ABSTRACT
Since cardiovascular disease (CVD) is one of the most common and debilitating disorders globally, risk factor modification is an urgent health priority. Interestingly, an increasing body of literature has suggested honey, and its by-products, can elicit a positive effect on CVD risk factors. Therefore, this systematic review aimed to summarize and discuss the outcomes of interventional studies in humans regarding the effects of bee products’ consumption on CVD risk factors. The Cochrane Collaboration tool was used for quality assessment of the included studies. A total of 23 studies met our inclusion criteria. Six studies used natural honey, seven used propolis, and ten administered royal jelly as the intervention. Natural honey consumption could improve lipid profile and anthropometric parameters, and propolis supplementation could enhance lipid profile and glycemic markers. Current evidence precludes conclusions being made regarding royal jelly and CVD risk factors. It seems that honey and propolis consumption could reduce CVD risk factors. Overall, in order to confirm the association between bee products and CVD risk factors, more clinical trials with adequate sample sizes and better methodology should be conducted in the future.
Introduction
Cardiovascular disease (CVD) may be described as a multifactorial disorder with large mortality rate. It is estimated that by 2030, CVD will remain a prominent cause of early mortality, affecting nearly 23.3 million individuals around the world.[Citation1] Therefore, a concerted effort is needed to decrease its prevalence; indeed, a plethora of disorders, including diabetes, hypertension, dyslipidemia, and obesity could contribute to CVD risk factors and its increased incidence .[Citation2] Moreover, nutritional and dietary factors appear to be independently associated with CVD, and it has been asserted that diet possesses a crucial role in CVD, whilst dietary changes represent a fundamental tenet in both the prevention and treatment of CVD and related risk factors .[Citation3]
Honey may be regarded as a complementary medicine, and the use of natural honey dates back to 2000 BC .[Citation4,Citation5] High fructose and glucose content, as well as small amounts of sucrose, are the main characteristics of honey and the reason for its sweet taste .[Citation6] Based on previous studies, the antioxidant, anti-bacterial, and healing effects of honey have been well established 4,5,6. Furthermore, honey by-products, including royal jelly and propolis, have garnered empirical attention during recent years. Propolis is a sticky, resinous material collected by honeybees from various plants, mixed with wax and other secretions .[Citation7] Biological properties of propolis include anticancer, antibacterial, antifungal, antioxidant, anti-inflammatory, and immune system modulatory effects .[Citation8] Royal jelly is produced by worker honeybees and containes various essential compounds with biological activities such as free amino acids, proteins, sugars, fatty acids, vitamins (thiamine, niacin, riboflavin), and minerals (mainly iron and calcium) .[Citation9] It has been reported to possess antioxidant, immunomodulatory, and vasoactive properties .[Citation10]
With regards to the above-mentioned conflicting evidence, and the increasing interest in the role of honey and its by-products in CVD prevention, this study synthesizes the available evidence to clarify the inconsistencies between studies regarding the relationship between bee products’ consumption and CVD risk factors. Therefore, to address these issues, we carried out this systematic review of interventional studies to evaluate the efficacy of honey and its by-products consumption on CVDs risk factors, including anthropometric indices, lipid profile, glycemic markers, and blood pressure in general population.
Methods
This systematic review methodology was performed based on the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) recommendations .[Citation11]
Search strategy
We selected papers published up to December 2020 by searching PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Scopus (http://www.scopus.com), ISI Web of Science (http://www.webofscience.com), Cochrane library (http://www.cochranelibrary.com), and Google Scholar (https://scholar.google.com). The search was performed using the following combination of terms: (“honey” or “propolis” or “bee glue” or “bee bread” or “royal jelly” OR “RJ”) AND (“diabetes mellitus” OR diabetes OR pre-diabetes OR diabetic OR “abnormal glucose homeostasis” OR “abnormal glucose tolerance” OR “glucose tolerance” OR “glucose homeostasis” OR “glucose intolerance” OR hyperglycemia OR “glycemic control” OR “insulin resistance” OR “blood glucose” OR “blood sugar” OR “fasting blood glucose” OR “fasting plasma glucose” OR “body weight” OR overweight OR obesity OR “body mass index” OR BMI OR “abdominal obesity” OR “morbid obesity” OR “Waist Circumference” OR “LDL cholesterol” OR LDL OR “HDL cholesterol” OR HDL OR “VLDL cholesterol” OR VLDL OR triglyceride OR TG OR “total cholesterol” OR cholesterol OR hyperlipidemia OR “abnormal lipid profile” OR “lipid profile” OR “blood pressure” OR hypertension OR “abnormal blood pressure” OR “high blood pressure”) (). No limitation was placed on the publication date or the language of the articles. In addition, the reference lists of the original reports and review articles were scrutinized for other relevant studies.
Table 1. Search terms
Study selection
After searching the above-mentioned databases, the results were exported to the reference manager software Endnote, Version X7 (Thomson Reuters, New York) to remove duplications. Then, publications were screened for eligibility in a step-wise manner. First, titles and abstracts were scanned to identify human RCTs that evaluated the effects of honey or its products. Second, the identified studies were checked to determine if at least one of the target outcomes including anthropometric indices (weight, body mass index [BMI] and waist circumference [WC]), lipid profile (total cholesterol [TC], triglyceride [TG], low-density lipoprotein cholesterol [LDL-C)], and high-density lipoprotein cholesterol [HDL-C]), glycemic indices (fasting blood glucose [FBG], 2-hour postprandial glucose [2-h PG] and hemoglobin A1C [HbA1C]), or blood pressure was reported. In the final step, the full text of the selected studies was carefully reviewed to determine the final eligible publications. Studies with intervention duration less than 2 weeks, or those that administrated honey products in combination with other compounds were excluded. Conference proceedings, study protocols, and duplicated reports were also rejected. All of these steps were performed by two independent investigators (A.H and A.A), and any disagreements were resolved by discussion with a third reviewer (N.R). Eligibility criteria for study selection was guided by the following components identified using the PICO (Population, Intervention, Comparison, Outcome) framework: P (Adult patients), I (honey, propolis, or royal jelly), C (placebo or control group), O (cardiometabolic risk factors including anthropometric indices, lipid profile, glycemic indices, and blood pressure).
