 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Akabare chilli (Capsicum chinese) is a high-value crop in the eastern hills of Nepal. Akabare growers faced the problem of its value decrement during transportation and marketing because of immediate post-harvest losses. This study was targeted for finding cheap post-harvest treatment to extend freshness and shelf life of fresh Akabare by applying post-harvest treatments like hydrocooling, refrigerated temperature storage, and perforation mediated plastic packaging. The chemical qualities (moisture, vitamin C and chlorophyll content) were studied on a regular interval of 4 days along with visual inspection for 20 days. The package modification included unperforated as well as perforated polypropylene bags having 2, 4 and 6 perforations of 2.5 mm diameter. Samples without any treatment remained fresh only for 2 days, while only hydrocooled samples retained moisture, vitamin C and chlorophyll content significantly (p < .05) higher than untreated, in both storage temperatures. None of the samples stored at room temperature retained freshness beyond 4 days. Thus, based on the better retention of moisture and vitamin C content, hydrocooling followed by packaging in polypropylene bag (four perforations) and storage at refrigerated temperature could be considered the best post-harvest treatment and packaging.
Introduction
There are 22–25 species of Capsicum out of which only five species are cultivated for use in food.[Citation1] Among these five, Capsicum annuum is the most commonly cultivated species for pungent (hot pepper) and non-pungent (sweet pepper/bell pepper) fruits and has worldwide commercial distribution. The hot pepper fruits are consumed in fresh, dried, or processed forms (e.g., after pickling in salt and vinegar). Fruit carotenoids, capsaicinoids and flavour extracts are used in food, feed, medicine and the cosmetic industries.[Citation2] Some varieties of Capsicum annuum (syn. chinense Jacqs) are highly prized for their noted hotness. One such variety, called Akabare khursani or dale khursani in Nepalese language, is taken to be one of the hottest chilli peppers (with Scoville heat unit ratings in the range of 100,000–350,000).[Citation3] The peculiar pungency and fiery hotness of Akabare increase appetite when taken as a meal adjunct.[Citation4] Akabare is grown in Sikkim, Nepal, Darjeeling and the surrounding regions.[Citation3] It is becoming a high-value crop in the eastern hilly region of Nepal (Tehrathum, Dhankuta, Panchthar and Ilam). It is also exported to India, Pakistan, Bangladesh and China. Its production has therefore become a very remunerative activity in the rural hills of eastern Nepal.[Citation4]
Akabare production, however, also has its own post-harvest problems, which stem mainly from the lack of technical knowledge for minimizing losses during handling, packaging, transportation and storage. Akabare loses moisture very rapidly. This causes not only weight loss (quantitative loss) but also shrivelling (qualitative loss), both to the detriment of the producer. The pepper is also very prone to microbial (mould and bacteria) decays.[Citation5] Several proven techniques have evolved for minimizing post-harvest losses in pepper[Citation6] but these largely apply to commercially important varieties. However, the literature on appropriate technology for the post-harvest handling of locally grown peppers in general and Akabare in particular, is relatively scarce and also, in the Nepalese rural context, the losses can be much higher than generalized in the literature. Therefore, this research intends to assess the effects of hydrocooling, packaging (use of perforated and unperforated plastic) and storage temperature (room and refrigerated) on the post-harvest quality (moisture and weight loss; vitamin C and chlorophyll content) and visual appearance of fresh Akabare.
Materials and methods
Fresh green Akabare (immediately after harvest) was collected in November 2019, from Pakhribas, Dhankuta, a hilly place situated in the eastern part of Nepal. The polypropylene (PP) plastic bags were purchased from the local market of Dharan, Nepal. The hand-plucked Akabare chillies of Habanero variety and same maturity (65 days from plantation) were packed loosely in plastic bags.
