galati, A.; Moschetti, G.; Francesca, N. Effect of the Lemon Essential Oils on the Safety and Sensory Quality of Salted Sardines (Sardina Pilchardus Walbaum 1792). Food Control. 2017, 73, 1265–1274. DOI: https://doi.org/10.1016/j.foodcont.2016.10.046.
- Falls, N.; Singh, D.; Anwar, F.; Verma, A.; Kumar, V. Amelioration of Neurodegeneration and Cognitive Impairment by Lemon Oil in Experimental Model of Stressed Mice. Biomed. Pharmacother. 2018, 106, 575–583. DOI: https://doi.org/10.1016/j.biopha.2018.06.160.
- Yazgan, H.; Ozogul, Y.; Kuley, E. Antimicrobial Influence of Nanoemulsified Lemon Essential Oil and Pure Lemon Essential Oil on Food-Borne Pathogens and Fish Spoilage Bacteria. Int. J. Food Microbiol. 2019, 306, 108266. DOI: https://doi.org/10.1016/j.ijfoodmicro.2019.108266.
- Pisoschi, A. M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. DOI: https://doi.org/10.1016/j.ejmech.2015.04.040.
- Das, S.; Kumar Mishra, B.; Hati, S. Techno-Functional Characterization of Indigenous Lactobacillus Isolates from the Traditional Fermented Foods of Meghalaya, India. Curr. Res. Food Sci. 2020, 3, 9–18. DOI: https://doi.org/10.1016/j.crfs.2020.01.002.
- Hashemi, S. M. B.; Mousavi Khaneghah, A.; Barba, F. J.; Nemati, Z.; Sohrabi Shokofti, S.; Alizadeh, F. Fermented Sweet Lemon Juice (Citrus Limetta) Using Lactobacillus Plantarum LS5: Chemical Composition, Antioxidant and Antibacterial Activities. J. Funct. Foods. 2017, 38, 409–414. DOI: https://doi.org/10.1016/j.jff.2017.09.040.
- Wang, C.-Y.; Chen, Y.-W.; Hou, C.-Y. Antioxidant and Antibacterial Activity of Seven Predominant Terpenoids. Int. J. Food Prop. 2019, 22(1), 230–238. DOI: https://doi.org/10.1080/10942912.2019.1582541.
- Lubinska-Szczygieł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T.; Shafreen, R. B.; Weisz, M.; Ezra, A.; Gorinstein, S. Quality of Limes Juices Based on the Aroma and Antioxidant Properties. Food Control. 2018, 89, 270–279. DOI: https://doi.org/10.1016/j.foodcont.2018.02.005.
- Park, Y. S.; Ham, K.-S.; Park, Y.-K.; Leontowicz, H.; Leontowicz, M.; Namieśnik, J.; Katrich, E.; Gorinstein, S. The Effects of Treatment on Quality Parameters of Smoothie-Type ‘Hayward’ Kiwi Fruit Beverages. Food Control. 2016, 70, 221–228. DOI: https://doi.org/10.1016/j.foodcont.2016.05.046.
- Hu, X. P.; Yin, F.-W.; Zhou, D.-Y.; Xie, H.-K.; Zhu, B.-W.; Ma, X.-C.; Tian, X.-G.; Wang, C.; Shahidi, F. Stability of Resveratrol Esters with Caprylic Acid during Simulated in Vitro Gastrointestinal Digestion. Food Chem. 2019, 276(June 2018), 675–679. DOI: https://doi.org/10.1016/j.foodchem.2018.10.062.
- Kandi, S.; Charles, A. L. Statistical Comparative Study between the Conventional DPPH Spectrophotometric and Dropping DPPH Analytical Method without Spectrophotometer: Evaluation for the Advancement of Antioxidant Activity Analysis. Food Chem. 2019, 287, 338–345. DOI: https://doi.org/10.1016/j.foodchem.2019.02.110.
- Mcharek, N.; Hanchi, B. Maturational Effects on Phenolic Constituents, Antioxidant Activities and LC-MS/MS Profiles of Lemon (Citrus Limon) Peels. Journal of Applied Botany and Food Quality. 2017, 90, 1–9.
- Hur, S. J.; OuLim, B.; Decker, E. A.; Julian McClements, D. In Vitro Human Digestion Models for Food Applications. Food Chem. 2011, 125(1), 1–12. DOI: https://doi.org/10.1016/j.foodchem.2010.08.036.
- Almalki, M. A.;. Exopolysaccharide Production by a New Lactobacillus Lactis Isolated from the Fermented Milk and Its Antioxidant Properties. J. King Saud Univ. Sci. 2020, 32(2), 1272–1277. DOI: https://doi.org/10.1016/j.jksus.2019.11.002.
- Liu, L.; Qu, X.; Xia, Q.; Wang, H.; Chen, P.; Li, X.; Wang, L.; Yang, W. Effect of Lactobacillus Rhamnosus on the Antioxidant Activity of Cheddar Cheese during Ripening and under Simulated Gastrointestinal Digestion. LWT. 2018, 95, 99–106. DOI: https://doi.org/10.1016/j.lwt.2018.04.053.
- Song, M. W.; Chung, Y.; Kim, K.-T.; Hong, W. S.; Chang, H. J.; Paik, H.-D. Probiotic Characteristics of Lactobacillus Brevis B13-2 Isolated from Kimchi and Investigation of Antioxidant and Immune-Modulating Abilities of Its Heat-Killed Cells. LWT. 2020, 128, 109452. DOI: https://doi.org/10.1016/j.lwt.2020.109452.
- Al-Qassabi, J.; Ali, S.; Mohammed Weli, A.; Hossain, M. A. Comparison of Total Phenols Content and Antioxidant Potential of Peel Extracts of Local and Imported Lemons Samples. Sustainable Chem. Pharm. 2018, 8, 71–75. DOI: https://doi.org/10.1016/j.scp.2018.03.001.
- Marin, F. R.; Martinez, M.; Uribesalgo, T.; Castillo, S.; Frutos, M. J. Changes in Nutraceutical Composition of Lemon Juices according to Different Industrial Extraction Systems. Food Chem. 2002, 78(3), 319–324. DOI: https://doi.org/10.1016/S0308-8146(02)00102-4.
- Park, Y.-S.; Cvikrová, M.; Martincová, O.; Ham, K.-S.; Kang, S.-G.; Park, Y.-K.; Namiesnik, J.; Rombolà, A. D.; Jastrzebski, Z.; Gorinstein, S. In Vitro Antioxidative and Binding Properties of Phenolics in Traditional, Citrus and Exotic Fruits. Food Res. Int. 2015, 74, 37–47. DOI: https://doi.org/10.1016/j.foodres.2015.04.021.
- Buttke, T. M.; Sandstrom, P. A. Oxidative Stress as a Mediator of Apoptosis. Immunol. Today 1994, 15(1), 7–10. DOI: https://doi.org/10.1016/0167-5699(94)90018-3.
- Abolaji, A. O.; Victoria Babalola, O.; Kehinde Adegoke, A.; Farombi, E. O. Hesperidin, a Citrus Bioflavonoid, Alleviates Trichloroethylene-Induced Oxidative Stress in Drosophila Melanogaster. Environ. Toxicol. Pharmacol. 2017, 55(August), 202–207. DOI: https://doi.org/10.1016/j.etap.2017.08.038.
- Zaidun, N. H.; Chi Thent, Z.; Abd Latiff, A. Combating Oxidative Stress Disorders with Citrus Flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. DOI: https://doi.org/10.1016/j.lfs.2018.07.017.