Data extraction
Characteristics of the studies and participants included first author’s last name, publication date, research location, study design, sample size in each group, participants’ demographics (mean age, gender, BMI, and health status), follow-up period, type and dosage of intervention, and mean and SD of outcome measures at baseline, post-intervention and/or changes in outcome measures from baseline to the end-of-trial. Data extraction procedures were performed by two independent reviewers (A.H and A.A) and differences were resolved through consensus. We contacted the corresponding authors via e-mail in case further information was required. In addition, if a study provided multiple data at different time points, only the latest were considered.
Quality assessment
The Cochrane Collaboration tool was used to assess the quality of the studies by a single investigator (A.H) .[Citation12] The quality assessment results were also confirmed by a second investigator (A.A). The following methodological domains were considered: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, selective reporting, and other biases. Each item was scored as a low, unclear, or high risk of bias.
Result
Search results
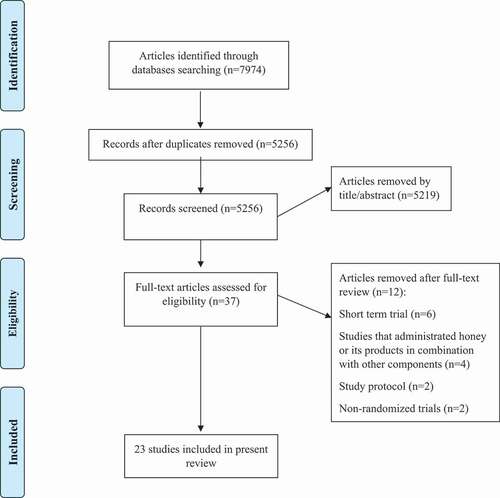
Our initial search identified 7974 articles, and after removing duplicates, the remaining 5256 papers were screened based upon title and abstracts by two independent reviewers. A total of 37 articles full-texts were retrieved and reviewed, and finally 23 publications met our inclusion criteria and were included in our systematic review. The PRISMA flow diagram summarizes the results of the study selection process for this systematic review ().
Overview of the included studies
Eligible studies were conducted between 2007 and 2020, and the mean age of the participants was between 22 and 63. Of 23 included articles, 6 recruited healthy subjects ,[Citation13–18] while 11 were conducted among diabetic or pre-diabetic patients ,[Citation8,Citation19–28] and others included patients with chronic kidney disease (CKD) ,[Citation29] dry eye disease ,[Citation30] hypercholesterolemic patients ,[Citation31] nonalcoholic fatty liver disease (NAFLD) ,[Citation32] and overweight subjects .[Citation33,Citation34] Studies were mostly conducted in Iran (n = 14)[Citation8,Citation16,Citation17,Citation19,Citation20,Citation22–28,,Citation32Citation33] or Japan (n = 4) ,[Citation13,Citation14,Citation21,Citation30] and the others were carried out in Brazil ,[Citation29] Chile ,[Citation15] Turkey ,[Citation18] Slovenia ,[Citation34] and Taiwan .[Citation31] Two studies used only female subjects ,[Citation23,Citation25] three recruited males ,[Citation16–18] and the remaining studies used both genders as participants. Regarding risk of bias of the included studies, most studies were ranked as good/fair and only two[Citation13,Citation20] were of poor quality. Characteristics of the included studies are detailed in .
Table 2. The characteristics of included studies
Effects of natural honey consumption
The effects of natural honey consumption on glycemic markers, anthropometric measures, lipid profile, or blood pressure were examined in 6 clinical trial studies .[Citation16,Citation17,Citation20,Citation26,Citation28,Citation33]
In 2009, Bahrami et al. conducted a randomized controlled clinical trial to evaluate the effects of natural honey consumption in diabetic patients. In this study, forty-eight diabetic patients were randomly assigned to the honey group and received natural honey orally (first 2 weeks, 1 g/kg/day; second 2 weeks, 1.5 g/kg/day; third 2 weeks, 2 g/kg/day; and last 2 weeks, 2.5 g/kg/day) for 8 weeks, and the control group did not consume honey. Results revealed significant improvements in weight, TC, LDL, and TG in the honey-administered group compared with the controls (P < .05) .[Citation20] Similarly, a randomized controlled crossover clinical trial was conducted on 53 patients with T2DM, who were randomly divided into groups of control (weight maintenance diet) or treatment (weight maintenance diet +50 g/day honey) for 8 weeks. Increased HbA1c and decreased WC of patients with T2DM were indicated following honey consumption .[Citation26] In a further study, the effects of natural honey on lipid profile, FBG, and weight in overweight individuals were investigated .[Citation33] In this RCT, 55 overweight/obese patients were randomly allocated to receive either 70 gr/day honey or sucrose for a maximum of 30 days, and the authors reported a mild reduction in weight and body fat in the intervention group .[Citation33] Further, honey consumption yielded improvements in FBG and lipid profile, particularly in subjects with elevated risk factors .[Citation33] In another study, sixty healthy subjects, aged 18 to 30 years, were randomly assigned to receive 70 gr/day honey or sucrose for a month, and FBG was shown to be decreased as a result of honey consumption (P < .05). However, this study failed to show any beneficial effects of honey intake on BP .[Citation16] Furthermore, there is evidence indicating that consumption of honey can lower TC, TG, and LDL, and lead to higher HDL in a normal population (P < .05) .[Citation17] The last study was done by Sadeghi et al. among 43 patients with T2DM using cross-over design .[Citation28] Consumption of 50 g/day of honey for 8 weeks did not change any of the lipid profile parameters including TC, TG, LDL, and HDL (P > .05).