Package designing
The package designing of Akabare was carried out by the method followed by Pandey and Goswami[Citation7] with slight modification. The PP bags of dimensions 12 cm × 12 cm were used and perforations at several levels (with each of 2, 4 and 6 holes) with the help of punching machine of 2.5 mm diameter were made. Each sample was subdivided into sachets of 51 ± 3 g which contained 15–16 chillies and stored at room temperature (25–27°C) and refrigerated temperature (8–10°C) having relative humidity (RH) of 70–75%. For storage at refrigerated temperature, refrigerator (Model: LG-GL-V292RVBN) was used and RH of 70–75% was maintained using a saturated sodium chloride solution.[Citation8] Maintenance of refrigerated condition and RH throughout the storage period was confirmed using thermometer and hygrometer (Model: J412CTH) regularly.
Experimental Design
Two experiments were conducted to determine the best storage condition for fresh Akabare: storage at room temperature (25–27°C) and refrigeration (8–10°C) and a total of 7 treatments for each were applied. The samples with treatments 1, 2, 3, 4, 5, 6 and 7 were coded as A, B, C, D, E, F and G, respectively. For hydrocooling, freshly harvested chillies were immersed in water at 5–10°C for 10 minutes.
Treatment 1: No treatment for the control sample (A)
Treatment 2: Only hydrocooled and no packaging (B)
Treatment 3: Hydrocooling of Akabare and unperforated plastic packaging (C)
Treatment 4: No Hydrocooling of Akabare and unperforated plastic packaging (D)
Treatment 5: Hydrocooling of Akabare and two perforations plastic packaging (E)
Treatment 6: Hydrocooling of Akabare and four perforations plastic packaging (F)
Treatment 7: Hydrocooling of Akabare and six perforations plastic packaging (G)
The methodological approach used was pre-and post-treatments, i.e., the chillies were analyzed before, during and after treatments. Before the commencement of the experiment, analyses were carried out for moisture content, vitamin C, and visual appearance. During the storage period, the visual analysis was carried out on each day whereas chemical analysis was carried out in every 4 days up to the end of the experiment. Firmness was measured in a descriptive way, i.e., textural changes were detected by touch. In this experiment, moisture content and vitamin C truly represented freshness and chlorophyll content represented the stage of ripening and quality of the chillies.
Analysis of Akabare
Geometric dimension, appearance and firmness
The physical parameters of Akabare such as length, breadth and weight were recorded with the help of Vernier callipers and electronic balance.[Citation9] Based on visual observations, the appearance was recorded. The observations included colour changes, loss of shine and gloss, and presence of shrinkage, withering and wilting of original chilli.[Citation10] Firmness changes rapidly during storage. These textural changes were detected by touch. Textural measurements were performed on harvest day and during post-harvest storage.[Citation11]
Moisture, vitamin C and chlorophyll content
Moisture content was determined as per the method reported by Ranganna[Citation12] by drying the sample in a hot air oven at 100 ± 3°C till constant mass was obtained. Ascorbic acid was determined by 2,6-dichlorophenol-indophenol visual titration method described by Ranganna.[Citation12] 10 g of the sample was macerated using 3% meta-phosphoric acid and volume was made up to 100 ml with meta-phosphoric acid. An aliquot of 5 ml of the extract was taken and titrated with the standard dye (2, 6-dichlorophenol indophenol) till pale pink end-point was observed which persisted for 15–20 s.
Chlorophyll content in chillies was determined by spectrophotometric method reported by Arnon.[Citation13] 1 g sample was taken and ground into fine pulp in mortar and pestle with about 10 ml of 80% acetone. The pulp was centrifuged for 5 min. It was extracted with 80% acetone till no perceptible green colour in the residue was seen. The absorbance of the extracts was read in a spectrophotometer at 663 and 645 nm using 80% acetone as blank. Using the absorption coefficients, the amount of chlorophyll is calculated using the empirical formula:
where A is the absorbance at a specific wavelength, V is the final volume of chlorophyll extract in 80% acetone, and W is the weight of tissue extracted.
Statistical analysis
Data were statistically processed by GenStat (12th edition) developed by VSN International Limited for Analysis of Variance (ANOVA). Means of the data was separated whether they are significant or not by using the Least Significant Difference (LSD) method at 5% level of significance. The analysis was carried out in sextuplicate.
Results and discussion
The shelf life of fresh Akabare chillies was observed by applying post-harvest treatments like hydrocooling, refrigerated temperature storage, and perforation mediated plastic packaging. Accordingly, seven treatments for each storage temperatures (room and refrigerated) were applied and the changes in moisture, vitamin C, and chlorophyll contents were measured and compared during the storage period.
Physical parameters of fresh Akabare
The average length of Akabare was found to be 2.7 ± 0.02 cm, breadth as 2.35 ± 0.02 cm, and weight as 3.45 ± 0.08 g. Similar results were obtained by Khanal.[Citation5]
Analysis of fresh Akabare before storage
Fresh green Akabare was analyzed before storage for moisture content, vitamin C and chlorophyll content and results obtained have been mentioned in .
Table 1. Chemical parameters of Akabare before storage
The chemical composition of chilli is influenced by many factors, such as genotype and fruit maturity stage, place, and cultivar[Citation6,Citation14] Thus, the chemical composition has a very wide range. The obtained values were closer to those obtained for hot peppers by Litoriya et al.[Citation15]
Effect of temperature on the storage stability of Akabare (based on visual appearance)
Maximum storage days of chillies under various treatments have been shown in .
Figure 1. Effect of storage temperature on storage time of Akabare chillies
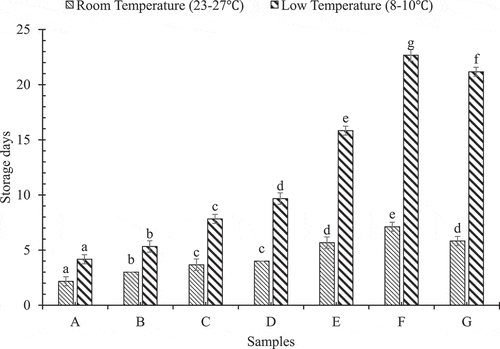
At room temperature (25–27°C), no treatment sample (sample A) was acceptable only for 2.17 ± 0.41 days and only hydrocooled sample (sample B) for 3 days whereas at refrigerated temperature (8–10°C), no treatment (sample A) and only hydrocooled (sample B) samples had storage lives of 4.17 ± 0.41 days and 5.33 ± 0.52 days, respectively; after which the samples seemed to fade and wither. The storage life of hydrocooled with unperforated packaged sample (sample C) was 3.67 ± 0.52 days and that of not hydrocooled with unperforated packaged sample (sample D) was 4 days at room temperature, as excessive rotting was observed after that. But in case of refrigerated temperature storage, unperforated packaged sample with hydrocooling (sample C) and without hydrocooling (sample D) had a storage life of 7.83 ± 0.41 and 9.67 ± 0.52 days respectively. This might be due to moisture condensation inside the package. Ben-Yehoshua[Citation16] also found a similar result in their experiment in which bell pepper in non-perforated plastic film promoted decay when water droplets condensed inside the package. In case of perforated packages, hydrocooled samples with packaging perforations of 2 (sample E), 4 (sample F) and 6 (sample G) were resistant to spoilage for 5.83 ± 0.41, 7.17 ± 0.41 and 5.67 ± 0.52 days, respectively, in room temperature and, 15.83 ± 0.41, 22.67 ± 0.52 and 21.17 ± 0.41 days, respectively, in refrigerated temperature after which, slight yellowing and rotting of the chillies were observed. The result agrees with the previous work done by de Jesús Ornelas-Paz et al.[Citation17]
At 8th day, nearly all chilli samples stored at room temperature were unmarketable in terms of colour and shape but at refrigerated temperature, storage life extended up to 20 days for sample F. Hence, during storage at either room or refrigerated temperature, it was observed that sample F was the most effective against spoilage. Once the spoilage was observed, the samples were considered unacceptable. Therefore, the use of refrigerated temperature had a significant effect on delaying the spoilage.