Effects of propolis supplementation
The effects of propolis supplementation on glycemic indices or lipid profile were examined in 7 clinical trials .[Citation8,Citation15,Citation19,Citation21,Citation27,Citation29,Citation32]
In 2019, Afsharpour et al. conducted a randomized, double-blind, placebo-controlled study and reported a significant reduction in the FBG and 2-h PG in T2DM patients treated with propolis (500 mg, 3 x daily for 8 weeks) compared with the placebo group (P < .05) .[Citation19] Furthermore, Mujica et al. in 2017, evaluated the efficacy of propolis supplementation on the modulation of lipids in a human population in Talca, Chile, and showed a significant improvement (P < .05) in HDL in those receiving propolis for 90 days .[Citation15] In another study, 66 patients with T2DM were recruited and randomly allocated to the intervention group to receive 300 mg propolis three times a day, or the control group who received similar capsules without propolis, on the same schedule for 12 weeks. The results yielded a significant reduction in FBG, HbA1c, TC, and LDL (P < .05) in patients compared to controls .[Citation8] Zakerkish et al. designed a study to evaluate the effects of Iranian propolis extract on glucose metabolism and lipid profile in patients with T2DM who were randomly divided into an Iranian propolis group (1000 mg/day) (n = 50) and a placebo group (n = 44) for 90 days. A significant decrease in the serum levels of HbA1c and 2-h PG, along with a notable elevation in serum HDL in the propolis group compared with the placebo group was documented .[Citation27] Thirty-two patients with CKD, 18–90 years of age, were randomly assigned to receive 12 months of Brazilian green propolis extract at a dose of 500 mg/day (n = 18) or placebo (n = 14) in a randomized, double-blind, placebo-controlled study done by Silveira et al. .[Citation29] However, the authors reported that no statistical differences between groups were observed during follow up in terms of HbA1c or mean systolic and diastolic blood pressure. Similarly, results of another study following a randomized, double-blind design failed to show any significant differences (P < .05) in parameters of glucose and lipid metabolism among 80 patients with T2DM who received 226.8 mg/day of Brazilian green propolis (n = 41) or placebo (n = 39) for 8 weeks .[Citation21] The last evidence among the Iranian population indicated that consumption of 500 mg/day propolis in 54 NAFLD patients did not improve any of the cardio-metabolic risk factors after 4 months .[Citation32]
Effects of royal jelly supplementation
The effects of royal jelly supplementation on glycemic indices or lipid profile were examined in 10 clinical trials .[Citation13,Citation18,Citation22–25,Citation30,Citation31,Citation34]
Mobasseri et al. conducted a pilot study to determine the efficacy of royal jelly supplementation to improve lipid profile in T2DM women. Fifty participants (30–65 years old) were randomly assigned to receive 1000 mg/day royal jelly soft gel or placebo for 2 months. In the treatment group, a significant reduction in the mean serum TG (P = .01) and TC levels (P = .004) was reported, while in the placebo group, the results were not statistically significant (P < .05) .[Citation23] In Chiu et al. study, the authors examined the hypo-cholesterolemic properties of royal jelly in healthy mild hyper-cholesterolemic adults [30]. 40 subjects in the experimental or placebo groups were prescribed to take nine capsules (350 mg/capsule) of royal jelly or placebo every day, respectively. Serum TC and LDL levels were significantly reduced (P < .05) after administration of royal jelly with no significant changes in anthropometric parameters, TG, or HDL levels. Guo et al. evaluated the benefits of royal jelly in healthy adults where fifteen volunteers were enrolled and randomly allocated to the intervention group (6 gr/d of royal jelly) or control for 4 weeks. They reported significant reductions (P < .05) in TC and LDL compared to the controls .[Citation13] In another attempt to determine the effects of royal jelly intake on serum glucose in patients with T2DM, fifty patients (20–65 years of age) were allocated in the intervention and control groups and given 1000 mg royal jelly or placebo 3 times a day for 8 weeks, respectively. Significant improvements (P = .011) in glucose concentrations due to royal jelly consumption were indicated as compared to the controls .[Citation22] Likewise, in 2012, Pourmoradian et al. assessed the efficacy of royal jelly supplementation in weight management among fifty female diabetic patients (aged 30–65 years) who were assigned into supplementation (n = 25) and placebo (n = 25) groups, given a daily dose of 1000 mg royal jelly soft gel or placebo, for 8 weeks, respectively. Results of the aforementioned study revealed significantly (P < .01) lower mean weight in the intervention group, in addition to an insignificant increase in the placebo group .[Citation25] Based on another investigation, royal jelly supplementation (1000 mg for 8 weeks) appeared to be efficacious in the reduction of FBG (P = .006) and systolic blood pressure (P = .02) in patients with type 2 diabetes .[Citation24] However, in another study, different levels of royal jelly were found to be ineffective (P < .05) on glucose, TC, HDL, LDL, and TC in 40 randomly selected male swimmers, aged 18 to 25 years, after receiving 500 mg, 1, and 2 g/day of royal jelly throughout the 30 day-exercise program .[Citation18] Similarly, there were no apparent effects of royal jelly on anthropometric measurements or serum lipids in 56 healthy volunteers (aged 42–83 years).[Citation14] Finally, a report by Petelin et al. among asymptomatic overweight adults revealed that consumption of royal jelly (2 g/day) for 8 weeks decreased TC significantly among the intervention group compared to the control (P < .05) .[Citation34]
Discussion
Diverse dietary patterns, dietary restrictions, and nutritional supplements are widely reported to be used by patients in order to reduce CVD risk factors, and many subjects claim beneficial effects from these dietary interventions .[Citation35] This systematic review has summarized the available literature on the efficacy of bee products, including honey, propolis, and royal jelly, on CVD risk factors, such as anthropometric indices, lipid profile, glycemic markers, and blood pressure in general population.
For studies investigating the effect of natural honey on CVD risk factors; the quality of included studies ranked from low[Citation20] to high .[Citation16,Citation17,Citation26,Citation33] It seems that natural honey consumption is more beneficial in the improvement of lipid profile and anthropometric measures, as compared to glycemic markers. Also, there is a lack of evidence to suggest a dose-dependent effects of honey consumption on these markers. There are some points which should be taken into account when interpreting the results. First, all of the studies were conducted in Iran, which precludes the generalization of the results. Second, honey has been administered mainly as a food item, and only two studies blinded participants. So, the beneficial properties of honey might be influenced somehow by systematic error (Bias). Third, included studies have involved different participants with different health conditions, which likely cause high heterogeneity. Fourth, the included studies have used honey with different flower origins, which makes their effectiveness variable based upon their species .[Citation36]
Natural honey contains several minerals and antioxidants that could elevate serum levels of antioxidants including ascorbic acid, beta-carotene, uric acid, glutathione reductase, and total phenolic content of plasma .[Citation37] These antioxidants, via exacerbation of diet-induced thermogenesis, could promote weight loss. This mechanism could represent a probable explanation for weight loss observed following honey consumption .[Citation20] Furthermore, honey contains oligosaccharides which can delay gastric emptying, slow digestion rate, and prolong satiety .[Citation38] Additionally, modulation of appetite-regulating hormones, such as ghrelin and peptide YY, may explain the anti-obesity effects of natural honey .[Citation39] It has been reported that honey consumption could reduce plasma and urinary prostaglandin E2, prostaglandin F2-alpha, and thromboxane B2 which could explain the anti-inflammatory effects of honey .[Citation40] Moreover, honey contains fructose, zinc, and copper, which are crucial for insulin and glucose metabolism .[Citation20] Indeed, in 1976, fructose was introduced as a remedy for diabetic patients ,[Citation41] although, further studies revealed negative effects in long term usage of fructose such as the development of insulin resistance, diabetes, obesity, and CVD; it may be concluded that components other than fructose, such as antioxidants, can contribute to beneficial effects of honey on glycemic markers since long term honey consumption does not exert negative effects comparable to fructose .[Citation42] Recently, the high content of fructose in western diets has been suggested to be involved in lipogenesis .[Citation43] Exposure of the liver to large amounts of fructose acts as stimuli for lipogenesis and triglyceride accumulation, which in turn leads to reduced insulin sensitivity and hepatic insulin resistance/glucose intolerance .[Citation20] So, it is conceivable that the hypolipidemic effects of honey could be attributed to some unknown substances. It has been proposed that natural honey might improve the lipid profile by its antioxidant properties .[Citation20,Citation44,Citation45] Other hypolipidemic effects of honey could be exerted through the following pathways: increased cholesterol secretion through bile, improvement in lipid metabolism, and its catabolism which have been attributed to its antioxidant content, and reduction in free radicals that decrease the accumulation of fat .[Citation20,Citation46] Furthermore, natural honey consumption could stimulate insulin secretion, which can increase lipid biosynthesis and reduce lipolysis, and as a result, lower serum levels of lipids are observed .[Citation17,Citation47] It should be mentioned that the ratio of fructose to glucose in natural honey varies depending on floral source and climate circumstances from 0.46 to 1.62. This ratio is an important factor in determining the usefulness of the honey and also explaining the probable inconsistencies between studies .[Citation26,Citation48]
For studies investigating the effects of propolis supplementation on CVD risk factors; all of the included studies were categorized as high quality, except one .[Citation49] It appears that propolis supplementation might improve glycemic and lipid profile parameters in a dose-dependent manner; where propolis supplementation of ≥1000 mg/day revealed greater improvements in glycemic and lipid profile parameters. However, the following points should be considered before making a final conclusion. All of the included articles were conducted in Asia and South America, which makes the generalizability of the findings difficult. Also, there appears to be heterogeneity among studies in terms of participants’ health status, study duration, administered dose of propolis, and its origins.
Propolis has been traditionally used to treat infections, but recent evidence has revealed its value as an antioxidant and/or in the management of non-communicable diseases such as diabetes, atherosclerosis, and cancer .[Citation15] Propolis is known as a good source of polyphenols and flavonoids, which are related to flora surrounding the hives. In other words, propolis composition and antioxidant activity vary based on geographical area, which should be taken into account when interpreting the results of the included studies .[Citation50,Citation51] Propolis has the ability to reduce reactive oxygen species (ROS) via two pathways, which explain its antioxidant activity. First, activation of transcription factor NrF2 by caffeic acid phenethyl ester (CAPE) .[Citation52] NrF2 is a modulatory protein linked with anti-oxidant protection and enhancement of antioxidant enzymes .[Citation15] The second mechanism could be mediated by the direct antioxidant activity of phenolic and flavonoid compounds like cinnamic acid, apigenin, quercetin, CAPE, p-vanillin, and p-coumaric acid .[Citation53] Experimental studies have suggested that propolis could reduce blood pressure through a nitric oxide pathway, acetylcholine-induced vasodilation, and antioxidant activity .[Citation29] In addition, the hypoglycemic properties of propolis could be attributed to flavonoids’ antioxidant activity and suppression of free radicals .[Citation54,Citation55] Glycemic control achieved through propolis supplementation might be elicited via decreased insulin resistance, modulation of oxidative stress, reducing the production of inflammatory elements, increasing the adiponectin levels, increasing glucose uptake by tissues, inhibition of carbohydrate digestive enzymes especially alpha-amylase and alpha-glycosidase .[Citation56] Indeed, further studies have supported some of these proposed mechanisms[Citation57]; for example, Elissa et al reported that a decrease in tumor necrosis factor-α level could result in decreased insulin resistance, ultimately lowering fasting blood glucose level .[Citation57] Furthermore, Elissa et al also reported that propolis could increase the activity of glucose transporter-4 by reducing insulin resistance .[Citation57] Finally, propolis could improve lipid profile parameters, including reduction of serum LDL and TC, and elevation of HDL. Propolis can enhance liver ATP-binding cassette transporters A1 and G1 (ABCA1 and ABCG1) protein expression, which is accompanied by cholesterol efflux from peripheral tissue; in addition to upregulating ApoA-1-mediated cholesterol efflux by macrophages .[Citation15]
For studies assessing the effect of royal jelly supplementation on CVD risk factors; the inconsistency in the reported results precludes any general conclusion. The methodological quality of the included studies was high except for one study .[Citation13] The included studies were variable in terms of health status, age, and BMI, which could influence the final results. Furthermore, studies also used diverse doses of royal jelly with different follow-up periods that render proper comparison impractical. Finally, most of the studies were conducted in Asian countries, which subsequently diminishes the external validity of the results.