Chemical characteristics during room temperature storage
It was observed that the samples kept at room temperature spoiled before 8 days of storage by visual inspection. Hence, analytical data of only 4 days could be collected. Also, the loss in moisture and vitamin C was excessive and unacceptable after 4 days of storage at room temperature.
Changes in moisture content
shows the pattern in which average moisture content in Akabare under various treatments has changed during the storage period at room temperature.
Figure 2. Pattern of change in moisture during storage at room temperature
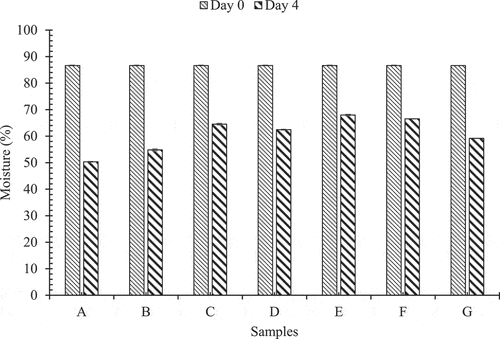
On 4th day of storage, the average moisture content was found to be 50.36 ± 0.17%, 54.83 ± 0.12%, 64.55 ± 0.39%, 62.46 ± 0.35%, 68.01 ± 0.14%, 66.56 ± 0.26% and 59.19 ± 0.02% on samples A, B, C, D, E, F and G, respectively. Hence, sample E retained higher moisture than other samples. But, loss of moisture below 10% of the original value is not acceptable.[Citation18] Also, all the chillies stored at room temperature were observed to be excessively withered, wilted and lost shine, thus making them undesirable and unmarketable. The results were similar to the findings of Mitcham et al.[Citation10] High temperature is supposed to increase respiration and enzymatic reaction rate by 2–3 fold.[Citation19] So, in terms of moisture content, storage at room temperature was found to be unacceptable.
Vitamin C content
shows the pattern in which average vitamin C content in Akabare under various treatments has changed during storage time at room temperature.
Figure 3. Pattern of change in vitamin C during storage at room temperature
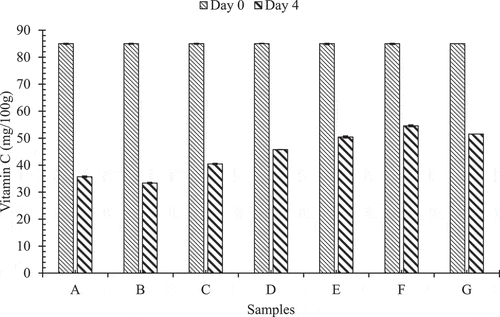
In samples A, B, C, D, E, F and G denote no any treatment, only hydrocooled, (hydrocooled, packaged but not perforated) and (not hydrocooled, packaged but not perforated), (hydrocooled, PP packaged with two perforations), (hydrocooled, PP packaged with four perforations), (hydrocooled, PP packaged with six perforations) conditions, respectively. On 4th day of storage, the vitamin C content (mg/100 g) was found to be 33.37 ± 0.15, 35.71 ± 0.28, 40.45 ± 0.25, 45.76 ± 0.22, 50.45 ± 0.02, 54.57 ± 0.37 and 51.53 ± 0.33 on samples A, B, C, D, E, F and G, respectively. Hence, sample F retained higher vitamin C than other samples. However, referring to freshness has already lost due to the reduction of moisture below 10% of the original value.
Total chlorophyll content
shows the pattern in which the average total chlorophyll content in Akabare under various treatments has changed during storage time at room temperature.