Hypolipidemic properties of royal jelly might be attributable to its protein content ,[Citation13] inhibition of pancreatic lipase, diminution of dietary fat absorption ,[Citation58] and reduction in the cholesterol hepatic biosynthesis and other sterols by reducing the expression of Squalene Epioxidase (SQLE), which is the key enzyme in cholesterol biosynthesis .[Citation59] Furthermore, reductions in sterol regulatory element-binding protein (SREB1) and increases in LDL-c and VLDL receptor gene expression in the liver upsurges cholesterol uptake by hepatocytes .[Citation13,Citation23] In addition to hypolipidemic effects, royal jelly could exert anti-oxidant, anti-inflammatory, and hypoglycemic effects; indeed, it has been reported that an anti-inflammatory component of royal jelly (Honey Bee Royal Jelly Anti-inflammatory Factor-HBRJ-AIF) might be beneficial in reducing pro-inflammatory cytokine secretions, such as interleukin-6 (IL-6), IL-2 and IL-4. It has also been suggested that major Royal Jelly Protein3 (MRJP3) is responsible for its anti-inflammatory effects .[Citation60,Citation61] Finally, it is demonstrable that insulin resistance is related to changes in oxidative stress status; royal jelly has protective effects against oxidative stress through its antioxidant peptides, and thus, can enhance insulin resistance via its antioxidant properties .[Citation22,Citation62]
It should be taken into account that HbA1c represents the mean value of blood glucose during the past three months. So it seems inappropriate to attribute the observed changes in this variable to bee products that were administered for lower than 12 weeks and this important point should be mentioned as a limitation of included studies.
The present study has some limitations that warrant consideration. First, significant heterogeneity was present between included studies that might be explained by different population characteristics, including age, BMI, health status, ethnicity, different dosages of administered bee products, different follow-up durations, and variation in bee products’ origin among studies. Second, most of the included studies were conducted in Asia, which, generally, precludes the generalization of the results. Third, despite several adjustments for different confounders in the included studies, residual confounding cannot be excluded. Despite our study limitations, this is the first systematic review to have assessed the effects of bee products on CVD risk factors.
Conclusion
It is apparent that natural honey consumption could improve lipid profile and anthropometric parameters, and propolis supplementation could enhance lipid profile and glycemic markers. Currently, available evidence precludes the establishment of a firm link between royal jelly and CVD risk factors. Finally, to ascertain an overall effect of the association between bee products and CVD risk factors, more clinical trials with adequate sample size and well-controlled methodologies should be conducted in the future.
Author contribution
A. Hadi and A. Arab: contributed in concept of MS
A. Arab, N. Rafie and A. Hadi contributed in writing and revising the manuscript.
Disclosure statement
The authors have no conflict of interests.
Additional information
Funding
References
- Bedani, R.; Rossi, E. A.; Cavallini, D. C.; Pinto, R. A.; Vendramini, R. C.; Augusto, E. M.; Abdalla, D. S.; Saad, S. M. Influence of daily consumption of synbiotic soy-based product supplemented with okara soybean by-product on risk factors for cardiovascular diseases. Food Res. Int. 2015 Jul 1;73:142-148.
- Torres, T.; Sales, R.; Vasconcelos, C.; Martins da Silva, B.; Selores, M. Framingham Risk Score Underestimates Cardiovascular Disease Risk in Severe Psoriatic Patients: Implications in Cardiovascular Risk Factors Management and Primary Prevention of Cardiovascular Disease. J. Dermatol. 2013, 40(11), 923–926. DOI: https://doi.org/10.1111/1346-8138.12267.
- Luís, Â.; Domingues, F.; Pereira, L. Association between Berries Intake and Cardiovascular Diseases Risk Factors: A Systematic Review with Meta-analysis and Trial Sequential Analysis of Randomized Controlled Trials. Food Funct. 2018, 9(2), 740–757.
- Rosdi, I. N.; Selvaraju, K.; Vikram, P.; Thevan, K.; Arifullah, M. Melissopalynological Analysis of Forest Honey from North Malaysia. J Trop Resour Sustain Sci. 2016, 4, 128–132.
- Erejuwa, O.; Nwobodo, N.; Akpan, J.; Okorie, U.; Ezeonu, C.; Ezeokpo, B.; Nwadike, K.; Erhiano, E.; Abdul Wahab, M.; Sulaiman, S.; et al. Nigerian Honey Ameliorates Hyperglycemia and Dyslipidemia in Alloxan-induced Diabetic Rats. Nutrients. 2016, 8(3), 95.
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27(6), 677–689. DOI: https://doi.org/10.1080/07315724.2008.10719745.
- Fuliang, H.; Hepburn, H.; Xuan, H.; Chen, M.; Daya, S.; Radloff, S. Effects of Propolis on Blood Glucose, Blood Lipid and Free Radicals in Rats with Diabetes Mellitus. Pharmacol. Res. 2005, 51(2), 147–152. DOI: https://doi.org/10.1016/j.phrs.2004.06.011.
- Samadi, N.; Mozaffari-Khosravi, H.; Rahmanian, M.; Askarishahi, M. Effects of Bee Propolis Supplementation on Glycemic Control, Lipid Profile and Insulin Resistance Indices in Patients with Type 2 Diabetes: A Randomized, Double-blind Clinical Trial. J. Integr. Med. 2017, 15(2), 124–134. DOI: https://doi.org/10.1016/S2095-4964(17)60315-7.
- Bincoletto, C.; Eberlin, S.; Figueiredo, C. A.; Luengo, M. B.; Queiroz, M. L. Effects Produced by Royal Jelly on Haematopoiesis: Relation with Host Resistance against Ehrlich Ascites Tumour Challenge. Int. Immunopharmacol. 2005, 5(4), 679–688. DOI: https://doi.org/10.1016/j.intimp.2004.11.015.
- Lambrinoudaki, I.; Augoulea, A.; Rizos, D.; Politi, M.; Tsoltos, N.; Moros, M.; Chinou, I.; Graikou, K.; Kouskouni, E.; Kambani, S.; et al. Greek-origin Royal Jelly Improves the Lipid Profile of Postmenopausal Women. Gynecol. Endocrinol. 2016, 32(10), 835–839.
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L. A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015 Dec 1; 4(1):1.
- Higgins, J. P.; Altman, D. G.; Gøtzsche, P. C.; Jüni, P.; Moher, D.; Oxman, A. D.; Savovic, J.; Schulz, K. F.; Weeks, L.; Sterne, J. A. C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. Bmj. 2011, 343(oct18 2), d5928.
- Guo, H.; Saiga, A.; Sato, M.; Miyazawa, I.; Shibata, M.; Takahata, Y.; Morimatsu F. Royal jelly supplementation improves lipoprotein metabolism in humans. J. Nutr. Sci. Vitaminol. 2007;53(4):345–348.
- Morita, H.; Ikeda, T.; Kajita, K.; Fujioka, K.; Mori, I.; Okada, H.; Uno, Y.; Ishizuka, T. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr. J. 2012 Dec 1;11(1):77.
- Mujica, V.; Orrego, R.; Pérez, J.; Romero, P.; Ovalle, P.; Zúñiga-Hernández, J.; Arredondo, M.; Leiva E. The Role of Propolis in Oxidative Stress and Lipid Metabolism: A Randomized Controlled Trial. Evid. Based Complement. Altern. Med. 2017, 2017, 1–11. DOI: https://doi.org/10.1155/2017/4272940.
- Rasad, H.; Dashtabi, A.; Khansari, M.; Chaboksavar, F.; Pahlavani, N.; Maghsoudi, Z.; Entezari, M. H. The Effect of Honey Consumption Compared with Sucrose on Blood Pressure and Fasting Blood Glucose in Healthy Young Subjects. Global J. Med. Res. Studies. 2014, 1(4), 117–121.
- Rasad, H.; Entezari, M. H.; Ghadiri, E.; Mahaki, B.; Pahlavani, N. The Effect of Honey Consumption Compared with Sucrose on Lipid Profile in Young Healthy Subjects (Randomized Clinical Trial). Clin. Nutr. ESPEN. 2018, 26, 8–12.
- Saritas, N.; Yildiz, K.; Büyükipekci, S.; Coskun, B. Effect of Different Levels of Royal Jelly on Biochemical Parameters of Swimmers. Afr. J. Biotechnol. 2011, 10(52), 10718–10723. DOI: https://doi.org/10.5897/AJB11.1862.
- Afsharpour, F.; Javadi, M.; Hashemipour, S.; Koushan, Y. Propolis Supplementation Improves Glycemic and Antioxidant Status in Patients with Type 2 Diabetes: A Randomized, Double-blind, Placebo-controlled Study. Complementary Ther. Med. 2019, 43, 283–288. DOI: https://doi.org/10.1016/j.ctim.2019.03.001.
- Bahrami, M.; Ataie-Jafari, A.; Hosseini, S.; Foruzanfar, M. H.; Rahmani, M.; Pajouhi, M. Effects of Natural Honey Consumption in Diabetic Patients: An 8-week Randomized Clinical Trial. Int. J. Food Sci. Nutr. 2009, 60(7), 618–626. DOI: https://doi.org/10.3109/09637480801990389.
- Fukuda, T.; Fukui, M.; Tanaka, M.; Senmaru, T.; Iwase, H.; Yamazaki, M.; Aoi, W.; Inui, T.; Nakamura, N.; Marunaka, Y.; et al. Effect of Brazilian Green Propolis in Patients with Type 2 Diabetes: A Double-blind Randomized Placebo-controlled Study. Biomed. Rep. 2015, 3(3), 355–360.
- Khoshpey, B.; Djazayeri, S.; Amiri, F.; Malek, M.; Hosseini, A. F.; Hosseini, S.; Shidfar, S.; Shidfar, F. Effect of Royal Jelly Intake on Serum Glucose, Apolipoprotein AI (Apoa-i), Apolipoprotein B (Apob) and ApoB/ApoA-I Ratios in Patients with Type 2 Diabetes: A Randomized, Double-blind Clinical Trial Study. Can. J. Diabetes. 2016, 40(4), 324–328.
- Mobasseri, M.; Pourmoradian, S.; Mahdavi, R.; Faramarzi, E. Effects of Royal Jelly Supplementation on Lipid Profile and High-sensitivity C-reactive Protein Levels in Type-2 Diabetic Women: A Pilot Study. Current Topics Nutraceutical Res. 2014, 12(3), 101–106.
- Mousavi, S. N.; Jazayeri, S.; Khoshpay, B.; Malek, M.; Hosseini, A. F.; Hosseini, S.; Shidfar, F. Royal Jelly Decreases Blood Pressure, Serum Glucose, and Interleukin-6 in Patients with Type 2 Diabetes on an Iso-caloric Diet. J. Nutr. Food Secur. 2017, 2(4), 300–307.
- Pourmoradian, S.; Mahdavi, R.; Mobasseri, M.; Faramarzi, E.; Mobasseri, M. Effects of Royal Jelly Supplementation on Body Weight and Dietary Intake in Type 2 Diabetic Females. Health Promotion Perspect. 2012, 2(2), 231. DOI: https://doi.org/10.5681/hpp.2012.028.
- Sadeghi, F.; Salehi, S.; Kohanmoo, A.; Akhlaghi, M. Effect of Natural Honey on Glycemic Control and Anthropometric Measures of Patients with Type 2 Diabetes: A Randomized Controlled Crossover Trial. Int. J. Preventive Med. 2019, 10. DOI: https://doi.org/10.4103/ijpvm.IJPVM_109_18.