Figure 4. Pattern of change in total chlorophyll during storage at room temperature
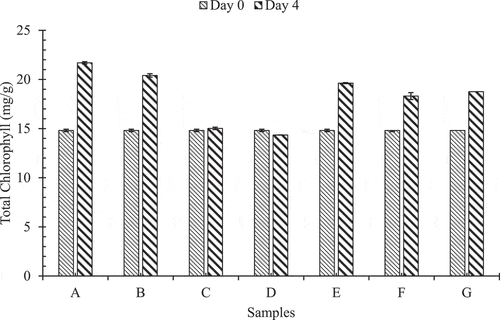
In samples A, B, C, D, E, F and G denote no any treatment, only hydrocooled, (hydrocooled, packaged but not perforated) and (not hydrocooled, packaged but not perforated), (hydrocooled, PP packaged with two perforations), (hydrocooled, PP packaged with four perforations), (hydrocooled, PP packaged with six perforations) conditions, respectively.
In general, increment in chlorophyll content was observed during storage of Akabare at room temperature. The values of total chlorophyll contents (mg/g) for samples A, B, C, D, E, F and G were 21.69 ± 0.08, 20.41 ± 0.09, 15.022 ± 0.07, 14.35 ± 0.07, 19.63 ± 0.01, 18.31 ± 0.03 and 18.76 ± 0.36, respectively, at 4th day analysis. Chlorophyll content might have increased because of the reduction in weight of the sample due to moisture loss.[Citation20] Thus, based on a visual inspection and chemical analysis the chillies stored at room temperature were considered unacceptable. Hence, the storage of chillies at room temperature for more than 2–3 days is not acceptable as rapid water loss and wilting were observed.
Effects of treatments on chemical characteristics during refrigerated temperature storage
Changes in moisture content
The pattern in which average moisture content in Akabare under various treatments has changed during storage time is shown in .
Figure 5. Pattern of change in moisture content during storage at refrigerated temperature
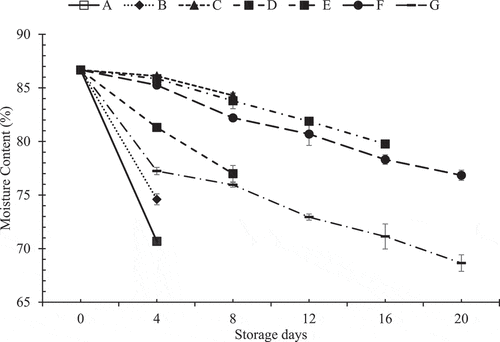
Only hydrocooled sample (sample B) had a moisture content of 74.6 ± 0.51% on 4th day itself whereas hydrocooled with unperforated packaged sample (sample C) had a moisture content of 84.29 ± 0.18% on 8th day. This means that hydrocooled with unperforated packaged sample (sample C) could retain high moisture for 8 days. But after 8 days, the rot was developed. Also, a sample with no hydrocooling and unperforated packaging (sample D) which had far lesser moisture than other treatments, developed rot. This could be since water droplets were seen to accumulate in samples packaged with no perforations, thereby facilitating microbial rot. This reason is justified by the fact that the samples with perforated packaging prevented rot as well as spoilage for a longer period than unperforated ones.[Citation16]
De[Citation21] reported that prepackaging of fresh fruits in perforated polyethene packages showed a reduction in water loss rates by 20 times as well as low microbial rot. For 16 days of the storage period, hydrocooled samples with packaging perforations of 2 (sample E), 4 (sample F) and 6 (sample G) well retained the moisture. On the 20th day, the hydrocooled samples with 4 perforations in the packaging (sample F) and 6 perforations in the packaging (sample G) prevented spoilage significantly compared to 2 perforations in the packaging (sample E), which could be attributed to the fact that sample E had higher moisture (79.77 ± 0.18%) inside the package on the 16th day itself and thereby caused microbial rot easily. However, comparing the initial moisture content (86.67 ± 0.10%) with the final moisture content, it was observed that on 20th day of storage, the moisture content of sample F was 76.85 ± 0.48% and sample G was 68.65 ± 0.77%. These values are significantly different (p < .05) from each other. Only sample F prevented moisture loss below 10% of the original fresh weight. Thus, sample F could be considered as ‘fresh’ at the end of 20th day.