- Zakerkish, M.; Jenabi, M.; Zaeemzadeh, N.; Hemmati, A. A.; Neisi, N. The Effect of Iranian Propolis on Glucose Metabolism, Lipid Profile, Insulin Resistance, Renal Function and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Sci. Rep. 2019, 9(1), 9. DOI: https://doi.org/10.1038/s41598-019-43838-8.
- Sadeghi, F.; Akhlaghi, M.; Salehi, S. Adverse Effects of Honey on Low-density Lipoprotein Cholesterol and Adiponectin Concentrations in Patients with Type 2 Diabetes: A Randomized Controlled Cross-over Trial. J. Diabetes Metab. Disord. 2020, 19(1), 373–380. DOI: https://doi.org/10.1007/s40200-020-00518-z.
- Mousavi, S. N.; Jazayeri, S.; Khoshpay, B.; Malek, M.; Hosseini, A. F.; Hosseini, S.; Shidfar, F. Effects of Brazilian Green Propolis on Proteinuria and Renal Function in Patients with Chronic Kidney Disease: A Randomized, Double-blind, Placebo-controlled Trial. BMC Nephrol. 2019, 20(1), 140.
- Inoue, S.; Kawashima, M.; Hisamura, R.; Imada, T.; Izuta, Y.; Nakamura, S.; Ito, M.; Tsubota, K. Clinical Evaluation of A Royal Jelly Supplementation for the Restoration of Dry Eye: A Prospective Randomized Double Blind Placebo Controlled Study and an Experimental Mouse Model. PloS One. 2017, 12(1), e0169069.
- Chiu, H. F.; Chen, B. K.; Lu, Y. Y.; Han, Y.C.; Shen, Y. C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C. K. Hypocholesterolemic Efficacy of Royal Jelly in Healthy Mild Hypercholesterolemic Adults. Pharm. Biol. 2017, 55(1), 497–502.
- Soleimani, D.; Rezaie, M.; Rajabzadeh, F.; Gholizadeh Navashenaq, J.; Abbaspour, M.; Miryan, M.; Razmpour, F.; Ranjbar, G.; Rezvani, R.; Jarahi, L.; et al. Protective Effects of Propolis on Hepatic Steatosis and Fibrosis among Patients with Nonalcoholic Fatty Liver Disease (NAFLD) Evaluated by Real-time Two-dimensional Shear Wave Elastography: A Randomized Clinical Trial. Phytotherapy Res. 2020. DOI:https://doi.org/10.1002/ptr.6937.
- Yaghoobi, N.; Al-Waili, N.; Ghayour-Mobarhan, M.; Parizadeh, S.; Abasalti, Z.; Yaghoobi, Z.; Yaghoobi, F.; Esmaeili, H.; Kazemi-Bajestani, S. M. R.; Aghasizadeh, R.; et al. Natural Honey and Cardiovascular Risk Factors; Effects on Blood Glucose, Cholesterol, Triacylglycerole, CRP, and Body Weight Compared with Sucrose. Sci. World J. 2008, 8, 463–469. DOI: https://doi.org/10.1100/tsw.2008.64.
- Petelin, A.; Kenig, S.; Kopinč, R.; Deželak, M.; Černelič Bizjak, M.; Jenko Pražnikar, Z. Effects of Royal Jelly Administration on Lipid Profile, Satiety, Inflammation, and Antioxidant Capacity in Asymptomatic Overweight Adults. Evid. Based Complement. Altern. Med. 2019, 2019, 1–11. DOI: https://doi.org/10.1155/2019/4969720.
- Getz, G. S.; Reardon, C. A. Nutrition and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27(12), 2499–2506. DOI: https://doi.org/10.1161/ATVBAHA.107.155853.
- Willix, D.; Molan, P. C.; Harfoot, C. A Comparison of the Sensitivity of Wound‐infecting Species of Bacteria to the Antibacterial Activity of Manuka Honey and Other Honey. J. Appl. Bacteriol. 1992, 73(5), 388–394. DOI: https://doi.org/10.1111/j.1365-2672.1992.tb04993.x.
- Schramm, D. D.; Karim, M.; Schrader, H. R.; Holt, R. R.; Cardetti, M.; Keen, C. L. Honey with High Levels of Antioxidants Can Provide Protection to Healthy Human Subjects. J. Agric. Food Chem. 2003, 51(6), 1732–1735. DOI: https://doi.org/10.1021/jf025928k.
- Erejuwa, O. O.; Sulaiman, S. A.; Ab Wahab, M. S. Honey-a Novel Antidiabetic Agent. Int. J. Bio. Sci. 2012, 8(6), 913. DOI: https://doi.org/10.7150/ijbs.3697.
- Larson-Meyer, D. E.; Willis, K. S.; Willis, L. M.; Austin, K. J.; Hart, A. M.; Breton, A. B, Alexander, B. M. Effect of Honey versus Sucrose on Appetite, Appetite-regulating Hormones, and Postmeal Thermogenesis. J. Am. Coll. Nutr. 2010, 29(5), 482–493.
- Al-Waili, N. S. Effects of Honey on the Urinary Total Nitrite and Prostaglandins Concentration. Int. Urol. Nephrol. 2005, 37(1), 107–111. DOI: https://doi.org/10.1007/s11255-004-0871-8.
- Mehnert, H. Sugar Substitutes in the Diabetic Diet. Internationale Zeitschrift Fur Vitamin-Und Ernahrungsforschung Beiheft. 1976, 15, 295–324.
- Basciano, H.; Federico, L.; Adeli, K. Fructose, Insulin Resistance, and Metabolic Dyslipidemia. Nutr. Metab. 2005, 2(1), 5. DOI: https://doi.org/10.1186/1743-7075-2-5.