Changes in vitamin C content
shows the pattern in which average vitamin C content in Akabare under various treatments has changed during storage time.
Figure 6. Pattern of change in vitamin C during storage at refrigerated temperature
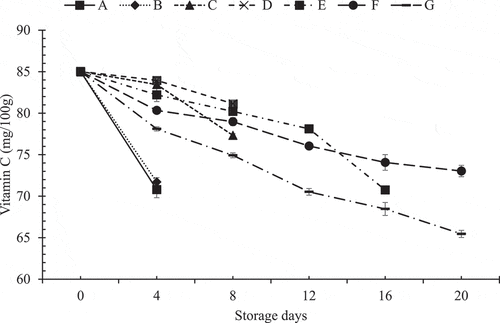
The ascorbic acid had shown a decreasing trend in all the samples during storage. In the decrement pattern of vitamin C, hydrocooled sample with four perforations in the packaging (sample F) has the least slope and the highest slope were observed in the sample with no treatment (sample A). On 4th day of storage, it can be easily observed that packaging had a significant effect (p < .05) on retention of vitamin C. Hydrocooled sample with six perforations in the packaging (sample G) had the least value of vitamin C on 4th day because of the higher number of pores. This might have attributed to high O2 and low CO2 concentration that may have caused oxidation of ascorbic acid into dehydroascorbic acid (DHA) leading to a subsequent decrease in ascorbic acid.[Citation22]
Hydrocooled with unperforated packaging sample (sample C) had higher vitamin C contents than hydrocooled samples with packaging perforations of 2 (sample E), 4 (sample F) and 6 (sample G) on 4th day of storage. However, sample C could not prevent spoilage until the 12th day of storage. On observing for 16 days of the storage period, the higher number of perforations caused the higher loss of vitamin C as sample G had lower vitamin C content than samples E and F. But, on 20th day of storage, vitamin C reduced significantly (p < .05) in sample E compared to F and only samples F and G remained unspoiled and had vitamin C content (mg/100 g) of 73.04 ± 0.68 and 65.46 ± 0.43, respectively. Thus, in the case of sample F, there were minimal changes in vitamin C content. This result agrees with the previous report by Koide and Shi,[Citation23] in which minimal minor changes in vitamin C were observed in intact green peppers that had been packed in perforated plastics and refrigerated.
Changes in chlorophyll content
shows the pattern in which the average total chlorophyll content in Akabare under various treatments has changed during storage time.
Figure 7. Pattern of change in total chlorophyll during storage at refrigerated temperature
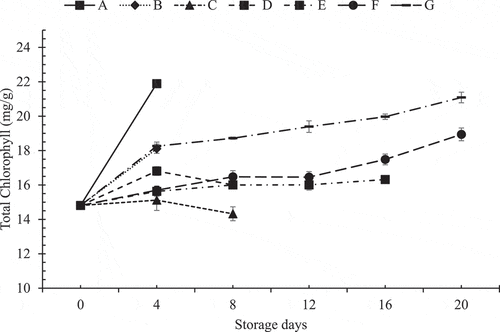
Chlorophyll content had increased over the storage period in all samples except C. On 8th day of storage, hydrocooled sample with six perforations in the packaging (sample G) was most effective in chlorophyll retention. However, considering the loss of moisture, sample G was unable to preserve freshness when compared to hydrocooled sample with four perforations in the packaging (sample F). Also, comparing the initial chlorophyll content (14.88 mg/g) with the final observation, it was observed that on 20th day of storage, the chlorophyll content of sample F was 18.94 mg/g and sample G was 21.09 mg/g. Moreover, there was no rot or withering seen in sample F possibly due to optimum moisture content. Hence, the sample F was considered superior. Thus, chlorophyll content was changed only due to moisture loss but was not affected by packaging or hydrocooling. On the other hand, chlorophyllase is only active at temperatures from 10 to 75°C; therefore, the activity of chlorophyll-degrading enzymes was low or non-existent in refrigerated peppers, favouring the stability of the green colour.[Citation24] Similar results were found by Kosson.[Citation25]
Conclusion
Storage of hydrocooled Akabare chillies at room temperature (25–27°C) resulted in high weight loss and rapid deterioration in vitamin C and chlorophyll change as compared to refrigerated temperature (8–10°C) storage having relative humidity (70–75%). The Akabare chillies stored in perforated polypropylene packages reduced water loss rates and showed less rotting and spoilage. The best-modified atmosphere was obtained with four perforation polypropylene packages at refrigerated storage temperature that facilitated storage of pepper up to 20 days without visual spoilage. Hence, this could represent a cost-effective alternative to preserve the quality of intact peppers for small-scale peasant farmers.
Acknowledgments
We would like to express our humble gratitude to the Central Department of Food Technology and Central Campus of Technology and all the teaching faculty and staffs for their co-operation and support. Also, we would like to give our sincere thanks to all the personnel directly or indirectly involved in this study as well as our friends and family. The authors declare that they do not have any conflict of interest. The study did not involve any human or animal testing.
References
- Basu, S. K.; De, A. K. Capsicum: Historical and Botanical Perspective. In Capsicum – The Genus Capsicum; De, A. K., Ed.; Taylor and Francis Group: London, 2003; pp 1–13.
- Kumar, S.; Kumar, R.; Singh, J. Cayenne/American Pepper. In Handbook of Herbs and Spices; Peter, K. V., Ed.; Woodhead Publishing Ltd: England, 2006; Vol. 3, pp 299–310.
- Bhutia, K. L.; Tombisana Meetei, N. G.; Khanna, V. K. In Vitro Regeneration of Dalle Khursani, an Important Chilli Cultivar of Sikkim, Using Various Explants. Agrotechnol. 2016, 5(1), 1–4. DOI: https://doi.org/10.4172/2168-9881.1000142.
- Rai, R.; Processing Effect on Oleoresin Retention of Akabare Chilli (Capsicum Chinese). B. Tech. Dissertation. Tribhuvan University, Nepal, 2017.
- Khanal, B.; Study on Postharvest Losses of Akabare Chilli (Capsicum Chinese) at Dharan Bazar. M. Tech. Dissertation. Tribhuvan University, Dharan, Nepal, 2009.
- Bosland, P. W.; Votava, E. J. Introduction. In Peppers: Vegetable and Spice Capsicums, 2nd Hulbert, S. Ed.; CAB International: UK, 2012; pp. 1–12.
- Pandey, S. K.; Goswami, T. K. An Engineering Approach to Design Perforated and Non Perforated Modified Atmospheric Packaging Unit for Capsicum. J. Food Process. Technol. 2012, 3(11), 3–6. DOI: https://doi.org/10.4172/2157-7110.1000187.
- Goffau, M. C. D.; Yang, X.; Dijl, J. M. V.; Harmsen, H. J. M. Bacterial Pleomorphism and Competition in a Relative Humidity Gradient. Environ. Microbiol. 2009, 11, 809–822. DOI: https://doi.org/10.1111/j.1462-2920.2008.01802.x.
- Rokayya, S.; Physical-mechanical, K. E. Estimation of Pepper (Capsicum Annuum L.) Fruit Varieties. J. Northeast Agric. Univ. 2016, 23(3), 61–69.