- Al-Waili, N. S. Natural Honey Lowers Plasma Glucose, C-reactive Protein, Homocysteine, and Blood Lipids in Healthy, Diabetic, and Hyperlipidemic Subjects: Comparison with Dextrose and Sucrose. J. Med. Food. 2004, 7(1), 100–107. DOI: https://doi.org/10.1089/109662004322984789.
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H. E.; Kaikkonen, J.; Zunft, H.-J. F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The Effect of Polyphenols in Olive Oil on Heart Disease Risk Factors: A Randomized Trial. Ann. Internal Med. 2006, 145(5), 333–341.
- Deyhim, F.; Lopez, E.; Gonzalez, J.; Garcia, M.; Patil, B. S. Citrus Juice Modulates Antioxidant Enzymes and Lipid Profiles in Orchidectomized Rats. J. Med. Food. 2006, 9(3), 422–426. DOI: https://doi.org/10.1089/jmf.2006.9.422.
- Alagwu, E.; Okwara, J.; Nneli, R.; Osim, E. Effect of Honey Intake on Serum Cholesterol, Triglycerides and Lipoprotein Levels in Albino Rats and Potential Benefits on Risks of Coronary Heart Disease. Nig. J. Physiol. Sci. 2011, 26, 161–165.
- Ebbert, J.; Jensen, M. Fat Depots, Free Fatty Acids, and Dyslipidemia. Nutrients. 2013, 5(2), 498–508. DOI: https://doi.org/10.3390/nu5020498.
- Erejuwa, O. O.; Sulaiman, S. A.; Wahab, M. S. A. Fructose Might Contribute to the Hypoglycemic Effect of Honey. Molecules. 2012, 17(2), 1900–1915. DOI: https://doi.org/10.3390/molecules17021900.
- Usman, A. N.; Abdullah, A. Z.; Hakim, B. A.; Hasan, N.; Ariyandi, A. The Effect of Propolis to Blood Glucose and Total Cholesterol of Prediabetes Patients. Proceedings of The Annual International Conference, Syiah Kuala University-Life Sciences & Engineering Chapter, 2013.
- Salatino, A.; Fernandes-Silva, C. C.; Righi, A. A.; Salatino, M. L. F. Propolis Research and the Chemistry of Plant Products. Nat. Prod. Rep. 2011, 28(5), 925–936. DOI: https://doi.org/10.1039/c0np00072h.
- Nina, N.; Quispe, C.; Jiménez-Aspee, F.; Theoduloz, C.; Feresín, G. E.; Lima, B.; Leiva, E.; Schmeda-Hirschmann, G. Antibacterial Activity, Antioxidant Effect and Chemical Composition of Propolis from the Región Del Maule, Central Chile. Molecules. 2015, 20(10), 18144–18167.
- Lee, Y.; Shin, D. H.; Kim, J. H.; Hong, S.; Choi, D.; Kim, Y. J.; Kwak, M. K.; Jung, Y. Caffeic Acid Phenethyl Ester-mediated Nrf2 Activation and IκB Kinase Inhibition are Involved in NFκB Inhibitory Effect: Structural Analysis for NFκB Inhibition. Eur. J. Pharmacol. 2010, 643(1), 21–28.
- Russo, A.; Longo, R.; Vanella, A. Antioxidant Activity of Propolis: Role of Caffeic Acid Phenethyl Ester and Galangin. Fitoterapia. 2002, 73, S21–S9. DOI: https://doi.org/10.1016/s0367-326x(02)00187-9.
- Hao, P. P.; Jiang, F.; Chen, Y. G.; Yang, J.; Zhang, K.; Zhang, M. X.; Zhang, C.; Zhao, Y. X.; Zhang, Y. Traditional Chinese Medication for Cardiovascular Disease. Nat. Rev. Cardiol. 2015, 12(2), 115.
- Hao, P.; Jiang, F.; Cheng, J.; Ma, L.; Zhang, Y.; Zhao, Y. Traditional Chinese Medicine for Cardiovascular Disease: Evidence and Potential Mechanisms. J. Am. Coll. Cardiol. 2017, 69(24), 2952–2966. DOI: https://doi.org/10.1016/j.jacc.2017.04.041.
- Liu, Y.; Liang, X.; Zhang, G.; Kong, L.; Peng, W.; Zhang, H. Galangin and Pinocembrin from Propolis Ameliorate Insulin Resistance in HepG2 Cells via Regulating Akt/mTOR Signaling. Evid. Based Complement. Altern. Med. 2018, 2018, 1–10. DOI: https://doi.org/10.1155/2018/7971842.
- Elissa, L. A.; Elsherbiny, N. M.; Magmomah, A. O. Propolis Restored Adiponectin Level in Type 2 Diabetes through PPARγ Activation. Egypt. J. Basic Appl. Sci. 2015, 2(4), 318–326. DOI: https://doi.org/10.1016/j.ejbas.2015.06.003.
- Lk H, M. K.; Takeshi, T.; Yoshiyuki, K. Anti-obesity Action of Royal Jelly (RJ) on Mice Fed a High-fat Diet. Jap Sci Technolo Agen. 2001, 63, 78–83.
- Kamakura, M.; Moriyama, T.; Sakaki, T. Changes in Hepatic Gene Expression Associated with the Hypocholesterolaemic Activity of Royal Jelly. J. Pharm. Pharmacol. 2006, 58(12), 1683–1689. DOI: https://doi.org/10.1211/jpp.58.12.0017.
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly Inhibits the Production of Proinflammatory Cytokines by Activated Macrophages. Biosci., Biotechnol., Biochem. 2004, 68(1), 138–145.
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major Royal Jelly Protein 3 Modulates Immune Responses in Vitro and in Vivo. Life Sci. 2003, 73(16), 2029–2045.
- Zamami, Y.; Takatori, S.; Goda, M.; Koyama, T.; Iwatani, Y.; Jin, X.; Takai-Doi, S.; Kawasaki, H. Royal Jelly Ameliorates Insulin Resistance in Fructose-drinking Rats. Biol. Pharm. Bull. 2008, 31(11), 2103–2107.