- Mitcham, B.; Cantwell, M.; Kader, A. Methods for Determining Quality of Fresh Commodities. Perishables Handling Q. 1996, 85, 1–5.
- Bourne, M. C.;. Food Texture and Viscosity, 2nd ed.; Academic Press: New York, 1982.
- Ranganna, S.;. Handbook of Analysis and Quality Control for Fruit and Vegetable Products, 2nd ed.; Tata McGraw-Hill Publishing Company: New Delhi, India, 1986.
- Arnon, D.;. Copper Enzymes in Isolated Chloroplasts Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24(1), 1–15.
- Mason, J.;. History and Science. In Growing and Using Capsicums and Chillies; Mason, J., Ed.; ACS Distance Education: Queensland, Australia, 2014; pp 6–10.
- Litoriya, N. S.; Gandhi, I. K.; Talati, J. G. Nutritional Composition of Different Chilli (Capsicum Annuum L.) Varieties. Indian J. Agri. Biochem. 2014, 27(1), 91–92.
- Ben-Yehoshua, S.;. Individual Seal Packaging of Fruits and Vegetables in Plastic Flims- New Postharvest Technique. Hortsci. 1985, 20, 32–37.
- de Jesús Ornelas-paz, J.; Castañeda-Jiménez, A. C.; Estrada-Alvarado, M. I.; Ramos-Aguilar, O. P.; Ibarra-Junquera, V.; Pérez-Martínez, J. D.; Escalante-Minakata, P.; Guevara-Arauza, J. C.; Ruiz-Cruz, S. Effect of the Perforation Level of recycled-LDPE Bags on the Modification of the Atmosphere Development, Bioactive Compounds Content, and Other Qualities of Jalapeño Peppers during Storage. J. Food Sci. Technol. 2015, 52, 6415–6424. DOI: https://doi.org/10.1007/s13197-015-1749-8.
- Kays, S. J.;. Postharvest Physiology of Perishable Plant Products; Exon Press: Athens, GA, 1997.
- Brecht, J. K.; Chau, K. V.; Fonseca, S. C.; Oliveira, F. A. R.; Silva, F. M.; Nunes, M. C. N.; Bender, R. J. Maintaining Optimal Atmosphere Conditions for Fruits and Vegetables Throughout the Postharvest Handling Chain. Postharvest Biology and Technology, 2003, 27(1), 87–101. DOI: https://doi.org/10.1016/S0925-5214(02)00185-0.
- King, V. A.; Liu, C.; Liu, Y. Chlorophyll Stability in Spinach Dehydrated by Freeze-drying and Controlled Low-temperature Vacuum Dehydration. Food Res. Int. 2001, 34, 167–175.
- De, A. K.;. Post-harvest Handling and Processing of Capsicums. In “Capsicum-The Genus Capsicum”; Prakash, V., Eipeson, W. E., Eds.; Taylor and Francis group: London, 2003; pp 161–174.
- Mahajan, P. V.; Caleb, O. J.; Singh, Z.; Watkins, C. B.; Geyer, M. Postharvest Treatments of Fresh Produce. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2014, 372. DOI: https://doi.org/10.1098/rsta.2013.0309.
- Koide, S.; Shi, J. Microbial and Quality Evaluation of Green Peppers Stored in Biodegradable Film Packaging. Food Control. 2007, 18(9), 1121–1125. DOI: https://doi.org/10.1016/j.foodcont.2006.07.013.
- Arkus, K. A.; Cahoon, E. B.; Jez, J. M. Mechanistic Analysis of Wheat Chlorophyllase. Arch. Biochem. Biophys. 2005, 438(2), 146–155. DOI: https://doi.org/10.1016/j.abb.2005.04.019.
- Kosson, R.;. Chlorophyll Fluorescence and Chilling Injury of Green Pepper as Affected by Storage Conditions. Acta Hortic. 2003, 628, 379–385. DOI: https://doi.org/10.17660/ActaHortic.2003.628.47.
